Abstract
There is an increased risk of acute rejection (AR) in HIV+ kidney transplant (KT) recipients. Induction immunosuppression is standard-of-care for those at high risk of AR; however, use in HIV+ patients is controversial given fears of increased infection rates. We sought to compare clinical outcomes between HIV+ KT recipients who were treated with 1) anti-thymocyte globulin (ATG), 2) interleukin-2 receptor antagonist (anti-IL2R) and 3) no induction. We studied 830 HIV+ KT recipients between 2000-2014 as captured in SRTR and compared rates of delayed graft function (DGF), AR, graft loss and death. Infections and hospitalizations were ascertained via ICD-9 codes in a subset of 308 with Medicare. Compared to no induction, neither induction agent was associated with an increased risk of infection (wHR=0.80, 95%CI: 0.55-1.18). HIV+ recipients who received induction spent fewer days in the hospital (wRR=0.70, 95%CI:0.52-0.95), had lower rates of DGF (wRR=0.66, 95%CI: 0.51-0.84), less graft loss (wHR=0.47, 95%CI:0.24-0.89), and a trend towards lower mortality (wHR=0.60, 95%CI: 0.24-1.28). Those who received induction with ATG had lower rates of acute rejection (wRR=0.59, 95%CI: 0.35-0.99). Induction in HIV+ KT recipients was not associated with increased infections; in fact, those receiving ATG, the most potent agent, had the lowest rates. In light of the high risk of acute rejection in this population, induction therapy should be strongly considered.
Introduction
It is estimated that over 30% of the 1.2 million HIV+ persons in the United States have chronic kidney disease and are at increased risk for end-stage renal disease (ESRD), and HIV is the third-leading cause of ESRD in African-Americans after diabetes and hypertension (1, 2). While HIV was initially a contraindication to kidney transplant (KT), over the past decade multiple studies have shown excellent patient and graft survival can be achieved both within multicenter prospective studies (3, 4) and outside the clinical trial setting (5-7). Use of this modality has been increasing, with over 800 HIV+ KTs performed since 2000 and 127 in the past year alone (8). However, a major challenge in this population is the high rate of acute rejection (AR), reported at 2-4 times that in HIV-uninfected recipients (5).
Currently, the vast majority of HIV- KT patients receive induction immunosuppression, shown to greatly reduce the risk of rejection and improve patient and graft survival (9). The two most commonly used induction agents are anti-thymocyte globulin (ATG), a potent polyclonal antibody that induces immunosuppression via long-term (months to years) depletion of T-lymphocytes and other immune cell populations, and an interleukin-2 receptor blocker (anti-IL2R), a less potent monoclonal antibody that blocks early T-cell activation without depleting the cell population (10). Recent guidelines from the Kidney Disease: Improving Global Outcomes transplant working group recommended anti-IL2R as the first line treatment for patients at low risk for rejection, and ATG for those at high risk (11).
While standard of care for most HIV- patients, the use of induction immunosuppression for HIV+ patients, particularly with ATG, remains controversial. On one hand, HIV+ patients have high rates of rejection and thus stand to benefit significantly from induction. However, the risks posed from prolonged lymphocyte depletion are of major concern given that HIV+ individuals are perceived to already have threatened T-cell populations and reduced immunity, states associated with an increased risk of opportunistic infections. A single-center non-randomized study of 20 patients published in 2006 reported more infections requiring hospitalization in 11 HIV+ KT recipients who received ATG; however, the indication for ATG was treatment for delayed graft function (DGF) or AR, not induction for the purposes of preventing AR (12). More recently, in the NIH multi-site study, HIV+ patients who received ATG in the first week after KT had 0.9 infections per year compared to 0.4 infections for those who received anti-IL2 or no induction; however, this was limited to 150 patients from a handful of centers, did not differentiate between ATG treatment for early rejection versus ATG for induction, and did not account for confounding or treatment selection bias (3).
These small studies, and the concerns outlined above, seem to have caused reluctance to use induction immunosuppression in HIV+ transplant recipients (13). Given the potential benefits of preventing rejection in this population yet concerns regarding risk of serious infections, we decided to investigate the role of induction in a large national cohort. The goals of our study were to 1) describe the national landscape of induction use for HIV+ KT recipients, and 2) compare the rates of adverse post-transplant outcomes including infections, hospitalizations, delayed graft function, acute rejection, graft loss, and death between those who received ATG or anti-IL2R and those who received no induction, using methods that best account for confounding and treatment selection bias.
Methods
Study Population
We studied 830 HIV+ patients aged>18 who received a KT between January 1, 2000 and December 1, 2014 as captured by the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data, submitted by the members of the Organ Procurement and Transplantation Network, on all donors, waitlisted candidates, and transplant recipients in the United States. The Health Resources and Services Administration of the U.S. Department of Health and Human Services provides oversight to the activities of Organ Procurement and Transplantation Network and SRTR contractors.
Induction Immunosuppression
Patients were divided into five categories of induction immunosuppression: those who received 1) no induction, 2) ATG only, 3) anti-IL2R only, 4) both ATG and anti-IL2R, and 5) other induction immunosuppression (including alemtuzumab). For each outcome of interest, we examined the effect of receiving any induction (groups 2-5) compared to no induction, then looked separately at the two most commonly used types of induction (ATG only and anti-IL2R only). To examine center-level patterns, we calculated the proportion of patients who received each type of induction at centers that had transplanted >5 HIV+ patients during the study period.
