Abstract
Objective
Transcranial magnetic stimulation (TMS) is a non-invasive tool used for studying cortical excitability and plasticity in the human brain. This review aims to quantitatively synthesize the literature on age-related differences in cortical excitability and plasticity, examined by TMS.
Methods
A literature search was conducted using MEDLINE, Embase, and PsycINFO from 1980 to December 2015. We extracted studies with healthy old (50–89 years) versus young (16–49 years) individuals that utilized the following TMS measures: resting motor threshold (RMT), short-interval cortical inhibition (SICI), short-latency afferent inhibition (SAI), cortical silent period (CSP), intracortical facilitation (ICF), and paired associative stimulation (PAS).
Results
We found a significant increase in RMT (g = 0.414, 95% confidence interval (CI) [0.284, 0.544], p<0.001), a significant decrease in SAI (g=0.778, 95% CI [0.478, 1.078], p<0.001), and a trending decrease in LTP-like plasticity (g=−0.528, 95% CI [−1.157, 0.100 p<0.1) with age.
Conclusions
Our findings suggest an age-dependent reduction in cortical excitability and sensorimotor integration within the human motor cortex.
Significance
Alterations in the ability to regulate cortical excitability, sensorimotor integration and plasticity may underlie several age-related motor deficits.
Keywords: Aging, Transcranial Magnetic Stimulation, Motor Cortex, Cortical Plasticity, Cortical Excitability, Gamma-Amino Butyric Acid
1 Introduction
Due to increases in life expectancy and aging of the baby boomer population, we will have more individuals reaching advanced old age than ever before. By 2050 the number of adults aged 65 years and over is estimated to reach nearly 1.5 billion world-wide, representing 16% of the world’s population (NIH, 2011). With a rapidly aging global population the economic, societal, and personal costs of neurodegenerative and neuropsychiatric diseases are expected to spike. This will present a significant burden to families, support workers, and health care providers. An enhanced understanding of the impact of aging on cortical functioning may help provide more insight into age-related illnesses.
Normal aging is characterized by neurophysiological and neuroanatomical changes of the brain. These changes are thought to underlie the decline in sensorimotor control and function that can accompany advancing age. An inability to modulate cortical excitability is suggested to underlie several motor deficits that healthy older adults may experience in daily life such as the deterioration of fine motor skills (Calautti et al. , 2001), impaired coordination skills (Swinnen et al. , 1998, Serrien et al. , 2000, Heuninckx et al. , 2004), and a decline in reaction times (Bedard et al. , 2002).
The primary inhibitory neurotransmitter in the brain is gamma-aminobutyric acid (GABA). GABA plays a central role in mediating cortical excitability (DeFelipe et al. , 1986, Schieber et al. , 1993). Cortical pyramidal cell activity is modulated by excitatory inputs, excitatory post-synaptic potentials (EPSPs), and inhibitory inputs, inhibitory post-synaptic potentials (IPSPs) (Krnjevic, 1997). The inhibitory inputs are produced by GABAergic interneurons that terminate on these cells (Krnjevic, 1997). The selective attenuation of cortical pyramidal activity by inhibitory GABA interneurons is termed cortical inhibition (Daskalakis et al. , 2007). Recent studies suggest a direct correlation between a decreased ability to modulate cortical inhibition and motor retardation in healthy older adults (Fujiyama et al. , 2012b, Heise et al. , 2013, Levin et al. , 2014).
GABAergic neurotransmission is also central to the induction and maintenance of neuroplasticity (Daskalakis et al. , 2007). The brain’s ability to adapt to internal and external stimuli is dependent on neuroplasticity; a process by which the brain reorganizes and generates new neural pathways. The induction and maintenance of neuroplasticity is contingent upon activity-dependent alterations in synaptic strength (van Mier et al. , 1998, Daskalakis et al. , 2008). The most extensively studied forms of neural plasticity are: long-term potentiation (LTP) and long-term depression (LTD). LTP, the strengthening of neuronal connections in highly activated pathways, increases the likelihood of synaptic firing to additional stimuli; conversely LTD, the weakening of poorly activated pathways, reduces the likelihood of synaptic firing (Hebb, 1949). Age-related deficits in LTP-like plasticity may underlie motor learning deficits observed in healthy older adults.
Transcranial magnetic stimulation (TMS) is a non-invasive brain stimulation tool used to assess cortical excitability and plasticity, in-vivo (Rossini et al. , 2015). Two TMS paradigms used to index cortical inhibition are: short-interval cortical inhibition (SICI) (Kujirai et al. , 1993) and cortical silent period (CSP) (Cantello et al. , 1992). SICI is a paired-pulse TMS paradigm that requires the paired delivery of two TMS stimuli, whereby a subthreshold conditioning stimulus precedes a suprathreshold test stimulus by 1–4 ms (Kujirai et al. , 1993). In contrast, CSP is a single-pulse paradigm that requires the delivery of a suprathreshold TMS pulse to the contralateral motor cortex during voluntary contraction of the target muscle (Cantello et al. , 1992). The suprathreshold TMS pulse evokes a motor-evoked potential (MEP) followed by a period of suppressed electromyography (EMG) activity. The duration of the silent period is measured from the onset of the MEP to the return of EMG activity (Cantello et al. , 1992).
An extensive body of literature suggests SICI and CSP represent GABAA and GABAB inhibitory neurotransmission respectively. For instance, the pharmacological profiles of SICI and CSP differ greatly. Benzodiazepines (e.g. lorazepam) act as positive allosteric modulators at GABAA receptors and reliably facilitate SICI. Conversely baclofen, a GABAB receptor agonist, prolongs CSP duration (Paulus et al. , 2008). In addition to differing pharmacological profiles, GABAA receptor-mediated IPSP peaks at 20ms while the GABAB receptor-mediated IPSP peaks at 150–200ms; this corresponds to the time course of SICI and CSP duration (McCormick, 1989, Davies et al. , 1990, Sanger et al. , 2001).
As well as cortical inhibition, TMS protocols are used to index cortical excitability. Cortical excitability can be assessed using the following TMS paradigms: resting motor threshold (RMT) and intracortical facilitation (ICF). The RMT measures general neuronal membrane excitability and is defined as the minimum intensity that evokes an MEP > 50μV in a muscle at rest in 5 out of 10 trials (Rossini et al. , 1994). ICF is a paired-pulse TMS paradigm that involves the paired delivery of a subthreshold conditioning stimulus preceding a suprathreshold test stimulus by 10–25ms, resulting in MEP facilitation (Kujirai et al. , 1993, Nakamura et al. , 1997). Pharmacological studies suggest ICF indexes N-methyl-D-aspartate (NMDA) glutamate-mediated excitatory neurotransmission (Paulus et al. , 2008).
Paired associative stimulation (PAS) is a TMS paradigm used to induce LTP and LTD-like cortical plasticity. PAS involves pairing repetitive, low-frequency peripheral median nerve stimulation (MNS) with TMS stimulation to the motor cortex. To induce potentiation of cortico-motor excitability (LTP-like plasticity) the MNS must precede the TMS stimulus by an interstimulus interval (ISI) of 25ms; to induce depression (LTD-like plasticity) the MNS must precede the TMS stimulus by 10ms (Stefan et al. , 2000, Weise et al. , 2013).
Glutamatergic NMDA receptors represent the molecular basis of LTP and LTD (Miyamoto, 2006, Zorumski et al. , 2012). PAS-induced facilitation of cortical excitability is critically dependent on NMDA receptor function. For example, blockade of NMDA receptors prevents PAS-induced facilitation of cortico-motor excitability (Ridding et al. , 2010). Therefore, PAS-induced cortical plasticity, LTP, and LTD are thought to rely on shared neuronal mechanisms (Luscher et al. , 2012). This review will quantitatively assess the effect of age on PAS-induced LTP-like plasticity.
The impact of age on sensorimotor integration can be evaluated using the TMS paradigm short-latency afferent inhibition (SAI). SAI requires median nerve stimulation paired with a single TMS pulse to the motor cortex. If the ISI is 20ms, the afferent nerve conditioning produces a marked decrease in EMG activity from the single TMS pulse (Classen et al. , 2000, Tokimura et al. , 2000). At the biological level, SAI is thought to primarily index cholinergic transmission. For example, scopolamine, a muscarinic acetylcholine receptor antagonist, selectively reduces the SAI cortical response in healthy subjects (Di Lazzaro et al. , 2000).
A limited number of TMS studies have examined the impact of healthy aging on cortical excitability and plasticity in healthy older adults. The trends in the current literature remain inconclusive and a synthesis of findings is lacking. Thus, we undertook a meta-analysis to quantitatively synthesize the literature on TMS measures of cortical excitability and plasticity in healthy older adults compared to younger adults - refer to Table 1 for an overview of the included TMS studies.
Table 1.
