Abstract
Objective
Identify the optimal number of pulses necessary to achieve reliable measures of motor evoked potentials (MEPs) in transcranial magnetic stimulation (TMS) studies.
Methods
Retrospective data was obtained from 54 healthy volunteers (30 men, mean age 61.7±13.1 years) who as part of prior studies had completed three blocks of 30 consecutive TMS stimuli using neuronavigation. Data from four protocols were assessed: single-pulse TMS for measures of amplitude and latency of MEPs; paired-pulse TMS for short-interval intracortical inhibition (sICI) and intracortical facilitation (ICF); and single-pulse TMS to assess the effects of intermittent theta burst stimulation (iTBS). Two statistical methods were used: an internal consistency analysis and probability of inclusion in the 95% confidence interval (CI) around the mean MEPs amplitude.
Results
For single-pulse TMS, the minimum number of pulses needed to achieve reliable amplitude and latency MEPs measures was 21 and 23, respectively. For paired-pulse TMS, the minimum number of pulses needed to achieve reliable sICI and ICF measures was 20 and 25, respectively. Finally, the minimum number of pulses needed to achieve reliable amplitude and latency MEPs measures after iTBS was 22 and 23, respectively.
Conclusions
This study provides guidelines regarding the minimum number of pulses needed to achieve reliable MEPs measurements in various study protocols using neuronavigated TMS.
Significance
Results from this study have the potential to increase the reliability and quality of future neuronavigated TMS studies.
Keywords: Transcranial magnetic stimulation, motor evoked potentials, intracortical inhibition, intracortical facilitation, reliability
1. Introduction
A single transcranial magnetic stimulation (TMS) pulse of adequate intensity can induce responses in muscles that receive corticomotor input from the stimulated motor cortical area (Barker et al., 1985). The action potentials induced by TMS travel along the corticospinal tract and peripheral motor nerve, resulting in muscle responses that can be recorded as motor evoked potentials (MEPs). MEPs are widely used to assess the integrity of the corticospinal and corticobulbar motor pathways in clinical neurophysiology and examine the influence of various interventions and factors (Rossini et al., 2015a). In addition, TMS can be used to investigate inhibitory and facilitatory interactions in the cortex with paired-stimuli based on a conditioning-test paradigm (Rossini et al., 2015a).
A basic parameter to assess TMS-induced MEPs is the cortical motor threshold (CMT) (Groppa et al., 2012). The CMT is defined as the minimal intensity of motor cortex stimulation required to elicit a reliable MEP of minimal amplitude in the target muscle. Two kinds of CMT have been used to study TMS-induced MEPs. One is the resting motor threshold (RMT), assessed with the target muscle at rest, and the other is the active motor threshold (AMT), assessed during a slight tonic contraction of the target muscle (Groppa et al., 2012). Different procedures for determining the CMT have been described and can be used depending on the setting and the available technical support (Rossini et al., 2015a). In addition, the amplitude and latency of MEPs have been used to assess corticomotor reactivity and conduction (Rossini and Rossi, 2007). However, these parameters show substantial variability and dependence on many technical factors. MEPs with the largest amplitude and shortest latency in a run of 5–6 consecutive MEPs can provide a good estimate of optimal corticomotor conduction in the clinical setting (Groppa et al., 2012). Others have advocated the use of average metrics of a block of MEPs (Jung et al., 2010; Kim et al., 2006; Vernet et al., 2014; Ziemann et al., 2001). In this approach, using a larger number of TMS pulses, while more accurate, requires more time for assessment. It also has been suggested that neuronavigation might increase the consistency of MEPs (Bashir et al., 2011; Gugino et al., 2001). However, there is no current consensus about the number of TMS pulses necessary to achieve reliable MEPs measurements.
The objective of the present study was to evaluate the optimal number of pulses to achieve reliable MEP parameters in neuronavigated TMS studies across a variety of common neurophysiological measurements in order to provide guidance for the design of experimental protocols.
2. Methods
2.1. Participants
For the present retrospective study, we used data obtained from various protocols that measured MEPs induced by neuronavigated TMS to assess corticomotor reactivity, corticospinal conduction and long-term potentiation-like plasticity in healthy participants. In all those studies, the motor hotspot had been identified as the scalp location of the TMS coil that evoked MEPs of greatest amplitude (consistent Rossini et al. 2015), but then the identified location had been marked on the individual’s MRI and neuronavigation (eXimia 3.1, Nexstim Ltd., Helsinki, Finland) used to identify and consistently target the hotspot across stimulation trials.