Ascertainment of Post-Transplant Infections
Analyses of post-KT infections and hospitalizations were limited to the subset of HIV+ patients who received a KT between 2000-2010 and had Medicare Part A and B as their primary form of insurance for two months prior to and one year following KT (n=308). This is a standard approach for studying Centers for Medicare and Medicaid Services' institutional claims data in KT patients (14-20). Diagnoses of the following infections within 1 year post-KT were ascertained via ICD-9 diagnosis codes: urinary tract infections, sepsis, oral candidiasis, pneumonia, respiratory viral infections, cytomegalovirus (CMV), herpes and adenovirus, Clostridium difficile, intestinal infections other than Clostridium difficile, and AIDS-defining illnesses (Candida esophagitis, cryptococcal meningitis, toxoplasmosis, pneumocystosis, CMV diseases, and cryptosporidiosis). Clinical Classification Software from the Agency for Healthcare Research and Quality was used to establish groups of ICD-9 codes for identification of each infection of interest (21). In addition, ICD-9 codes used for each infection category were independently reviewed by two infectious disease physicians (CD and RA).
Our outcome of interest was time to first infection of each type; we built separate Cox proportional hazards models to evaluate associations between induction category and each infectious outcome. To account for possible confounding by indication (treatment selection bias), patients were weighted by the inverse probability of receiving each induction type (see details of weighting below).
Inverse probability of treatment weights
Inverse probability of treatment weights (IPTW) were used to account for potential confounders in all statistical models. Briefly, IPTWs are constructed by modeling the probability of receiving a given treatment. Models of the outcome of interest are then adjusted for the inverse probability of receiving the treatment rather than for individual covariates. This approach was advantageous in this setting because many of the outcomes were relatively rare, thus modeling, and then adjusting for, the probability of the exposure allowed us to account for more confounders than would have been possible using a traditional regression approach. To construct the weights, we modeled the probability of receiving each treatment compared to receiving no induction given the patient's age, gender, race, number of HLA mismatches, panel reactive antibody, HCV status, donor age, donor type (live or deceased), expanded criteria donor, and donation after cardiac death. Weights were stabilized; a robust sandwich estimator was used to prevent underestimation of the variance. Good balance was achieved on all confounders (standardized absolute mean difference <0.2 for all covariates, and overall <0.1 for all models) (22).
Ascertainment of Days Spent Hospitalized
Length of stay (LOS for the transplant hospitalization was obtained from the KT recipient worksheet collected by the OPTN. For the subset of patients who had Medicare as their primary insurance, we also calculated the total days spent in the hospital in the first year post-KT using methods previously described (14-17, 23). Negative binomial regression with IPTW was used to examine associations between induction type and 1) KT LOS, and 2) hospitalized day in the year post-KT.
Ascertainment of Early Hospital Readmission
Hospital admissions were identified from Medicare claims data using methods previously described.(14-17, 23) We calculated the rate of early hospital readmission (a readmission occurring within 30 days of the transplant) and used modified Poisson regression with IPTW to examine associations between induction and early hospital readmission.
Delayed Graft Function and Acute Rejection in the First Year
Delayed graft function (DGF) and AR were ascertained from the adult KT recipient follow-up worksheet collected by the OPTN; as such, these analyses were not restricted to patients with Medicare. DGF was defined as requiring dialysis within the first week post-transplant; AR as any report of an AR episode in the first year. As a sensitivity analysis, we also examined those with biopsy confirmed AR only. We used modified Poisson regression with IPTW to examine associations between induction and these outcomes (24).
Death Censored Graft Loss and Mortality
We compared the cumulative incidence of 1) death-censored graft loss and 2) mortality, both within the first year post-KT and by end of study (December 1, 2014) between induction categories using Cox proportional hazards models, weighted with IPTW as described above. As with DGF and AR, these outcomes were based on data reported to the OPTN as well as SRTR linkage to CMS and the Social Security Death Master File (to augment graft loss and mortality ascertainment), and thus these analyses were also not restricted to patients with Medicare. Weighted survival curves were generated comparing those receiving any induction to those receiving no induction. For both graft loss and mortality there were ≤ 10 events in the first year in the ATG and anti-IL2R induction categories; as such, only the combined estimate including all induction categories is shown.
Sensitivity Analyses
We repeated the primary analyses of infection, death-censored graft loss, and mortality 1) additionally adjusting for use of a calcineurin-inhibitor based maintenance regimen, and 2) stratified by era (2000-2007 versus 2008-2014) to explore whether inferences differed after the introduction of integrase inhibitors in 2008.
Statistical Methods
All statistical tests used a two-sided alpha of 0.05. Confidence intervals are reported per the method of Louis and Zeger (25). All analyses were performed using multiprocessor Stata version 13.0/MP for Linux (Stata-Corp, College Station, Texas).
