Abstract
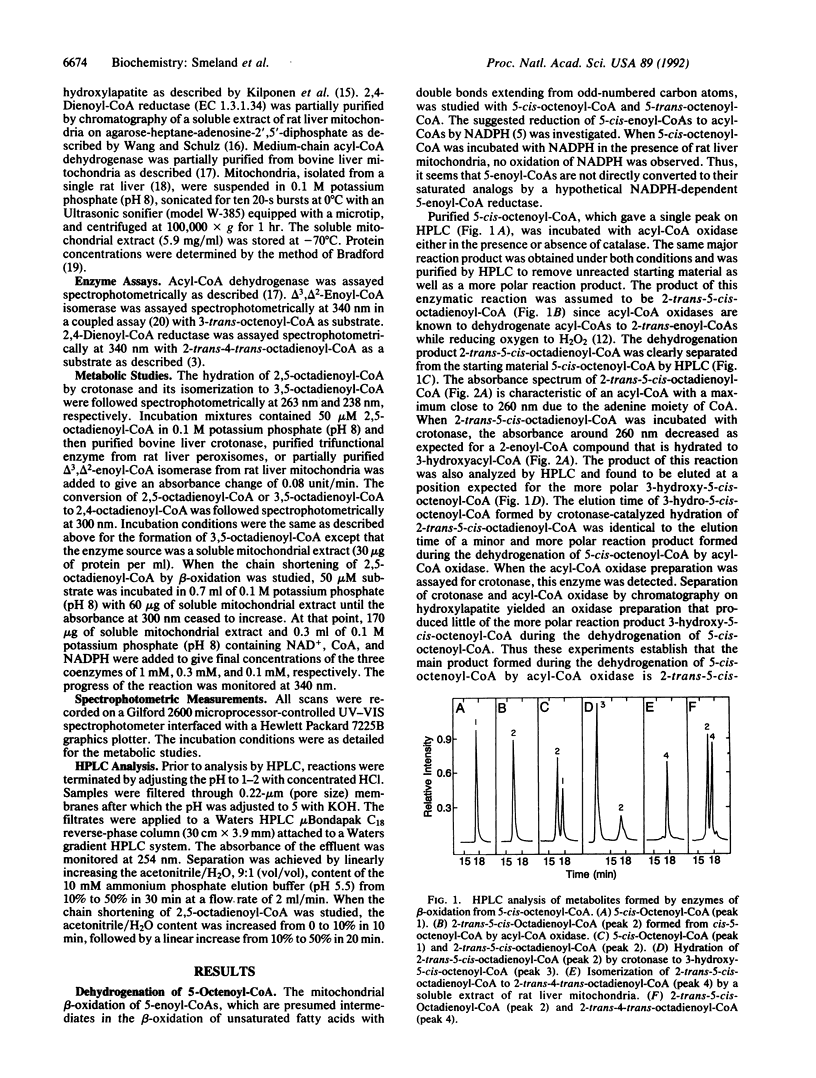
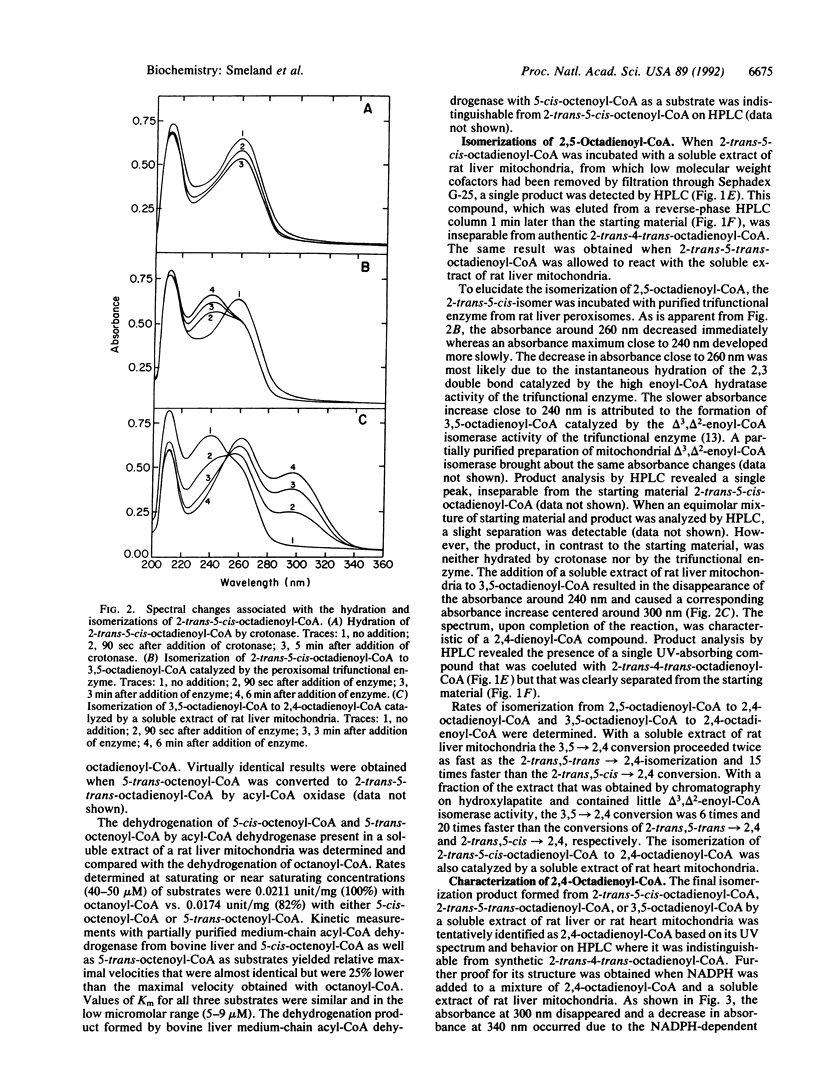
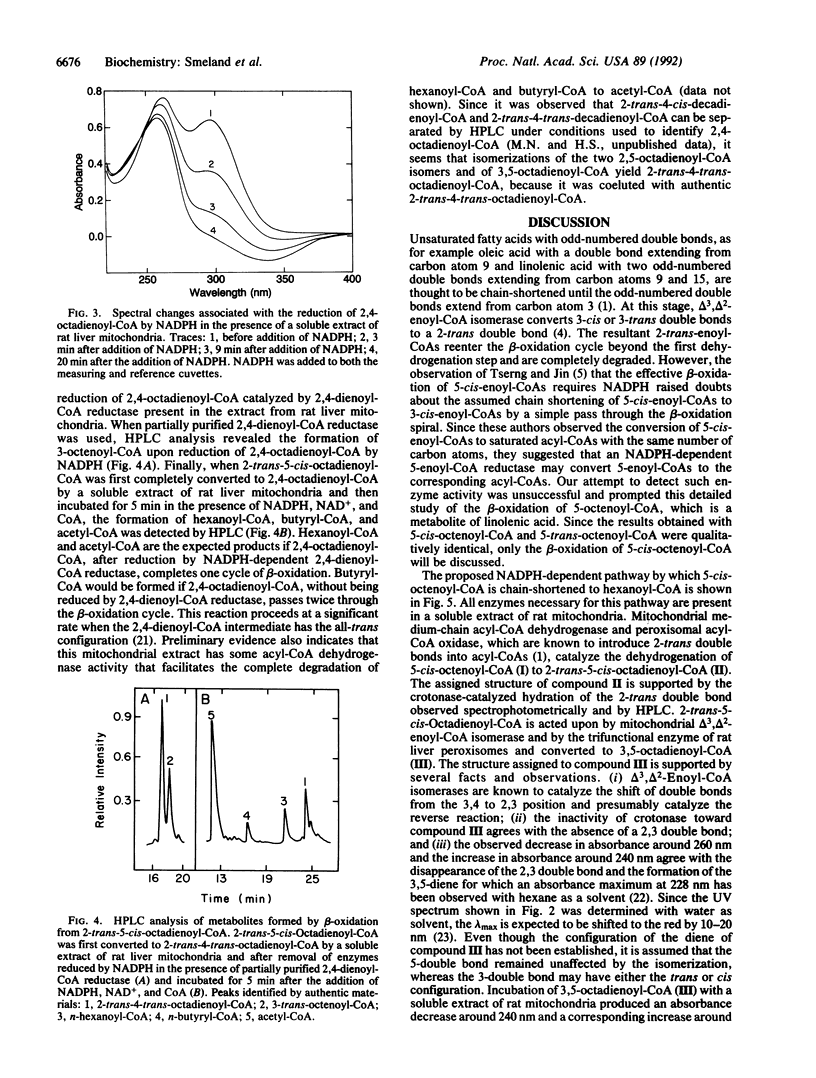
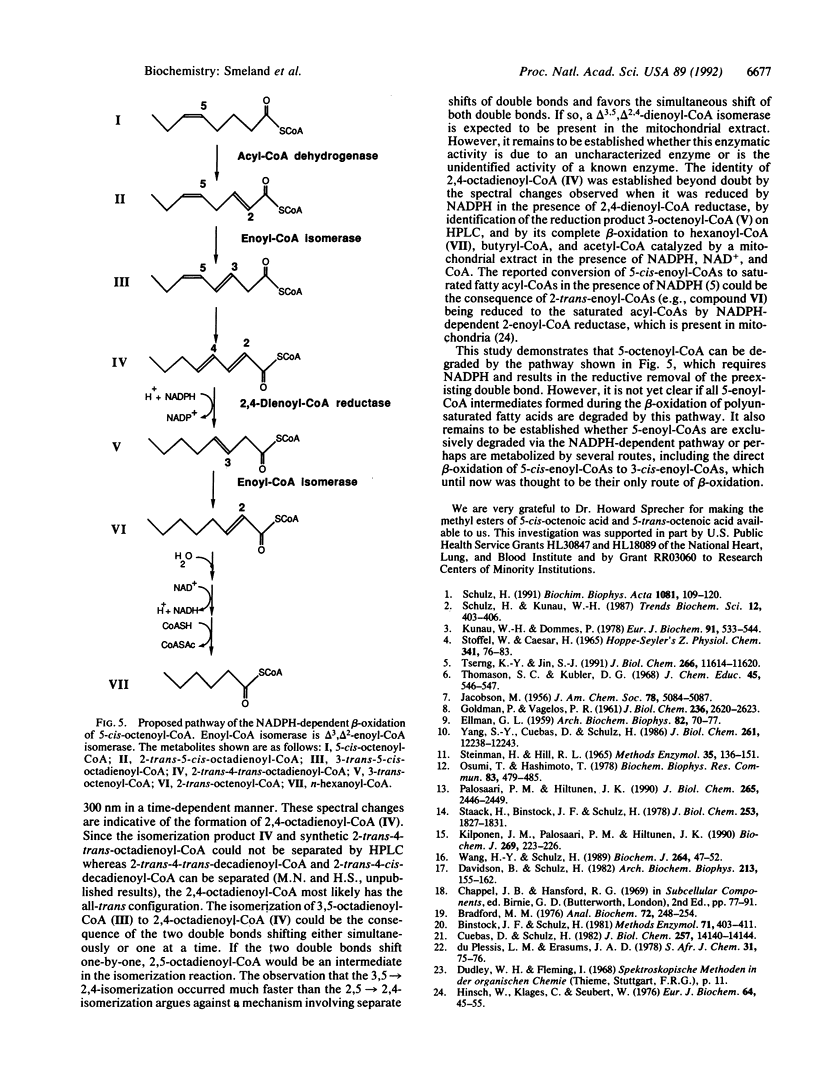
The mitochondrial metabolism of 5-enoyl-CoAs, which are formed during the beta-oxidation of unsaturated fatty acids with double bonds extending from odd-numbered carbon atoms, was studied with mitochondrial extracts and purified enzymes of beta-oxidation. Metabolites were identified spectrophotometrically and by high performance liquid chromatography. 5-cis-Octenoyl-CoA, a putative metabolite of linolenic acid, was efficiently dehydrogenated by medium-chain acyl-CoA dehydrogenase (EC 1.3.99.3) to 2-trans-5-cis-octadienoyl-CoA, which was isomerized to 3,5-octadienoyl-CoA either by mitochondrial delta 3,delta 2-enoyl-CoA isomerase (EC 5.3.3.8) or by peroxisomal trifunctional enzyme. Further isomerization of 3,5-octadienoyl-CoA to 2-trans-4-trans-octadienoyl-CoA in the presence of soluble extracts of either rat liver or rat heart mitochondria was observed and attributed to a delta 3,5,delta 2,4-dienoyl-CoA isomerase. Qualitatively similar results were obtained with 2-trans-5-trans-octadienoyl-CoA formed by dehydrogenation of 5-trans-octenoyl-CoA. 2-trans-4-trans-Octadienoyl-CoA was a substrate for NADPH-dependent 2,4-dienoyl-CoA reductase (EC 1.3.1.34). A soluble extract of rat liver mitochondria catalyzed the isomerization of 