Abstract
MicroRNAs (miRNAs) play important roles in the pathogenesis of pulmonary arterial hypertension (PAH). However, the pathways targeted by miRNAs in PAH have not been systematically investigated. We aim to identify dysregulated miRNAs for patients with idiopathic PAH (IPAH).
miRNA profiling was performed on lung tissue total RNA from eight IPAH patients and eight control subjects. Real-time quantitative RT-PCR (qRT-PCR) was used for validation of miRNA and mRNA expression levels in 14 IPAH patients and 14 control subjects.
Pathway enrichment analysis showed Wnt/β-catenin signaling is among the top PAH related pathways enriched in target genes of dysregulated miRNAs. We confirmed the significant increased expression levels of 5 miRNAs (let-7a-5p, miR-26b-5p, miR-27b-3p, miR-199a-3p and miR-656) targeting major PAH-related pathways. Moreover, qRT-PCR validation of Wnt/β-catenin pathway activation indicated multiple genes including receptors (FZD4, FZD5), core molecule (CTNNB1), and downstream targets (CCND1, VEGFA and AXIN2) were significantly upregulated. The expression level of miR-199b-5p was positively correlated with patients’ hemodynamics (PVR: p=0.038) and pulmonary vascular remodeling (muscularization: p=0.021). We confirmed overexpression of miR-199b-5p in hypoxic pulmonary arterial endothelial cells that negatively regulates GSK3B expression.
In summary, miRNAs influence the pathogenesis of PAH by regulating major PAH related pathways including Wnt/β-catenin in end-stage IPAH.
Keywords: microRNA, Wnt/β-catenin, idiopathic pulmonary arterial hypertension, microarray
INTRODUCTION
Idiopathic pulmonary arterial hypertension (idiopathic PAH, IPAH) is a debilitating disease characterized by elevated pulmonary artery pressure (PAP) and pulmonary vascular resistance (PVR). Although the incidence of IPAH is low [1], it is often life-threatening, causing right heart failure. The main pathological changes of IPAH are pulmonary vascular remodeling located in but not limited to small pulmonary arteries, including intima and media thickening, small artery muscularization, plexiform lesion, perivascular inflammation and in situ thrombosis [2–4]. The biological behaviors of IPAH are similar to those of cancer. Traits of cancer, such as monoclonal cell proliferation [5], apoptosis resistance [6], Warburg effect [7] and angiogenesis [8] are found in IPAH.
Wnt signaling pathways consist of Wnt/β-catenin pathway (the canonical Wnt pathway), the noncanonical planar cell polarity pathway (Wnt/PCP pathway) and the noncanonical Wnt/calcium pathway. Wnt pathways have been identified to be critical in embryonic development, cell proliferation, and carcinogenesis. Recent studies have revealed Wnt also plays a role in the pathogenesis of PAH. In the context of human lung tissue, both the Wnt/β-catenin [9] and Wnt/PCP pathways [10] are activated in pulmonary vasculature from patients with PAH. Thus, the Wnt signaling pathways have emerged as attractive treatment targets in PAH given their role in the preservation of pulmonary vascular homeostasis and the recent development of Wnt-specific compounds and biological therapies capable of modulating Wnt pathway activity.
microRNAs (miRNAs or miRs), a type of non-coding RNA, are key regulators in gene post-transcriptional regulation [11]. Previous studies have uncovered dysregulation of miRNAs in both tissue and plasma that are correlated with PAH severity and prognosis [12,13]. Systematic approaches combining transcriptomic data with the powerful predictive methods of network biology are warranted to identify essential pathways in the control of PAH and ultimately novel therapeutic interventions.
We hypothesized that an unbiased approach using miRNA profiling followed by pathway enrichment analysis would help identify dysregulated miRNAs and major pathways involved in the pathogenesis of PAH. Furthermore, these miRNAs would be correlated with hemodynamic and pathologic severity of the disease.
METHODS
Patient enrolment and tissue sample preparation
A total of 22 IPAH patients and 22 control subjects were enrolled in this study. An initial miRNA expression profiling was performed with the total RNA isolated from lung tissue of eight IPAH patients and eight age-matched controls (Screening cohort). In miRNA expression validation, the cohort of 14 IPAH patients and 14 control subjects were included (Validation cohort), and the entire cohort was used for gene expression validation. The IPAH tissue samples were obtained from explanted lungs of patients undergoing lung transplantation, and control tissue samples were from the lungs of failed organ donors (FD). All subjects were enrolled in the Pulmonary Hypertension Breakthrough Initiative (PHBI), and all lung tissue was collected according to the PHBI standardized tissue-processing protocol [4]. The diagnosis of IPAH was based on standard criteria with confirmation by right heart catheterization (RHC) and exclusion of other forms of pulmonary hypertension as per the most recent guidelines of diagnosis [14,15]. Lung tissue collection was approved by each Institutional Review Board at all lung transplant sites. Written informed consent from all subjects or their legal guardians was obtained upon enrolment.
miRNA microarray screening
Isolated RNA samples were hybridized to a Human miRNA Microarray V3 (G4872A, Agilent Technologies) platform according to the manufacturer’s instructions in the JHMI Deep Sequencing and Microarray Core Facility (see the Supplementary Methods). The data from this study have been deposited in the National Centre for Biotechnology Information’s Gene Expression Omnibus (GEO) and are accessible through GEO Series accession number GSE67597.
miRNA target gene exploration and pathway analysis
To identify biological functions and canonical pathways in which the significantly dysregulated miRNAs are involved, Ingenuity® Pathway Analysis (IPA) (QIAGEN, http://www.ingenuity.com/products/ipa) was applied. Utilizing the “microRNA Target Filter” tool in IPA, target genes experimentally observed and predicted with high confidence were obtained. Pathway enrichment analysis of these validated and putative target genes was performed by IPA’s “Core Analysis” tool.
Selection of miRNAs targeting major PAH pathways for technical validation
A pathway-based target gene-to-miRNA searching approach was adopted to select the miRNAs for technical validation. First, miRSystem (http://mirsystem.cgm.ntu.edu.tw/), an online integrated miRNA target gene prediction tool was employed to find the Wnt-specific miRNAs targeting the 73 Wnt/β-catenin pathway genes identified by IPA (Table S1). Eleven dysregulated miRNAs identified by array analysis, were found to be targeting the 73 Wnt/β-catenin pathway genes. Next, search of the literature indicated 7 out of these 11 Wnt-targeting miRNAs (let-7a-5p, miR-26b-5p, miR-27b-3p, miR-199a-3p, miR-199b-5p, miR-205-5p and miR-656) were linked to at least one of the following 3 major PAH-related pathways: hypoxia, inflammation or TGF-β signaling. Thus, these 7 miRNAs were selected for further miRNA qRT-PCR validation (Table S2, see the Supplementary Methods).
LNA-ISH for miR-199b-5p and miR-656
We employed techniques for miRNA localization to reveal the spatial distribution of the dysregulated miRNAs. Locked nucleic acid (LNA) - in situ hybridization was performed on formalin-fixed paraffin embedded (FFPE) tissue samples according to the manufacturer’s protocol (see the Supplementary Methods).
miRNA validated target gene search and network construction
First, experimentally validated gene targets of these miRNAs (let-7a-5p, miR-26b-5p, miR-27b-3p, miR-199a-3p, miR-199b-5p and miR-656) were obtained from a publicly available database: miRWalk (http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/mirnatargetpub.html). Then, genes in all of the 4 major PAH-related pathways (hypoxia, inflammation, TGF-β and Wnt) were obtained from the NCBI website (http://www.ncbi.nlm.nih.gov/gene/). Synonyms of each pathway were used for search. The overlapping genes of the above two lists were then subjected to network construction. Direct interactions between network constituents based on published literature were obtained using the “My Pathway” tool in IPA. Genes with multiple interactions with others in the network, so-called “gene hubs,” were validated by qRT-PCR.
