Summary
Macrophages are a crucial component of the innate immune system in sensing pathogens and promoting local and systemic inflammation. RIPK1 and RIPK3 are homologous kinases, previously linked to activation of necroptotic death. In this study we have described roles for these kinases as master regulators of pro-inflammatory gene expression induced by lipopolysaccharide, independent of their well-documented cell death functions. In primary macrophages, this regulation was elicited in the absence of caspase-8 activity, required the adaptor molecule TRIF, and proceeded in a cell autonomous manner. RIPK1 and RIPK3 kinases promoted sustained activation of Erk, cFos and NFκB, which were required for inflammatory changes. Utilizing genetic and pharmacologic tools, we showed that RIPK1 and RIPK3 account for acute inflammatory responses induced by lipopolysaccharide in vivo; notably, this regulation did not require exogenous manipulation of caspases. These findings identified a new pharmacologically accessible pathway that may be relevant to inflammatory pathologies.
Introduction
RIPK1 and RIPK3 are homologous Ser/Thr kinases, which attracted interest as central inducers of regulated necrotic cell death, termed “necroptosis” (Christofferson and Yuan, 2010; Degterev et al., 2005; Linkermann and Green, 2014; Pasparakis and Vandenabeele, 2015; Silke et al., 2015). A range of triggers, including extracellular factors linked to innate immune regulation, such as ligands for tumor necrosis factor receptor (TNFR), interferon-alpha receptor (IFNαR), and Toll-like receptor (TLR) families, as well as viral infection and genotoxic stressors have been associated with RIPK1 and RIPK3 activation. These stimuli have been shown to induce necroptosis in vitro under conditions in which the apical apoptotic cysteine protease, caspase-8, is inhibited pharmacologically or genetically (Degterev et al., 2005; He et al., 2011; Kaiser et al., 2013; Tenev et al., 2011; Thapa et al., 2013; Upton et al., 2010, 2012). Necroptosis is best understood in the context of TNFα signaling and the kinase activity of RIPK3 has been shown to play a central role in its activation (Cho et al., 2009; He et al., 2009; Zhang et al., 2009). RIPK3 functions within an insoluble amyloid-like RIPK1 and RIPK3 “necrosome” complex, formation of which requires catalytic activities of both kinases (Cho et al., 2009), and serves as a signaling platform for the activation of a downstream pseudo-kinase MLKL, a critical mediator of cell lysis and necroptotic death (Wang et al., 2014).
In contrast to the well-established roles for kinase activities of RIPK1 and RIPK3 in directing cell death, evidence also hints at the possibility for additional roles for RIPK1 and RIPK3 in the direct regulation of pro-inflammatory signaling (Christofferson et al., 2012; Lukens et al., 2013; McNamara et al., 2013; Wong et al., 2014). Among various inducers of RIPK1 and RIPK3, signaling by TLR3 and TLR4 members of the TLR family of Pattern Recognition Receptors (PRRs) in macrophages engages the RIPK1, RIPK3, and MLKL pathway directly in a manner that is dependent on the RHIM-containing adaptor protein TRIF in the presence of the pan-caspase inhibitor zVAD.fmk (zVAD) (He et al., 2011; Kaiser et al., 2013; Schworer et al., 2014). Upregulation of inflammatory gene expression by TLR4 is an important component of both physiologic innate immune responses to gram negative bacterial cell membrane component, lipopolysaccharide (LPS), and a contributor to various inflammatory pathologies (Kawai and Akira, 2006, 2010). Given the challenge of separating pro-inflammatory from pro-necroptotic consequences of RIPK1 and RIPK3 activation, we reasoned that the former regulation might have been overlooked in the previous works.
Here, we report roles for kinase activities of RIPK1 and RIPK3 in LPS-induced expression of inflammatory cytokines that manifest independently from the pro-death functions of these molecules. We have demonstrated that activation of RIPK1 in macrophages by LPS in the presence of zVAD robustly increased expression of a broad range of inflammatory molecules. RIPK3 also contributed to the RIPK1 kinase-dependent inflammation, but in a context dependent manner. We have shown that this regulation is independent of necroptosis and occurs in Mlkl−/− macrophages protected from cell death. We have further established that prolonged activation of Erk1/2 MAPK, which may occur through direct engagement by RIPK1 and RIPK3 “necrosome”-like aggregates, is a critical step in directing RIPK1 and RIPK3 kinase-mediated inflammation. Finally, we have extended our observations in vivo, affirming the importance of RIPK1 and RIPK3 kinases in the global regulation of acute inflammation induced by a sub-lethal dose of LPS in the absence of exogenous caspase-8 inhibition.
