Abstract
By the middle of this century, racial/ethnic minority populations will collectively constitute 50% of the US population. This temporal shift in the racial/ethnic make-up of the US population demands a close look at the race/ethnicity-specific burden of morbidity and premature mortality among childhood cancer survivors. To optimize targeted long-term follow-up care, it is essential to understand whether the burden of morbidity borne by survivors of childhood cancer differs by race/ethnicity. This is challenging because the number of minority participants is often limited in current childhood cancer survivorship research, resulting in a paucity of race/ethnicity-specific recommendations and/or interventions. We show that while the overall childhood cancer incidence increased between 1973 and 2003, the mortality rate declined; however these changes did not differ appreciably by race/ethnicity. We speculate that any racial/ethnic differences in outcome are likely to be multifactorial, and draw upon data from the Childhood Cancer Survivor Study to illustrate the various contributors (socioeconomic characteristics, health behaviors and comorbidities) that could explain any observed differences in key treatment-related complications. Finally, we outline challenges in conducting race/ethnicity-specific childhood cancer survivorship research, showing that there are limited absolute numbers of children who are diagnosed and survive cancer in any one racial/ethnic minority population, precluding a rigorous evaluation of adverse events among specific primary cancer diagnoses and treatment exposure groups.
The past four decades have seen significant temporal shifts in the demographic characteristics of the US population, resulting in the projection that by 2042, the proportion of individuals belonging to a racial/ethnic background other than non-Hispanic white (NHW) will exceed 50%. Race and ethnicity categories (developed in 1997 by the Office of Management and Budget and described in detail in the Supplement) are used to describe groups to which individuals belong or identify with.1 Individuals are asked to designate ethnicity as Hispanic or not Hispanic. With respect to race. Individuals are asked to indicate one or more races that apply mong the following: American Indian or Alaskan, Asian, African American, Pacific Islander and white. The primary driver of recent changes in the racial and ethnic composition of the US population is immigration from Latin America and Asia.2 In fact, US Census data3,4 indicate that the proportion reporting Hispanic origin increased from <5% (1970) to 16% (2010), and the proportion reporting their race as Asian/Pacific Islander increased from 1% (1970) to 5% (2010) (Figure 1A). The population reporting black race on the other hand has been largely static at about 12% over this time period. Furthermore, the greatest increase in the minority population over this period has occurred among children (Figure 1B).3 As race and ethnicity are important determinants of health in the US, these demographic shifts necessitate a close look at the impact of this change in demographics in the US on the health of children. In this position paper we do so in the context of childhood cancer.
Figure 1A. Temporal trends in the US Population by race/ethnicity – Source U.S. Census Bureau.
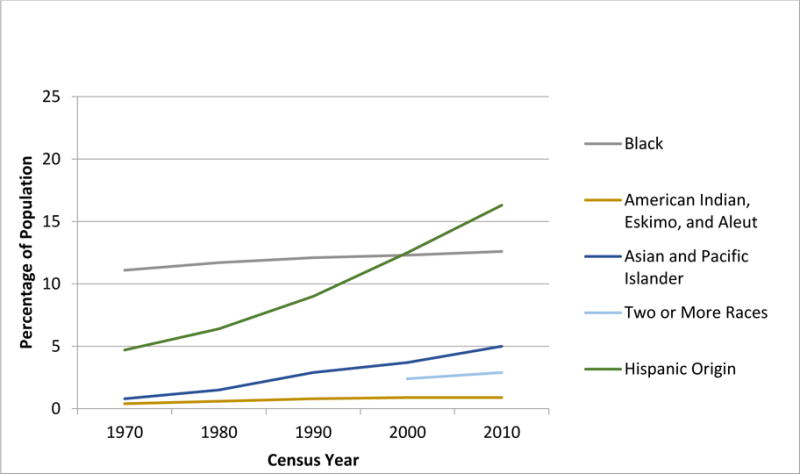
Note: Respondents were able to identify more than one race starting in 2000
Note: Hispanic ethnicity is indicated separately from the race categories (not mutually exclusive)
Figure 1B. Temporal trends in the US Population age 18 and under, by race/ethnicity – Source U.S. Census Bureau.
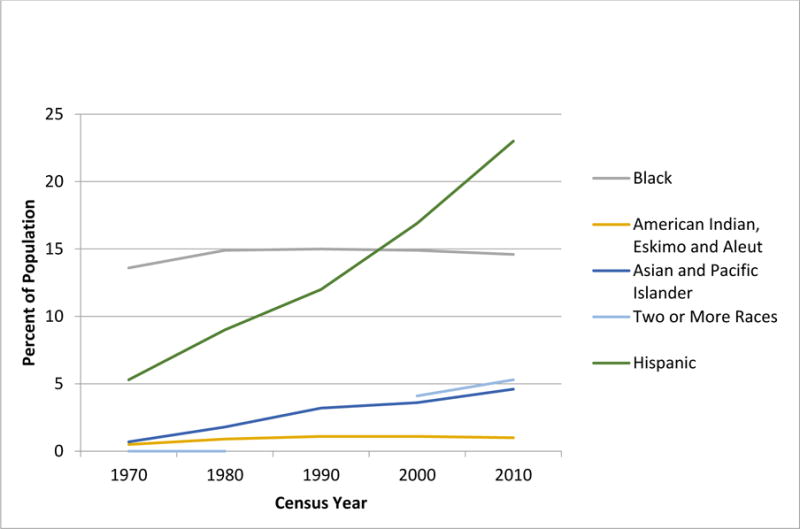
Note: Respondents were able to identify more than one race starting in 2000
Note: Hispanic ethnicity is indicated separately from the race categories (not mutually exclusive)
Five-year survival rates for childhood cancer have improved substantially over the past four decades.5 Unfortunately, the improvement in survival is often accompanied by significant long-term morbidity and premature mortality.6,7 A large clinic-based study demonstrated that the cumulative prevalence of severe/disabling or life-threatening conditions approaches 80% by age 45.8 These chronic health conditions are directly related to treatment of the primary cancer, and place childhood cancer survivors at increased risk of premature death.9,10 Given this high burden of morbidity borne by childhood cancer survivors6,8, the documented racial/ethnic disparity in survival11, and the changing demographics of the US population (Figures 1A, 1B), a close examination of the role of race and ethnicity in long-term cancer outcomes is needed. Unfortunately, this issue has not been addressed adequately, and the paucity of published literature on this topic represents a critical gap since the knowledge gained from survivorship research may not be generalizable to minority populations that are under-represented in published studies. This is particularly important if the burden of morbidity differs by race/ethnicity, because of a need for race/ethnicity-specific recommendations and/or interventions designed to reduce morbidity. Studies addressing these issues are challenging because minority populations are often under-represented in cancer survivorship research. Ideally, a cohort of survivors of childhood cancer with sufficiently large numbers from the various racial/ethnic groups would allow rigorous investigation of race/ethnicity-specific risk for adverse events. If racial/ethnic differences in outcome do exist, then such an investigation would permit an examination of the biologic, socioeconomic and/or therapeutic contributors to observed racial/ethnic differences.
In the following sections, we first highlight racial/ethnic differences in childhood cancer incidence and mortality (using data from the population-based Surveillance Epidemiology and End Results [SEER] program).12 We next explore the potential role for socioeconomic (income, education, insurance) and behavioral (tobacco, alcohol, physical inactivity) contributors as well as the presence of comorbidities, to the racial/ethnic differences in the burden of morbidity and premature mortality using data from the hospital-based Childhood Cancer Survivor Study (CCSS)13. Finally, we outline challenges in conducting this type of research, specifically drawing upon the historical experience of Children’s Oncology Group to illustrate the challenges in constructing cohorts with adequate size of different racial/ethnic groups.