Gene expression analysis
Expression levels of microRNA target genes were detected by TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA) on the Applied Biosystems 7300 Real-Time PCR System (see the Supplementary Methods).
Hypoxia treatment of cells and transfection of oligonucleotides for miR-199b-5p
Primary human pulmonary arterial endothelial cells (HPAECs) were purchased and propagated in EGM-2 cell culture media (Lonza). Experiments were performed at passages 4–9. HPAECs were exposed for 24 or 48 hours either to standard non-hypoxic cell-culture conditions (21% O2, 5% CO2, with N2 balance at 37°C) or to hypoxia (4% O2, 5% CO2, with N2 balance at 37°C), in a modular hypoxia chamber.
miR-199b-5p mimic (to mimic the mature form of miR-199b-5p), miR-199b-5p inhibitor (hairpin loop oligonucleotides that carry specific antisense sequences) and negative control molecules were obtained from Dharmacon (USA) and transfected into HPAECs, using Geneporter B reagent (GeneTherapy Systems), at a final concentration of 60 nM for the mimic and 100 nM for the inhibitor, according to the manufacturer’s protocol. Transfected cells were allowed to recover in normal growth media for 24 hours prior to hypoxia treatment (24 hours).
Statistical analyses
For microarray analysis, the filtering criteria of fold change (FC, increase or decrease) ≥ 1.5 and p-value <0.05 were employed. An unpaired t-test or Mann-Whitney U test was applied to the comparisons of relative miRNA or mRNA expression levels between control subjects and IPAH patients, and a p-value of less than 0.05 was considered significant.
The correlations between −ΔΔCt values of miRNAs, genes and hemodynamics or histopathological parameters in IPAH patients were assessed by Pearson correlation. The hemodynamics at both diagnosis and the last RHC before transplantation were analyzed. Mean right atrial pressure (mRAP), mean PAP (mPAP), cardiac output (CO) and PVR were available as hemodynamic measurements. Pulmonary vascular pathological parameters, including scores for the pulmonary artery (PA) fractional thicknesses (Intima, Media and Adventitia), muscularization, Intima thickening (INTTHICK), Medial thickening (MEDTHICK) and Proliferation (PROLIF) were assessed [4]. Receiver operating characteristic (ROC) curve assessment of cut-off was employed to evaluate the diagnostic value of miRNAs. Calculations were performed with SPSS 19.0 (IBM, Armonk, NY).
An overview of our study design and work flow is summarized in Figure S1.
RESULTS
Patient characteristics
We have listed the demographic features, hemodynamic parameters (at diagnosis), six-minute walk distance (6MWD), WHO functional classification (WHO FC) and initial treatment of the screening cohort, validation cohort and entire cohort in Table 1. The entire cohort was composed predominantly of females (N=15/22, 68%) with a mean age of 46±12.4 years. Upon presentation, most patients were categorized as WHO FC III and IV (N=17/18, 94.4%) and had documented mean 6MWD of 347±81 m. All patients underwent RHC that showed an average mRAP of 15.0±6.0 mmHg, a median mPAP of 52 (49, 64) mmHg, mean CO of 4.36±1.51 L/min and median PVR of 11.41 (8.02, 15.63) Wood Units. Of note, there were no carriers of either BMPR2 or SMAD9 genetic mutation.
Table 1.
Subject demographics, histopathological parameters and clinical information.
| Screening cohort | Validation cohort | Entire cohort | ||||
|---|---|---|---|---|---|---|
| Clinical Variable | Control Group (N=8) |
IPAH (N=8) |
Control Group (N=14) |
IPAH (N=14) |
Control Group (N=22) |
IPAH (N=22) |
|
Age at transplant, year |
43.9 (14.8) | 43.8 (14.1) | 46.7 (11.3) | 47.3 (11.7) | 45.7 (12.4) | 46.0 (12.4) |
| Gender, n | ||||||
| Female | 2 | 4 | 7 | 11 | 9 | 15 |
| Male | 6 | 4 | 7 | 3 | 13 | 7 |
| Race, n | ||||||
| White | 8 | 6 | 13 | 12 | 21 | 18 |
| AA | 0 | 2 | 1 | 2 | 1 | 4 |
| mRAP, mmHg | NA | 16 (6) | NA | 14 (6) | NA | 15 (6) |
| PCWP, mmHg | NA | 11 (3) | NA | 9 (3) | NA | 10 (3) |
| mPAP, mmHg | NA | 55 (51, 75) | NA | 54 (10) | NA | 52 (49, 64) |
| CO, L/min | NA | 3.81 (1.26) | NA | 4.67 (1.59) | NA | 4.36 (1.51) |
| PVR, Wood Units | NA | 12.08 (9.91, 17.02) |
NA | 10.96 (4.73) | NA | 11.41 (8.02, 15.63) |
| 6MWD, m | NA | 307 (76) | NA | 369 (76) | NA | 347 (81) |
|
WHO FC, I/II/III/IV* |
NA | 0/0/2/4* | NA | 0/1/8/3* | NA | 0/1/10/7* |
|
Morphological data |
||||||
| Intima fractional thickness |
0.196 (0.080) |
0.307 (0.109) |
0.160 (0.070) |
0.347 (0.138) |
0.176 (0.074) |
0.329 (0.124) |
| Media fractional thickness |
0.198 (0.044) |
0.241 (0.042) |
0.204 (0.044) |
0.235 (0.047) |
0.201 (0.042) |
0.238 (0.044) |
| Adventitia fractional thickness |
0.178 (0.032) |
0.167 (0.045) |
0.152 (0.043) |
0.170 (0.053) |
0.164 (0.039) |
0.169 (0.048) |
| Muscularization | 0.17 (0.00, 1.00) |
1.83 (1.38, 2.00) |
0.00 (0.00, 0.73) |
1.75 (1.19, 2.58) |
0.00 (0.00, 0.83) |
1.83 (1.27, 2.00) |
| Targeted therapy, n | ||||||
| Epoprostenol | NA | 5 | NA | 2 | NA | 7 |
| ERA | NA | 4 | NA | 5 | NA | 9 |
| PDE-5 inhibitor | NA | 6 | NA | 4 | NA | 10 |
| Prostacyclin analog |
NA | 1 | NA | 2 | NA | 3 |
| Combined therapy |
NA | 7 | NA | 4 | NA | 11 |
| CCB** | NA | 0 | NA | 0 | NA | 0 |
| Anticoagulated, n | NA | 6 | NA | 11 | NA | 17 |
Data are presented as number, mean (standard deviation) or median (25th percentile, 75th percentile). AA=African American; mRAP=mean right atrial pressure; PCWP= pulmonary capillary wedge pressure; mPAP=mean pulmonary artery pressure; CO=cardiac output; PVR=pulmonary vascular resistance; 6MWD= six-minute walk distance; WHO FC=World Health Organization functional classification; ERA=endothelin receptor antagonist; PDE-5=phosphodiesterase type 5; CCB=calcium channel blocker; NA=not available.
WHO FC data is available for 18 IPAH patients
CCB for treatment of pulmonary arterial hypertension with positive acute vascular test
Identifying PAH related pathways
Of 2,006 miRNAs screened, 21 were differentially expressed in the lung of IPAH patients compared to controls including 13 downregulated and 8 upregulated (Figure S2).