Results
Induction Use in HIV+ Compared to HIV- Patients
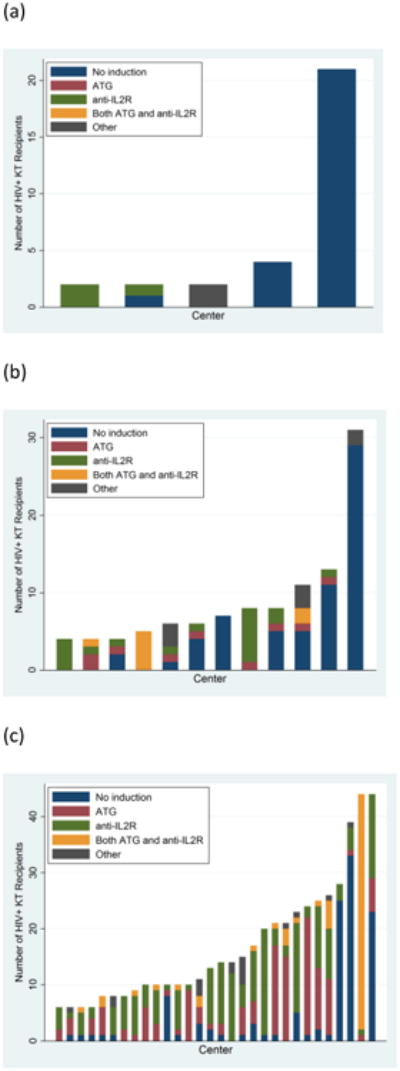
Among HIV+ KT recipients, 30.4% received no induction, 32.3% received anti-IL2R only, 22.8% received ATG only, 9.6% received both ATG and anti-IL2R, and 4.9% received alemtuzumab or other forms of induction, compared to 20.8%, 17.2%, 42.1%, 4.2%, and 15.7% in a matched cohort of HIV- patients. 101 centers transplanted at least 1 HIV+ recipient during the study period, and 32 centers transplanted >5. There was wide variation in induction use for HIV+ patients, with some centers almost exclusively using no induction and others preferentially using ATG or anti-IL2R (Figure 1). The number of centers transplanting HIV+ patients has increased significantly since 2000, and centers are increasingly using ATG and anti-IL2R. However, even in recent years several high volume centers use no induction almost exclusively for HIV+ patients.
Figure 1. Center Level Patterns in Induction Use for HIV+ Kidney Transplant Recipients (a) 2000-2003, (b) 2004-2007, (c) 2008-2014.
Characteristics of HIV+ Patients, by Induction Category
HIV+ patients who received ATG, anti-IL2R, and no induction were similar in regards to gender, race, number of zero-HLA mismatches and HBsAg positivity; they received a similar proportion of live donors, and, for those who received deceased donors, a similar percentage were classified as expanded criteria or donation after cardiac death (Table 1). Those who received ATG were slightly older (mean 49.6 years for ATG vs. 47.8 and 47.1 for those were received anti-IL2R or no induction, respectively, p=0.09), had a higher peak PRA (mean 23.2 vs. 15.3 and 17.3, p=0.02), and had longer CIT (mean 15.9 hours vs 13.2 and 13.9 hours, p <0.001). ATG and anti-IL2R recipients were less often HCV-antibody positive (22.2% for ATG and 18.3% for anti-IL2R, compared to 31.3% of those who received no induction, p<0.001).
Table 1. Characteristics of HIV+ kidney transplant recipients, by induction type.
| None (N=252) | ATG (N=189) | Anti-IL2R (N=268) | Both (n=80) | Other (n=41) | p-value | |
|---|---|---|---|---|---|---|
| Age (y, mean±SD) | 47.1 ± 9.5 | 49.6 ± 9.5 | 47.7 ± 7.3 | 47.7±7.3 | 47.3±9.0 | 0.09 |
| Female sex (%) | 20.2 | 26.5 | 21.6 | 25.0 | 14.6 | 0.4 |
| Recipient race (%) | 0.7 | |||||
| White | 18.3 | 19.6 | 17.5 | 17.5 | 17.1 | |
| African American | 70.2 | 69.3 | 75.7 | 76.3 | 70.7 | |
| Hispanic/Latino | 8.7 | 9.5 | 6.0 | 3.8 | 9.8 | |
| Other/multi-racial | 2.8 | 1.6 | 0.7 | 2.5 | 2.4 | |
| Zero HLA mismatches (%) | 9.1 | 6.3 | 6.0 | 8.8 | 4.9 | 0.6 |
| Peak PRA (mean±SD) | 17.3 ± 27.0 | 23.2 ± 32.4 | 15.3 ± 25.2 | 20.1±29.0 | 26.9±35.1 | 0.02 |
| HCV positive (%) | 31.3 | 22.2 | 18.3 | 8.8 | 17.1 | <0.001 |
| HBsAg positive (%) | 6.7 | 4.8 | 3.0 | 6.3 | 4.9 | 0.4 |
| Donor age (y, mean±SD) | 38.6 ± 14.1 | 37.5 ± 15.5 | 37.3±14.6 | 35.9±13.7 | 39.8±15.5 | 0.5 |
| Live donor (%) | 22.6 | 21.2 | 28.4 | 28.7 | 26.8 | 0.3 |
| ECD (%) | 10.8 | 8.7 | 13.0 | 1.8 | 16.7 | 0.1 |
| DCD (%) | 14.4 | 15.4 | 10.4 | 7.0 | 10.0 | 0.4 |
| CIT (hr, mean±SD) | 13.9 ± 10.6 | 16.9 ± 11.1 | 13.2 ± 9.8 | 24.9±23.7 | 16.5±14.4 | <0.001 |
PRA = Panel Reactive Antibody
HCV = Hepatitis C Virus
HBsAg = Hepatitis B Surface Antigen
ECD = Expanded Criteria Donor
DCD= Donation After Cardiac Death
CIT = Cold ischemic time
Infections in HIV+ Patients, by Induction Category
For most infections, rates were similar between induction categories, and slightly lower than in those who received no induction (Figure 2). Among HIV+ patients, 52.8% of ATG recipients, 52.5% of anti-IL2R recipients, and 55.7% of those receiving no induction developed at least one infection in the first year post-KT. UTIs were the most common infection across all induction categories (37.7% of ATG, 32.3% of anti-IL2R, and 31.3% of no induction), followed by sepsis (15.1% of ATG, 13.1% anti-IL2R, and 19.1% of no induction), AIDS-defining infections (9.4% of ATG, 12.1% of anti-IL2R, and 9.6% of no induction), Clostridium difficile (1.9% of ATG, 6.1% of anti-IL2R, and 6.1% of no induction), pneumonia (5.7% of ATG, 12.1% of anti-IL2R, and 13.9% of no induction), CMV (3.8% of ATG, 10.1% of anti-IL2R, and 7.0% of no induction), oral candidiasis (3.8% of ATG, 7.1% of anti-IL2R, 3.5% of no induction), herpes and adenovirus (0% of ATG, 2.0% of anti-IL2R, and 2.6% of no induction), and respiratory viral infections (0% of ATG, 2.0% of anti-IL2R, and 1.7% of no induction).