For the present analysis we included data from a total of 54 healthy participants (30 men, mean age 61.7±13.1 years). Their baseline characteristics are summarized in Table 1. The local institutional review board had approved all the trials at which data were collected, as well as this retrospective combined analysis. All participants provided written informed consent.
Table 1.
Baseline characteristics of participants
| Characteristics | Values |
|---|---|
| Age (y, mean ± SD, range) | 61.7 ± 13.1 (20 – 80) |
| Sex (M :F) | 30 : 24 |
| Handedness (Right : Left) | 53 : 1 |
| Resting motor threshold (%, mean ± SD, range) | 53.9 ± 13.0 (31 – 83) |
| Active motor threshold (%, mean ± SD, range) | 44.7 ± 8.9 (29 – 63) |
Inclusion criteria for the present study were: (1) healthy participants (as evidenced by a medical examination of general health, neurologic and cognitive function); (2) age greater than 18 years.
Exclusion criteria for the present study were: (1) clinical evidence or suspicion of vascular disease including cardiovascular disease, peripheral vascular disease, stroke, or microvascular disease; (2) current or history of any neurological disorder affecting cognitive function, including dementia, epilepsy, stroke, brain lesions, or multiple sclerosis; (3) history of neurosurgical procedures or head trauma that resulted in neurological impairment; (4) current or history of major depression, bipolar or psychotic disorders, or any other major psychiatric condition; and (5) any ongoing medications with known TMS contraindications. In addition, at all source study protocols, participants with any risk factors or contraindications to TMS as per current recommendations endorsed by The International Federation of Clinical Neurophysiology (IFCN) (Rossi et al., 2009; Rossini et al., 2015b) had been excluded.
2.2 Source Data
In the experiments generating the source data, the following TMS methodologies and experimental protocol were used:
2.2.a. Experimental Set-up
For single and paired-pulse stimulation, a Magstim Super Rapid Stimulator (Magstim Ltd., Withland, Wales, UK) was used to deliver biphasic pulses with current flowing in the brain in an antero-posterior and then a postero-anterior (AP–PA) direction. For intermittent theta burst stimulation (iTBS), a MagPro Stimulator (MagVenture A/S, Farum, Denmark) was used to deliver biphasic pulses with current flowing in AP–PA direction. An infrared-based MRI-guided navigation system (Nexstim Ltd., Helsinki, Finland) was used to ensure that the same cortical location was targeted in each study. During stimulation, surface electromyography (EMG) was recorded and monitored continuously online. Active electrodes were attached to the skin overlying the belly of first dorsal interosseous (FDI) muscle. A reference electrode was placed over the metacarpophalangeal joint. A ground electrode was placed over either the ulnar styloid process or the ipsilateral forearm. The EMG signals were filtered (8–500 Hz), amplified, displayed, and stored for off-line analysis. The TMS system delivered triggered pulses that synchronized the TMS and EMG systems. The participants were also monitored for drowsiness and asked to keep their eyes open throughout the experiment. Relaxation of the measured muscle was controlled by continuous visual EMG monitoring.
2.2.b. Single-pulse TMS
Each participant was seated in a comfortable chair with a headrest, elbows positioned at approximately 90°, and hands resting on a pillow on his or her lap. The optimal scalp location for activation of the FDI in the dominant hand was determined as the location at which TMS consistently elicited MEPs of maximum peak-to-peak amplitude. Once the optimal location was identified, a marker was placed in the individual participant’s MRI using the navigation system. This allowed the TMS coil to be placed systematically in the same location and with the same orientation and tilt in each session.
CMT was determined according to the recommendations of the International Federation for Clinical Neurophysiology (Groppa et al., 2012). RMT was defined as the lowest stimulus intensity capable of inducing MEPs ≥50 μV peak-to-peak amplitude in at least five of 10 consecutive trials. EMG monitoring was used to assure that the target muscle was at rest. Finally, 30 consecutive TMS pulses were administered every 5–6s (random jitter) at 120% of RMT in order to determine the amplitude and latency of MEPs.