Overview of TMS studies on the effects of aging on motor cortical neurophysiology.
| Study | Demographic characteristics | TMS protocol | Mean±SD | Hedge’s g | ||
|---|---|---|---|---|---|---|
| Authors | Group | No. of subjects [m/f] | Age (years±SD) | |||
| Rossini et al., 1992 | YA OL |
25 [10/15] 40 [14/26] |
39.4±3.5 43.9±6.4 |
RMT | 39.40±3.5 43.90±6.4 |
0.811 |
| Matsunaga et al., 1998 | YA OL |
22 [11/11] 21 [9/12] |
54.3±7.3 61.4±11.2 |
RMT | 54.30±7.3 61.40±11.2 |
0.741 |
| Kossev et al., 2002 | YA OL |
11 [m/f] 11 [m/f] |
53.67±8.9 51.73±6.6 |
RMT | 53.670±8.9 51.730±6.6 |
−0.238 |
| Sale et al., 2005 | YA OL |
10 [5/5] 10 [5/5] |
40±3.16 42±6.32 |
RMT | 40.00±3.16 42.00±6.32 |
0.383 |
| CSP | 185.70±24.10 160.10±31.90 |
−0.867 | ||||
| Hortobagyi et al., 2006 | YA OL |
6 [2/4] 6 [1/5] |
42±6.1 42.3±10.5 |
RMT | 42.00±6.1 42.30±10.5 |
0.032 |
| Tecchio et al., 2008 | YA OL |
25 [12/13] 25 [12/13] |
45.9±7 62.3±9 |
RMT | 45.90±7.0 62.30±9.0 |
2.002 |
| Post-PAS 10 | 0.856±0.44 0.443±0.11 |
−1.627 | ||||
| Mueller-Dahlhaus et al., 2008 | YA OL |
14 7 |
41.6±6.36 45.3±10.32 |
RMT | 41.60±6.36 45.30±10.32 |
0.454 |
| Post-PAS 10 | −0.050±0.54 0.130±0.50 |
0.332 | ||||
| Pellicari et al., 2009 | YA OL |
16 [8/8] 16 [8/8] |
52.5±7.2 58.3±8.8 |
RMT | 52.50±7.2 58.30±8.8 |
0.703 |
| Rogasch et al., 2009 | YA OL |
14 [8/6] 14 [8/6] |
43.71±7.23 44.14±3.74 |
RMT | 43.71±7.23 44.14±3.74 |
0.073 |
| SICI | 0.646±0.24 0.407±0.31 |
−0.835 | ||||
| Smith et al., 2009 | YA OL |
13 [13/0] 16 [16/0] |
36.45±7.1 39.35±6.6 |
RMT | 36.45±7.10 39.35±6.60 |
0.413 |
| SICI | 0.850±0.56 0.512±0.26 |
−0.784 | ||||
| ICF | 1.167±0.30 1.164±0.47 |
−0.007 | ||||
| Fujiyama et al., 2009 | YA OL |
15 [6/9] 15 [6/9] |
39.73±4.34 41.33±3.64 |
RMT | 39.73±4.34 41.33±3.64 |
0.389 |
| CSP | 150.080±19.25 150.580±26.38 |
0.021 | ||||
| Fathi et al., 2010 | YA OL |
16 [14/2] 16 [11/5] |
56±12 50±8 |
RMT | 56.00±12.0 50.00±8.0 |
−0.574 |
| Cirillo et al., 2010 | YA OL |
12 [5/7] 14 [7/7] |
45.08±8.59 44.86±6.86 |
RMT | 45.08±8.59 44.86±6.86 |
−0.028 |
| SICI | 0.517±0.36 0.404±0.36 |
−0.303 | ||||
| Smith et al., 2011(a) | YA OL |
13 18 |
35.46±7.01 37.67±7.52 |
RMT | 35.46±7.01 37.67±7.52 |
0.294 |
| Levin et al., 2011 | YA OL |
6 5 |
44.8±5.6 46±9.4 |
RMT | 44.80±5.6 46.00±9.4 |
0.146 |
| Clark et al., 2011 | YA OL |
27 [8/19] 27 [12/15] |
49.3±7.1 49.1±11.4 |
RMT | 49.30±7.10 49.10±11.4 |
−0.021 |
| Cirillo et al., 2011 | YA OL |
16 [7/9] 16 [7/9] |
41.63±9.99 47.38±8.22 |
RMT | 41.63±9.99 47.38±8.22 |
0.613 |
| SICI | 0.496±0.04 0.499±0.03 |
0.086 | ||||
| Saisanen et al., 2011 | YA OL |
11 29 |
60.1±7.8 66.2±16.4 |
RMT | 60.10±7.8 66.20±16.4 |
0.409 |
| SICI | 0.63±0.59 1.30±1.56 |
0.478 | ||||
| ICF | 1.79±1.40 2.44±1.60 |
0.411 | ||||
| Smith et al., 2011(b) | YA OL |
15 [15/0] 15 [15/0] |
44.5±10 44.7±9.2 |
RMT | 44.50±10.0 44.70±9.2 |
0.02 |
| SICI | 0.618±0.33 0.613±0.27 |
−0.016 | ||||
| ICF | 1.375±0.39 1.206±0.36 |
−0.438 | ||||
| Fujiyama et al., 2011 | YA OL |
13 [4/9] 13 [4/9] |
43.75±1.47 47.08±2.57 |
RMT | 43.75±1.47 47.08±2.57 |
1.54 |
| SICI | 0.508±0.14 0.486±0.11 |
−0.167 | ||||
| Marneweck et al., 2011 | YA OL |
25 [12/13] 24 [11/13] |
18–29 59–88 |
SICI | 0.30±0.41 0.61±0.65 |
0.564 |
| Degardin et al., 2011 | YA OL |
14 [8/6] 14 [6/8] |
26.4±2.7 62.4±7.1 |
RMT | 41.1±22.45 44.1±23.57 |
0.127 |
| SAI | 65.5±50.89 52.8±20.21 |
−0.318 | ||||
| CSP | 98.1±42.77 103.6±45.65 |
0.121 | ||||
| Bernard et al., 2012 | YA OL |
16 17 |
57.6±4.91 62.75±11.83 |
RMT | 57.60±4.91 62.75±11.83 |
0.549 |
| Fujiyama et al., 2012(a) | YA OL |
15 [7/8] 15 [6/9] |
41.8±6.32 42.6±7.09 |
RMT | 41.80±6.32 42.60±7.09 |
0.116 |
| CSP | 140.470±37.64 134.160±27.09 |
−0.187 | ||||
| Fujiyama et al., 2012(b) | YA OL |
13 [3/10] 13 [3/10] |
46.77±13.02 45.85±12.93 |
RMT | 46.77±13.02 45.85±12.93 |
−0.069 |
| SICI | 0.470±0.28 0.570±0.28 |
0.351 | ||||
| Young-Bernier et al., 2012(b) | YA OL |
24 [11/13] 31 [13/18] |
22.67±3.49 70.29±3.81 |
RMT | 66.00±11.55 72.55±12.71 |
0.528 |
| SAI | 18.12±15.74 51.36±34.62 |
1.168 | ||||
| Hinder et al., 2013 | YA OL |
15 [7/8] 15 [5/10] |
49.3±8.50 49.7±7.90 |
RMT | 49.30±8.50 49.70±7.90 |
0.047 |
| SICI | 0.510±0.67 0.770±0.91 |
0.316 | ||||
| Cuypers et al., 2013 | YA OL |
14 [6/8] 10 [2/8] |
39.6±4.5 51.8±4.8 |
RMT | 39.60±4.50 51.80±4.80 |
2.547 |
| Opie et al., 2014 | YA OL |
22 18 |
46.36±7.31 48.17±10.74 |
RMT | 46.36±7.31 48.17±10.74 |
0.196 |
| SICI | 0.443±0.28 0.597±0.51 |
0.379 | ||||
| Young-Bernier et al., 2014 | YA OL |
20 [7/13] 18 [9/9] |
22.3±3.2 70.1±5.6 |
RMT | 42.6±9.3 46.2±11.2 |
0.464 |
| SAI | 19.43±12.13 42.07±34.79 |
0.880 | ||||
| Young-Bernier et al., 2015 | YA OL |
33 [13/20] 31 [13/18] |
22.4±3.2 70.2±4.9 |
SAI | 20.43±13.12 74.83±80.31 |
0.949 |
YA healthy young adults; OA healthy older adults; RMT resting motor threshold, SICI short-interval cortical inhibition; SAI short-latency afferent inhibition; CSP cortical silent period; ICF intracortical facilitation; PAS paired-associative stimulation
2 Methods
2.1 Data Sources
A literature search was conducted using MEDLINE, EMBASE, and PsycINFO from 1980 through December 2015. This search was supported by a hand search of bibliographies. Please refer to the Supplementary File for a description of the exact terms used in the literature search.
2.2. Inclusion Criteria
Selected studies had to meet the subsequent inclusion criteria:
Studies assessed one or more of the following TMS measures in the left motor cortex: RMT, SICI, SAI, CSP, ICF, and/or PAS.
To be consistent, we used studies that used the right hand muscles for EMG recordings.
Studies included a healthy old (≥ 50 years) and healthy young (< 50 years) group.
Studies provided adequate data to perform a Hedge’s g analysis: mean, standard deviation (SD), and sample size of groups.
The manuscript was written in English and had a minimum sample size of more than three participants in order to calculate the effect size.
There is no universal definition of old age because the definition is context-dependent. The majority of the included studies used a cut off between 50 and 60 years of age to delineate young versus old adults. Due to the limited number of studies on TMS and normal aging, we chose to delineate young and old adults at the age of 50 to include the maximum number of studies in our analyses.
2.3 Study Exclusion
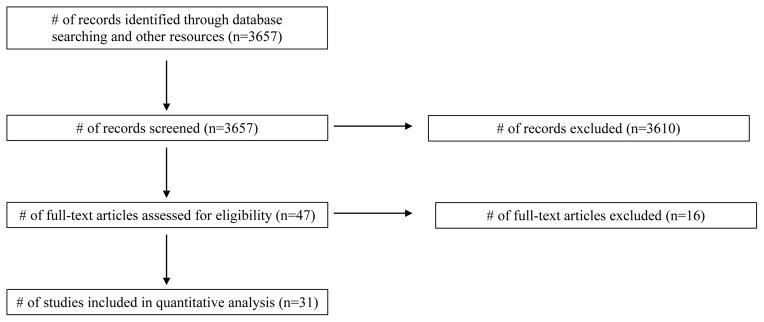
The number of studies and reasons for exclusion were as follows: insufficient data (8), EMG recording from leg muscles (3), tested the right hemisphere (2), no young comparison group (1), and did not meet age range inclusion criteria (2). Refer to Figure 1 for the PRISMA flow diagram. We chose to exclude TMS studies examining interhemispheric inhibition to primarily focus on the effects of aging on the left motor cortex.
Fig. 1.
PRISMA diagram.