To identify the top pathways enriched in target genes of differentially expressed miRNAs in IPAH, we retrieved 4,664 gene targets of the 21 dysregulated miRNAs. These genes were either validated by previous experiments or predicted in silico with high confidence. Pathway enrichment analysis of these target genes revealed that ‘Molecular Mechanisms of Cancer’, ‘HGF’, ‘PTEN’, ‘TGF-β’, ‘Wnt/β-catenin’ and ‘Cardiac Hypertrophy’ signaling were the top canonical pathways enriched in these target genes (Table S1 and Figure S3). Given the emerging role of Wnt/β-catenin signaling in the pathogenesis of PAH and other cardiovascular diseases [16], and the potential of recently developed Wnt-specific compounds and biological therapies capable of modulating Wnt pathway activity [17], we have focused on the Wnt/β-catenin pathway in the following validations.
qRT-PCR validation confirmed the dysregulation of five miRNAs
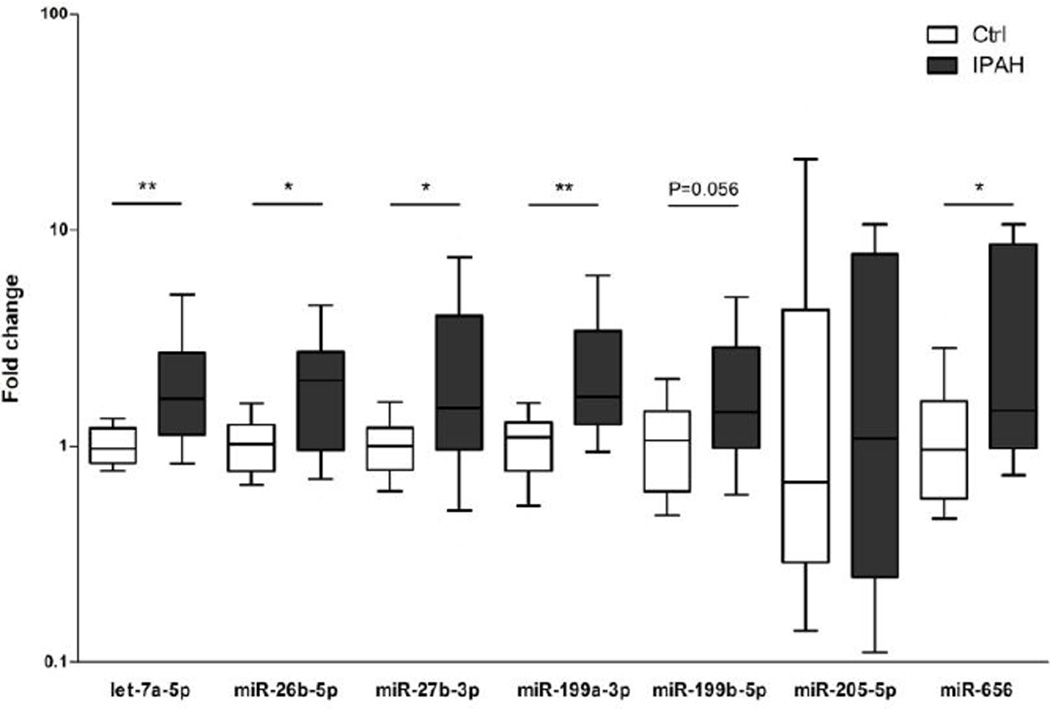
By target gene-to-miRNA prediction from miRSystem, we found 11 dysregulated miRNAs to be targeting the 73 Wnt/β-catenin pathway genes (6 out of 11 were shown in Table S3), and 7 of them (let-7a-5p, miR-26b-5p, miR-27b-3p, miR-199a-3p, miR-199b-5p, miR-205-5p and miR-656) were also linked to at least one of the 3 additional major PAH-related pathways: hypoxia, inflammation or TGF-β signaling (Table S4). Significant upregulation of 5 of these miRNAs (let-7a-5p, miR-26b-5p, miR-27b-3p, miR-199a-3p and miR-656) was confirmed by qRT-PCR validation (Figure 1). miR-199b-5p also showed a trend toward significance in both the validation cohort (p=0.056) and the entire cohort (p=0.046).
Figure 1.
Validation of selected miRNAs in lung tissue samples of IPAH patients and controls by qRT-PCR. Expression levels of miR-656, let-7a-5p, miR-26b-5p, miR-27b-3p and miR-199b-5p were significantly increased in IPAH patients (N=14) compared with the controls (N=14). In all experiments, miRNA levels were normalized to U6 snRNA. Data are expressed as median and 25th/75th (boxes) and 10th/90th percentiles (whiskers). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
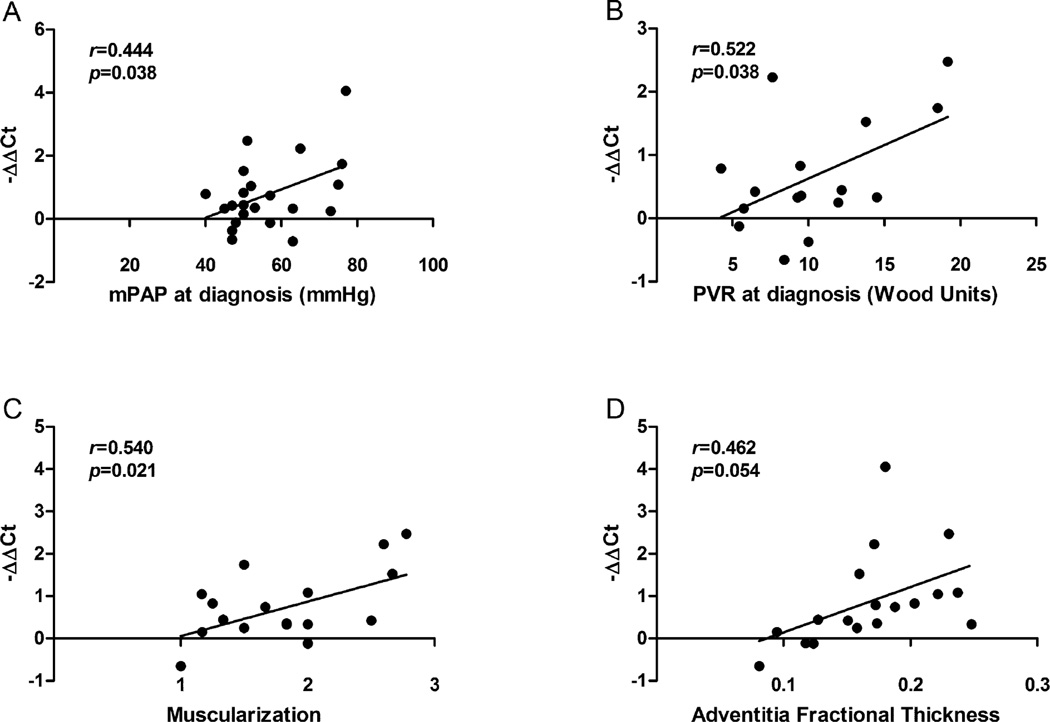
Expression levels of miRNAs were correlated with hemodynamics and histopathological parameters
The expression level of miR-199b-5p was significantly correlated with both mPAP (r=0.444, p=0.038) and PVR (r=0.522, p=0.038), a key determinant of PAH prognosis [18] as well as muscularization (r=0.540, p=0.021). Expression level of miR-199b-5p also had a borderline correlation with adventitia fractional thickness (r=0.462, p=0.054) (Figure 2). Expression level of miR-656 had significant positive correlations with intima thickening, medial thickening, and proliferation (Figure S4) despite that the abnormal scores for these 3 parameters were observed only in very few patients. In addition, we found expression levels of miR-199a-3p, miR-26b-5p and miR-27b-3p were correlated with RAP at diagnosis; expression levels of miR-656 were correlated with RAP before transplantation (Figure S5).
Figure 2.