Racial/ethnic Trends in Childhood Cancer Incidence and Mortality in the United States
The SEER registry was used to calculate the racial/ethnic trends in childhood cancer (≤18 years at diagnosis) incidence rates. Age-adjusted incidence rates of childhood cancer14 are shown by race for the period from 1973 to 2012 (Figure 2A), and by ethnicity for the period 1992 to 2012 (Figure 2B). Overall, cancer incidence increased from 1973 to 2012 at a constant rate of 0.7 (95% CI 0.6–0.8) percent per year; the rate of increase did not differ between black and white children. These analyses confirm previous observations that the incidence continues to be highest among white children.14 There were no differences in incidence rates or incidence trends when comparing Hispanic children to non-Hispanic children.15,16 Similar increases in childhood cancer incidence since the 1960s and 1970s have been reported in Europe and other parts of the world.17–20 We also examined trends in race-/ethnicity-specific mortality rates between 1973 and 2003 using the SEER data.12 Similar to previous reports21, we found that mortality rates declined over time, and the rate of decline was similar for children from all racial/ethnic backgrounds (Figures 2A and 2B)14.
Figure 2A. Age-adjusted yearly incidence and mortality rates for children 19 years of age or younger for the period 1973 to 2012 – Source SEER 9 Registry.
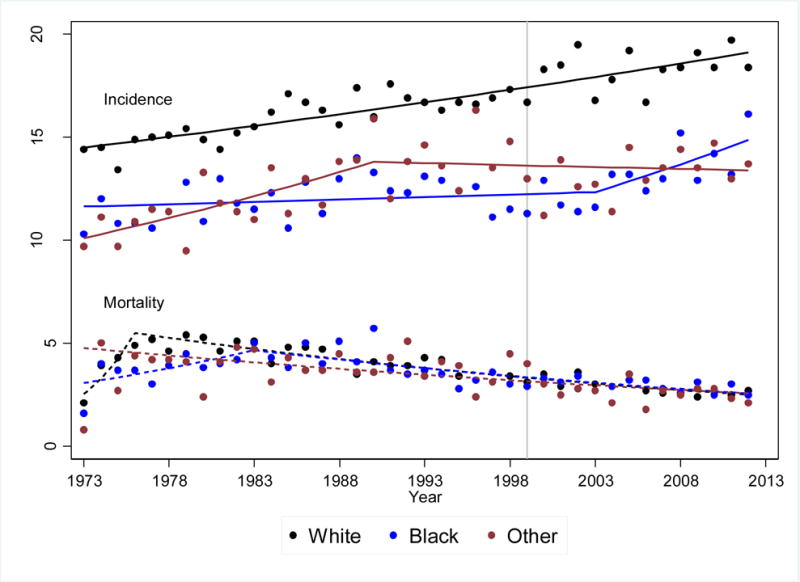
This figure used diagnosis categories (International Classification of Diseases for Oncology v 3 (ICDO-3) codes) consistent with categories reported in the CCSS (Supplemental Table 1).
Figure 2B.
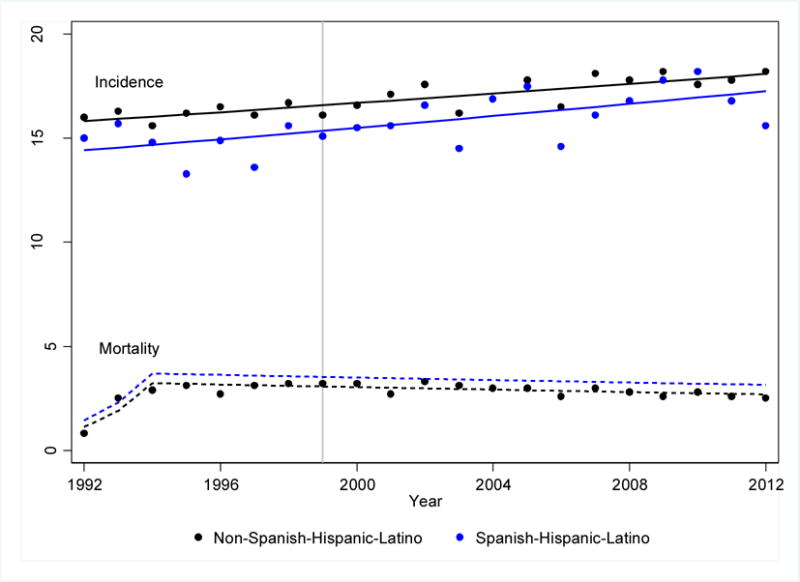
Age-adjusted yearly incidence and mortality rates for children 19 years of age or younger for the period 1973 to 2012 by ethnicity – Source SEER 13 Registry
Contributors to Race/ethnicity-specific Burden of Morbidity in Childhood Cancer Survivors
Understanding the factors that contribute to racial/ethnic differences in the burden of morbidity in childhood cancer survivors would inform targeted follow-up recommendations and risk-reducing interventions. We utilized the expanded CCSS to explore this issue, according to the framework proposed in Figure 3. The CCSS is a retrospective cohort of 35,923 childhood cancer survivors and includes participants diagnosed over three decades, from 1970 to 1999, a time period during which the population distribution of individuals from different racial and ethnic backgrounds in the US changed dramatically.13 Data from this cohort have been used to characterize the burden of morbidity6, premature mortality7,22, and associations between specific therapeutic exposures and key adverse outcomes among childhood cancer survivors10,23. We acknowledge that the CCSS may not be entirely representative of the general US population24, yet it is the single largest cohort of clinically-annotated childhood cancer survivors, and therefore represents a viable option to evaluate race/ethnicity-specific health of long-term survivors. Using the original cohort from CCSS (5+ year survivors of children diagnosed with cancer between 1974 and 1986) we have recently demonstrated that the higher rates of all-cause mortality in NHBs were abrogated after adjusting for SES.25 We also showed that both NHB and Hispanic survivors were more likely to report diabetes and NHBs were more likely to report cardiac conditions; these risks persisted after adjusting for SES and cardiovascular risk factors. However, by and large, NHB and Hispanic childhood cancer survivors experienced a comparable burden of morbidity and mortality to their NHW counterparts. The few differences in risk were explained by racial/ethnic differences in SES and comorbidities.
Figure 3.
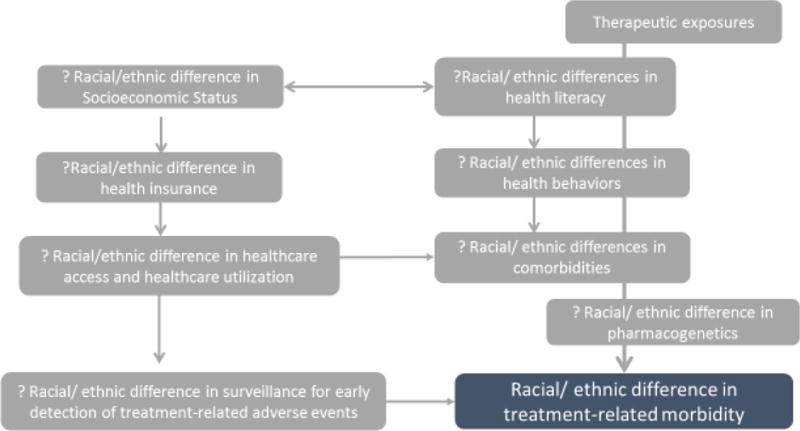
Proposed model for difference in morbidity by race/ethnicity
These findings suggest that potential contributors to racial/ethnic differences in morbidity/mortality experienced by childhood cancer survivors include socioeconomic characteristics (annual household income, education and insurance status), risky health behaviors (tobacco and alcohol use and physical inactivity), patterns of healthcare utilization, and surveillance for long-term toxicities and presence of comorbidities (obesity and hypertension). In this report, drawing upon the expanded CCSS cohort, we use select treatment-related outcomes (diabetes, stroke and all-cause late mortality) to illustrate how these modifiers could help explain the observed racial/ethnic differences.
Risky Health Behaviors
Risky health behavior included tobacco use, alcohol consumption (binge drinking) and physical inactivity. Tobacco use was dichotomized as current/former versus never smoker. Physical activity was evaluated as inactivity versus reporting participation in any physical activity within the last month. Binge drinking was defined as an average of >4 drinks per day for women, or >5 drinks per day for men.