2.4 Handedness
The majority of the summarized studies included participants who were right-handed, thereby left-hemisphere dominant. Five of the 31 studies (Rossini et al. , 1992, Fujiyama et al. , 2011, Opie et al. , 2014, Young-Bernier et al. , 2014, Young-Bernier et al. , 2015) did not explicitly state the handedness of the participants. A total of three studies included both right and left-handed participants (Matsunaga et al. , 1998, Fujiyama et al. , 2009, Young-Bernier et al. , 2012b).
2.5 Data extraction
The data obtained from each study was as follows: (1) number of healthy old and young participants, (2) the mean and SD of the outcome measure. If the published content provided insufficient data for the quantitative analysis, the corresponding authors were contacted to request additional data.
2.6 Quantitative Analysis
2.6.1 Hedge’s G
We used the Hedge’s g, a standardized meta-analytic technique, to perform the quantitative analysis. For all included TMS measures the Hedge’s g effect size, p-value, and 95% confidence interval (CI) were calculated (healthy older versus young adults) in a fixed effects model. The Comprehensive Meta-Analysis Version 3.0 (Biostat, Englewood, New Jersey) was used to conduct the analyses. The sample size of the individual studies influenced the weight given to their means and SDs in the analyses.
2.6.2 Test of Publication Bias
The N fail-safe value represents the amount of non-significant unpublished studies that would render the effect size non-significant (Møller et al. , 2001). For all analyses, a p-value of 0.05, 2-tailed was used. A minimum of three studies were required for the N fail-safe analysis.
2.6.3 Test of Heterogeneity
Clinical heterogeneity and methodological heterogeneity contribute to statistical heterogeneity. Statistical heterogeneity results in a greater difference between intervention effects than one would anticipate due to chance alone (2011). From here on statistical heterogeneity will be referred to as heterogeneity. We utilized the Cochran Q, p-value and I2 test to determine whether there are actual differences underlying the studies’ results (heterogeneity), or whether the differences between findings were due to random error (homogeneity) alone. The Q statistic informs us of the presence versus absence of heterogeneity across studies (Huedo-Medina et al. , 2006). The power of the Cochran’s Q is low when a small number of studies is used. In these circumstances, the I2 value is a more reliable measure for analyzing the heterogeneity in the included studies’ results (Higgins et al. , 2003). The I2 statistic quantifies the degree of heterogeneity present across studies (Higgins et al. , 2002, Higgins et al. , 2003). The I2 value is a percentage and ranges from no heterogeneity (0%) to high heterogeneity (100%) (Ried, 2006).
3 Results
3.1 Impact of age on RMT
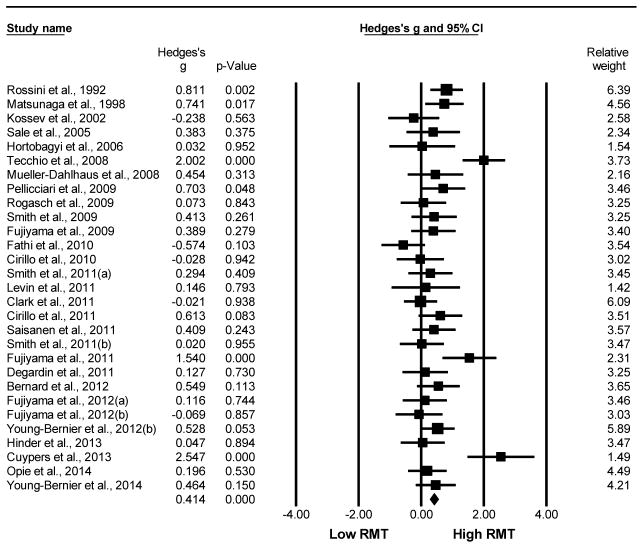
Older adults demonstrated significantly greater RMT values compared to young adults. This analysis compared 485 older to 453 young adults from a total of 29 studies. The Hedge’s g value was g=0.414, 95% CI [0.284, 0.544], p<0.001. The test of heterogeneity reached statistical significance (Q=70.101, df(Q)=28, p<0.001, I2=60.06), indicating that the variation across studies was mostly due to heterogeneity not chance. The N-failsafe value was 269 unpublished studies. Refer to Fig. 2 for the Hedge’s g forest plot for RMT comparing older versus young adults (Rossini et al. , 1992, Matsunaga et al. , 1998, Kossev et al. , 2002, Sale et al. , 2005, Hortobagyi et al. , 2006, Muller-Dahlhaus et al. , 2008, Tecchio et al. , 2008, Fujiyama et al. , 2009, Pellicciari et al. , 2009, Rogasch et al. , 2009, Smith et al. , 2009, Cirillo et al. , 2010, Fathi et al. , 2010, Cirillo et al. , 2011, Clark et al. , 2011, Degardin et al. , 2011, Fujiyama et al. , 2011, Levin et al. , 2011, Saisanen et al. , 2011, Smith et al. , 2011a, Smith et al. , 2011b, Bernard et al. , 2012, Fujiyama et al. , 2012a, Fujiyama et al. , 2012b, Young-Bernier et al. , 2012a, Cuypers et al. , 2013, Hinder et al. , 2013, Opie et al. , 2014, Young-Bernier et al. , 2014).
Fig. 2.
Forest plot of the Hedge’s g analysis comparing the RMT of older to young adults.
3.2 Impact of age on SICI
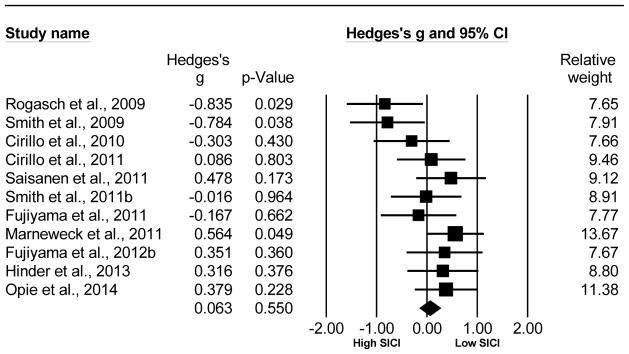
Older adults showed a slight reduction in SICI compared to young adults, however this was not statistically significant. This analysis compared 187 older to 169 young adults from a total of 11 studies (Rogasch et al. , 2009, Smith et al. , 2009, Cirillo et al. , 2010, Cirillo et al. , 2011, Fujiyama et al. , 2011, Marneweck et al. , 2011, Saisanen et al. , 2011, Smith et al. , 2011b, Fujiyama et al. , 2012b, Hinder et al. , 2013, Opie et al. , 2014). The Hedge’s g was g=0.063, 95% CI [−0.145, 0.271], p=0.550. Refer to Fig. 3 for the forest plot for the SICI Hedge’s g analysis comparing older versus young adults. Although the Cochran’s Q barely reached statistical significance (Q= 18.383, df(Q)= 10, p= 0.049, the I2 value (45.60) indicates that the inconsistency among studies was moderately large. Since there were no significant differences observed between the two groups for SICI, the N-failsafe value was 0 unpublished studies.
Fig. 3.
Forest plot of the Hedge’s g analysis comparing SICI in older to young adults.
3.3 Impact of age on SAI
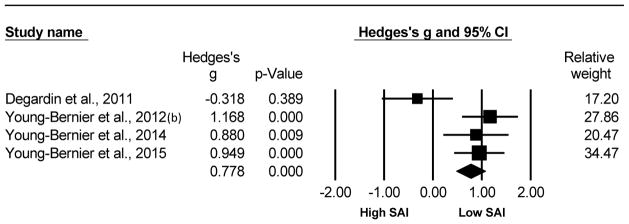
Older adults exhibited significantly reduced levels of SAI compared to young adults. This analysis compared 93 older and 91 young adults from 4 studies. The Hedge’s g was g=0.778, 95% CI [0.478, 1.078], p<0.001. The test of heterogeneity reached significance (Q=11.138, df(Q)= 3, p=0.011, I2=73.07) indicating the variation across studies was largely due to heterogeneity. The N-failsafe value was 19 unpublished studies. Refer to Fig. 4 for the forest plot of the for the SAI analysis comparing older versus young adults (Degardin et al. , 2011, Young-Bernier et al. , 2012b, Young-Bernier et al. , 2014, Young-Bernier et al. , 2015).
Fig. 4.
Forest plot of the Hedge’s g analysis comparing the SAI of older versus young adults.
3.4 Impact of age on CSP
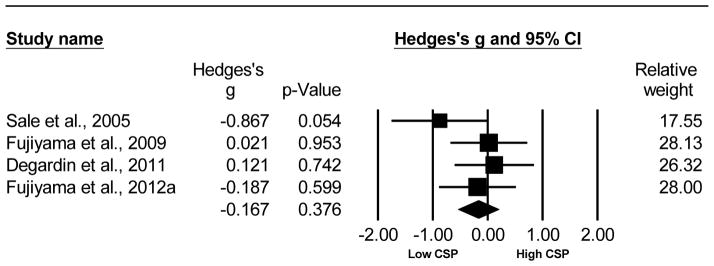
The CSP duration between the older and young adults was not significantly different. This analysis compared 54 older to 54 young adults from 4 studies (Sale et al. , 2005, Fujiyama et al. , 2009, Degardin et al. , 2011, Fujiyama et al. , 2012a). The Hedge’s g was g=−0.167, 95% CI [−0.536, 0.202], p= 0.376. The test of heterogeneity did not reach significance (Q= 3.322, df(Q)= 3, p= 0.345, I2= 9.68), meaning the variance among studies can be explained mostly by chance. The N-failsafe value was 0 unpublished studies. Refer to Fig. 5 for the Hedge’s g analysis for CSP of older versus young adults.
Fig. 5.
Forest plot of the Hedge’s g analysis comparing the CSP of older versus young adults.