Figure 2. Proportion of HIV Positive Patients Who Developed Infections in the First Year Post Kidney Transplant, by Induction.
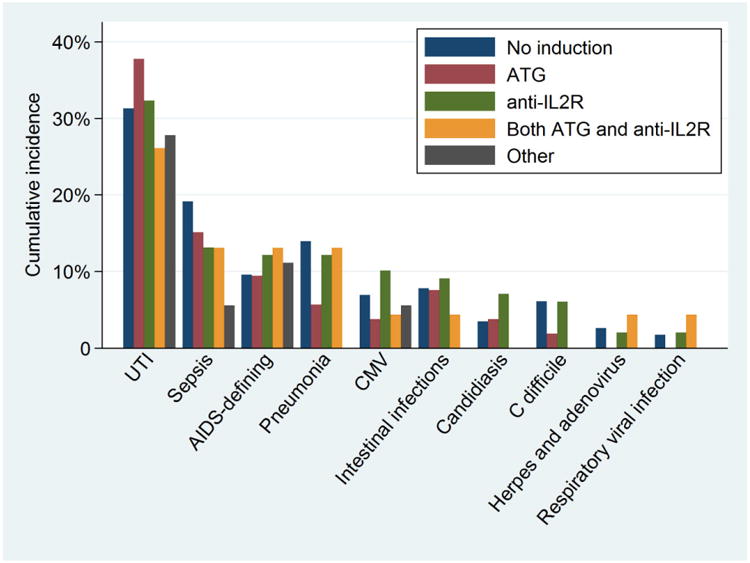
The majority of AIDS-defining infections were CMV-related; however, CMV is a common post-transplant complication in HIV- KT recipients.(26) Non-CMV AIDS-defining infections were rare and occurred at similar rates between induction groups: 3 cases of PCP (2 in the anti-IL2R group, 1 in the no induction group), 1 case of coccidioidomycosis (ATG group), 1 case of TB (anti-IL2R group), 1 case of Kaposi's sarcoma (ATG group) and 8 cases of Candida esophagitis (3 in the ATG group, 2 in the anti-IL2R group, and 3 in the no induction group). In adjusted analyses, ATG recipients had lower or equivalent rates of all infections compared to those who did not receive induction.
Days Spent Hospitalized and Early Hospital Readmissions in HIV+ Patients, by Induction Category
HIV+ patients who received ATG or anti-IL2 spent 8.1 and 7.4 days in the hospital before discharge from KT admission, respectively, compared to 10.8 days for those who did not receive induction (wRR any induction=0.750.901.08, wRR ATG=0.730.891.08, wRR anti-IL2R=0.680.820.98, Table 2). In the year post-KT, those who received ATG or anti-IL2R induction spent on average 22.7 and 21.8 days in the hospital, versus 31.4 days for those who did not receive induction (wRR any induction=0.520.700.95, wRR ATG=0.450.691.07, wRR anti-IL2R=0.470.691.00). Of those receiving ATG or anti-IL2, 37.3% and 42.5% had an early hospital readmission (readmission within 30 days of transplant), compared to 45.3% of those who did not receive induction (wRR any induction=0.630.851.15, wRR ATG=0.520.781.16, wRR anti-IL2R=0.710.981.36).
Table 2. Hazard of (a) Infection, (b) Hospitalization, and (c) Delayed Graft Function and Acute Rejection within the First Year Post Kidney Transplant Among HIV Infected Recipients, by Induction Therapy.