2.2.c. Paired-Pulse TMS
Paired-pulse paradigms following the protocol introduced by Kujirai et al. (Kujirai et al., 1993) were recorded at two inter-stimulus intervals: 3 ms to assess short-interval intracortical inhibition (sICI) and 12 ms to assess intracortical facilitation (ICF). The conditioning stimulus was applied at an intensity of 80% of RMT, while the test-stimulus intensity was set at 120% of RMT; these intensities were maintained in all paired-pulse TMS trials. For each sICI and ICF measure, 30 consecutive paired-pulse TMS pulses were administered every 5–6s (random jitter).
2.2.d. TMS after iTBS
Prior to iTBS, AMT was determined, using the same MagPro Stimulator, as the minimum single-pulse TMS intensity required to produce MEPs ≥200 μV in at least five of 10 consecutive trials while participants contracted the target muscle (FDI in dominant hand) at approximately 20% of the maximal voluntary contraction (Vernet et al., 2014). The iTBS protocol was applied about 3–5 min after the end of the AMT measurement procedures; the relaxation of hand muscles was monitored continuously by the experimenters during and after the stimulation.
iTBS was applied using parameters similar to those used by Huang et al. (Huang et al., 2005): bursts of three pulses at 50 Hz repeated at intervals of 200 ms for a total of 2 seconds (one train); each train was repeated every 10 seconds for 20 instances, for a total of 600 stimuli. The intensity was fixed at 80% of AMT. MEPs were then measured using the same method of applying single-pulse TMS at 5 min after iTBS in order to assess changes in amplitude and latency.
2.3. Data and Statistical analysis
To determine the amplitude of MEPs, continuous EMG was sampled in epochs starting 50–100 ms before a TMS pulse and ending 300–400 ms after. MEPs were analyzed using MegaWin (Mega Electronics Ltd, Kuopio, Finland) or Scope software (ADInstruments, Colorado Springs, USA). The latencies and peak-to-peak amplitudes were assessed automatically.
SPSS version 21.0 (IBM SPSS, Chicago, IL, USA) was used for statistical analyses. Before conducting statistical analyses to determine optimal number of pulses to achieve reliable MEP parameters, we assessed the stability of MEP parameters by comparing the mean parameters of the first, second, and third sets of ten MEPs using repeated-measures analysis of variance (ANOVA). All measurements of 30 consecutive TMS pulses were included in the analysis irrespective of whether a proper response was elicited or not. Two different statistical methods were used: internal-consistency analysis and probability of inclusion in the 95% confidence interval (CI) (Cuypers et al., 2014). For each participant, the average parameter was calculated for subsets of consecutive stimuli. For internal-consistency analysis, an average of 30 TMS pulses was defined to be the true value. The analysis for each parameter was then conducted between the average of the experimental number of pulses and the average of 30 TMS pulses. Excellent internal consistency was defined as more than 0.990 of Cronbach’s alpha (Kline, 2000). The probability of inclusion in the 95% CI was applied to acquire a reliable estimate of MEPs using single-pulse TMS as applied by Cuypers et al. (Cuypers et al., 2014). A 95% CI was calculated using all 30 stimuli for each participant. Using both these statistical analyses, it was determined whether the average value was included in the CI, yielding a binary variable (0 = not included in the CI, 1 = included in the CI). A p-value <0.05 was considered to be statistically significant.
3. Results
3.1. Stability of parameters
There was no difference in the stability across the baseline single-pulse TMS, paired-pulse TMS and the single-pulse measures after iTBS (Table 2).
Table 2.
Stability of parameters
| Mean value
|
F-, p-values | |||
|---|---|---|---|---|
| 1st to 10th pulses | 11th to 10th pulses | 21st to 30th pulses | ||
| Baseline | ||||
| Amplitude (uV) | 1152.94 ±1176.00 | 1013.46 ±1099.75 | 1048.53 ±1044.09 | F1.7,87.9=2.036, p=0.145 |
| Latency (ms) | 22.80 ±2.77 | 22.75 ±3.35 | 23.03 ±2.97 | F2,106=0.580, p=0.562 |
| sICI (uV) | 537.92 ±531.11 | 423.47 ±378.03 | 427.08 ±367.32 | F1.3,59.0=3.574, p=0.054 |
| ICF (uV) | 1470.31 ±1179.92 | 1552.95 ±1339.17 | 1367.63 ±826.92 | F1.6,77.0=1.083, p=0.333 |
| After iTBS | ||||
| Amplitude (uV) | 1485.61 ±1261.14 | 1399.24 ±1380.78 | 1365.65 ±1132.94 | F2,102=0.974, p=0.381 |
| Latency (ms) | 23.36 ±3.38 | 23.93 ±2.99 | 23.46 ±3.25 | F2,102=1.334, p=0.268 |
Values are mean±SD.