3.5 Impact of age on ICF
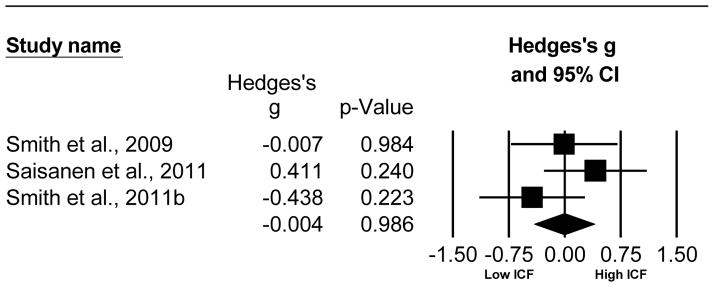
Old adults demonstrate a slight reduction in ICF compared to young adults which did not reach significance. This analysis compared 60 old to 39 young adults from 3 studies (Smith et al. , 2009, Saisanen et al. , 2011, Smith et al. , 2011b). The Hedge’s g was g = −0.004, 95% CI [−0.408, 0.401], p=0.986. The Cochran’s Q failed to reach significance (Q=2.860, df(Q)=2, p=0.239, I2= 30.08), but the I2 value indicates a moderate effect of heterogeneity. The N-failsafe value was 0 unpublished studies. Refer to Fig. 6 for the forest plot of the ICF analysis comparing older adult to young adults.
Fig. 6.
Forest plot of the Hedge’s g analysis comparing ICF of older versus young adults.
3.6 Impact of age on PAS
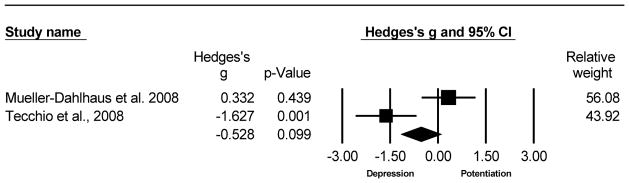
This analysis compared the MEP change at Post-PAS 10 minutes for 20 older versus 29 young adults from 2 studies (Muller-Dahlhaus et al. , 2008, Tecchio et al. , 2008). There was a trending decrease in LTP-like plasticity with age. The Hedge’s g was g=-0.528, 95% CI [−1.157, 0.100], p=0.099. The test of heterogeneity was statistically significant (Q=9.183, df(Q)=1, p=0.002, I2=89.11) because this analysis only included 2 published studies, the N-failsafe value was incalculable. Refer to Fig. 7 for the forest plot of the Hedge’s g analysis for Post-PAS 10 minutes of older compared to young adults.
Fig. 7.
Forest plot of the Hedge’s g analysis comparing the MEP change (Post-PAS MEP/Pre- PAS MEP) 10 minutes after PAS for older versus young adults.
4 Discussion
To our knowledge, this is the first quantitative summary of the impact of age on cortical excitability and LTP-like plasticity assessed by TMS. We found that older adults demonstrated a significant reduction in the RMT compared to young adults. No significant differences were observed for SICI, CSP, and ICF with age. However, older adults exhibited a significant reduction in SAI compared to young adults. We also observed a trending age-related decrease in PAS-induced LTP-like plasticity.
Our finding of an increased RMT with age is consistent with the majority of the literature (Rossini et al. , 1992, Peinemann et al. , 2001, Levin et al. , 2011, Cuypers et al. , 2013). An age-related increase in RMT suggests hypo-excitability in older adults. The cause of this hypo-excitability is multi-faceted with contributing factors such as central nervous system (CNS) decline and age-related anatomical and functional integrity changes (Oliviero et al. , 2006).
I-waves are produced by high frequency, repetitive discharge of corticospinal fibers. They are thought to originate in the motor cortex through the activation of cortico-cortical projections terminating on corticospinal neurons (Ziemann et al. , 2000). An age-related reduction in the synchronization of I-waves, deficits in recruiting later I-waves in the descending volley, and loss of both cortical and spinal motoneurons may underlie the observed age-related decrease in cortical excitability (Eisen et al. , 1996, Kimura, 2001, Pitcher et al. , 2003).
In addition to CNS changes, alterations in the peripheral nervous system (PNS) may also contribute to the observed hypo-excitability in older adults. It has been established that the neuromuscular system undergoes structural and functional alterations with age. Aging is generally associated with a loss of motor units and muscle mass atrophy. Motor unit loss has been proposed as the principal mechanism for decreased muscle strength and mass in older adults (Doherty et al. , 1993, Mesrati et al. , 2004). However, compensatory collateral innervation by the remaining motor units has been observed in older adults to counterbalance the loss of motor units (Doherty et al. , 1993, Mesrati et al. , 2004). A decrease in the motor units innervating the target muscle may contribute, in part, to the age-related decrease in RMT. Future studies assessing the impact of age on TMS measures should assess age-related PNS changes that may impact interpretation of said measures. For example, the Hoffman reflex (H-reflex) is often used as an estimate of the number of motor neurons capable of activation in a given state. Additionally, the compound muscle action potential (M-max) is used to assess the entire motor neuron pool, i.e. maximum muscle activation. Both the H-reflex and M-max have shown a gradual decrease with age suggesting a general age-related decrease in spinal pathway excitability (Scaglioni et al. , 2003, Kido et al. , 2004).
Gray matter reductions and widespread cortical thinning with age have been reported in several brain regions, such as the primary motor cortex (Salat et al. , 2004, Lemaitre et al. , 2005, Giorgio et al. , 2010, Clark et al. , 2011). In contrast, Bashir et al. found no difference in cortical thickness between young and old adults (Bashir et al. , 2014). RMT values are affected by the cortex-to-coil distance (Stokes et al. , 2005). Congruent with previous findings (Kozel et al. , 2000, McConnell et al. , 2001), Stokes et al. (2005) demonstrate a steep linear relationship between RMT and cortex-to-scalp distance in healthy participants. The studies included here did not control for age-related motor cortical thinning. An increased cortex-to-coil distance may partially underlie the decreased excitability observed in older adults.
Congruent with the majority of the literature, we found no significant age effect on ICF (Peinemann et al. , 2001, Smith et al. , 2009). However, McGinley et al. have demonstrated a decrease in ICF with age (McGinley et al. , 2010). In line with this study, several animal studies using rat and monkey aging models not only show an age-related loss of NMDA receptor binding in multiple neocortical and subcortical regions (Wenk et al. , 1991), but highlight the association between the loss of NMDA receptors and decline in motor function (Ossowska et al. , 2001). Further investigations are needed to elucidate the age-related changes in glutamatergic neurotransmission within the human motor cortex and their functional consequences.
Previous TMS studies primarily investigated SAI effects on individuals diagnosed with Alzheimer’s, Parkinson’s disease and dysexecutive syndromes, using healthy older adults as “normal” age-matched controls. However, due to the absence of a young comparison group, the possible age effects on SAI were not accounted for in these studies. To date, a small handful of studies have examined the effect of age on SAI. Oliviero et al. did not find any significant impact of aging on SAI (Oliviero et al. , 2006). More recently, Degardin et al. demonstrated a slightly greater SAI in older compared to young adults; however, this result was was not statistically significant (Degardin et al. , 2011). However, Young-Bernier’s group have shown that older adults have a selective decrease in MEP inhibition at an ISI of 20ms (Young-Bernier et al. , 2012a, Young-Bernier et al. , 2012b, Young-Bernier et al. , 2014, Young-Bernier et al. , 2015), suggesting a decrease in central cholinergic function in normal aging. Congruent with these findings, our analysis revealed significantly decreased MEP inhibition in older compared to younger adults.
Age-related dysfunctional modulation of cholinergic transmission may underlie the observed age-related decrease in SAI. The cholinergic hypothesis of aging was proposed over two decades ago and hypothesized that disturbed cholinergic neurotransmission occurs in normal aging and plays a critical role in memory and cognitive disturbances associated with increased age (Bartus et al. , 1982). Increased aging is associated with a reduction in the choline acetyltransferase (CAT) enzyme, which synthesizes acetylcholine (Perry, 1980). There is inconsistency with regards to muscarinic receptor binding changes with age. For instance, some studies show reduced muscarinic receptor binding with age (White et al. , 1977) but other do not (Davies et al. , 1978, Perry, 1980). Moreover, the motor cortex is densely innervated by cholinergic inputs from the nucleus basalis of Meynert, which has shown significant neuronal loss with aging (McGeer et al. , 1984).
There is general consensus in the literature for a strong association between cholinergic dysfunction, memory loss and cognitive deficits. However, the association between cholinergic deficits and motor performance is less clear. Young-Bernier et al. provide the first set of evidence for the impact of age on cholinergic transmission and decline in motor performance (Young-Bernier et al. , 2012a). SAI predicted motor performance for three complex motor tasks assessing dexterity and processing speed. These tasks are inextricably linked with executive control which is critically dependent on cholinergic modulation.
SAI is critically dependent on intact cortico-cortical connections between the somatosensory and primary motor cortices. Alongside CNS alterations, age-related PNS changes may account for the observed disruption of sensorimotor integration. For example, a decrease in SAI may be due to a reduction or loss of sensory fibers and/or a decrease in axonal conduction speed (Degardin et al. , 2011).
A role for GABAA receptor-mediated inhibitory neurotransmission in SAI’s neurophysiological response has been suggested through pharmacological studies. For example lorazepam, a GABAA-receptor antagonist, decreases (Di Lazzaro et al. , 2005b) and diazepam, GABA agonist, enhances (Di Lazzaro et al. , 2005a) SAI effects. Since GABAA receptors mediate both SAI and SICI, they are vulnerable to age-related deficits in GABAergic neurotransmission (Young-Bernier et al. , 2012a). However, our analysis did not demonstrate age-related changes in SICI or CSP.
The above finding is consistent with several TMS studies investigating cortical inhibition using the same TMS paradigms (Rogasch et al. , 2009, Cirillo et al. , 2010, Smith et al. , 2011a, Opie et al. , 2014). Yet, there are a number of conflicting results within the literature. For example, Peinemann et al. and Marneweck et al. reported a reduction in SICI with age (Peinemann et al. , 2001, Marneweck et al. , 2011). In contrast, Smith et al. and McGinley et al. reported an increase in SICI with age (Smith et al. , 2009, McGinley et al. , 2010).