2-trans-5-cis-octadienoyl-CoA to 2-trans-4-trans-octadienoyl-CoA, which upon addition of NADPH, NAD+, and CoA was chain-shortened to hexanoyl-CoA, butyryl-CoA, and acetyl-CoA. Thus we conclude that odd-numbered double bonds, like even-numbered double bonds, can be reductively removed during the beta-oxidation of polyunsaturated fatty acids.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binstock J. F., Schulz H. Fatty acid oxidation complex from Escherichia coli. Methods Enzymol. 1981;71(Pt 100):403–411. doi: 10.1016/0076-6879(81)71051-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cuebas D., Schulz H. Evidence for a modified pathway of linoleate degradation. Metabolism of 2,4-decadienoyl coenzyme A. J Biol Chem. 1982 Dec 10;257(23):14140–14144. [PubMed] [Google Scholar]
- Davidson B., Schulz H. Separation, properties, and regulation of acyl coenzyme A dehydrogenases from bovine heat and liver. Arch Biochem Biophys. 1982 Jan;213(1):155–162. doi: 10.1016/0003-9861(82)90450-7. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- GOLDMAN P., VAGELOS P. R. The specificity of triglyceride synthesis from diglycerides in chicken adipose tissue. J Biol Chem. 1961 Oct;236:2620–2623. [PubMed] [Google Scholar]
- Hinsch W., Klages C., Seubert W. On the mechanism of malonyl-CoA-independent fatty-acid synthesis. Different properties of the mitochondrial chain elongation and enoylCoA reductase in various tissues. Eur J Biochem. 1976 Apr 15;64(1):45–55. doi: 10.1111/j.1432-1033.1976.tb10273.x. [DOI] [PubMed] [Google Scholar]