Healthcare Utilization
Healthcare utilization reported by survivors in a 2-year period was examined as the following two outcomes26: (1) general physical examination (how many of the visits to a physician’s office involved a complete physical exam), and (2) cancer-related medical visit (how many of the visits to a physician’s office were related to their previous cancer). These outcomes were not mutually exclusive.
Surveillance for Long-term Toxicities
The Institute of Medicine recommends that all childhood cancer survivors have regular risk-adapted medical care.27 The Children’s Oncology Group (COG) has developed a series of risk-based guidelines for targeted surveillance and early detection of chronic health conditions, with the goal to mitigate long-term morbidity and consequent mortality after cancer.28 As an example, the COG guidelines recommend annual mammograms for early detection of radiation-related breast cancer, starting at age 25 years for girls exposed to chest radiation for an unrelated cancer. COG guidelines also recommend periodic echocardiographic evaluation after anthracycline exposure for early detection of cardiomyopathy. We examined surveillance for screening echocardiogram (yes vs. no/don’t know) and screening mammography (yes vs. no/don’t know) within the last 2 years.
Comorbidities
Self-reported height and weight were used to calculate BMI; survivors with a BMI ≥30 Kg/m2 were categorized as “obese.” Hypertension was self-reported by the study participants.
Logistic regression models were used to estimate odds ratios (OR) and 95% confidence intervals (CI) for the association between race/ethnicity and key mediators/moderators of the racial/ethnic difference in selected outcomes (all-cause mortality, diabetes and stroke). These mediators/moderators included socioeconomic characteristics, risky health behaviors, healthcare utilization, surveillance for long-term outcomes and comorbidities. These models were adjusted for attainted age (effects modeled by natural cubic splines) and sex. For the analyses focusing on the selected outcomes, separate piecewise exponential models were used to assess the effect of race/ethnicity on all-cause mortality rates, rates of diabetes and stroke, adjusting for attainted age and sex, using the logarithm of person-years as the offset, and the effect was quantified as relative rates (RR). Socioeconomic characteristics, risky health behaviors, and, for non-mortality analysis, comorbidities were added to the age- and sex-adjusted model, to examine if they explain, at least partially, the race/ethnicity effects in the age/sex-adjusted model.
The results are detailed in Table 1, along with the variables included in each multivariable model; highlights are summarized here.
Table 1.
Racial/Ethnic differences in Potential Contributors to Long-term Morbidity
| NHW | Hispanics | African Americans | ||||
|---|---|---|---|---|---|---|
| Outcomes* | N, % | OR, 95% CI | N, % | OR, 95% CI | N, % | OR, 95% CI |
| Socioeconomic status | ||||||
| Annual household income <20,000 | 2783, 15.6% | ref | 369, 24.4% | 1.6 (1.5–1.8), p<0.001 | 493, 40.2% | 3.5 (3.1–3.9), p<0.001 |
| Education < high school | 3007, 15.6% | ref | 420, 24.0% | 1.3 (1.1–1.5), p<0.001 | 350, 23.9% | 1.5 (1.3–1.7), p<0.001 |
| With health insurance | 17228, 87.4% | ref | 1201, 73.6% | 0.5 (0.5–0.6), p<0.001 | 1036, 76.5% | 0.7 (0.6–0.8), p<0.001 |
| Risky health behaviors, adjusted for education and income | ||||||
| Current smokers | 2797, 16.4% | ref | 196, 13.5% | 0.6 (0.6–0.8), p<0.001 | 162, 13.1% | 0.5 (0.5–0.6), p<0.001 |
| Alcohol consumption (binge drinking) | 6344, 39.6% | ref | 535, 39.4% | 1.1 (1.0–1.2), p=0.07 | 264, 23.7% | 0.5 (0.5–0.6), p <0.001 |
| Physically inactive# | 4175, 47.4% | ref | 207, 48.5% | 1.0 (0.8–1.2) p=0.8 | 213, 60.0% | 1.6 (1.3–2.0), p <0.001 |
| Healthcare utilization, adjusted for education, income, and insurance | ||||||
| General Physical exam | 12511, 71.8% | ref | 1101, 70.4% | 1.1 (1.0–1.2) p=0.1 | 918, 71.3% | 1.2 (1.0–1.4), p=0.02 |
| Cancer-related care | 7376, 41.7% | ref | 641, 43.6% | 1.0 (0.9–1.1) p=0.8 | 461, 39.1% | 0.7 (0.6–0.8), p <0.001 |
| Surveillance for long-term toxicities, adjusted for education, income, and insurance | ||||||
| Mammography in chest-irradiated women | 1043, 65.0% | ref | 67, 52.3% | 0.9 (0.5–1.4) p=0.6 | 29, 40.3% | 0.6 (0.3–1.1), p=0.09 |
| Echo in anthracycline-exposed survivors | 4845, 79.5% | ref | 503, 78.8% | 1.0 (0.8–1.1) p=0.6 | 365, 75.9% | 0.8 (0.6–0.9), p=0.01 |
| Comorbidities | ||||||
| Obesity | 3735, 19.8% | ref | 426, 25.1% | 1.6 (1.4–1.7), p<0.001 | 381, 26.9% | 1.6 (1.4–1.8), p<0.001 |
| Hypertension | 2906, 14.9% | ref | 234, 13.1% | 1.1 (0.9–1.2), 0.3 | 259, 17.1% | 1.3 (1.2–1.5), p<0.001 |
| Key Outcomes | ||||||
| Diabetes | 647, rate=2.0 | ref | 74, rate=2.9 | 1.7 (1.4–2.1), p<0.001 | 83, rate=3.9 | 2.3 (1.9–2.9), p<0.001 |
| DiabetesΩ | 647, rate=2.0 | ref | 74, rate=2.9 | 1.4 (1.1–1.8), p=0.003 | 83, rate=3.9 | 1.9 (1.5–2.3), p<0.001 |
| Long-term outcomes | ||||||
| Stroke | 382, rate=1.2 | ref | 30, rate=1.2 | 0.9, (0.6–1.3), p=0.6 | 44, rate=2.0 | 1.9, (1.4–2.5), p=0.01 |
| StrokeΩ | 382, rate=1.2 | ref | 30, rate=1.2 | 0.8, (0.5–1.1), p=0.2 | 44, rate=2.0 | 1.5, (1.1–2.0), p=0.01 |
| Mortality† | ||||||
| All-cause late mortality | 2087, rate=6.5 | ref | 160, rate=6.6 | RR=1.0, 95% CI, 0.9–1.2, p=0.9 | 163, rate=7.7 | RR=1.2, 95% CI, 1.0–1.4, p=0.01 |
| All-cause late mortalityδ | 2087, rate=6.5 | ref | 160, rate=6.6 | RR=0.9, 95%CI, 0.8–1.0, p=0.1 | 163, rate=7.7 | RR=1.0, 95% CI, 0.8–1.1, p=0.7 |
Besides the adjustments specified in the table, all models additionally adjusted for age and sex.
Used only original cohort who had detailed measurement on physical activity in Fu2003 and Fu2007.
Rate=number of events per 1000 person years, RR=rate ratio.
adjusted for socioeconomic characteristics, risky health behaviors, healthcare utilization and obesity
adjusted for socioeconomic characteristics, health behaviors, and healthcare utilization
Socioeconomic Characteristics
When compared with NHWs, non-Hispanic black (NHB) and Hispanic survivors from CCSS were more likely to report lower income (NHBs: OR=3.5, 95%CI, 3.1–3.9; Hispanics: OR=1.6, 95%CI, 1.5–1.8) and lower education (NHBs: OR=1.5, 95%CI, 1.3–1.7; Hispanics: OR=1.3, 95%CI, 1.1–1.5). Further, NHBs and Hispanics were less likely to have health insurance coverage when compared with NHW survivors (NHBs: OR=0.7, 95%CI, 0.6–0.8; Hispanics: OR=0.5, 95%CI, 0.5–0.6).