Animal models have been used to explore the impact of age on GABAergic neurotransmission. A large number of animal studies show an age-related decline of GABA-mediated inhibition. This was indexed by findings such as a decline in the total number of GABAergic neurons (Hua et al. , 2008), alterations in GABAA receptor subunit composition and function (Caspary et al. , 1999, Yu et al. , 2006, Schmidt et al. , 2010), loss of the amount of GABA neurotransmitter, and a reduction in glutamic acid decarboxylase (GAD) (Ling et al. , 2005). The majority of these studies have investigated GABA in the prefrontal, primary visual and auditory cortices. Variations in neurotransmitter distribution, receptor density, and cortical architecture between cortical regions outside of and those comprising the motor system hinder the translatability of these results to the motor cortex (Hasan et al. , 2013). Therefore, there is ongoing uncertainty around the effect of aging on motor cortical GABAergic neurotransmission that remains unresolved based on the studies included in this analysis and amongst other studies that have employed related neurophysiological measures.
Older adults have consistently demonstrated a reduction in the ability to coordinate movements (Greene et al. , 1996, Serrien et al. , 2000, Heuninckx et al. , 2004) and slowed reaction times (Salthouse, 1991, Morgan et al. , 1994, Salthouse, 1996, Hunter et al. , 2001). To effectively perform coordinated movements successful inhibition of conflicting neuronal outputs is necessary (Baldissera et al. , 2005, Fujiyama et al. , 2009). There is growing evidence for a relationship between impairments in modulating cortical inhibition and age-related decline in motor function. One study suggests a correlation between resting state GABA neurotransmission and older adults’ modulatory capacity of cortical inhibition (Heise et al. , 2013). Studies on functional changes in inhibitory TMS measures after behavioral tasks may produce greater changes between older and younger adults.
We observed a trending decrease in PAS-induced motor cortical response with age. This finding is congruent with previous TMS reports of an age-related decline in LTP-like plasticity (Muller-Dahlhaus et al. , 2008, Tecchio et al. , 2008, Fathi et al. , 2010, Todd et al. , 2010, Freitas et al. , 2011). Indirect evidence for reduced plasticity with age has also been brought to light using related neurophysiological measures investigating use-dependent plasticity (Sawaki et al. , 2003) and motor cortical excitability changes (Rogasch et al. , 2009).
Changes in motor cortical excitatory and inhibitory neurotransmission may provide one possible mechanism for reduced LTP-like plasticity with age. PAS-induced LTP-like plasticity may, to some extent, be driven by an increase in excitability of either excitatory interneurons or corticospinal neurons at the post-synaptic level (Wolters et al. , 2003). This suggests that deficient excitatory neurotransmission may underlie deficient LTP-like plasticity. This is in line with our RMT results wherein older adults demonstrated a reduction in cortical excitability indexed by an increase in RMT. However, these results should be interpreted with caution as further research is needed to determine the causal link, or lack thereof, between increased RMT and deficient PAS. With regards to GABA, pharmacological studies demonstrate conflicting results for GABAB receptor mediated modulation of LTP. For instance, GABAB antagonists can produce both facilitation (Olpe et al. , 1990, Olpe et al. , 1993) and reduction of LTP (Davies et al. , 1991, Olpe et al. , 1993). Likewise, baclofen, a GABAB agonist, can enhance LTP (Mott et al., 1990) and decrease PAS-induced LTP-like plasticity (McDonnell et al. , 2007). In summary, our analysis did not demonstrate a significant age-related change in GABAergic neurotransmission.
As well as changes in excitatory and inhibitory neurotransmission, dysfunctional sensorimotor integration may also play a role in the age-related decline of LTP-like plasticity. Direct communication between the sensory afferent signal with motor cortex output requires intact projections from the somatosensory cortex into the motor cortex (Fathi et al. , 2010). For PAS, synchronization of the electrical afferent input and TMS stimulus is imperative. A disruption of these projections may disrupt the synchronization, thereby reducing or completely abolishing LTP-like plasticity induction.
Instead of studying age-related cortical changes due to single neurotransmitter systems, it is necessary to investigate the interaction of several neurotransmitters in the aging brain. In addition to GABA and glutamate, dopamine, acetylcholine, and norepinephrine influence the induction and maintenance of synaptic plasticity. Dopamine is a major hetero-synaptic modulator of synaptic plasticity (Jay, 2003) and is essential for motor memory encoding and motor skill acquisition (Jay, 2003, Floel et al. , 2005, Molina-Luna et al. , 2009). Dopaminergic neurotransmission deteriorates with age in the human brain. It is possible that disruption of the basal ganglia-thalamocortical loop, due to an age-related decrease in striatal dopamine, may underlie age-related reductions in LTP-like plasticity. Additionally, acetylcholine and norepinephrine are crucial in facilitating use-dependent plasticity within the motor cortex (Butefisch et al. , 2002, Sawaki et al. , 2002, Meintzschel et al. , 2006). Use-dependent plasticity and LTP appear to share the same neurobiological basis. Further research is required to explore the impact of age-related changes in dopaminergic, cholinergic and adrenergic neurotransmission on LTP-like motor cortical plasticity.
5 Limitations
This study has several limitations. First, the number of published studies assessing the impact of age on cortical excitation, inhibition, and LTP-like plasticity were limited and their sample sizes were small. The broad age range of the included studies is a limitation and may obscure differences between young and old subjects. To fully understand the effects of aging, an individual subject level meta-analysis that stratified based on different age subgroups (early, mid, and late-life), would be required. In order to summarize the findings, we focused on resting baseline measures. Functional changes in these measures after behavioral tasks were beyond the scope of the analysis, but such studies may yield greater changes between older and younger adults. This study focused on TMS measures limited to the motor cortex. However, other brain regions play a more central role in motor learning, skill acquisition and, execution. Brain regions such as the prefrontal cortex, basal ganglia, and cerebellum are recruited and contribute to maintenance of balance, gait, coordination, reaction time, and fine and gross motor skills. An issue of “supply and demand” arises as these areas are the most susceptible to the impact of age (Seidler et al. , 2010).
The tests of heterogeneity for the majority of the TMS paradigms indicate that the differences between the studies’ results were mostly due to moderate to high heterogeneity, not chance. CSP is unique in the fact that the variability among studies can be explained mostly by chance (I2=19.6%), not heterogeneity. The high heterogeneity in our analyses may be due to inter-individual variability among subjects caused by factors such as gender, physical activity, education level, skull thickness, and history of synaptic activation.
Human error, specifically unintentional coil movement, is also a limitation of the reviewed studies (Saisanen et al. , 2011). Even slight shifts in coil orientation may result in activation of different, unintended neuronal populations. Neuronavigation allows for highly accurate coil placement and any shifts in coil orientation can be monitored and adjusted in real time, significantly reducing the possibility of human error.
For PAS, Mueller-Dahlhaus et al. report only two-thirds of young healthy subjects demonstrate an LTP-like enhancement in MEP amplitude. Orientation differences of sulci, gyri and/or motor cortical neurons have been speculated to contribute to this variability (Muller-Dahlhaus et al. , 2008). Our PAS analysis was further limited by the data we were able to acquire. We were only able to analyze MEP amplitude changes at 10 minutes post-PAS. Further studies looking at MEP amplitude changes at several time intervals post-PAS (e.g. 0, 15, 30, 60 minutes) are required in order to develop a more concrete understanding of age-related alterations in PAS-induced LTP-like plasticity.
The last and most significant limitation is the large variability in TMS methodologies between studies. A number of measures could be standardized to facilitate a more accurate comparison of TMS data across studies. SICI and ICF should be measured using a conditioning stimulus of 80% RMT that precedes a suprathreshold test stimulus at an intensity that evokes a peak-to-peak MEP amplitude of 1mV (Kujirai et al. , 1993). SICI measurements should use interstimulus intervals of 2ms and 4ms and ICF should be evaluated at interstimulus intervals of 10, 15, and 20ms (Kujirai et al. , 1993, Nakamura et al. , 1997). CSP should be evaluated using a test stimulus of 140% RMT while the contralateral muscle is active at 20% of maximum contraction (Cantello et al. , 1992). PAS should be evaluated by low-frequency electrical stimulation of the peripheral median nerve preceding a test stimulus to the contralateral motor cortex at an intensity that evokes peak-to-peak MEP amplitude of 1mV. The electrical stimulus should precede the TMS stimulus by either 25ms to evoke LTP-like plasticity or by 10ms to induce LTD-like plasticity (Stefan et al. , 2000, Weise et al. , 2013). A consistent approach would allow for more pooling of data across research groups.
6 Conclusion
Despite these limitations, this meta-analysis provides a current summary of studies conducted to date and a framework for future motor cortical studies that compare old and young adults. TMS is a unique tool that allows for the in-vivo examination of cortical physiology. With the aging population an enhanced understanding of the impact of age on cortical functioning is necessary and may provide insight into age-related illnesses.
Supplementary Material
Highlights.
TMS measures of motor cortical excitation, inhibition and plasticity were assessed in young vs older adults.
Age-related motor cortical hypo-excitability and a trending decrease in LTP-like plasticity observed.
Older adults showed deficits in sensorimotor integration in comparison to young adults.
Acknowledgments
We gratefully acknowledge the assistance and contribution of Dr.s John Semmler, Hakuei Fujiyama, Julia Pitcher, Laura Saisanen, Franca Tecchio, Friedhelm C. Hummel, Mark Hinder, Michelle Marneweck, Geoff Hammond and Oron Levin for providing additional data.