| (a) Infections* | ||||
|---|---|---|---|---|
| No Induction | Any Induction | ATG | Anti-IL2R | |
| UTI | Reference | 0.530.881.46 | 0.601.061.88 | 0.591.031.79 |
| Sepsis | Reference | 0.250.511.04 | 0.310.721.70 | 0.290.641.43 |
| Pneumonia | Reference | 0.250.611.53 | 0.100.381.35 | 0.330.791.91 |
| AIDS-Defining | Reference | 0.350.952.61 | 0.300.932.84 | 0.641.674.36 |
| Other Intestinal Infections | Reference | 0.441.062.58 | 0.341.153.86 | 0.681.975.71 |
| CMV | Reference | 0.220.782.79 | 0.100.502.47 | 0.631.966.06 |
| Oral Candidiasis | Reference | 0.883.6815.36 | 0.482.8016.23 | 1.616.4425.75 |
| C. Difficile | Reference | 0.220.661.98 | 0.050.383.18 | 0.521.886.79 |
| Any Infection | Reference | 0.550.801.18 | 0.540.871.39 | 0.701.081.67 |
| (b) Hospitalization** | ||||
| LOS for KT | Reference | 0.750.901.08 | 0.740.891.08 | 0.680.820.98 |
| Days in hospital | Reference | 0.520.700.95 | 0.450.691.07 | 0.470.691.00 |
| Early Hospital Readmission | Reference | 0.630.851.15 | 0.520.781.16 | 0.710.981.36 |
| (c) Delayed Graft Function and Acute Rejection*** | ||||
| DGF | Reference | 0.510.660.84 | 0.550.730.97 | 0.520.700.94 |
| Acute Rejection | Reference | 0.600.901.34 | 0.350.590.99 | 0.490.771.21 |
| Biopsy-Confirmed | Reference | 0.550.861.36 | 0.310.561.03 | 0.510.841.40 |
Subscript indicates upper and lower bounds of a 95% confidence interval
Bold indicates statistical significance at p <0.05 level
Weighted hazard ratios derived from Cox proportional hazards models
Weighted relative rates derived from negative binomial regression (mean LOS and time hospitalized), and modified Poisson regression (EHR)
Weighted relative rates derived from modified Poisson regression models
ATG = anti-thymocyte globulin
CMV = cytomegalovirus
UTI = urinary tract infection
ATG = anti-thymocyte globulin
LOS = length of stay
EHR = early hospital readmissions
DGF = Delayed Graft Function
Delayed Graft Function and Acute Rejection in HIV+ Patients, by Induction Category
Those who received induction were 34% less likely to have DGF (wRR =0.510.660.84, Table 2). This finding persisted in analyses limited to ATG recipients (wRR =0.550.730.97), and to anti-IL2R recipients (wRR=0.520.700.94). Overall, those who received induction did not have significantly lower rates of acute rejection (wRR = 0.600.901.34); however, when stratified by induction group, those who received ATG had over 40% lower rates of both reported and biopsy-confirmed AR (wRR = 0.350.590.99 and wRR = 0.310.561.03, respectively). Anti-IL2R recipients had 23% and 16% lower rates of reported and biopsy confirmed AR; however, these reductions were not statistically significant (wRR =0.490.771.21 and wRR =0.510.841.40, respectively).
Death-Censored Graft Loss and Mortality in HIV+ Patients, by Induction Category
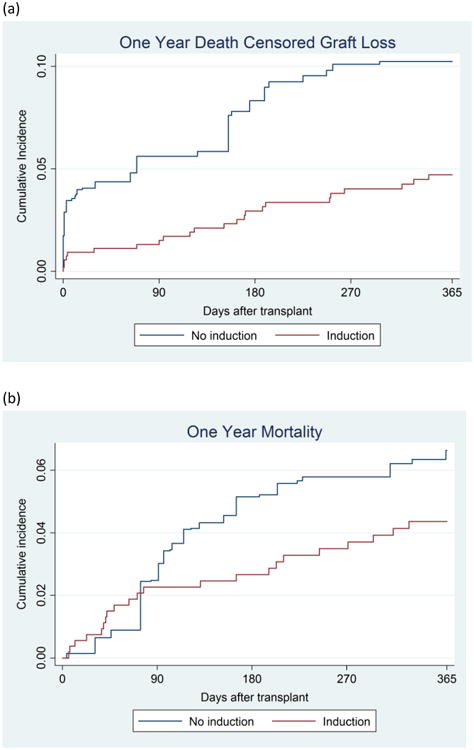
The median follow-up time for patients in our study was 3.4 years. Induction recipients had less than half the hazards of death-censored graft failure in the year post-KT compared to those who received no induction (wHR = 0.240.470.89, Figure 3A). Furthermore, induction recipients had 0.60-fold lower hazards of death in the first year post-KT (wHR =0.290.601.28, Figure 3B); however, this was not statistically significant. Inferences were similar in analyses censored at end of study rather than 1 year (wHR = 0.490.690.97 and wHR = 0.651.021.62.), for death-censored graft loss and death, respectively.
Figure 3. Hazard of (a) Death-Censored Graft Loss, and (b) Mortality in the First Year After Kidney Transplant, HIV+ Patients Who Receive Induction Compared to Those who Receive No Induction.
Sensitivity Analyses
In analyses adjusting for calcineurin inhibitor use and stratifying by era (2000-2007 and 2008-2014) results were similar, with ATG recipients having lower or equivalent rates of all infections compared to those who received no induction (data not shown). Induction use was associated with a 0.81-fold lower hazard of death-centered graft loss between 2000-2007 (wHR = .0.32 0.812.04)), and a 0.41-fold lower hazard between 2008-2014 (wHR = 0.18 0.410.90)), adjusting for calcineurin inhibitor use. There was a trend towards lower mortality in both eras; however, this was not statistically significant (wHR = .0.180.541.66 and wHR = .0.250.651.70, 2000-2007 and 2008-2014, respectively).
Discussion
In a large, national cohort of 830 HIV+ KT recipients, we found wide variation in the use of induction immunosuppression, with more than 30% HIV+ patients receiving no induction, compared with only 20% of their HIV- counterparts. Contrary to conventional wisdom, use of induction, including the lymphocyte depleting agent ATG, was not associated with an increased risk of infections. Despite the fact that induction recipients were of higher risk phenotypes for AR, we observed significantly lower rates of DGF, AR, graft loss, and days spent hospitalized in the first year post-KT, as well as a trend towards lower mortality.