Risky Health Behaviors
NHB (OR=0.5, 95%CI, 0.5–0.6) and Hispanic (OR=0.6, 95%CI, 0.6–0.8, p<0.001) survivors were less likely to be current smokers, when compared with NHW survivors. NHBs were less likely to report binge drinking (OR=0.5, 95%CI, 0.5–0.6), but Hispanic survivors were as likely (OR=1.1, 95%CI, 1.0–1.2) as NHW survivors. While NHB survivors were more likely to be physically inactive (OR=1.6, 95%CI, 1.3–2.0), Hispanics reported similar levels of physical activity (OR=1.0, 95%CI, 0.8–1.2) as NHWs.
Healthcare Utilization
NHBs were more likely to report general physical examinations (OR=1.2, 95%CI, 1.0–1.4), but less likely to report cancer-related care (OR=0.7, 95%CI, 0.6–0.8) when compared with NHWs. Hispanic cancer survivors, however, were equally as likely to receive general physical examinations (OR=1.1, 95%CI, 1.0–1.2) and cancer-related care (OR=1.0, 95%CI, 0.9–1.1) as NHWs.
Surveillance for Long-Term Toxicities
Among chest-irradiated female survivors from the CCSS who were 25 years of age or older, the odds of reporting a mammographic evaluation were lower, but not statistically significantly different for NHBs (OR=0.6, 95%CI, 0.3–1.1, p=0.09), or Hispanics (OR=0.9, 95%CI, 0.5–1.4, p=0.6) when compared with NHWs. Among anthracycline-exposed childhood cancer survivors, odds of having an echocardiographic screening evaluation relative to NHW survivors were lower for NHBs (OR=0.8, 95%CI, 0.6–0.9), but comparable for Hispanics (OR=1.0, 95%CI, 0.8–1.1).
Comorbidities
NHBs (OR=1.6, 95%CI, 1.4–1.8, p<0.001) and Hispanics (OR=1.6, 95%CI, 1.4–1.7, p<0.001) were more likely to be obese, when compared with NHWs. NHBs were more likely to report hypertension (OR=1.3, 95%CI 1.2–1.5, p<0.001), but odds of reporting hypertension were comparable between Hispanics and NHWs (OR=1.1, 95%CI, 0.9–1.2, p=0.3).
Role of Modifiers/Mediators in Racial/ethnic Difference to Key Outcomes
In an analysis adjusted for age and sex, NHB and Hispanic survivors were more likely to report diabetes (NHBs: RR=2.3, 95%CI, 1.9–2.9, p<0.001; Hispanics: RR=1.7, 95%CI, 1.4–2.1, p<0.001) when compared with NHW survivors. Inclusion of socioeconomic characteristics, health behaviors, healthcare utilization and comorbidities mitigated the magnitude of this association slightly (NHBs, OR=1.9, 95%CI, 1.5–2.3, p<0.001; Hispanics: OR=1.4, 95%CI, 1.1–1.8, p=0.003). In an analysis adjusted for age and sex, NHB survivors were more likely to report having had stroke (RR=1.9, 95%CI, 1.4–2.5, p<0.01). Inclusion of socioeconomic characteristics, health behaviors, healthcare utilization and comorbidities mitigated the difference (OR=1.5, 95%CI, 1.1–2.0, p=0.01).
Late Mortality
The age- and sex-adjusted risk of all-cause mortality in the CCSS population was 1.2-fold higher among NHBs (RR=1.2, 95%CI, 1.0–1.4, p=0.01) when compared with NHWs. However, adjustment for socioeconomic characteristics, health behaviors, and healthcare utilization abrogated the difference in mortality (RR=1.0, 95%CI, 0.8–1.1, p=0.7). The age- and sex-adjusted risk of premature death for Hispanic childhood cancer survivors was comparable to that observed among the NHW survivors (RR=0.9, 95%CI, 0.8–1.0, p=0.1).
The racial/ethnic differences observed in the CCSS cohort are aligned with those observed in the general population in US. Thus, NHBs and Hispanics are more likely to live in poverty29 and have lower levels of educational attainment30. The prevalence of current use of a tobacco product was 21.9% for Hispanics, 27.3% for NHBs and 29.5% for NHWs.31 NHWs are generally more likely than other racial/ethnic groups to report current use of alcohol than NHBs or Hispanics.32 In the general population, NHBs are more likely, but Hispanics are less likely to receive preventive screening, when compared with NHWs.33
With respect to treatment-related outcomes, NHB survivors were more likely to report diabetes and stroke when compared with NHWs, and adjustment for the variables listed above, there was mitigation of the magnitude of excess risk among NHBs, showing that the excess risk is at least partially attributed to these modifiers/mediators. The excess late mortality observed among NHBs was also abrogated after adjustment of key these moderators/mediators. Hispanic survivors were more likely to report diabetes and the magnitude of this risk was mitigated after adjustment for the moderators/mediators listed above.
Examination of the CCSS data highlights racial/ethnic differences in key contributors (moderators/mediators) to long-term morbidity in childhood cancer survivors, and demonstrates that the racial/ethnic differences in these contributors help explain (in part) the observed differences in long-term morbidity by race/ethnicity. These analyses were not aimed to be comprehensive or all-encompassing; instead, this exercise serves as a demonstration of both modifiable and some non-modifiable contributors to racial/ethnic differences in outcomes that need to be addressed when we look to mitigate disparities in long-term cancer outcomes. Another important issue that we have not yet addressed is the racial/ethnic differences in the molecular underpinnings of long-term treatment toxicities. In the general population, race/ethnicity-specific response to β-blockers and ACE inhibitors for hypertension are perhaps the mostly widely-recognized examples.34 Among childhood cancer survivors, it is increasingly recognized that for a given therapeutic exposure, heterogeneity exists in the prevalence and severity of adverse outcomes. Emerging data suggest that genetic susceptibility could play a role in modifying individual response to therapeutic exposures.35–38 While understanding the molecular underpinning of treatment-related adverse events will allow a better understanding of the pathogenesis of these life-threatening complications, it will be equally important to explore whether any racial/ethnic differences exist in the frequencies of genetic variants that would place a sub-population at a particularly high risk of complications, potentially impacting long-term survival.
Challenges to Establishment of Minority Survivor Populations
The CCSS cohort consists largely of NHWs (82.1%), with NHBs (6.3%), Hispanics (7.4%) and others (4.2%) constituting a small minority. With the growing racial/ethnic diversity of the US population, it is imperative to understand whether lessons learned from the CCSS can be applied to racial/ethnic minority populations. Major challenges exist in establishment of large, racially/ethnically diverse cohorts of childhood cancer survivors that would facilitate detailed evaluation of race/ethnicity-specific health-related outcomes. These challenges include: 1) availability of a population of sufficient size to evaluate race/ethnicity-specific outcomes within specific cancer diagnoses and treatment exposure groups; and 2) the relatively lower rates of participation experienced in cohort studies for certain minority populations compared to NHWs.
Availability of a Large Representative Sample of Childhood Cancer Survivors
While the number of individuals from racial/ethnic minority populations as an aggregate is approaching that of NHWs in the general population, research on long-term survivors of childhood cancer is inherently dependent on cancer incidence and survival among minorities in previous eras. CCSS has enrolled five-year survivors of childhood cancer diagnosed over three decades (1970–1999). Using SEER data (Table 2) for children ages 0 to 20 years,39 we estimate that 89,540 white children in the US were diagnosed with cancer in the 1970s, compared to 11,851 black children.
Table 2.