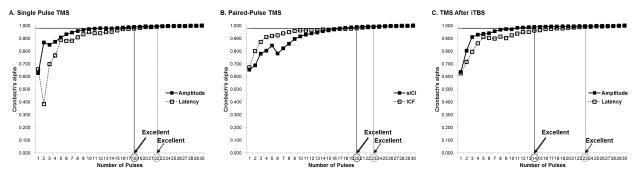
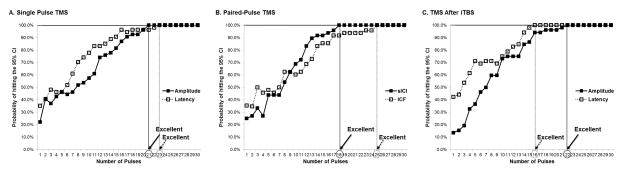
3.2. Single-pulse TMS
Using the internal consistency analysis, the minimum number of pulses needed to achieve excellent reliability of amplitude and latency of MEPs was 18 and 22, respectively (Table 3, Fig. 1-A). With the exploratory data analysis of the probability of inclusion in the 95% CI, at least 21 and 23 consecutive stimuli were required to reach a probability of 100.0% in amplitude and latency of MEPs, respectively (Table 4, Fig. 2-A). Supplementary Table S1 shows Cronbach’s alpha and probability for each number of consecutive stimuli in single-pulse TMS.
Table 3.
Minimum pulses needed to achieve reliable parameters according to internal consistency analysis
| Cronbach’s alpha
|
|||
|---|---|---|---|
| 0.900 | 0.950 | 0.990 | |
| Baseline | |||
| Amplitude | 5 | 8 | 18 |
| Latency | 9 | 14 | 22 |
| sICI | 10 | 14 | 20 |
| ICF | 4 | 8 | 23 |
| After iTBS | |||
| Amplitude | 3 | 7 | 14 |
| Latency | 5 | 13 | 23 |
Values are the minimum number of pulses.
sICI, short interval intracortical inhibition; ICF, intracortical facilitation; iTBS, intermittent theta burst stimulation
Figure 1.
Results of internal consistency analysis.
TMS, transcranial magnetic stimulation; iTBS, intermittent theta burst stimulation.
Table 4.
Minimum pulses needed to achieve reliable parameters according to probability analysisf
| Probability of hitting the 95% CI
|
|||
|---|---|---|---|
| 90.0% | 95.0% | 100.0% | |
| Baseline | |||
| Amplitude | 17 | 20 | 21 |
| Latency | 15 | 18 | 23 |
| sICI | 14 | 17 | 18 |
| ICF | 17 | 23 | 25 |
| After iTBS | |||
| Amplitude | 16 | 18 | 22 |
| Latency | 14 | 15 | 16 |
Values are the minimum number of pulses.
CI, confidence interval; sICI, short interval intracortical inhibition; ICF, intracortical facilitation; iTBS, intermittent theta burst stimulation
Figure 2.
Results of exploratory data analysis based on probability of inclusion.
TMS, transcranial magnetic stimulation; iTBS, intermittent theta burst stimulation.
3.3. Paired-Pulse TMS
The data of 48 participants was analyzed, as paired-pulse TMS measures were not available in six of the 54 healthy participants. Using the internal consistency analysis, the minimum number of pulses needed to achieve excellent reliability of the sICI and the ICF was found to be 20 and 23, respectively (Table 3, Fig. 1-B). With the exploratory data analysis of the probability of inclusion in the 95% CI, at least 18 and 25 consecutive pulses were required to reach a probability of 100.0% in the sICI and the ICF, respectively (Table 4, Fig. 2-B). Supplementary Table S2 shows Cronbach’s alpha and probability for each number of consecutive stimuli in paired-pulse TMS.