Pearson correlation between expression levels of miR-199b-5p and (A) mean pulmonary artery pressure (mPAP) at diagnosis (N=22, r=0.444, p=0.038), (B) pulmonary vascular resistance (PVR) at diagnosis (N=16, r=0.522, p=0.038), (C) muscularization (N=18, r=0.540, p=0.021), and (D) adventitia fractional thickness (N=18, r=0.462, p=0.054).
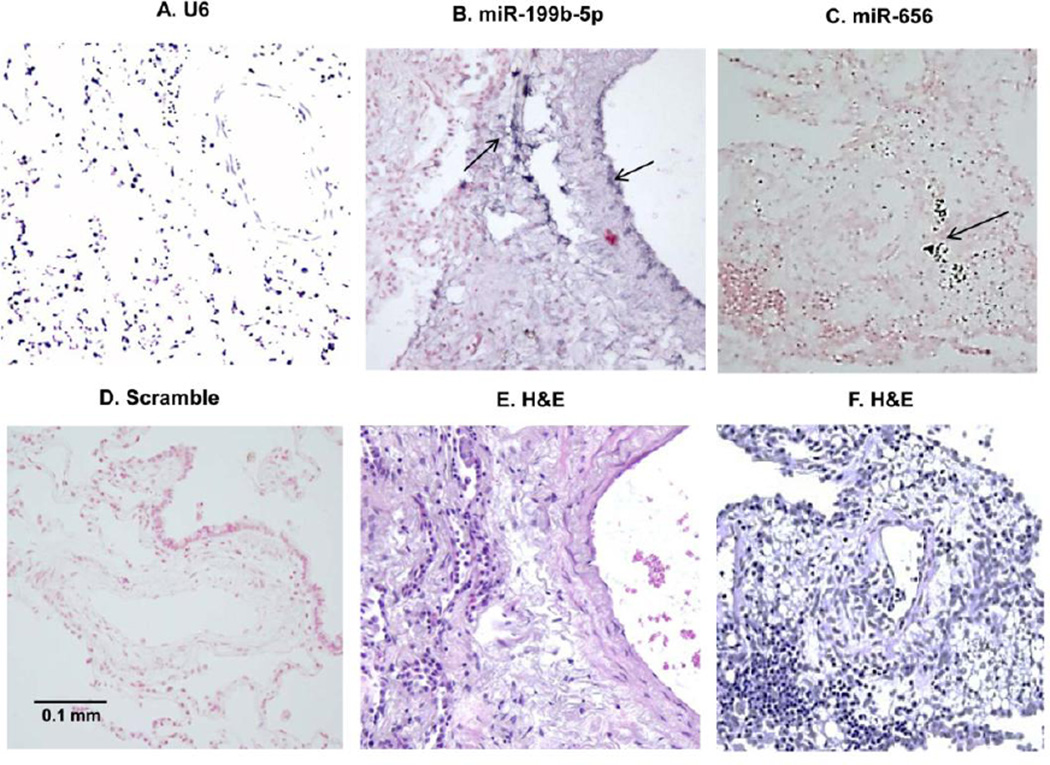
The strong positive correlation between overexpression of miR-199b-5p and miR-656 and various features of vascular remodeling prompted us to pursue an in-depth investigation of their tissue distribution. We found intriguing specificity and intensity of expression of both miRNAs in and surrounding small and medium-sized vessels. miR-199b-5p displayed expression in the pulmonary vascular endothelium and adventitia; and miR-656 showed detectable but very low-abundance expression (Figure 3).
Figure 3.
In situ hybridization of U6 snRNA (A, positive control, to determine ISH sensitivity, control subject was used), miR-199b-5p (B) and miR-656 (C) with DIG-labeled LNA probe (LNA-ISH) in human IPAH lung. The scrambled (negative control, D) and matching H&E staining for miR-199b-5p (E) and miR-656 (F) are also shown. For LNA-ISH, positive staining is dark blue (arrows). Magnification: 20×, using Olympus BX 51 Microscope. Scale bar = 0.1 mm.
Diagnostic value of validated miRNAs for IPAH
Expression levels of let-7a-5p, miR-26b-5p, miR-27b-3p, miR-199a-3p, miR-199b-5p and miR-656 were associated with the diagnosis of IPAH on ROC analysis. Subjects with higher tissue levels of these six miRNAs are more likely to be IPAH patients. Area under curve (AUC) and cut-off values from ROC analyses of these miRNAs are shown in Table 2 and Figure S6.
Table 2.
Area under curve (AUC), significance, cutoff value, sensitivity and specificity of 6 dysregulated miRNAs from ROC analyses.
| miRNA | AUC | 95% CI | p-value | Cutoff value |
Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| let-7a-5p | 0.841 | 0.720–0.962 | 0.0001 | 1.385 | 0.727 | 0.864 |
| miR-26b-5p | 0.736 | 0.579–0.892 | 0.0075 | 1.703 | 0.545 | 1.000 |
| miR-27b-3p | 0.833 | 0.706–0.959 | 0.0002 | 1.440 | 0.773 | 0.864 |
| miR-199a-3p | 0.859 | 0.752–0.966 | 0.0001 | 1.426 | 0.773 | 0.810 |
| miR-199b-5p | 0.706 | 0.551–0.860 | 0.0210 | 1.153 | 0.727 | 0.619 |
| miR-656 | 0.797 | 0.662–0.931 | 0.001 | 1.301 | 0.789 | 0.727 |
Activation of Wnt/ β-catenin pathway in end-stage IPAH lung tissue
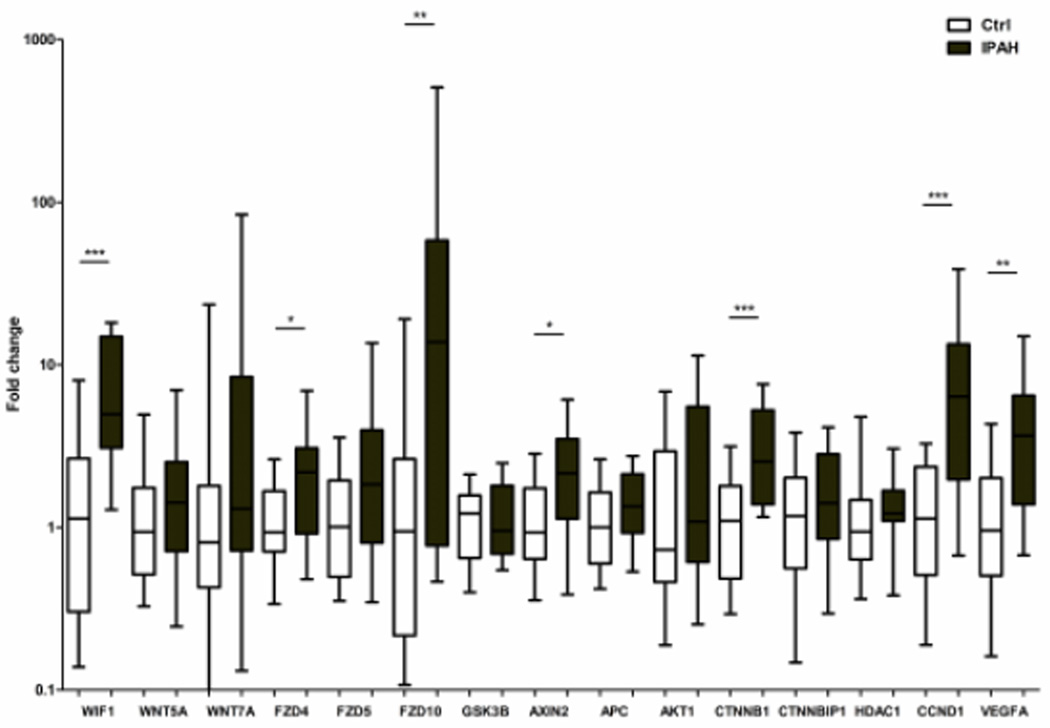
Expression levels of Wnt ligand inhibitor (WIF1), major ligands (WNT5A, WNT7A and WNT16), receptors (FZD4, FZD5 and FZD10), components of the Wnt inhibition complex (APC and GSK3B), core gene β-catenin (CTNNB1), β-catenin inhibitors (CTNNBIP1 and HDAC1) and downstream target genes (CCND1, VEGFA and AXIN2) were analyzed by qRT-PCR. We found Wnt inhibitor (WIF1), receptors (FZD4, FZD10), core gene (CTNNB1) and its downstream targets (CCND1, VEGFA and AXIN2) were significantly upregulated in lung tissue from patients with IPAH (Figure 4 and Table S5).