Estimated number of children ages 0–20 years in the US population, with cancer, and with cancer death by race and ethnicity and by decade
| 1970–1979 | 1980–1989 | 1990–1999 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| US Populationa | Cancerb | Cancer Deathc | US Populationd | Cancerb | Cancer Deathc | US Populationd | Cancerb | Cancer Deathc | |
| Race (N, %) | |||||||||
| White | 635,038,817 84.1% |
89,540 87.0% |
27,942 86.8% |
575,951,922 81.1% |
91,576 84.8% |
26,494 82.2% |
600,975,673 79.0% |
100,363 83.9% |
16,827 80.2% |
| Black | 105,813,966 14.0% |
11,851 11.5% |
3,703 11.5% |
106,891,549 15.1% |
13,041 12.0% |
4,703 14.6% |
120,653,352 15.9% |
14,358 12.1% |
3,137 14.9% |
| American Indian | – | – | – | – | – | – | 9,497,426 1.3% |
950 0.8% |
180 0.9% |
| Asian | – | – | – | – | – | – | 29,121,923 3.8% |
3,961 3.3% |
845 4.0% |
| Otherf | 14,650,538 1.9% |
1,538 1.5% |
542 1.7% |
26,897,675 3.8% |
3,335 3.1% |
1,049 3.3% |
– | – | – |
| Hispanic Ethnicity (N, %) | |||||||||
| Yes | NAe | 337 5.0% |
NAe | 75,059,415 10.6% |
670 6.5% |
NAe | 108,426,466 14.3% |
15,939 13.1% |
3,361 16.0% |
| No | NAe | 6375 95.0% |
NAe | 634,681,801 89.4% |
9543 93.5% |
NA3 | 651,821,908 85.7% |
105,595 86.9% |
17,599 84.0% |
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Populations — Total U.S. (1969–2009) <Katrina/Rita Adjustment> — Linked To County Attributes — Total U.S., 1969–2009 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released November 2010.
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence — SEER 9 Regs Research Data (with SEER Delay Factors), Nov 2013 Sub (1973–2011) <Katrina/Rita Population Adjustment> — Linked To County Attributes — Total U.S., 1969–2012 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014, based on the November 2013 submission.
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Mortality — Al l COD, Aggregated With State, Total U.S. (1969–2011) <Katrina/Rita Population Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released July 2014. Underlying mortality data provided by NCHS (www.cdc.gov/nchs).
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Populations — Total U.S. (1981–2012), State-Level, by Expanded Race <Single Ages to 85+, Katrina/Rita Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released December 2013.
Population or SEER data for Ethnicity (Hispanic yes.no) not available (NA)
Includes American Indian, Alaska Native, Asian/Pacific Islander until 1990
NE=Not estimated.
In the 1990s, the absolute number of white children diagnosed with cancer was estimated to be 100,363 in contrast to 14,358 black children. Knowledge of the true number of Hispanic survivors across this era is restricted by the lack of available data for Hispanics. In the 1990’s, there were an estimated 15,939 Hispanic children diagnosed with cancer. Thus, the primary barrier to race/ethnicity-specific research among aging adult survivors of childhood cancer is the limited absolute number of children who were diagnosed and survived cancer in any one racial/ethnic minority population, precluding a rigorous evaluation of adverse events among specific primary cancer diagnoses and treatment exposure groups.
With the existing gaps in knowledge regarding long-term health outcomes among racial/ethnic minority survivors, the CCSS has considered available options to enrich the cohort for minority participants. One approach considered was to expand ascertainment of minority survivors beyond the existing CCSS hospital-base by establishing a distinct minority cohort. The feasibility of recruiting a minority cohort comparable to the recently completed CCSS cohort expansion population (five-year survivors, diagnosed between 1987 and 1999) was evaluated. Applying SEER rates to the US population, it is estimated that 18,047 NHB children and 19,783 Hispanic children were diagnosed during this period (Table 3). Assuming a 70% survival and a 55% participation rate, approximately 7,000 NHB survivors and 7,600 Hispanic survivors could potentially be enrolled into a nationwide cohort. Unfortunately, as contact information for persons in the SEER registry (or for those estimated in the general population from the SEER registry) is not readily available, this approach was considered to be logistically prohibitive, and we turned our attention to consideration of alternative approaches.
Table 3.
Estimates of the potential size of a childhood 5-year cancer survivor minority cohort diagnosed and treated between 1987–1999: population-based, SEER-based and Children’s Oncology Group-based estimates
| Population-based Cohort | |||||
|---|---|---|---|---|---|
| Diagnosed in US 1987–1999 | Estimate 70% survival | Estimate 65% Participation In Minority Cohort |
Estimate 55% Participation In Minority Cohort |
||
| Racea,b | |||||
| Black | 18,047 | 12,633 | 8,211 | 6,948 | |
| American Indian | 1,183 | 828 | 538 | 455 | |
| Asian/Pacific Islander | 4,912 | 3,438 | 2,235 | 1,891 | |
| Ethnicitya,c | |||||
| Hispanic | 19,783 | 13,848 | 9,001 | 7,616 | |
| SEER-based Cohortd | |||||
| Diagnosed in US 1987–1999 | Know five-year Survivors | Estimate 65% Participation In Minority Cohort |
Estimate 55% Participation In Minority Cohort |
||
| Race | |||||
| Black | 1,578 | 1,099 | 714 | 604 | |
| American Indian | 172 | 117 | 76 | 65 | |
| Asian/Pacific Islander | 1,155 | 836 | 543 | 460 | |
| Unknown | 129 | 121 | 79 | 66 | |
| Ethnicity | |||||
| Hispanic | 1,440 | 1,055 | 686 | 580 | |
| COG-based Cohort | |||||
| Diagnosed in US 1987–1999 | Estimate 70% survival | Estimate 65% Participation In Minority Cohort |
Estimate 55% Participation In Minority Cohort |
||
| Race | |||||
| Black | 5,446 | 3,812 | 2,478 | 2,096 | |
| American Indian | 219 | 153 | 100 | 84 | |
| Asian/Pacific Islander | 1219 | 853 | 555 | 470 | |
| Unknown Race | 19,147 | ||||
| Ethnicity | |||||
| Hispanic | 5,731 | 4,011 | 2,607 | 2,206 | |
| Unknown Ethnicity | 9,591 | ||||
Race and ethnicity specific rates for 1992–1999 are from the following: Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence — SEER 13 Regs Research Data, Nov 2013 Sub (1992–2011) <Katrina/Rita Population Adjustment> — Linked To County Attributes — Total U.S., 1969–2012 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014, based on the November 2013 submission.
Race specific population estimates for 1987–1999 for persons < 20 years of age are from: Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Populations — Total U.S. (1981–2011), State-Level, by Expanded Race <Single Ages to 85+, Katrina/Rita Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released October 2012.
Ethnicity specific population estimates for 1987–1999 for persons < 20 years of age are from: Surveillance, Epidemiology, and End Results (SEER) Program www.seer.cancer.gov) SEER*Stat Database: Populations — Total U.S. (1981–2012), State-Level, by Race (White, Non-White)/Ethnicity <Single Ages to 85+, Katrina/Rita Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released December 2013.
SEER Counts for 1987–1999 are from the following: Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence — SEER 9 Regs Research Data (with SEER Delay Factors), Nov 2013 Sub (1973–2011) <Katrina/Rita Population Adjustment> — Linked To County Attributes — Total U.S., 1969–2012 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014, based on the November 2013 submission.
COG and its legacy groups have been the primary resource for clinical trials in childhood cancer since the 1970s. Review of COG clinical trial enrollment between 1987 and 1999 identified 5,446 NHB and 5,731 Hispanic children, but also a very high number (n=19,147) of patients with unknown or missing race/ethnicity (Table 3). Taking into account survival (70%) and participation (55%) in a long-term follow-up study, we estimated that only 2,096 NHB and 2,206 Hispanic participants could be successfully enrolled in a minority survivor cohort; a proportion of these survivors are already participating in CCSS. Considering the high cost of establishing/expanding a cohort, and the modest number of additional patients from racial/ethnic minorities that could be added, expansion through COG could not be justified. For example, such a cohort would yield less than 150 NHB CNS tumors survivors who received cranial radiotherapy and less than 140 Hispanic HL survivors who received chest-directed radiotherapy, limiting evaluation of dose-response effects for these high risk populations.