- Kilponen J. M., Palosaari P. M., Hiltunen J. K. Occurrence of a long-chain delta 3,delta 2-enoyl-CoA isomerase in rat liver. Biochem J. 1990 Jul 1;269(1):223–226. doi: 10.1042/bj2690223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunau W. H., Dommes P. Degradation of unsaturated fatty acids. Identification of intermediates in the degradation of cis-4-decenoly-CoA by extracts of beef-liver mitochondria. Eur J Biochem. 1978 Nov 15;91(2):533–544. doi: 10.1111/j.1432-1033.1978.tb12707.x. [DOI] [PubMed] [Google Scholar]
- Osumi T., Hashimoto T. Acyl-CoA oxidase of rat liver: a new enzyme for fatty acid oxidation. Biochem Biophys Res Commun. 1978 Jul 28;83(2):479–485. doi: 10.1016/0006-291x(78)91015-x. [DOI] [PubMed] [Google Scholar]
- Palosaari P. M., Hiltunen J. K. Peroxisomal bifunctional protein from rat liver is a trifunctional enzyme possessing 2-enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase, and delta 3, delta 2-enoyl-CoA isomerase activities. J Biol Chem. 1990 Feb 15;265(5):2446–2449. [PubMed] [Google Scholar]
- Schulz H. Beta oxidation of fatty acids. Biochim Biophys Acta. 1991 Jan 28;1081(2):109–120. doi: 10.1016/0005-2760(91)90015-a. [DOI] [PubMed] [Google Scholar]