Our results suggest that the benefits of induction immunosuppression to prevent graft rejection in HIV+ KT recipients far outweigh the perceived risk for increased infections. While smaller previous studies suggested an increased risk of infections and graft loss,(3, 12) our study addresses a number of limitations of these previous studies. We have the largest sample size to date, and our cohort is nationally representative rather than a select study population. Furthermore, we accounted for confounding and treatment selection bias, which previous studies did not do, and we did this using IPTW, a method that allowed us to adjust for many clinical and demographic factors even when modeling relatively rare outcomes such as graft loss and death. Finally, we studied a wide range of transplant outcomes and found a consistent pattern across all of them. If serious infections were truly more common among ATG or anti-IL2R recipients, we would expect this to lead to elevated rates of hospitalizations, graft loss, and death: the fact that all these adverse events are less common among both induction groups provides strong evidence that induction is not harmful and may be beneficial.
Our finding that induction immunosuppression with a lymphocyte depleting agent was associated with lower rates of certain infections may initially seem counterintuitive, and is certainly contrary to conventional wisdom. However, this could be explained by the known association between graft rejection and risk of subsequent infection (27-30). In other words, with the development of AR, high-dose immunosuppressive agents must be administered to prevent graft failure; thus treatment of AR often leads to secondary infectious complications. Our observation that HIV+ KT recipients who did not receive induction had higher rates of infections suggests that preventing AR up front via induction rather than treating it after the fact may be the best strategy to minimize post-transplant infections in HIV+ patients. Preventing rejection may particularly protect against CMV, which occurred at half the rate in ATG recipients, a finding also reported in HIV- patients (31).
While both ATG and anti-IL2R were associated with lower rates of DGF, only ATG was associated with a lower rate of AR. This is consistent with recent guidelines from the Kidney Disease: Improving Global Outcomes (KDIGO) transplant working group who recommended anti-IL2R as the first line treatment for patients at low risk for rejection, but ATG as the first line treatment for those at high risk (11). Our results suggest these recommendations can safely be extended to patients with well-controlled HIV infection.
While some centers used multiple induction types, there were several high-volume centers that strongly favored no induction for HIV+ patients, and a number of lower volume centers that strongly favored either ATG or anti-IL2R. This suggests that the choice of induction may be driven more by center-level preference than individual patient characteristics. While this would minimize potential confounding by indication, it is possible that differences in center-level practices might contribute to observed differences in outcomes by induction type. Because the majority of centers performed 5 or fewer HIV+ transplants, we did not have sufficient power to explore center-level differences.
Our study has several limitations that merit consideration. Since participants were not randomized, it is possible that those who received induction were different from those who did not. As discussed previously, induction choice may reflect center-level preference more than individual characteristics. However, we suspect that at centers using multiple induction types, ATG was more likely to be given to those with risk factors for AR. Consistent with this, patients in our study who received ATG had higher peak PRAs and received kidneys with longer CITs. As such, we would expect our findings might underestimate the effectiveness of induction at reducing the risk of AR and DGF; however, we still observed much lower rates among the induction group, despite this potential bias. It is also possible that those who did not receive induction had more poorly controlled HIV; however, this seems unlikely as current practice guidelines require that HIV+ KT candidates are somewhat homogeneous in terms of HIV control, with undetectable HIV viral loads on treatment and reconstituted immune systems with CD4 counts > 200 cells/uL (32). Unfortunately it is impossible to know what drove decision making regarding induction and thus our hypothesis about the direction of bias is speculative. We did not have data on opportunistic infections prior to KT or whether patients were placed on prophylactic regimens; thus we were unable to assess whether this impacted induction choice. Infections were ascertained via ICD-9 codes rather than directly observed; however, the rates and distributions of infection types were similar to what was reported in the NIH multisite study, and any bias would only result if there were differential errors in ICD-9 coding between those who received induction and those who did not. Finally, analyses of infections and hospitalizations were limited to patients who had Medicare as their primary form of insurance. While patients without Medicare may have different rates of infections, a bias would only result if the relationship between induction therapy and infections differed by insurance type. Furthermore, our results were consistent across multiple outcomes (including those such as AR, DGF, death, and graft loss) that were captured for all patients and not limited to the subset with Medicare.
Our findings that induction immunosuppression in HIV+ KT recipients did not increase rates of infections and improves transplant outcomes are plausibly due to the fact that AR is the most common complication observed after transplant in this population. The initial reports of increased rejection were surprising to many in the field who hypothesized that rejection would be lower in the HIV+ population due to impaired T cell immunity. Given the 2-4 fold increased risk of AR, (3, 5) a remaining question is whether ATG use should be considered for all HIV+ KT recipients. The answer largely depends on the mechanism for elevated rejection rates which is an area of intense investigation. Proposed explanations include drug interactions between HIV medications and immunosuppressants (33, 34) versus a biologic predisposition to AR in HIV+ individuals due to immune dysregulation (35, 36). Practice guidelines have addressed the first possibility by recommending avoidance of ritonavir-boosted protease inhibitors in HIV+ transplant recipients to prevent underexposure to the calcineurin inhibitor class of immunosuppressants (32). However, HIV+ individuals may be at high risk for AR even when problematic drug interactions are avoided due to enhanced allosensitization and an increased memory T cell phenotype (37, 38).