Based on the above, we conclude that the limited number of available survivors from specific racial/ethnic minority groups is a major barrier to the conduct of outcomes research comparable to what can be and is being carried-out with the larger existing number of NHW survivors. Nonetheless, research among minority survivor populations should continue to be a priority, while recognizing the limitations imposed by sample size and the accompanying statistical power.
Minority Populations and Participation Bias
Among aging survivors of childhood cancer it is plausible that participants in long-term follow-up research studies may not represent the larger underlying population of survivors in the US, potentially providing biased estimates of rates and risks that are not generalizable to all survivors. There is contradictory evidence related to differences in participation rates in observational studies by race/ethnicity.40–43 Differences in recruitment rates vary widely based on the methodological approaches;42 higher minority participation rates are observed where specific strategies are used to maximize minority participation.41,42,44
To further understand how race/ethnicity-specific participation rates within the CCSS cohort compared to other large NIH-funded observational research studies, we reviewed the literature describing forty-three studies and found that few cohorts describe race/ethnicity-specific participation rates (Table 4). While methodological approaches varied widely, community-based recruitment approaches had the capacity to achieve >70% minority participation, rates comparable to those of NHWs.42–83 However, the Multi-Ethnic Cohort, which utilized survey methodology (i.e., US Mail) for recruitment and survey distribution, demonstrated low overall response among minorities (Hispanic 17%, NHBs, 21%) and reduced rates of participation relative to whites (34%).44 By comparison, CCSS, which augments US mail distribution with phone follow-up and phone- and web-based survey completion has achieved superior participation rates (Hispanics: 67%, NHBs: 57% and NHWs: 70%). For many studies description of demographic and treatment-related factors associated with non-participation is not complete because characteristics of non-participants are not fully known, compounding the inability to comprehensively assess for participation bias. To further explore participation rates specifically among studies of cancer survivors that are more comparable to the CCSS, we reviewed NIH-sponsored, survey-based outcome studies restricted to survivors of adult and/or pediatric cancer (Table 4).42–45 In general, these studies had low overall participation rates and, in most instances, even larger gaps in participation between minority and non-minority survivors compared to those observed by CCSS.
Table 4.
Participation rates by race and ethnicity among selected NIH-funded cohorts and survey-based cancer survivor studies
| Participation rates by race and ethnicity among NIH funded cohort studies* | ||||||||
|---|---|---|---|---|---|---|---|---|
| Recruitment Methodology | Eligible (N) | Participating (N, % of Eligible) | ||||||
| Cohort size | Hispanic | Non-Hispanic white | Non-Hispanic black | Asian/Pacific Islander | Native American | |||
| Childhood Cancer Survivor Study (CCSS, Expanded Cohort) | US Mail/phone | 15,233 | 10,004 (66%) | 1,063 (67%) | 7615 (70%) | 842 (57%) | 198 (61%) | – |
| The Multiethnic Cohort (MEC)44 | US Mail | 854,613 | 218,780 (30%) | 281,990 (17%) | 136,142 (34%) | 170,694 (21%) | 137,812 (44%) | – |
| Adventist Health Study 2 (AHS-2)45,46 | Community-based | 350,000 | 96,163 (28%) | – | 71,865 (28%) | 25,087 (28%) | – | – |
| Genetic and Epidemiology Study of Cardiovascular Disease in Alaska Natives (GOCADAN)47,48 | Community-based | 7,7000 | 1,214 (74%) | – | – | – | – | 1,214 (74%) |
| Jackson Heart Study (JHS)49 | Community-based | 76,420 | 5,302 (51%) | – | – | 5,302 (51%) | – | – |
| National Health and Nutrition Examination Survey II (NHANES II)50 | 27,801 | 25,286 (91%) | – | 21,359 (91%) | 3,389 (93%) | – | – | |
| National Health and Nutrition Examination Survey III (NHANES III)51 | 39.695 | 33,994# (82%) | 9,751 (87%) | 13,085 (80%) | 9,627 (86%) | – | – | |
| Strong Heart Study (SHS, American Indian)52 | Community-based | 7,305 | 4,529# (62%) | – | – | – | – | 4,529# (62%) |
| Participation rates by race and ethnicity among NIH funded, survey-based cancer survivor studiesˆ | ||||||||
| Recruitment Methodology | Eligible (N) | Participating (N, % of Eligible) | ||||||
| TOTAL | Hispanic | White, Non-Hispanic | Black, Non-Hispanic | Asian/Pacific Islander | Native American | |||
| Childhood Cancer Survivor Study (CCSS, Expanded Cohort) | US Mail/phone | 15,233 | 10,004 (66%) | 1,063 (67%) | 7615 (70%) | 842 (57%) | 198 (61%) | – |
| Project Forward Study53 | US Mail | 470 | 235 (50%) | 121 (49%) | 80 (58%) | – | – | – |
| Adolescent and Young Adult Health Outcomes and Patient Experience Study (AYA-HOPE)54 | US Mail/phone/internet | 1,208 | 524 (43%) | 97 (35%) | 331 (47%) | 42 (38%) | – | – |
| Experience of Care and Health Outcomes of Survivors of Non-Hodgkin Lymphoma (ECHOS-NHL)55 | US Mail | 744 | 408 (55%) | 89 (45%) | 290 (59%) | 29 (49%) | – | – |
| Health, Eating, Activity and Lifestyle Study (HEAL)56 | US Mail/In-person Interview | 3,070 | 856 (29%) | 429 (37%) | 671 (27%) | 1 (2%) | 14 (19%) | |
Clearly, strategies that maximize overall participation and reduce differential participation by race/ethnicity are needed. Recruitment materials that encourage minority participation, provision of surveys in Spanish and other languages, and use of race/ethnicity-specific telephone interviewers that are culturally-sensitive may improve participation. However, thus far, only use of incentives has shown an improvement in participation rates among racial/ethnic minority populations in survey-based research.84 Further research to identify effective strategies is certainly needed.
In summary, by the middle of this century, racial/ethnic minority populations will constitute ~50% of the US population. This temporal shift demands a close look at race/ethnicity-specific burden of morbidity and mortality among childhood cancer survivors. Ideally, a large cohort of minority survivors of childhood cancer would allow rigorous investigation of race/ethnicity-specific risk factors for adverse events. Large cohorts, enriched for minority populations, are difficult to construct because of smaller absolute numbers of eligible individuals and lower participation rates. These challenges notwithstanding, there remains a need to employ innovative strategies to improve participation rates in order to ensure adequate representation of survivors from the various racial/ethnic backgrounds. It is equally as important to ensure that all possible contributors of racial/ethnic differences in outcomes are measured adequately, such that targeted interventions can be developed to mitigate these differences.
Supplementary Material
Acknowledgments
Supported in part by Grant No. CA55727 from the National Cancer Institute
Footnotes
Author Contribution: The statistical analyses were conducted by TMG, KKN, QL, YY and SB conducted or supervised data. All coauthors contributed to the interpretation of the analysis and creation of this position paper.
Conflicts of Interest: No conflicts of interest were identified for any of the authors.
References
- 1.Colby SL. Projections of the size and composition of the U.S. Population: 2014 to 2060. United States Census Bureau; 2015. [Google Scholar]
- 2.Perez AD, Hirschman C. The Changing Racial and Ethnic Composition of the US Population: Emerging American Identities. Popul Dev Rev. 2009;35:1–51. doi: 10.1111/j.1728-4457.2009.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hobbs F, Stoops N. Demographic Trends in the 20th Century. Washington, DC: U.S. Government Printing Office; 2002. (Census 2000 Special Reports USCB, Series CENSR-4). [Google Scholar]
- 4.2010 Census Briefs. Overview of Race and Hispanic Origin 2010. at www.census.gov/library/publications/2011/dec/c2010br-02.html.)