3.4. TMS After iTBS
The data of 52 healthy participants was analyzed, as iTBS was not available for two of the 54 participants. Using the internal consistency analysis, the minimum number of pulses needed to achieve excellent reliability of the amplitude and the latency of MEPs after iTBS was found to be 14 and 23, respectively (Table 3, Fig. 1-C). With the exploratory data analysis of the probability of inclusion in the 95% CI, at least 22 and 16 consecutive pulses were required to reach a probability of 100.0% in the amplitude and the latency of MEPs after iTBS, respectively (Table 4, Fig. 2-C). Supplementary Table S3 shows Cronbach’s alpha and probability for each number of consecutive pulses after iTBS.
4. Discussion
The primary objective of this study was to determine the optimal number of TMS pulses necessary for reliable MEP measurements in an effort to help design experimental protocols for assessment of the functional state of the corticospinal pathway. Two kinds of analyses were applied on data obtained using consistent methodology in prior studies: an internal consistency analysis and the probability of inclusion in the 95% CI around the mean MEP amplitude. Combining both methods, this study conservatively recommends that the minimum number of pulses needed to achieve reliable MEP parameters in neuronavigated TMS protocols be 21, 23, 20, and 25 for amplitude, latency of MEPs, sICI, and ICF, respectively. As often all these outcome measures are collected and analyzed as part of the same experimental protocol, we would recommend that a minimum 25 trials be collected ensure consistent results whilst making protocols easier to implement by having consistent number of trials.
Previous studies have shown that the amplitude of MEPs is highly variable within participants (Darling et al., 2006; Jung et al., 2010; Kiers et al., 1993). The oscillation of the graphs before reaching the required threshold reflects the high variability of MEPs parameters. Previous studies have used between five and 30 consecutive TMS pulses to assess cortical excitability with single-pulse TMS-induced MEPs (Jung et al., 2010; Kim et al., 2006; Vernet et al., 2014; Ziemann et al., 2001). Several TMS measures, other than single-pulse TMS-induced MEPs, have been used to assess the functional state of the cortical pathway (Rossini and Rossi, 2007). Inhibitory and facilitatory interactions in the cortex can be studied with the widely used paired-pulse TMS, combining a subthreshold conditioning stimulus with a suprathreshold test stimulus at different, short (1–20 ms) inter-stimulus intervals through the same TMS coil (Kobayashi and Pascual-Leone, 2003; Kujirai et al., 1993). sICI reflects a GABA-mediated corticocortical inhibition, and ICF is assumed to reflect a glutamatergic-mediated corticocortical facilitation (Rossini and Rossi, 2007). These different measures allow for a comprehensive evaluation of the functional state of the corticospinal pathway in both physiologic and pathologic conditions (Kobayashi and Pascual-Leone, 2003; Rossini and Rossi, 2007). Because TMS measurements can be influenced by the previous pulse, the frequency of consecutive TMS pulses is recommended to be less than 0.2 Hz to avoid carryover effects (Groppa et al., 2012). For this reason, if single-pulse and paired-pulse TMS are applied over both motor cortices with 30 consecutive pulses, the study protocol typically takes more than 20 minutes to complete. It has been suggested that the use of neuronavigation decreases the variability of MEPs, thus resulting in fewer number of pulses needed to achieve reliable results while decreasing the duration of a study protocol (Bashir et al., 2011; Gugino et al., 2001).
The parameters of TMS-induced MEPs might vary during consecutive TMS pulses due to numerous reasons (Darling et al., 2006; Kiers et al., 1993). For example, the interval between TMS pulses could influence the corticospinal excitability. In light of evidence suggesting that rTMS at frequencies <0.9 Hz has no lasting effect on MEP amplitude in the hand area (Fitzgerald et al., 2006; Ziemann et al., 2008), we chose to deliver TMS pulses every 5–6s, to minimize train effects. In addition, we jittered the interval between TMS pulses in order to avoid train effects. Finally, we compared the values of the first, second, and third sets of ten pulses for each parameter to assess the possibility consistent changes over the course of the collection block. There was no significant variation in any of the MEP parameters during 30 consecutive TMS pulses in this study. These results indicate that the MEP parameters were relatively stable during the 30 consecutive pulses.