In the era of effective HAART, while AIDS-related infections are declining, HIV+ individuals remain at risk for chronic diseases. High levels of inflammation due to persistent immunologic abnormalities have been postulated as one mechanism for the high burden of heart, liver, kidney, bone, and neurodegenerative diseases observed in HIV+ patients. This persistent immune dysregulation may also drive the high rejection rates seen in HIV+ KT recipients. In our study, use of a potent immunosuppressant decreased morbidity from immunologic complications of transplant without increasing infections. More broadly, our results suggest that fear of infections consequences should not deter the use of immunosuppressive drugs for inflammatory conditions in patients with well-controlled HIV.
Acknowledgments
Lauren Kucirka and Dorry Segev had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
This work was supported by grant numbers K24DK101828 and F30DK095545 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Abbreviations
- AR
acute rejection
- ATG
anti-thymocyte globulin
- KT
kidney transplant
- Anti-IL2R
anti-interleukin-2 receptor
- DGF
delayed graft function
- SRTR
scientific registry of transplant recipients
- HIV
Human Immunodeficiency Virus
- ESRD
end-stage renal disease
- IPTW
inverse probability of treatment weights
- CMS
Center for Medicare and Medicaid Services
- LOS
length of stay
- OPTN
Organ Procurement and Transplant Network
- CMV
cytomegalovirus
- wRR
weighted relative risk
- wHR
weighted hazard ratio
- KDIGO
Kidney Disease: Improving Global Outcomes transplant working group
- HAART
Highly active anti-retroviral therapy
Footnotes
Disclaimer: The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Contributor Information
Lauren M Kucirka, Email: lauren@jhmi.edu.
Christine M Durand, Email: christinedurand@jhmi.edu.
Sunjae Bae, Email: sbae4@jhu.edu.
Robin K Avery, Email: ravery4@jhmi.edu.
Jayme E Locke, Email: jlocke@uabmc.edu.
Babak J Orandi, Email: borandi1@jhmi.edu.
Mara McAdams-DeMarco, Email: mmcadam4@jhmi.edu.
Morgan E Grams, Email: mgrams2@jhmi.edu.
References
- 1.Monahan M, Tanji N, Klotman PE. HIV-associated nephropathy: an urban epidemic. Semin Nephrol. 2001;21(4):394–402. doi: 10.1053/snep.2001.23771. [DOI] [PubMed] [Google Scholar]
- 2.Szczech LA, Gange SJ, van der Horst C, Bartlett JA, Young M, Cohen MH, et al. Predictors of proteinuria and renal failure among women with HIV infection. Kidney Int. 2002;61(1):195–202. doi: 10.1046/j.1523-1755.2002.00094.x. [DOI] [PubMed] [Google Scholar]
- 3.Stock PG, Barin B, Murphy B, Hanto D, Diego JM, Light J, et al. Outcomes of kidney transplantation in HIV-infected recipients. N Engl J Med. 2010;363(21):2004–14. doi: 10.1056/NEJMoa1001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harbell J, Fung J, Nissen N, Olthoff K, Florman SS, Hanto DW, et al. Surgical complications in 275 HIV-infected liver and/or kidney transplantation recipients. Surgery. 2012;152(3):376–81. doi: 10.1016/j.surg.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locke JE, James NT, Mannon RB, Mehta SG, Pappas PG, Baddley JW, et al. Immunosuppression regimen and the risk of acute rejection in HIV-infected kidney transplant recipients. Transplantation. 2014;97(4):446–50. doi: 10.1097/01.TP.0000436905.54640.8c. [DOI] [PubMed] [Google Scholar]
- 6.Locke JE, Mehta S, Reed RD, MacLennan P, Massie A, Nellore A, et al. A National Study of Outcomes among HIV-Infected Kidney Transplant Recipients. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Locke JE, Montgomery RA, Warren DS, Subramanian A, Segev DL. Renal transplant in HIV-positive patients: long-term outcomes and risk factors for graft loss. Arch Surg. 2009;144(1):83–6. doi: 10.1001/archsurg.2008.508. [DOI] [PubMed] [Google Scholar]
- 8.OPTN. Scientific Registry of Transplant Recipients Standard Analysis Files. 2015 [Google Scholar]
- 9.Cai J, Terasaki PI. Induction immunosuppression improves long-term graft and patient outcome in organ transplantation: an analysis of United Network for Organ Sharing registry data. Transplantation. 2010;90(12):1511–5. doi: 10.1097/TP.0b013e3181fecfcb. [DOI] [PubMed] [Google Scholar]
- 10.Gabardi S, Martin ST, Roberts KL, Grafals M. Induction immunosuppressive therapies in renal transplantation. Am J Health Syst Pharm. 2011;68(3):211–8. doi: 10.2146/ajhp090636. [DOI] [PubMed] [Google Scholar]
- 11.KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 12.Carter JT, Melcher ML, Carlson LL, Roland ME, Stock PG. Thymoglobulin-associated Cd4+ T-cell depletion and infection risk in HIV-infected renal transplant recipients. Am J Transplant. 2006;6(4):753–60. doi: 10.1111/j.1600-6143.2006.01238.x. [DOI] [PubMed] [Google Scholar]
- 13.Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582–9. doi: 10.1038/nrneph.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAdams-Demarco MA, Grams ME, Hall EC, Coresh J, Segev DL. Early hospital readmission after kidney transplantation: patient and center-level associations. Am J Transplant. 2012;12(12):3283–8. doi: 10.1111/j.1600-6143.2012.04285.x. [DOI] [PubMed] [Google Scholar]