- Staack H., Binstock J. F., Schulz H. Purification and properties of a pig heart thiolase with broad chain length specificity and comparison of thiolases from pig heart and Escherichia coli. J Biol Chem. 1978 Mar 25;253(6):1827–1831. [PubMed] [Google Scholar]
- Steinman H. M., Hill R. L. Bovine liver crotonase (enoyl coenzyme A hydratase). EC 4.2.1.17 L-3-hydroxyacyl-CoA hydrolyase. Methods Enzymol. 1975;35:136–151. doi: 10.1016/0076-6879(75)35149-5. [DOI] [PubMed] [Google Scholar]
- Stoffel W., Caesar H. Der Stoffwechsel der ungesättigten Fettsäuren. V. Zur beta-Oxydation der Mono- und Polyenfettsäuren. Der Mechanismus der enzymatischen Reaktionen an delta-2-cis-Enoyl-CoA-Verbindungen. Hoppe Seylers Z Physiol Chem. 1965;341(1):76–83. [PubMed] [Google Scholar]
- Tserng K. Y., Jin S. J. NADPH-dependent reductive metabolism of cis-5 unsaturated fatty acids. A revised pathway for the beta-oxidation of oleic acid. J Biol Chem. 1991 Jun 25;266(18):11614–11620. [PubMed] [Google Scholar]
- Wang H. Y., Schulz H. Beta-oxidation of polyunsaturated fatty acids with conjugated double bonds. Mitochondrial metabolism of octa-2,4,6-trienoic acid. Biochem J. 1989 Nov 15;264(1):47–52. doi: 10.1042/bj2640047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. Y., Cuebas D., Schulz H. 3-Hydroxyacyl-CoA epimerases of rat liver peroxisomes and Escherichia coli function as auxiliary enzymes in the beta-oxidation of polyunsaturated fatty acids. J Biol Chem. 1986 Sep 15;261(26):12238–12243. [PubMed] [Google Scholar]