Abbreviations
- TMS
Transcranial Magnetic Stimulation
- GABA
Gamma-aminobutyric acid
- RMT
Resting motor threshold
- SICI
Short-interval cortical inhibition
- SAI
Short-latency afferent inhibition
- CSP
Cortical silent period
- ICF
Intracortical facilitation
- PAS
Paired associative stimulation
- LTP
Long-term potentiation
- LTD
Long-term depression
- NMDA
N-methyl-D-aspartate
- EMG
Electromyography
- ISI
interstimulus interval
- CI
confidence interval
- SD
standard deviation
- CNS
central nervous system
- PNS
peripheral nervous system
Footnotes
Disclosures
AB and FF report no financial disclosures.
During the course of this work, NR was supported by an Ontario Mental Health Foundation (OMHF) research studentship.
TKR receives research support from Brain Canada, Brain and Behavior Research Foundation, Canadian Foundation for Innovation, Canadian Institutes of Health Research (CIHR), Ontario Ministry of Health and Long-Term Care, Ontario Ministry of Research and Innovation, the US National Institute of Health (NIH), and the W. Garfield Weston Foundation. TKR reports no competing interests.
BHM currently receives research support from the Canadian Institutes of Health Research (CIHR), the US National Institute of Health (NIH), Brain Canada, the CAMH Foundation, Bristol-Myers Squibb (medications for a NIH-funded clinical trial) and Pfizer (medications for a NIH-funded clinical trial). He directly own stocks of General Electric (less than $5,000). Within the past five years, he has also received some travel support from Roche.
DMB receives research support from the Canadian Institutes of Health Research (CIHR), National Institutes of Health (NIH), Brain Canada, and Temerty Family through the Centre for Addiction and Mental Health (CAMH) Foundation and the Campbell Family Research Institute. He receives non-salary operating funds and in-kind equipment support from Brain Research and Development Services Ltd. for an investigator-initiated study. He is the site principal investigator for several sponsor-initiated clinical trials from Brain Research and Development Services Ltd. He receives in-kind equipment support from Tonika/Magventure for an investigator-initiated study.
In the last 5 years, ZJD received research and equipment in-kind support for an investigator-initiated study through Brainsway Inc. ZJD has also served on the advisory board for Hoffmann-La Roche Limited and Merck and received speaker support from Eli Lilly. This work was supported by the Ontario Mental Health Foundation (OMHF), the Canadian Institutes of Health Research (CIHR), the Brain and Behaviour Research Foundation and the Temerty Family and Grant Family and through the Centre for Addiction and Mental Health (CAMH) Foundation and the Campbell Institute.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. What is heterogeneity? [Google Scholar]
- Baldissera F, Esposti R. Postural constraints to coupling of ipsilateral hand-foot movements. Neuroreport. 2005;16:1615–9. doi: 10.1097/01.wnr.0000181586.49130.48. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–14. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Bashir S, Perez JM, Horvath JC, Pena-Gomez C, Vernet M, Capia A, et al. Differential effects of motor cortical excitability and plasticity in young and old individuals: a Transcranial Magnetic Stimulation (TMS) study. Front Aging Neurosci. 2014;6:111. doi: 10.3389/fnagi.2014.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard AC, Nichols S, Barbosa JA, Schachar R, Logan GD, Tannock R. The development of selective inhibitory control across the life span. Dev Neuropsychol. 2002;21:93–111. doi: 10.1207/S15326942DN2101_5. [DOI] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD. Evidence for motor cortex dedifferentiation in older adults. Neurobiol Aging. 2012;33:1890–9. doi: 10.1016/j.neurobiolaging.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butefisch CM, Davis BC, Sawaki L, Waldvogel D, Classen J, Kopylev L, et al. Modulation of use-dependent plasticity by d-amphetamine. Ann Neurol. 2002;51:59–68. doi: 10.1002/ana.10056. [DOI] [PubMed] [Google Scholar]
- Calautti C, Serrati C, Baron JC. Effects of age on brain activation during auditory-cued thumb-to-index opposition: A positron emission tomography study. Stroke. 2001;32:139–46. doi: 10.1161/01.str.32.1.139. [DOI] [PubMed] [Google Scholar]
- Cantello R, Gianelli M, Civardi C, Mutani R. Magnetic brain stimulation: the silent period after the motor evoked potential. Neurology. 1992;42:1951–9. doi: 10.1212/wnl.42.10.1951. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Holder TM, Hughes LF, Milbrandt JC, McKernan RM, Naritoku DK. Age-related changes in GABA(A) receptor subunit composition and function in rat auditory system. Neuroscience. 1999;93:307–12. doi: 10.1016/s0306-4522(99)00121-9. [DOI] [PubMed] [Google Scholar]
- Cirillo J, Rogasch NC, Semmler JG. Hemispheric differences in use-dependent corticomotor plasticity in young and old adults. Exp Brain Res. 2010;205:57–68. doi: 10.1007/s00221-010-2332-1. [DOI] [PubMed] [Google Scholar]
- Cirillo J, Todd G, Semmler JG. Corticomotor excitability and plasticity following complex visuomotor training in young and old adults. Eur J Neurosci. 2011;34:1847–56. doi: 10.1111/j.1460-9568.2011.07870.x. [DOI] [PubMed] [Google Scholar]
- Clark BC, Taylor JL. Age-related changes in motor cortical properties and voluntary activation of skeletal muscle. Curr Aging Sci. 2011;4:192–9. doi: 10.2174/1874609811104030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen J, Steinfelder B, Liepert J, Stefan K, Celnik P, Cohen LG, et al. Cutaneomotor integration in humans is somatotopically organized at various levels of the nervous system and is task dependent. Exp Brain Res. 2000;130:48–59. doi: 10.1007/s002210050005. [DOI] [PubMed] [Google Scholar]
- Cuypers K, Thijs H, Duque J, Swinnen SP, Levin O, Meesen RL. Age-related differences in corticospinal excitability during a choice reaction time task. Age (Dordr) 2013;35:1705–19. doi: 10.1007/s11357-012-9471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Chen R. Dysfunctional neural plasticity in patients with schizophrenia. Arch Gen Psychiatry. 2008;65:378–85. doi: 10.1001/archpsyc.65.4.378. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Fitzgerald PB, Christensen BK. The role of cortical inhibition in the pathophysiology and treatment of schizophrenia. Brain Res Rev. 2007;56:427–42. doi: 10.1016/j.brainresrev.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Davies CH, Davies SN, Collingridge GL. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 1990;424:513–31. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CH, Starkey SJ, Pozza MF, Collingridge GL. GABA autoreceptors regulate the induction of LTP. Nature. 1991;349:609–11. doi: 10.1038/349609a0. [DOI] [PubMed] [Google Scholar]
- Davies P, Verth AH. Regional distribution of muscarinic acetylcholine receptor in normal and Alzheimer-type dementia brains. Brain Res. 1977;138:385–92. doi: 10.1016/0006-8993(77)90758-2. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Conley M, Jones EG. Long-range focal collateralization of axons arising from corticocortical cells in monkey sensory-motor cortex. J Neurosci. 1986;6:3749–66. doi: 10.1523/JNEUROSCI.06-12-03749.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degardin A, Devos D, Cassim F, Bourriez JL, Defebvre L, Derambure P, et al. Deficit of sensorimotor integration in normal aging. Neurosci Lett. 2011;498:208–12. doi: 10.1016/j.neulet.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Marra C, et al. Neurophysiological predictors of long term response to AChE inhibitors in AD patients. J Neurol Neurosurg Psychiatry. 2005a;76:1064–9. doi: 10.1136/jnnp.2004.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Di Giovanni S, Zito G, et al. Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp Brain Res. 2000;135:455–61. doi: 10.1007/s002210000543. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Saturno E, Dileone M, Pilato F, Nardone R, et al. Effects of lorazepam on short latency afferent inhibition and short latency intracortical inhibition in humans. J Physiol. 2005b;564:661–8. doi: 10.1113/jphysiol.2004.061747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty TJ, Vandervoort AA, Brown WF. Effects of ageing on the motor unit: a brief review. Can J Appl Physiol. 1993;18:331–58. doi: 10.1139/h93-029. [DOI] [PubMed] [Google Scholar]
- Eisen A, Entezari-Taher M, Stewart H. Cortical projections to spinal motoneurons: changes with aging and amyotrophic lateral sclerosis. Neurology. 1996;46:1396–404. doi: 10.1212/wnl.46.5.1396. [DOI] [PubMed] [Google Scholar]
- Fathi D, Ueki Y, Mima T, Koganemaru S, Nagamine T, Tawfik A, et al. Effects of aging on the human motor cortical plasticity studied by paired associative stimulation. Clin Neurophysiol. 2010;121:90–3. doi: 10.1016/j.clinph.2009.07.048. [DOI] [PubMed] [Google Scholar]
- Floel A, Breitenstein C, Hummel F, Celnik P, Gingert C, Sawaki L, et al. Dopaminergic influences on formation of a motor memory. Ann Neurol. 2005;58:121–30. doi: 10.1002/ana.20536. [DOI] [PubMed] [Google Scholar]
- Freitas C, Perez J, Knobel M, Tormos JM, Oberman L, Eldaief M, et al. Changes in cortical plasticity across the lifespan. Front Aging Neurosci. 2011;3:1–8. doi: 10.3389/fnagi.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama H, Garry MI, Levin O, Swinnen SP, Summers JJ. Age-related differences in inhibitory processes during interlimb coordination. Brain Res. 2009;1262:38–47. doi: 10.1016/j.brainres.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Fujiyama H, Hinder MR, Schmidt MW, Garry MI, Summers JJ. Age-related differences in corticospinal excitability and inhibition during coordination of upper and lower limbs. Neurobiol Aging. 2012a;33:1484, e1–14. doi: 10.1016/j.neurobiolaging.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Fujiyama H, Hinder MR, Schmidt MW, Tandonnet C, Garry MI, Summers JJ. Age-related differences in corticomotor excitability and inhibitory processes during a visuomotor RT task. J Cogn Neurosci. 2012b;24:1253–63. doi: 10.1162/jocn_a_00201. [DOI] [PubMed] [Google Scholar]