- 15.McAdams-Demarco MA, Grams ME, King E, Desai NM, Segev DL. Sequelae of early hospital readmission after kidney transplantation. Am J Transplant. 2014;14(2):397–403. doi: 10.1111/ajt.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAdams-DeMarco MA, Law A, Salter ML, Boyarsky B, Gimenez L, Jaar BG, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61(6):896–901. doi: 10.1111/jgs.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McAdams-DeMarco MA, Law A, Salter ML, Chow E, Grams M, Walston J, et al. Frailty and early hospital readmission after kidney transplantation. Am J Transplant. 2013;13(8):2091–5. doi: 10.1111/ajt.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong SP, Kreuter W, Curtis JR, Hall YN, O'Hare AM. Trends in In-Hospital Cardiopulmonary Resuscitation and Survival in Adults Receiving Maintenance Dialysis. JAMA Intern Med. 2015 doi: 10.1001/jamainternmed.2015.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ball AM, Gillen DL, Sherrard D, Weiss NS, Emerson SS, Seliger SL, et al. Risk of hip fracture among dialysis and renal transplant recipients. JAMA. 2002;288(23):3014–8. doi: 10.1001/jama.288.23.3014. [DOI] [PubMed] [Google Scholar]
- 20.Schnitzler MA, Hollenbeak CS, Cohen DS, Woodward RS, Lowell JA, Singer GG, et al. The economic implications of HLA matching in cadaveric renal transplantation. N Engl J Med. 1999;341(19):1440–6. doi: 10.1056/NEJM199911043411906. [DOI] [PubMed] [Google Scholar]
- 21.Healthcare Cost and Utilization Project: A Federal State-Industry Partnership in Health Data: Clinical Classifications Software 2014. Agency for Healthcare and Research Quality. 2014 [Google Scholar]
- 22.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–60. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Grams ME, McAdams Demarco MA, Kucirka LM, Segev DL. Recipient age and time spent hospitalized in the year before and after kidney transplantation. Transplantation. 2012;94(7):750–6. doi: 10.1097/TP.0b013e31826205b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 25.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics. 2009;10(1):1–2. doi: 10.1093/biostatistics/kxn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cordero E, Casasola C, Ecarma R, Danguilan R. Cytomegalovirus disease in kidney transplant recipients: incidence, clinical profile, and risk factors. Transplant Proc. 2012;44(3):694–700. doi: 10.1016/j.transproceed.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 27.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357(25):2601–14. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 28.Hibberd PL, Tolkoff-Rubin NE, Conti D, Stuart F, Thistlethwaite JR, Neylan JF, et al. Preemptive ganciclovir therapy to prevent cytomegalovirus disease in cytomegalovirus antibody-positive renal transplant recipients. A randomized controlled trial. Ann Intern Med. 1995;123(1):18–26. doi: 10.7326/0003-4819-123-1-199507010-00002. [DOI] [PubMed] [Google Scholar]
- 29.Conti DJ, Freed BM, Singh TP, Gallichio M, Gruber SA, Lempert N. Preemptive ganciclovir therapy in cytomegalovirus-seropositive renal transplants recipients. Arch Surg. 1995;130(11):1217–21. doi: 10.1001/archsurg.1995.01430110075014. discussion 1221-2. [DOI] [PubMed] [Google Scholar]
- 30.Portela D, Patel R, Larson-Keller JJ, Ilstrup DM, Wiesner RH, Steers JL, et al. OKT3 treatment for allograft rejection is a risk factor for cytomegalovirus disease in liver transplantation. J Infect Dis. 1995;171(4):1014–8. doi: 10.1093/infdis/171.4.1014. [DOI] [PubMed] [Google Scholar]
- 31.Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355(19):1967–77. doi: 10.1056/NEJMoa060068. [DOI] [PubMed] [Google Scholar]
- 32.Blumberg EA, Rogers CC. Human immunodeficiency virus in solid organ transplantation. Am J Transplant. 2013;13(4):169–78. doi: 10.1111/ajt.12109. [DOI] [PubMed] [Google Scholar]
- 33.Marfo K, Greenstein S. Antiretroviral and immunosuppressive drug-drug interactions in human immunodeficiency virus-infected liver and kidney transplant recipients. Transplant Proc. 2009;41(9):3796–9. doi: 10.1016/j.transproceed.2009.06.186. [DOI] [PubMed] [Google Scholar]
- 34.Frassetto LA, Browne M, Cheng A, Wolfe AR, Roland ME, Stock PG, et al. Immunosuppressant pharmacokinetics and dosing modifications in HIV-1 infected liver and kidney transplant recipients. Am J Transplant. 2007;7(12):2816–20. doi: 10.1111/j.1600-6143.2007.02007.x. [DOI] [PubMed] [Google Scholar]
- 35.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–55. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10(1):87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brehm MA, Markees TG, Daniels KA, Greiner DL, Rossini AA, Welsh RM. Direct visualization of cross-reactive effector and memory allo-specific CD8 T cells generated in response to viral infections. J Immunol. 2003;170(8):4077–86. doi: 10.4049/jimmunol.170.8.4077. [DOI] [PubMed] [Google Scholar]