Figure 4.
Evaluation of mRNA expression levels of major Wnt/ β-catenin pathway genes by qRT-PCR. Expression levels of WIF1, FZD4, FZD10, AXIN2, CTNNB1, CCND1, and VEGFA were up-regulated in IPAH patients (N=22) compared with the controls (N=22). In all experiments, level of miRNAs was normalized to GAPDH. Data are expressed as median and 25th/75th (boxes) and 10th/90th percentiles (whiskers). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Network construction and validation
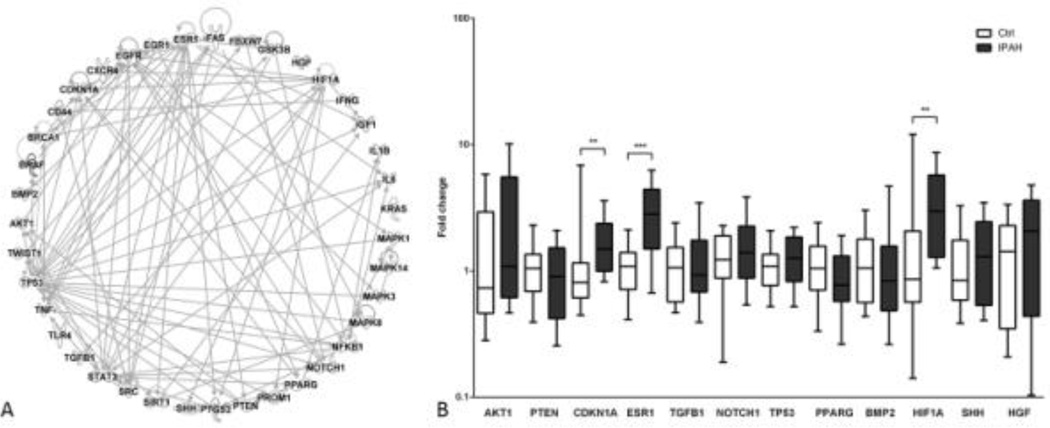
Given the importance of these miRNAs at the crucial intersection of hypoxia, inflammation, TGF-β, and Wnt signaling in PAH, we evaluated the miRNA’s broader effects using a network approach. We retrieved 439 experimentally validated gene targets of the 6 validated miRNAs (Table S6). A total number of 71 genes were ‘common’ genes involved in all 4 major PAH-related pathways (Figure S7). Among them, 39 were ‘core’ genes, not only involved in all 4 pathways (Figure S8), but also being targeted by all the qRT-PCR verified miRNAs, except for miR-656 (Figure S9). We further constructed a network consisting of these genes and their direct interactions using IPA. In this network, an edge between two nodes means these two genes have experimentally observed direct interactions in human (Figure 5A). We also constructed 5 separate networks for each of the 5 miRNAs, let-7a-5p, miR-26b-5p, miR-27b-3p, miR-199a-3p, and miR-199b-5p (Figure S10). Gene expression levels of the 12 out of 39 core genes were selected for validation by qRT-PCR and we observed significant upregulation of genes CDKN1A, ESR1 and HIF1A (Figure 5B). Interestingly, expression levels of ESR1, which encodes an estrogen receptor alpha, were significantly higher in male IPAH patients than in female patients. However, we did not observe this difference between genders among the controls (Figure S11).
Figure 5.
Network analysis of 39 validated target genes for the 6 miRNAs and the qRT-PCR validation of selected network constituents (gene hubs). (A) Gene-gene interaction network showing experimentally observed direct interactions between gene targets. (B) Expression levels of selected genes of the network. CDKN1A, ESR1 and HIF1A were upregulated in IPAH patients compared to the controls. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
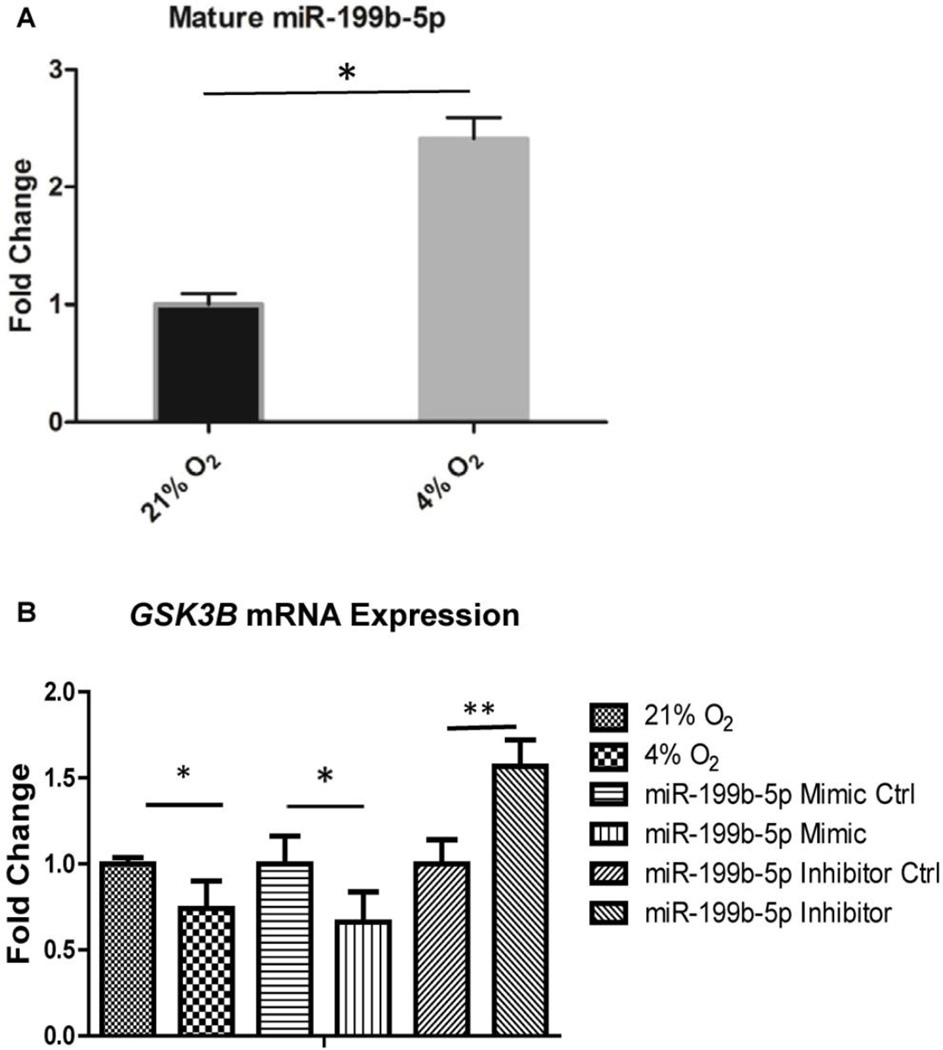
Overexpression of miR-199b-5p by hypoxia and negative regulation of GSK3B in HPAECs
We observed overexpression of miR-199b-5p in the lung of IPAH patients, which was correlated with features of vascular remodeling. Given that it was localized to the endothelium, as suggested by the in situ hybridization, we further validated miR-199b-5p expression in HPAECs. Expression of mature miR-199b-5p was increased 2.29 fold after exposure of the cells to 4% O2 for 48 hours (p=0.037; Figure 6A), consistent with its substantial contribution to the dysregulated pathophenotype observed in PAH. Through target prediction analyses using miRSystem, GSK3B, which encodes the multifunctional kinase GSK3-β, a protein involved in signal transduction from the Wnt/β-catenin pathway, was identified as a putative target gene of miR-199b-5p (Table S3). To determine whether this potential binding can translate into efficient GSK3B regulation in HPAECs, we transfected oligonucleotides regulating miR-199b-5p expression to test this hypothesis. Interestingly, we found hypoxic HPAECs displayed decreased levels of GSK3B (fold change=0.74, p=0.01; Figure 6B, left), as assessed by RT-PCR in HPAECs. Moreover, forced expression of miR-199b-5p by mimic displayed a significant downregulation of GSK3B expression (fold change=0.66, p=0.024; middle). In contrast, the miR-199b-5p inhibitor upregulated GSK3B expression (fold change=1.57, p=0.032; right). Together, these data demonstrate the antagonistic function of GSK3B on β-catenin signaling is reduced by miR-199b-5p, potentially constituting a pathological feed forward mechanism, affecting activation status of downstream positive effectors of β-catenin.