- 5.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 6.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 7.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–79. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–81. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inskip PD, Robison LL, Stovall M, et al. Radiation dose and breast cancer risk in the childhood cancer survivor study. J Clin Oncol. 2009;27:3901–7. doi: 10.1200/JCO.2008.20.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neglia JP, Robison LL, Stovall M, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98:1528–37. doi: 10.1093/jnci/djj411. [DOI] [PubMed] [Google Scholar]
- 11.Bhatia S. Disparities in cancer outcomes: lessons learned from children with cancer. Pediatr Blood Cancer. 2011;56:994–1002. doi: 10.1002/pbc.23078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Surveillance, Epidemiology, and End Results Program. at http://seer.cancer.gov/registries/terms.html.)
- 13.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–18. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Cancer Institute SEER*Stat software. at seer.cancer.gov/seerstat.)
- 15.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 16.Joinpoint Regression Program, Version 4.2.0. National Cancer Institute; 2015. [Google Scholar]
- 17.Baade PD, Youlden DR, Valery PC, et al. Trends in incidence of childhood cancer in Australia, 1983–2006. Br J Cancer. 2010;102:620–6. doi: 10.1038/sj.bjc.6605503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stiller CA, Marcos-Gragera R, Ardanaz E, et al. Geographical patterns of childhood cancer incidence in Europe, 1988–1997 Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:1952–60. doi: 10.1016/j.ejca.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Kaatsch P, Steliarova-Foucher E, Crocetti E, Magnani C, Spix C, Zambon P. Time trends of cancer incidence in European children (1978–1997): report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:1961–71. doi: 10.1016/j.ejca.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Steliarova-Foucher E, Stiller C, Kaatsch P, et al. Geographical patterns and time trends of cancer incidence and survival among children and adolescents in Europe since the 1970s (the ACCISproject): an epidemiological study. Lancet. 2004;364:2097–105. doi: 10.1016/S0140-6736(04)17550-8. [DOI] [PubMed] [Google Scholar]
- 21.Linabery AM, Ross JA. Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975–1999. Cancer. 2008;113:2575–96. doi: 10.1002/cncr.23866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armstrong GT, Chen Y, Yasui Y, et al. Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer. N Engl J Med. 2016 doi: 10.1056/NEJMoa1510795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatti P, Veiga LH, Ronckers CM, et al. Risk of second primary thyroid cancer after radiotherapy for a childhood cancer in a large cohort study: an update from the childhood cancer survivor study. Radiat Res. 2010;174:741–52. doi: 10.1667/RR2240.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24:653–63. doi: 10.1158/1055-9965.EPI-14-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Q, Leisenring W, Ness KK, et al. Racial/ethnic differences in adverse outcomes among childhood cancer survivors: The Childhood Cancer Survivor Study. J Clin Oncol. 2016 doi: 10.1200/JCO.2015.66.3567. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oeffinger KC, Mertens AC, Hudson MM, et al. Health care of young adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Fam Med. 2004;2:61–70. doi: 10.1370/afm.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hewitt M, Weiner SL, Simone JV, editors. Childhood Cancer Survivorship: Improving Care and Quality of Life. Washington (DC): 2003. [PubMed] [Google Scholar]
- 28.Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. 2014 Version 4.0. at http://www.survivorshipguidelines.org.)
- 29.Statistics. With Chartbook on trends in the Health of Americans; 2006. [Google Scholar]
- 30.Bureau USC. Current Population Survey. Annual Social and Economic Supplement. 2003 [Google Scholar]
- 31.Prevention CfDCa. The health consequences of smoking: A report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2004. [PubMed] [Google Scholar]
- 32.Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: HHS Publication No. (SMA) 11-4658; 2011. (NSDUH Series H-41). [Google Scholar]
- 33.Corbie-Smith G, Flagg EW, Doyle JP, O’Brien MA. Influence of usual source of care on differences by race/ethnicity in receipt of preventive services. J Gen Intern Med. 2002;17:458–64. doi: 10.1046/j.1525-1497.2002.10733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson JA. Ethnic differences in cardiovascular drug response: potential contribution of pharmacogenetics. Circulation. 2008;118:1383–93. doi: 10.1161/CIRCULATIONAHA.107.704023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Best T, Li D, Skol AD, et al. Variants at 6q21 implicate PRDM1 in the etiology of therapy-induced second malignancies after Hodgkin’s lymphoma. Nat Med. 2011;17:941–3. doi: 10.1038/nm.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhatia S. Genetic variation as a modifier of association between therapeutic exposure and subsequent malignant neoplasms in cancer survivors. Cancer. 2015;121:648–63. doi: 10.1002/cncr.29096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blanco JG, Sun CL, Landier W, et al. Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes–a report from the Children’s Oncology Group. J Clin Oncol. 2012;30:1415–21. doi: 10.1200/JCO.2011.34.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Liu W, Sun CL, et al. Hyaluronan synthase 3 variant and anthracycline-related cardiomyopathy: a report from the children’s oncology group. J Clin Oncol. 2014;32:647–53. doi: 10.1200/JCO.2013.50.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2011. Bethesda, MD: National Cancer Institute; 2014. [Google Scholar]
- 40.Klein DJ, Elliott MN, Haviland AM, et al. Understanding nonresponse to the 2007 Medicare CAHPS survey. Gerontologist. 2011;51:843–55. doi: 10.1093/geront/gnr046. [DOI] [PubMed] [Google Scholar]
- 41.Ofstedal MB, Weir DR. Recruitment and retention of minority participants in the health and retirement study. Gerontologist. 2011;51(Suppl 1):S8–20. doi: 10.1093/geront/gnq100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sykes LL, Walker RL, Ngwakongnwi E, Quan H. A systematic literature review on response rates across racial and ethnic populations. Can J Public Health. 2010;101:213–9. doi: 10.1007/BF03404376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wendler D, Kington R, Madans J, et al. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2006;3:e19. doi: 10.1371/journal.pmed.0030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steffen AD, Kolonel LN, Nomura AM, Nagamine FS, Monroe KR, Wilkens LR. The effect of multiple mailings on recruitment: the Multiethnic Cohort. Cancer Epidemiol Biomarkers Prev. 2008;17:447–54. doi: 10.1158/1055-9965.EPI-07-2576. [DOI] [PubMed] [Google Scholar]
- 45.Butler TL, Fraser GE, Beeson WL, et al. Cohort profile: The Adventist Health Study-2 (AHS-2) Int J Epidemiol. 2008;37:260–5. doi: 10.1093/ije/dym165. [DOI] [PubMed] [Google Scholar]
- 46.Herring RP, Butler T, Hall S, Montgomery SB, Fraser GE. Recruiting black Americans in a large cohort study: the Adventist Health Study-2 (AHS-2) design, methods and participant characteristics. Ethn Dis. 2010;20:437–43. [PMC free article] [PubMed] [Google Scholar]
- 47.Howard BV, Devereux RB, Cole SA, et al. A genetic and epidemiologic study of cardiovascular disease in Alaska natives (GOCADAN): design and methods. Int J Circumpolar Health. 2005;64:206–21. doi: 10.3402/ijch.v64i3.17985. [DOI] [PubMed] [Google Scholar]
- 48.Annuzzi G, Rivellese AA, Wang H, et al. Lipoprotein subfractions and dietary intake of n-3 fatty acid: the Genetics of Coronary Artery Disease in Alaska Natives study. Am J Clin Nutr. 2012;95:1315–22. doi: 10.3945/ajcn.111.023887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fuqua SR, Wyatt SB, Andrew ME, et al. Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis. 2005;15:S6-18–29. [PubMed] [Google Scholar]