The previously recommended optimal number of pulses needed to obtain the most reliable amplitude of single-pulse TMS-induced MEPs is 30 consecutive stimuli (Cuypers et al., 2014). Recently, Goldsworthy et al. (Goldsworthy et al., 2016) also reported that, without neuronavigation, 29 trials were necessary to reach a probability of 1 for inclusion of the estimates of MEP amplitude in the 95% CI of all trials. Results of the present study show that the minimum number of pulses needed to achieve a reliable amplitude of single-pulse TMS-induced MEPs when employing neuronavigation can be reduced to 21, thus requiring 30% less pulses than that found in the previous study. A study comparing non-navigated and MRI-guided navigated TMS reported that MRI-guided navigated TMS resulted in significantly lower MEP variability (Julkunen et al., 2009). In addition, Bashir et al. (Bashir et al., 2011) reported that navigated repetitive TMS (rTMS) leads to more robust modulation of physiological and behavioral effects compared with non-navigated rTMS. In the present study, a navigation system was used to ensure the consistency of the stimulated cortical location during TMS procedures. While the present study did not directly compare navigated and non-navigated TMS, the results suggest that navigated TMS reduces the time needed for TMS procedures by as much as 30% compared to non-navigated TMS (Cuypers et al., 2014).
The minimum number of pulses required to achieve reliable parameters after iTBS was similar to the baseline of single-pulse TMS-induced MEPs. These results indicate that there was no definitive fluctuation of TMS-induced MEPs parameters between trials, in spite of the large variability in responses to noninvasive brain stimulation (Wassermann, 2002; Wiethoff et al., 2014). The physiology of MEP amplitude variability remains unclear, but experimental fluctuations in TMS-inducedMEP parameters may be caused by rapid fluctuating changes in corticospinal excitability (Ellaway et al., 1998; Funase et al., 1999) and further work is needed to investigate this issue.
All the MEPs analyzed in the present study were triggered by TMS pulses at 120% of RMT. It is plausible that other stimulation intensities (e.g., 110 or 150% of RMT) might affect the optimal number of pulses. Systematic studies are required to study the effect of stimulation intensity on the optimal number of pulses for various TMS protocols. In addition, even though we analyzed MEPs that were recorded according to the current recommended guidelines (Groppa et al., 2012), hardware limitation and variability across equipment (including TMS and EMG systems) should be considered before generalizing the present results. Nonetheless, our findings provide guidance for studies employing iTBS protocols to characterize the efficacy of the mechanisms of cortical plasticity (Silvanto and Pascual-Leone, 2008). Future studies using real-time integration of TMS with electroencephalography may provide further insights into the source of corticomotor variability.
It is worth noting that the analyzed data included all measurements of 30 consecutive TMS pulses, irrespective of whether of a proper response was elicited or not. Therefore, trials with no response were also included in the calculation of the average estimate in this study. If only the TMS pulses that elicited proper responses were included in the analysis, the optimal number of pulses might be further reduced. However, as mentioned before, electrophysiology measures such as MEPs from TMS are prone to variability from many sources. In the present study, we were interested in capturing and assessing the variability responses that were realistic in terms of the data collected across different research studies. In addition, by doing so, it is likely that we minimized bias introduced by the influence of selective decision-making by an experimenter.
Supplementary Material
Highlights.
The minimum number of pulses for reliable amplitude and latency of motor evoked potentials was 21 and 23 in response to neuronavigated single-pulse TMS, respectively.
The minimum number of pulses for reliable short-interval intracortical inhibition and intracortical facilitation was 20 and 25, respectively.
Navigated transcranial magnetic stimulation might reduce the number of pulses necessary for reliable measurements.
Acknowledgments
This study was supported in part by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2014R1A2A1A01005128), the Sidney R. Baer Jr. Foundation, the National Institutes of Health (R01HD069776, R21 NS082870, R01NS073601, R21 MH099196, R21 NS085491, R21 HD07616), and Harvard Catalyst | The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH, UL1 RR025758). The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Institutes of Health, or the Sidney R. Baer Jr. Foundation.