Figure 6.
Assessing the regulation of miR-199b-5p expression and effects on GSK3B in cultured HPAECs. (A) miR-199b-5p is upregulated by hypoxia, as measured by reverse transcription polymerase chain reaction (RT-PCR), in human pulmonary arterial endothelial cells (HPAECs). (B) Left, GSK3B expression is decreased in HPAECs after chronic exposure to 4% O2 for 48 hours. Right, inhibition of miR-199b-5p expression by miR-199b-5p inhibitor upregulates GSK3B expression. In contrast, forced expression of miR-199b-5p reciprocally downregulated GSK3B expression (middle panel). Conditions utilized as control in each comparison are assigned a fold change of 1, with which other conditions are compared. Error bars reflect SEM. *, p < 0.05; **, p < 0.01; n≥3 in each group.
DISCUSSION
This is the first study utilizing miRNA profiling as an unbiased approach to identify dysregulated miRNAs in lung tissue of IPAH patients, providing specific epigenetic information regarding the pathophysiology of PAH.
miRNAs are small non-coding RNAs which regulate gene expression by binding to the 3’-untranslated region (UTR) of gene transcripts and causing mRNA degradation or inhibition of translation [19]. Current miRNA studies in PAH revealed dysregulation of several miRNA families in PAH, including miR-17/92 cluster [20], miR-21 [21–23], miR-130/301 cluster [24,25], miR-143/145 cluster [26–28], miR-204 [13,29] and miR-424/503 cluster [30,31]. Recent reports found that miR-223 was down-regulated in human PAH lungs, in both the right heart and lungs from rodent models of PH. Downregulation of miR-223 triggers PARP-1 and insulin-like growth factor 1 receptor (IGF-1R) overexpression, subsequent pathologic DNA damage repair, and increased proliferation [32, 33]. The relationship between miRNAs and target genes in vivo is a complex network. Thus far, very few studies looked into the systematic interactions between miRNA and gene signaling pathways. In the present study, we performed pathway enrichment analysis utilizing unbiased miRNA microarray data, and have identified top canonical pathways highly relevant to the pathogenesis of PAH, including the Wnt/β-catenin signaling. Further validation by qRT-PCR confirmed activation of the Wnt/β-catenin pathway in IPAH.
The Wnt/β-catenin pathway is highly conserved among species, regulating stem cell pluripotency and cell fate decisions during development [34]. Wnt/β-catenin pathway activation triggers displacement of GSK-3β from a regulatory APC/Axin/GSK-3β-complex and inhibits degradation of β-catenin. Translocation of β-catenin into the nucleus activates transcription of downstream targets, e.g., cyclin D1, VEGF and survivin. Wnt/β-catenin signaling is essential in vascular development and remodeling [35]. Previous studies have discovered that Wnt pathways, both canonical and noncanonical, are operative in PAH [9,10,36,37]. We observed increased expression of cell membrane receptors (FZD4, FZD10), core gene (CTNNB1) and downstream targets (CCND1, VEGFA and AXIN2) in lung tissue from patients with IPAH. These findings argue for the existence of a coordinated regulatory cascade in which the selective upregulation of these miRNAs activates β-catenin via membrane receptors and modulators (e.g., suppression of the GSK-3β inhibition complex), and the induction of downstream positive effectors (e.g., CCND1 and VEGFA), leading to vascular remodeling and poor outcome of PAH.
We validated and confirmed the dysregulation of 5 miRNAs: let-7a-5p, miR-26b-5p, miR-27b-3p, miR-199a-3p and miR-656. The let-7 miRNA family is the first known human miRNA and is highly conserved across species; its family members are induced by hypoxia and involved in angiogenesis [38]. let-7f was downregulated during the development of PAH in both rat chronic hypoxia and MCT models but let-7a was reduced only in MCT [39]. miR-26b-5p shares the same seed region with miR-26a. The expression level of miR-26a was decreased in both the MCT-PAH rat model and PAH patients’ plasma, and was positively correlated with patients’ 6MWD suggesting it could serve as a potential biomarker for PAH [40]. miR-27b-3p has the same seed region as miR-27a. We confirmed upregulation of miR-27b-3p, which is consistent with previous findings from Courboulin et al done on PASMCs [13]. A subsequent study found that overexpression of miR-27a promoted human pulmonary artery endothelial cell proliferation, and miR-27a and PPARγ repressed each other in hypoxia-induced PH [41]. Upregulation of both miR-199a-3p and miR-199b-5p was first discovered in a murine RVH model [42].
Among the miRNAs we validated, tissue levels of miR-199b-5p had positive correlations with patients’ hemodynamics. We further examined target in Wnt/β-catenin signaling with artificial reconstitution of miR-199b-5p expression changes. Notably, our data show for the first time that miR-199b-5p impairs Wnt/β-catenin signaling in HPAECs through negative regulation of GSK3B. However, further experiments are warranted to demonstrate the presence of a functional conserved miR-199b-5p seed region in the 3’ UTR of GSK3B, and miR-199b-5p silences GSK3B expression via 3′UTR binding.
We further constructed gene networks for the 39 common core genes shared by the 4 major PAH-related pathways and targeted by the 5 relevant validated miRNAs let-7a-5p, miR-26b-5p, miR-27b-3p, miR-199a-3p, miR-199b-5p. Interestingly, we found only 3 genes were dysregulated in the qRT-PCR validation of network components (HIF1A, ESR1 and CDKN1A). HIF1A, targeted by miR-199b-5p, encodes the alpha subunit of hypoxia-inducible factor-1 (HIF-1). Recent evidence shows HIF-1A in smooth muscle contributes to pulmonary vascular remodeling and PH in chronic hypoxia [43]. ESR1 encodes estrogen receptor alpha which is essential for sexual development and involved in pathological processes of cancers. Rajkumar et al discovered upregulation of ESR1 in patients with PAH [44]. Interestingly, we found the expression level of ESR1 in male IPAH patients was significantly higher than that in female patients. CDKN1A encodes a cyclin-dependent kinase inhibitor. Recent study reveals CDKN1A is a direct target of miR-130a. Hypoxia has been seen to induce miR-130a expression and thus repress CDKN1A translation [45]. These findings suggest experimental validation at the post-transcriptional level is necessary to confirm the role(s) of target genes of dysregulated miRNAs.