- Fujiyama H, Tandonnet C, Summers JJ. Age-related differences in corticospinal excitability during a Go/NoGo task. Psychophysiology. 2011;48:1448–55. doi: 10.1111/j.1469-8986.2011.01201.x. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N, et al. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage. 2010;51:943–51. doi: 10.1016/j.neuroimage.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LS, Williams HG. Aging and coordination from the dynamic pattern perspective. In: Ferrandez AM, Teasdale N, editors. Changes in Sensory Motor Behavior in Aging. Amsterdam: Elsevier; 1996. pp. 89–131. [Google Scholar]
- Hasan A, Wobrock T, Rajji T, Malchow B, Daskalakis ZJ. Modulating neural plasticity with non-invasive brain stimulation in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2013;263:621–31. doi: 10.1007/s00406-013-0446-8. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The organization of behaviour. New York (NY): Wiley; 1949. [Google Scholar]
- Heise KF, Zimerman M, Hoppe J, Gerloff C, Wegscheider K, Hummel FC. The aging motor system as a model for plastic changes of GABA-mediated intracortical inhibition and their behavioral relevance. J Neurosci. 2013;33:9039–49. doi: 10.1523/JNEUROSCI.4094-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S, Debaere F, Wenderoth N, Verschueren S, Swinnen SP. Ipsilateral coordination deficits and central processing requirements associated with coordination as a function of aging. J Gerontol B Psychol Sci Soc Sci. 2004;59:P225–32. doi: 10.1093/geronb/59.5.p225. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinder MR, Carroll TJ, Summers JJ. Inter-limb transfer of ballistic motor skill following non-dominant limb training in young and older adults. Exp Brain Res. 2013;227:19–29. doi: 10.1007/s00221-013-3481-9. [DOI] [PubMed] [Google Scholar]
- Hortobagyi T, del Olmo MF, Rothwell JC. Age reduces cortical reciprocal inhibition in humans. Exp Brain Res. 2006;171:322–9. doi: 10.1007/s00221-005-0274-9. [DOI] [PubMed] [Google Scholar]
- Hua T, Kao C, Sun Q, Li X, Zhou Y. Decreased proportion of GABA neurons accompanies age- related degradation of neuronal function in cat striate cortex. Brain Res Bull. 2008;75:119–25. doi: 10.1016/j.brainresbull.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Huedo-Medina T, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Thompson W, Roger DA. Reaction time, strength, and physical activity in women aged 20–89 years. J Aging Phys Activity. 2001;9:32–42. [Google Scholar]
- Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol. 2003;69:375–90. doi: 10.1016/s0301-0082(03)00085-6. [DOI] [PubMed] [Google Scholar]
- Kido A, Tanaka N, Stein RB. Spinal excitation and inhibition decrease as humans age. Can J Physiol Pharmacol. 2004;82:238–248. doi: 10.1139/y04-017. [DOI] [PubMed] [Google Scholar]
- Kimura J. Oxford University Press, editor. Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice. 3. 2001. Principles and variation of nerve conduction studies; pp. 91–129. [Google Scholar]
- Kossev AR, Schrader C, Dauper J, Dengler R, Rollnik JD. Increased intracortical inhibition in middle-aged humans; a study using paired-pulse transcranial magnetic stimulation. Neurosci Lett. 2002;333:83–6. doi: 10.1016/s0304-3940(02)00986-2. [DOI] [PubMed] [Google Scholar]
- Kozel FA, Nahas Z, deBrux C, Molloy M, Lorberbaum JP, Bohning DE, et al. How coil-cortex distance relates to age, motor threshold, and antidepressant response to repetitive transcranial magnetic stimulation. J Neuropsychiatry Clin Neurosci. 2000;12:376–84. doi: 10.1176/jnp.12.3.376. [DOI] [PubMed] [Google Scholar]
- Krnjevic K. Role of GABA in cerebral cortex. Can J Physiol Pharmacol. 1997;75:439–51. [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–19. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre H, Crivello F, Grassiot B, Alperovitch A, Tzourio C, Mazoyer B. Age- and sex-related effects on the neuroanatomy of healthy elderly. Neuroimage. 2005;26:900–11. doi: 10.1016/j.neuroimage.2005.02.042. [DOI] [PubMed] [Google Scholar]
- Levin O, Cuypers K, Netz Y, Thijs H, Nuttin B, Helsen WF, et al. Age-related differences in human corticospinal excitability during simple reaction time. Neurosci Lett. 2011;487:53–7. doi: 10.1016/j.neulet.2010.09.072. [DOI] [PubMed] [Google Scholar]
- Levin O, Fujiyama H, Boisgontier MP, Swinnen SP, Summers JJ. Aging and motor inhibition: a converging perspective provided by brain stimulation and imaging approaches. Neurosci Biobehav Rev. 2014;43:100–17. doi: 10.1016/j.neubiorev.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Ling LL, Hughes LF, Caspary DM. Age-related loss of the GABA synthetic enzyme glutamic acid decarboxylase in rat primary auditory cortex. Neuroscience. 2005;132:1103–13. doi: 10.1016/j.neuroscience.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD) Cold Spring Harb Perspect Biol. 2012;4(6) doi: 10.1101/cshperspect.a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marneweck M, Loftus A, Hammond G. Short-interval intracortical inhibition and manual dexterity in healthy aging. Neurosci Res. 2011;70:408–14. doi: 10.1016/j.neures.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Matsunaga K, Uozumi T, Tsuji S, Murai Y. Age-dependent changes in physiological threshold asymmetries for the motor evoked potential and silent period following transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1998;109:502–7. doi: 10.1016/s1388-2457(98)00020-0. [DOI] [PubMed] [Google Scholar]
- McConnell KA, Nahas Z, Shastri A, Lorberbaum JP, Kozel FA, Bohning DE, et al. The transcranial magnetic stimulation motor threshold depends on the distance from coil to underlying cortex: a replication in healthy adults comparing two methods of assessing the distance to cortex. Biol Psychiatry. 2001;49:454–9. doi: 10.1016/s0006-3223(00)01039-8. [DOI] [PubMed] [Google Scholar]
- McCormick DA. GABA as an inhibitory neurotransmitter in human cerebral cortex. J Neurophysiol. 1989;62:1018–27. doi: 10.1152/jn.1989.62.5.1018. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. Suppression of LTP-like plasticity in human motor cortex by the GABAB receptor agonist baclofen. Exp Brain Res. 2007;180:181–6. doi: 10.1007/s00221-006-0849-0. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG, Suzuki J, Dolman CE, Nagai T. Aging, Alzheimer's disease, and the cholinergic system of the basal forebrain. Neurology. 1984;34:741–5. doi: 10.1212/wnl.34.6.741. [DOI] [PubMed] [Google Scholar]
- McGinley M, Hoffman RL, Russ DW, Thomas JS, Clark BC. Older adults exhibit more intracortical inhibition and less intracortical facilitation than young adults. Exp Gerontol. 2010;45:671–8. doi: 10.1016/j.exger.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintzschel F, Ziemann U. Modification of practice-dependent plasticity in human motor cortex by neuromodulators. Cereb Cortex. 2006;16:1106–15. doi: 10.1093/cercor/bhj052. [DOI] [PubMed] [Google Scholar]
- Mesrati F, Vecchierini MF. F-waves: neurophysiology and clinical value. Neurophysiol Clin. 2004;34:217–43. doi: 10.1016/j.neucli.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Miyamoto E. Molecular mechanism of neuronal plasticity: induction and maintenance of long-term potentiation in the hippocampus. J Pharmacol Sci. 2006;100:433–42. doi: 10.1254/jphs.cpj06007x. [DOI] [PubMed] [Google Scholar]
- Molina-Luna K, Pekanovic A, Rohrich S, Hertler B, Schubring-Giese M, Rioult-Pedotti M, et al. Dopamine in Motor Cortex Is Necessary for Skill Learning and Synaptic Plasticity. PLoS One. 2009;4:e7082. doi: 10.1371/journal.pone.0007082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller AP, Jennions MD. Testing and adjusting for publication bias. Trends Ecol Evol. 2001;16:580–6. [Google Scholar]
- Morgan M, Phillips JG, Bradshaw JL, Mattingley JB, Iansek R, Bradshaw JA. Age-related motor slowness: simply strategic? J Gerontol. 1994;49:M133–9. doi: 10.1093/geronj/49.3.m133. [DOI] [PubMed] [Google Scholar]
- Mott DD, Lewis DV, Ferrari CM, Wilson WA, Swartzwelder HS. Baclofen facilitates the development of long-term potentiation in the rat dentate gyrus. Neurosci Lett. 1990;113:222–6. doi: 10.1016/0304-3940(90)90307-u. [DOI] [PubMed] [Google Scholar]
- Muller-Dahlhaus JF, Orekhov Y, Liu Y, Ziemann U. Interindividual variability and age-dependency of motor cortical plasticity induced by paired associative stimulation. Exp Brain Res. 2008;187:467–75. doi: 10.1007/s00221-008-1319-7. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol. 1997;498:817–23. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH. Global Health and Aging. National Institutes of Health; 2011. p. 32. [Google Scholar]
- Oliviero A, Profice P, Tonali PA, Pilato F, Saturno E, Dileone M, et al. Effects of aging on motor cortex excitability. Neurosci Res. 2006;55:74–7. doi: 10.1016/j.neures.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Olpe HR, Karlsson G. The effects of baclofen and two GABAB-receptor antagonists on long-term potentiation. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:194–7. doi: 10.1007/BF00166964. [DOI] [PubMed] [Google Scholar]
- Olpe HR, Worner W, Ferrat T. Stimulation parameters determine role of GABAB receptors in long-term potentiation. Experientia. 1993;49:542–6. doi: 10.1007/BF01955159. [DOI] [PubMed] [Google Scholar]
- Opie GM, Semmler JG. Age-related differences in short- and long-interval intracortical inhibition in a human hand muscle. Brain Stimul. 2014;7:665–72. doi: 10.1016/j.brs.2014.06.014. [DOI] [PubMed] [Google Scholar]