Footnotes
Conflict of interest
Dr. A. Pascual-Leone serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, Axilum Robotics, Magstim Inc., and Neosync; and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation with electroencephalography and magnetic resonance imaging. The other authors have no conflicts of interest to declare. The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–7. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- Bashir S, Edwards D, Pascual-Leone A. Neuronavigation increases the physiologic and behavioral effects of low-frequency rTMS of primary motor cortex in healthy subjects. Brain Topogr. 2011;24:54–64. doi: 10.1007/s10548-010-0165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers K, Thijs H, Meesen RL. Optimization of the transcranial magnetic stimulation protocol by defining a reliable estimate for corticospinal excitability. PLoS One. 2014;9:e86380. doi: 10.1371/journal.pone.0086380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling WG, Wolf SL, Butler AJ. Variability of motor potentials evoked by transcranial magnetic stimulation depends on muscle activation. Exp Brain Res. 2006;174:376–85. doi: 10.1007/s00221-006-0468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway PH, Davey NJ, Maskill DW, Rawlinson SR, Lewis HS, Anissimova NP. Variability in the amplitude of skeletal muscle responses to magnetic stimulation of the motor cortex in man. Electroencephalogr Clin Neurophysiol. 1998;109:104–13. doi: 10.1016/s0924-980x(98)00007-1. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117:2584–96. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- Funase K, Miles TS, Gooden BR. Trial-to-trial fluctuations in H-reflexes and motor evoked potentials in human wrist flexor. Neurosci Lett. 1999;271:25–8. doi: 10.1016/s0304-3940(99)00467-x. [DOI] [PubMed] [Google Scholar]
- Goldsworthy MR, Hordacre B, Ridding MC. Minimum number of trials required for within- and between-session reliability of TMS measures of corticospinal excitability. Neuroscience. 2016;320:205–9. doi: 10.1016/j.neuroscience.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, et al. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2012;123:858–82. doi: 10.1016/j.clinph.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugino LD, Romero JR, Aglio L, Titone D, Ramirez M, Pascual-Leone A, et al. Transcranial magnetic stimulation coregistered with MRI: a comparison of a guided versus blind stimulation technique and its effect on evoked compound muscle action potentials. Clin Neurophysiol. 2001;112:1781–92. doi: 10.1016/s1388-2457(01)00633-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–6. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Julkunen P, Saisanen L, Danner N, Niskanen E, Hukkanen T, Mervaala E, et al. Comparison of navigated and non-navigated transcranial magnetic stimulation for motor cortex mapping, motor threshold and motor evoked potentials. Neuroimage. 2009;44:790–5. doi: 10.1016/j.neuroimage.2008.09.040. [DOI] [PubMed] [Google Scholar]
- Jung NH, Delvendahl I, Kuhnke NG, Hauschke D, Stolle S, Mall V. Navigated transcranial magnetic stimulation does not decrease the variability of motor-evoked potentials. Brain Stimul. 2010;3:87–94. doi: 10.1016/j.brs.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Kiers L, Cros D, Chiappa KH, Fang J. Variability of motor potentials evoked by transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1993;89:415–23. doi: 10.1016/0168-5597(93)90115-6. [DOI] [PubMed] [Google Scholar]
- Kim YH, You SH, Ko MH, Park JW, Lee KH, Jang SH, et al. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke. 2006;37:1471–6. doi: 10.1161/01.STR.0000221233.55497.51. [DOI] [PubMed] [Google Scholar]
- Kline P. The handbook of psychological testing. 2. London; New York: Routledge; 2000. p. vii.p. 744. [Google Scholar]
- Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–56. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–19. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A Safety of TMSCG. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee Clin Neurophysiol. 2015a;126:1071–107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee Clin Neurophysiol. 2015b;126:1071–107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Rossi S. Transcranial magnetic stimulation: diagnostic, therapeutic, and research potential. Neurology. 2007;68:484–8. doi: 10.1212/01.wnl.0000250268.13789.b2. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Pascual-Leone A. State-dependency of transcranial magnetic stimulation. Brain Topogr. 2008;21:1–10. doi: 10.1007/s10548-008-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet M, Bashir S, Yoo WK, Oberman L, Mizrahi I, Ifert-Miller F, et al. Reproducibility of the effects of theta burst stimulation on motor cortical plasticity in healthy participants. Clin Neurophysiol. 2014;125:320–6. doi: 10.1016/j.clinph.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM. Variation in the response to transcranial magnetic brain stimulation in the general population. Clin Neurophysiol. 2002;113:1165–71. doi: 10.1016/s1388-2457(02)00144-x. [DOI] [PubMed] [Google Scholar]
- Wiethoff S, Hamada M, Rothwell JC. Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul. 2014;7:468–75. doi: 10.1016/j.brs.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain. 2001;124:1171–81. doi: 10.1093/brain/124.6.1171. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Nitsche MA, Pascual-Leone A, Byblow WD, Berardelli A, et al. Consensus: Motor cortex plasticity protocols. Brain Stimul. 2008;1:164–82. doi: 10.1016/j.brs.2008.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.