Surprisingly, some known dysregulated genes in PAH didn’t show significant change by qRT-PCR validation in our study, including: TGFB1, PPARG, NOTCH1 and TP53. This may be due to posttranscriptional regulation and modification. Many microRNA exert an effect on translation, we only analyzed the mRNA levels of the genes in our study thus would not have seen protein-level dysregulations.
There remain limitations to our study. First, the microarray screening served solely as a discovery tool. This may explain in part the discrepancy observed between microarray and qRT-PCR validation for miR-656. Second, all tissue samples from IPAH patients were isolated post-transplant. Using lung explants, we cannot trace dynamic changes in lung miRNA profiles during PAH initiation and propagation. Third, lung tissue homogenate was used in this study for miRNA profiling and validations of miRNAs and their target genes. miRNA biosynthesis and function are highly regulated and this regulation may be cell type specific. Examination of miRNA levels in other relevant tissue (e.g., right ventricle) and cell-types (PASMCs and lung fibroblasts) is warranted to investigate the consequences of miRNA expression changes that relate to PAH pathobiology. Finally, investigation of circulating miRNAs is necessary to explore the clinical utility of these miRNAs as novel biomarkers for PAH because lung biopsy is invasive, and particularly risky for patients with PAH.
In summary, this is the first report utilizing miRNA profiling in lung tissue samples from patients with IPAH. We confirmed the dysregulation of 6 miRNAs targeting major PAH-related pathways including Wnt/β-catenin. The wide-ranging activation of Wnt/β-catenin pathway genes in our study substantiates the significance of this pathway in the pathogenesis of PAH and strongly supports further investigation of the Wnt/β-catenin pathway in PAH.
Supplementary Material
Key Messages.
It is the first miRNA profiling study in lung tissue from end-stage idiopathic PAH.
We identified dysregulated miRNAs and major pathways (e.g., Wnt signaling) in IPAH.
Levels of miRNA expression were correlated with hemodynamics and pathological changes.
We observed aberrant expression of target genes in the Wnt/β-catenin pathway.
miRNAs influence the pathogenesis of PAH by regulating major PAH related pathways.
Acknowledgments
Acknowledgements and Funding
The authors thank the JHMI Deep Sequencing and Array Facility for performing the work on miRNA arrays. The authors thank Brian Graham for pathology assistance and Alan Berger for helpful discussions. Tissue samples were provided by the Genomics Core and Tissue Core under the Pulmonary Hypertension Breakthrough Initiative (PHBI). Funding for the PHBI is provided by the Cardiovascular Medical Research and Education Fund (CMREF). This work was supported by a Johns Hopkins University Microarray Grant to L.G. from Agilent Technologies, and National Institutes of Health (NIH) grant 1R03HL114937 to L.G., K.C.B. and P.M.H. In addition, LG was supported in part by the Gilead Sciences Research Scholars Program in Pulmonary Arterial Hypertension.
Footnotes
Disclosure: The authors declare that they have no competing interests.
Authors’ contributions
D.W. performed molecular experiments, statistical analysis and contributed to the manuscript, C.T. performed statistical analysis of the miRNA array data and contributed to the manuscript, Q.L. performed LNA-ISH, R.L.D performed the forced expression and inhibition of miRNA experiment, Z.J. provided intellectual contributions to the manuscript regarding the role of estrogen and receptors, R.T. performed histopathological analysis and contributed to the manuscript, K.C.B. and P.M.H. provided intellectual contributions to the design of the experiments and contributed to the manuscript, and L.G. conceived the project, performed experiments, data analysis, and contributed to the manuscript. All authors have read and approved the final manuscript.
REFERENCES
- 1.Rubin LJ. Primary pulmonary hypertension. N Engl J Med. 1997;336(2):111–117. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- 2.Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM, Reid LM, Tuder RM. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol. 2004;43(12 Suppl S):25S–32S. doi: 10.1016/j.jacc.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 3.Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin Chest Med. 2007;28(1):23–42. vii. doi: 10.1016/j.ccm.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, Jessup M, Grizzle WE, Aldred MA, Cool CD, et al. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186(3):261–272. doi: 10.1164/rccm.201201-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SD, Shroyer KR, Markham NE, Cool CD, Voelkel NF, Tuder RM. Monoclonal endothelial cell proliferation is present in primary but not secondary pulmonary hypertension. J Clin Invest. 1998;101(5):927–934. doi: 10.1172/JCI1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293(3):L548–L554. doi: 10.1152/ajplung.00428.2006. [DOI] [PubMed] [Google Scholar]
- 7.Cottrill KA, Chan SY. Metabolic dysfunction in pulmonary hypertension: the expanding relevance of the Warburg effect. Eur J Clin Invest. 2013;43(8):855–865. doi: 10.1111/eci.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuder RM, Voelkel NF. Angiogenesis and pulmonary hypertension: a unique process in a unique disease. Antioxid Redox Signal. 2002;4(5):833–843. doi: 10.1089/152308602760598990. [DOI] [PubMed] [Google Scholar]
- 9.West JD, Austin ED, Gaskill C, Marriott S, Baskir R, Bilousova G, Jean JC, Hemnes AR, Menon S, Bloodworth NC, et al. Identification of a common Wnt-associated genetic signature across multiple cell types in pulmonary arterial hypertension. Am J Physiol Cell Physiol. 2014;307(5):C415–C430. doi: 10.1152/ajpcell.00057.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laumanns IP, Fink L, Wilhelm J, Wolff JC, Mitnacht-Kraus R, Graef-Hoechst S, Stein MM, Bohle RM, Klepetko W, Hoda MA, et al. The noncanonical WNT pathway is operative in idiopathic pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2009;40(6):683–691. doi: 10.1165/rcmb.2008-0153OC. [DOI] [PubMed] [Google Scholar]
- 11.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 12.Rhodes CJ, Wharton J, Boon RA, Roexe T, Tsang H, Wojciak-Stothard B, Chakrabarti A, Howard LS, Gibbs JS, Lawrie A, et al. Reduced microRNA-150 is associated with poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;187(3):294–302. doi: 10.1164/rccm.201205-0839OC. [DOI] [PubMed] [Google Scholar]
- 13.Courboulin A, Paulin R, Giguere NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Cote J, et al. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med. 2011;208(3):535–548. doi: 10.1084/jem.20101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30(20):2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 15.Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, Langleben D, Manes A, Satoh T, Torres F, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D42–D50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 16.Cohen ED, Tian Y, Morrisey EE. Wnt signaling: an essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development. 2008;135(5):789–798. doi: 10.1242/dev.016865. [DOI] [PubMed] [Google Scholar]