- 50.Forthofer RN. Investigation of nonresponse bias in NHANES II. Am J Epidemiol. 1983;117:507–15. doi: 10.1093/oxfordjournals.aje.a113568. [DOI] [PubMed] [Google Scholar]
- 51.Woteki CE, Briefel R, Hitchcock D, Ezzati T, Maurer K. Selection of nutrition status indicators for field surveys: the NHANES III design. J Nutr. 1990;120(Suppl 11):1440–5. doi: 10.1093/jn/120.suppl_11.1440. [DOI] [PubMed] [Google Scholar]
- 52.Lee ET, Welty TK, Fabsitz R, et al. The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132:1141–55. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 53.Milam JE, Meeske K, Slaughter RI, et al. Cancer-related follow-up care among Hispanic and non-Hispanic childhood cancer survivors: The Project Forward study. Cancer. 2015;121:605–13. doi: 10.1002/cncr.29105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harlan LC, Lynch CF, Keegan TH, et al. Recruitment and follow-up of adolescent and young adult cancer survivors: the AYA HOPE Study. J Cancer Surviv. 2011;5:305–14. doi: 10.1007/s11764-011-0173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arora NK, Hamilton AS, Potosky AL, et al. Population-based survivorship research using cancer registries: a study of non-Hodgkin’s lymphoma survivors. J Cancer Surviv. 2007;1:49–63. doi: 10.1007/s11764-007-0004-3. [DOI] [PubMed] [Google Scholar]
- 56.McTiernan A, Rajan KB, Tworoger SS, et al. Adiposity and sex hormones in postmenopausal breast cancer survivors. J Clin Oncol. 2003;21:1961–6. doi: 10.1200/JCO.2003.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galai N, Safaeian M, Vlahov D, Bolotin A, Celentano DD, Study A. Longitudinal patterns of drug injection behavior in the ALIVE Study cohort, 1988–2000: description and determinants. Am J Epidemiol. 2003;158:695–704. doi: 10.1093/aje/kwg209. [DOI] [PubMed] [Google Scholar]
- 58.Alavanja MC, Sandler DP, McDonnell CJ, et al. Characteristics of pesticide use in a pesticide applicator cohort: the Agricultural Health Study. Environ Res. 1999;80:172–9. doi: 10.1006/enrs.1998.3888. [DOI] [PubMed] [Google Scholar]
- 59.Bernstein L, Allen M, Anton-Culver H, et al. High breast cancer incidence rates among California teachers: results from the California Teachers Study (United States) Cancer Causes Control. 2002;13:625–35. doi: 10.1023/a:1019552126105. [DOI] [PubMed] [Google Scholar]
- 60.Tsilidis KK, Erlinger TP, Rifai N, et al. C-reactive protein and colorectal adenoma in the CLUE II cohort. Cancer Causes Control. 2008;19:559–67. doi: 10.1007/s10552-008-9117-x. [DOI] [PubMed] [Google Scholar]
- 61.Helzlsouer KJ, Alberg AJ, Huang HY, et al. Serum concentrations of organochlorine compounds and the subsequent development of breast cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:525–32. [PubMed] [Google Scholar]
- 62.Splansky GL, Corey D, Yang Q, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–35. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 63.Boyer BB, Mohatt GV, Plaetke R, et al. Metabolic syndrome in Yup’ik Eskimos: the Center for Alaska Native Health Research (CANHR) Study. Obesity (Silver Spring) 2007;15:2535–40. doi: 10.1038/oby.2007.302. [DOI] [PubMed] [Google Scholar]
- 64.Tomeo CA, Field AE, Berkey CS, Colditz GA, Frazier AL. Weight concerns, weight control behaviors, and smoking initiation. Pediatrics. 1999;104:918–24. doi: 10.1542/peds.104.4.918. [DOI] [PubMed] [Google Scholar]
- 65.Grobbee DE, Rimm EB, Giovannucci E, Colditz G, Stampfer M, Willett W. Coffee, caffeine, and cardiovascular disease in men. N Engl J Med. 1990;323:1026–32. doi: 10.1056/NEJM199010113231504. [DOI] [PubMed] [Google Scholar]
- 66.Rausch SM, Millay S, Scott C, et al. Health behaviors among cancer survivors receiving screening mammography. Am J Clin Oncol. 2012;35:22–31. doi: 10.1097/COC.0b013e318200598e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–8. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 68.Blair A, Stewart PA, Hoover RN. Mortality from lung cancer among workers employed in formaldehyde industries. Am J Ind Med. 1990;17:683–99. doi: 10.1002/ajim.4700170604. [DOI] [PubMed] [Google Scholar]
- 69.Harris KM, Perreira KM, Lee D. Obesity in the transition to adulthood: predictions across race/ethnicity, immigrant generation, and sex. Arch Pediatr Adolesc Med. 2009;163:1022–8. doi: 10.1001/archpediatrics.2009.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Madans JH, Cox CS, Kleinman JC, et al. 10 years after NHANES I: mortality experience at initial followup, 1982–84. Public Health Rep. 1986;101:474–81. [PMC free article] [PubMed] [Google Scholar]
- 71.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions : the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154:1119–25. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 72.Barton J, Bain C, Hennekens CH, et al. Characteristics of respondents and non-respondents to a mailed questionnaire. Am J Public Health. 1980;70:823–5. doi: 10.2105/ajph.70.8.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Toniolo PG, Levitz M, Zeleniuch-Jacquotte A, et al. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. J Natl Cancer Inst. 1995;87:190–7. doi: 10.1093/jnci/87.3.190. [DOI] [PubMed] [Google Scholar]
- 74.Khawaja O, Sarwar A, Albert CM, Gaziano JM, Djousse L. Sleep duration and risk of atrial fibrillation (from the Physicians’ Health Study) Am J Cardiol. 2013;111:547–51. doi: 10.1016/j.amjcard.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matise TC, Ambite JL, Buyske S, et al. The Next PAGE in understanding complex traits: design for the analysis of Population Architecture Using Genetics and Epidemiology (PAGE) Study. Am J Epidemiol. 2011;174:849–59. doi: 10.1093/aje/kwr160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andriole GL, Reding D, Hayes RB, Prorok PC, Gohagan JK, Committee PS. The prostate, lung, colon, and ovarian (PLCO) cancer screening trial: Status and promise. Urol Oncol. 2004;22:358–61. doi: 10.1016/j.urolonc.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 77.White E, Patterson RE, Kristal AR, et al. VITamins And Lifestyle cohort study: study design and characteristics of supplement users. Am J Epidemiol. 2004;159:83–93. doi: 10.1093/aje/kwh010. [DOI] [PubMed] [Google Scholar]
- 78.Herd P, Carr D, Roan C. Cohort profile: Wisconsin longitudinal study (WLS) Int J Epidemiol. 2014;43:34–41. doi: 10.1093/ije/dys194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hays J, Hunt JR, Hubbell FA, et al. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 80.Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the Women’s Health Study. J Womens Health Gend Based Med. 2000;9:19–27. doi: 10.1089/152460900318911. [DOI] [PubMed] [Google Scholar]
- 81.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–25. [PubMed] [Google Scholar]
- 82.McCarty CA, Giampietro PF, Wesbrook SD, Caldwell MD. Marshfield Clinic Personalized Medicine Research Project (PMRP): design, methods and recruitment for a large poplulation-based biobank. Per Med. 2005;2:49–79. doi: 10.1517/17410541.2.1.49. [DOI] [PubMed] [Google Scholar]
- 83.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 84.Satia JA, Galanko JA, Rimer BK. Methods and strategies to recruit African Americans into cancer prevention surveillance studies. Cancer Epidemiol Biomarkers Prev. 2005;14:718–21. doi: 10.1158/1055-9965.EPI-04-0132. [DOI] [PubMed] [Google Scholar]
- 85.Rosenberg L, Palmer JR, Rao RS, Adams-Campbell LL. Correlates of postmenopausal female hormone use among black women in the United States. Obstet Gynecol. 1998;91:454–8. doi: 10.1016/s0029-7844(97)00699-6. [DOI] [PubMed] [Google Scholar]
- 86.Weinberg CR, Shore DL, Umbach DM, Sandler DP. Using risk-based sampling to enrich cohorts for endpoints, genes, and exposures. Am J Epidemiol. 2007;166:447–55. doi: 10.1093/aje/kwm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