- Ossowska K, Wolfarth S, Schulze G, Wardas J, Pietraszek M, Lorenc-Koci E, et al. Decline in motor functions in aging is related to the loss of NMDA receptors. Brain research. 2001;907:71–83. doi: 10.1016/s0006-8993(01)02601-4. [DOI] [PubMed] [Google Scholar]
- Paulus W, Classen J, Cohen LG, Large CH, Di Lazzaro V, Nitsche M, et al. State of the art: Pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimul. 2008;1:151–63. doi: 10.1016/j.brs.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Lehner C, Conrad B, Siebner HR. Age-related decrease in paired-pulse intracortical inhibition in the human primary motor cortex. Neurosci Lett. 2001;313:33–6. doi: 10.1016/s0304-3940(01)02239-x. [DOI] [PubMed] [Google Scholar]
- Pellicciari MC, Miniussi C, Rossini PM, De Gennaro L. Increased cortical plasticity in the elderly: changes in the somatosensory cortex after paired associative stimulation. Neuroscience. 2009;163:266–76. doi: 10.1016/j.neuroscience.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Perry EK. The cholinergic system in old age and Alzheimer's disease. Age Ageing. 1980;9:1–8. doi: 10.1093/ageing/9.1.1. [DOI] [PubMed] [Google Scholar]
- Pitcher JB, Ogston KM, Miles TS. Age and sex differences in human motor cortex input-output characteristics. J Physiol. 2003;546:605–13. doi: 10.1113/jphysiol.2002.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Ziemann U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J Physiol. 2010;588:2291–304. doi: 10.1113/jphysiol.2010.190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ried K. Interpreting and understanding meta-analysis graphs--a practical guide. Aust Fam Physician. 2006;35:635–8. [PubMed] [Google Scholar]
- Rogasch NC, Dartnall TJ, Cirillo J, Nordstrom MA, Semmler JG. Corticomotor plasticity and learning of a ballistic thumb training task are diminished in older adults. J Appl Physiol (1985) 2009;107:1874–83. doi: 10.1152/japplphysiol.00443.2009. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126:1071–107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Desiato MT, Caramia MD. Age-related changes of motor evoked potentials in healthy humans: non-invasive evaluation of central and peripheral motor tracts excitability and conductivity. Brain Res. 1992;593:14–9. doi: 10.1016/0006-8993(92)91256-e. [DOI] [PubMed] [Google Scholar]
- Saisanen L, Julkunen P, Niskanen E, Hukkanen T, Mervaala E, Karhu J, et al. Short- and intermediate-interval cortical inhibition and facilitation assessed by navigated transcranial magnetic stimulation. J Neurosci Methods. 2011;195:241–8. doi: 10.1016/j.jneumeth.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–30. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Sale MV, Semmler JG. Age-related differences in corticospinal control during functional isometric contractions in left and right hands. J Appl Physiol (1985) 2005;99:1483–93. doi: 10.1152/japplphysiol.00371.2005. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Theoretical perspective on cognitive aging. Hillside, NJ: Erlbaum; 1991. [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–28. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530:307–17. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaki L, Boroojerdi B, Kaelin-Lang A, Burstein AH, Butefisch CM, Kopylev L, et al. Cholinergic influences on use-dependent plasticity. J Neurophysiol. 2002;87:166–71. doi: 10.1152/jn.00279.2001. [DOI] [PubMed] [Google Scholar]
- Sawaki L, Yaseen Z, Kopylev L, Cohen LG. Age-dependent changes in the ability to encode a novel elementary motor memory. Ann Neurol. 2003;53:521–4. doi: 10.1002/ana.10529. [DOI] [PubMed] [Google Scholar]
- Scaglioni G, Narici MV, Maffiuletti NA, Pensini M, Martin A. Effect of ageing on the electrical and mechanical properties of human soleus motor units activated by the H reflex and M wave. J Physiol. 2003;548:649–661. doi: 10.1113/jphysiol.2002.032763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH, Hibbard LS. How somatotopic is the motor cortex hand area? Science. 1993;261:489–92. doi: 10.1126/science.8332915. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Redecker C, Bruehl C, Witte OW. Age-related decline of functional inhibition in rat cortex. Neurobiol Aging. 2010;31:504–11. doi: 10.1016/j.neurobiolaging.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, et al. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34:721–33. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrien DJ, Swinnen SP, Stelmach GE. Age-related deterioration of coordinated interlimb behavior. J Gerontol B Psychol Sci Soc Sci. 2000;55:P295–303. doi: 10.1093/geronb/55.5.p295. [DOI] [PubMed] [Google Scholar]
- Smith AE, Ridding MC, Higgins RD, Wittert GA, Pitcher JB. Age-related changes in short-latency motor cortex inhibition. Exp Brain Res. 2009;198:489–500. doi: 10.1007/s00221-009-1945-8. [DOI] [PubMed] [Google Scholar]
- Smith AE, Ridding MC, Higgins RD, Wittert GA, Pitcher JB. Cutaneous afferent input does not modulate motor intracortical inhibition in ageing men. Eur J Neurosci. 2011b;34:1461–9. doi: 10.1111/j.1460-9568.2011.07869.x. [DOI] [PubMed] [Google Scholar]
- Smith AE, Sale MV, Higgins RD, Wittert GA, Pitcher JB. Male human motor cortex stimulus-response characteristics are not altered by aging. J Appl Physiol. 2011a;110:206–12. doi: 10.1152/japplphysiol.00403.2010. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–84. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Stokes MG, Chambers CD, Gould IC, Henderson TR, Janko NE, Allen NB, et al. Simple Metric For Scaling Motor Threshold Based on Scalp-Cortex Distance: Application to Studies Using Transcranial Magnetic Stimulation. J Neurophysiol. 2005;94:4520–7. doi: 10.1152/jn.00067.2005. [DOI] [PubMed] [Google Scholar]
- Swinnen SP, Verschueren S, Bogaerts H, Dounskaia N, Lee T, Stelmach G, et al. Age-related deficits in motor learning and differences in feedback processing during the production of a bimanual coordination pattern. Cogn Neuropsychol. 1998;15:439–66. doi: 10.1080/026432998381104. [DOI] [PubMed] [Google Scholar]
- Tecchio F, Zappasodi F, Pasqualetti P, De Gennaro L, Pellicciari MC, Ercolani M, et al. Age dependence of primary motor cortex plasticity induced by paired associative stimulation. Clin Neurophysiol. 2008;119:675–82. doi: 10.1016/j.clinph.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Todd G, Kimber TE, Ridding MC, Semmler JG. Reduced motor cortex plasticity following inhibitory rTMS in older adults. Clin Neurophysiol. 2010;121:441–7. doi: 10.1016/j.clinph.2009.11.089. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, et al. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol. 2000;2:503–13. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Mier H, Tempel LW, Perlmutter JS, Raichle ME, Petersen SE. Changes in brain activity during motor learning measured with PET: effects of hand of performance and practice. J Neurophysiol. 1998;80:2177–99. doi: 10.1152/jn.1998.80.4.2177. [DOI] [PubMed] [Google Scholar]
- Weise D, Mann J, Ridding M, Eskandar K, Huss M, Rumpf JJ, et al. Microcircuit mechanisms involved in paired associative stimulation-induced depression of corticospinal excitability. J Physiol. 2013;591:4903–20. doi: 10.1113/jphysiol.2013.253989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk GL, Walker LC, Price DL, Cork LC. Loss of NMDA, but not GABA-A, binding in the brains of aged rats and monkeys. Neurobiology of aging. 1991;12:93–8. doi: 10.1016/0197-4580(91)90047-n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P, Hiley CR, Goodhardt MJ, Carrasco LH, Keet JP, Williams IE, et al. Neocortical cholinergic neurons in elderly people. Lancet. 1977;1:668–71. doi: 10.1016/s0140-6736(77)92114-6. [DOI] [PubMed] [Google Scholar]
- Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, et al. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–45. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- Young-Bernier M, Davidson PS, Tremblay F. Paired-pulse afferent modulation of TMS responses reveals a selective decrease in short latency afferent inhibition with age. Neurobiol Aging. 2012a;33:835, e1–11. doi: 10.1016/j.neurobiolaging.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Young-Bernier M, Kamil Y, Tremblay F, Davidson PS. Associations between a neurophysiological marker of central cholinergic activity and cognitive functions in young and older adults. Behav Brain Funct. 2012b;8:17. doi: 10.1186/1744-9081-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Bernier M, Tanguay AN, Davidson PS, Tremblay F. Short-latency afferent inhibition is a poor predictor of individual susceptibility to rTMS-induced plasticity in the motor cortex of young and older adults. Front Aging Neurosci. 2014;6:182. doi: 10.3389/fnagi.2014.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Bernier M, Tanguay AN, Tremblay F, Davidson PS. Age Differences in Reaction Times and a Neurophysiological Marker of Cholinergic Activity. Can J Aging. 2015;34:471–80. doi: 10.1017/S0714980815000409. [DOI] [PubMed] [Google Scholar]
- Yu ZY, Wang W, Fritschy JM, Witte OW, Redecker C. Changes in neocortical and hippocampal GABAA receptor subunit distribution during brain maturation and aging. Brain Res. 2006;1099:73–81. doi: 10.1016/j.brainres.2006.04.118. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC. I-waves in motor cortex. J Clin Neurophysiol. 2000;17:397–405. doi: 10.1097/00004691-200007000-00005. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Izumi Y. NMDA receptors and metaplasticity: mechanisms and possible roles in neuropsychiatric disorders. Neurosci Biobehav Rev. 2012;36:989–1000. doi: 10.1016/j.neubiorev.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.