- 17.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13(1):11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 18.Howard LS. Prognostic factors in pulmonary arterial hypertension: assessing the course of the disease. Eur Respir Rev. 2011;20(122):236–242. doi: 10.1183/09059180.00006711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5(7):522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 20.Chen T, Zhou G, Zhou Q, Tang H, Ibe JC, Cheng H, Gou D, Chen J, Yuan JX, Raj JU. Loss of microRNA-17 approximately 92 in smooth muscle cells attenuates experimental pulmonary hypertension via induction of PDZ and LIM domain 5. Am J Respir Crit Care Med. 2015;191(6):678–692. doi: 10.1164/rccm.201405-0941OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parikh VN, Jin RC, Rabello S, Gulbahce N, White K, Hale A, Cottrill KA, Shaik RS, Waxman AB, Zhang YY, et al. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation. 2012;125(12):1520–1532. doi: 10.1161/CIRCULATIONAHA.111.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang S, Banerjee S, Freitas A, Cui H, Xie N, Abraham E, Liu G. miR-21 regulates chronic hypoxia-induced pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2012;302(6):L521–L529. doi: 10.1152/ajplung.00316.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarkar J, Gou D, Turaka P, Viktorova E, Ramchandran R, Raj JU. MicroRNA-21 plays a role in hypoxia-mediated pulmonary artery smooth muscle cell proliferation and migration. Am J Physiol Lung Cell Mol Physiol. 2010;299(6):L861–L871. doi: 10.1152/ajplung.00201.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertero T, Cottrill K, Krauszman A, Lu Y, Annis S, Hale A, Bhat B, Waxman AB, Chau BN, Kuebler WM, et al. The microRNA-130/301 family controls vasoconstriction in pulmonary hypertension. J Biol Chem. 2015;290(4):2069–2085. doi: 10.1074/jbc.M114.617845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertero T, Lu Y, Annis S, Hale A, Bhat B, Saggar R, Saggar R, Wallace WD, Ross DJ, Vargas SO, et al. Systems-level regulation of microRNA networks by miR-130/301 promotes pulmonary hypertension. J Clin Invest. 2014;124(8):3514–3528. doi: 10.1172/JCI74773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis-Dusenbery BN, Chan MC, Reno KE, Weisman AS, Layne MD, Lagna G, Hata A. Down-regulation of Kruppel-like factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-beta and bone morphogenetic protein 4. J Biol Chem. 2011;286(32):28097–28110. doi: 10.1074/jbc.M111.236950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caruso P, Dempsie Y, Stevens HC, McDonald RA, Long L, Lu R, White K, Mair KM, McClure JD, Southwood M, et al. A role for miR-145 in pulmonary arterial hypertension: evidence from mouse models and patient samples. Circ Res. 2012;111(3):290–300. doi: 10.1161/CIRCRESAHA.112.267591. [DOI] [PubMed] [Google Scholar]
- 28.Bockmeyer CL, Maegel L, Janciauskiene S, Rische J, Lehmann U, Maus UA, Nickel N, Haverich A, Hoeper MM, Golpon HA, et al. Plexiform vasculopathy of severe pulmonary arterial hypertension and microRNA expression. J Heart Lung Transplant. 2012;31(7):764–772. doi: 10.1016/j.healun.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Meloche J, Pflieger A, Vaillancourt M, Paulin R, Potus F, Zervopoulos S, Graydon C, Courboulin A, Breuils-Bonnet S, Tremblay E, et al. Role for DNA damage signaling in pulmonary arterial hypertension. Circulation. 2014;129(7):786–797. doi: 10.1161/CIRCULATIONAHA.113.006167. [DOI] [PubMed] [Google Scholar]
- 30.Kim J, Kang Y, Kojima Y, Lighthouse JK, Hu X, Aldred MA, McLean DL, Park H, Comhair SA, Greif DM, et al. An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat Med. 2013;19(1):74–82. doi: 10.1038/nm.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J, Hwangbo C, Hu X, Kang Y, Papangeli I, Mehrotra D, Park H, Ju H, McLean DL, Comhair SA, et al. Restoration of impaired endothelial myocyte enhancer factor 2 function rescues pulmonary arterial hypertension. Circulation. 2015;131(2):190–199. doi: 10.1161/CIRCULATIONAHA.114.013339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi L, Kojonazarov B, Elgheznawy A, Popp R, Dahal BK, Bohm M, Pullamsetti SS, Ghofrani HA, Godecke A, Jungmann A, et al. miR-223-IGF-IR signalling in hypoxia- and load-induced right-ventricular failure: a novel therapeutic approach. Cardiovasc Res. 2016 doi: 10.1093/cvr/cvw065. [DOI] [PubMed] [Google Scholar]
- 33.Meloche J, Le Guen M, Potus F, Vinck J, Ranchoux B, Johnson I, Antigny F, Tremblay E, Breuils-Bonnet S, Perros F, et al. miR-223 reverses experimental pulmonary arterial hypertension. Am J Physiol Cell Physiol. 2015;309(6):C363–C372. doi: 10.1152/ajpcell.00149.2015. [DOI] [PubMed] [Google Scholar]
- 34.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodwin AM, D'Amore PA. Wnt signaling in the vasculature. Angiogenesis. 2002;5(1–2):1–9. doi: 10.1023/a:1021563510866. [DOI] [PubMed] [Google Scholar]
- 36.Fantozzi I, Huang W, Zhang J, Zhang S, Platoshyn O, Remillard CV, Thistlethwaite PA, Yuan JX. Divergent effects of BMP-2 on gene expression in pulmonary artery smooth muscle cells from normal subjects and patients with idiopathic pulmonary arterial hypertension. Exp Lung Res. 2005;31(8):783–806. doi: 10.1080/01902140500461026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rai PR, Cool CD, King JA, Stevens T, Burns N, Winn RA, Kasper M, Voelkel NF. The cancer paradigm of severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178(6):558–564. doi: 10.1164/rccm.200709-1369PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Z, Lai TC, Jan YH, Lin FM, Wang WC, Xiao H, Wang YT, Sun W, Cui X, Li YS, et al. Hypoxia-responsive miRNAs target argonaute 1 to promote angiogenesis. J Clin Invest. 2013;123(3):1057–1067. doi: 10.1172/JCI65344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caruso P, MacLean MR, Khanin R, McClure J, Soon E, Southgate M, MacDonald RA, Greig JA, Robertson KE, Masson R, et al. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol. 2010;30(4):716–723. doi: 10.1161/ATVBAHA.109.202028. [DOI] [PubMed] [Google Scholar]
- 40.Schlosser K, White RJ, Stewart DJ. miR-26a linked to pulmonary hypertension by global assessment of circulating extracellular microRNAs. Am J Respir Crit Care Med. 2013;188(12):1472–1475. doi: 10.1164/rccm.201308-1403LE. [DOI] [PubMed] [Google Scholar]
- 41.Kang BY, Park KK, Green DE, Bijli KM, Searles CD, Sutliff RL, Hart CM. Hypoxia mediates mutual repression between microRNA-27a and PPARgamma in the pulmonary vasculature. PLoS One. 2013;8(11):e79503. doi: 10.1371/journal.pone.0079503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reddy S, Zhao M, Hu DQ, Fajardo G, Hu S, Ghosh Z, Rajagopalan V, Wu JC, Bernstein D. Dynamic microRNA expression during the transition from right ventricular hypertrophy to failure. Physiol Genomics. 2012;44(10):562–575. doi: 10.1152/physiolgenomics.00163.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ball MK, Waypa GB, Mungai PT, Nielsen JM, Czech L, Dudley VJ, Beussink L, Dettman RW, Berkelhamer SK, Steinhorn RH, et al. Regulation of hypoxia-induced pulmonary hypertension by vascular smooth muscle hypoxia-inducible factor-1alpha. Am J Respir Crit Care Med. 2014;189(3):314–324. doi: 10.1164/rccm.201302-0302OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajkumar R, Konishi K, Richards TJ, Ishizawar DC, Wiechert AC, Kaminski N, Ahmad F. Genomewide RNA expression profiling in lung identifies distinct signatures in idiopathic pulmonary arterial hypertension and secondary pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2010;298(4):H1235–H1248. doi: 10.1152/ajpheart.00254.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brock M, Haider TJ, Vogel J, Gassmann M, Speich R, Trenkmann M, Ulrich S, Kohler M, Huber LC. The hypoxia-induced microRNA-130a controls pulmonary smooth muscle cell proliferation by directly targeting CDKN1A. Int J Biochem Cell Biol. 2015;61:129–137. doi: 10.1016/j.biocel.2015.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.