Results
RIPK1 and RIPK3 kinases promote LPS-induced inflammatory gene expression in the absence of caspase-8 activation
Previously published work suggests that kinase activity of RIPK1 does not contribute to the pro-inflammatory regulation by TLR4 in response to LPS (Cusson-Hermance et al., 2005; Meylan et al., 2004; Newton et al., 2004; Vivarelli et al., 2004). However, the activation of RIPK1 kinase activity and necroptosis also does not occur in LPS-treated macrophages, unless caspases are inhibited using the pan-caspase inhibitor zVAD (Berger et al., 2014; Kaiser et al., 2013; Schworer et al., 2014). Abrogation of the response by a highly selective RIPK1 inhibitor, Nec-1s (also known as 7-Cl-O-Nec-1) (Degterev et al., 2013), and in BMDMs from Ripk1D138N/D138N mice, carrying a targeted mutation inactivating the kinase function of RIPK1 (Polykratis et al., 2014), confirmed the response specificity (Fig. 1A, S1A). Additionally, zVAD was required for LPS-induced accumulation of RIPK1, RIPK3 and phosphorylated MLKL in the NP40-insoluble cellular fraction (Fig. 1B), reflecting previously reported formation of detergent-insoluble RIPK1 and RIPK3 necrosomes (Moquin et al., 2013; Ofengeim et al., 2015). Collectively, these observations confirmed the requirement for caspase inhibition for the induction of RIPK1 kinase activity in LPS-treated BMDMs.
Figure 1. Caspase-8 inhibition promotes LPS-induced cytokine gene expression that is dependent on RIPK1 and RIPK3 kinases in macrophages.
(A) Autophosphorylation of RIPK1 at Ser166 in BMDMs treated for 4 hrs. p-Ser166/total RIPK1 measured by ELISA. (B) Western of NP40-soluble and NP40-insoluble fractions from wild type BMDMs treated for 4 hrs. (C, D) TNF mRNA expression (C) and protein release (D) in BMDMs treated for 7 hrs. (E) Affymetrix gene array expression analysis of inflammatory genes increased by 7 hrs treatment with LPS or LPS with zVAD in BMDMs. (F) Cell viability of BMDMs treated for 24 hrs. (G) Western of NP40-soluble and NP40-insoluble fractions from BMDMs treated for 4 hrs. (H) TNF mRNA expression in BMDMs treated for 7 hrs. (I) Cell viability of BMDMs treated with LPS alone for 24 hrs. (J) TNF mRNA expression BMDMs treated for 7 hrs.
Values represent Mean ± SD. *p<0.05. BMDMs were treated with LPS=10 ng/mL, zVAD=50 µM and Nec-1s=30 µM in all experiments. Use of Nec-1 refers to Nec-1s in this and all other figures. Also see figure S1.
Having verified a system to evaluate catalytic function of RIPK1 in BMDMs, we re-examined the role of kinase activity of RIPK1 in LPS-induced inflammatory gene expression. Notably, addition of zVAD robustly increased TNFα mRNA expression and protein secretion in BMDMs treated with LPS (Fig. 1C,D), while only a slight effect was observed when BMDMs were treated with zVAD alone (Fig. S1B). The increase in TNFα synthesis by LPS with zVAD, but not LPS alone was abolished in RIPK1 D138N cells and upon treatment with Nec-1s (Fig. 1C,D). Examining the kinetics, we observed enrichment of RIPK1, RIPK3, and p-MLKL in the NP40-insoluble cellular fraction reaching a maximum ~1.5 hr after addition of LPS with zVAD (Fig. S1C), followed by increases in TNFα mRNA expression (~2-6 hr) (Fig. S1D,E), which preceded on-set of cell death (>7 hr) (Fig. S1F). We also observed comparable RIPK1 kinase-dependent induction of TNFα mRNA by LPS with zVAD in a different lineage of primary macrophages – thioglycollate-elicited peritoneal macrophages (Fig. S1G,H).
To comprehensively evaluate the RIPK1 kinase-dependent inflammatory profile, we performed a genome-wide microarray analysis on BMDMs stimulated with LPS or LPS with zVAD which revealed a large panel of inflammatory genes whose expression was augmented by zVAD and inhibited by Nec-1s (Fig. 1E). Analysis of a number of these genes in D138N RIPK1 BMDMs confirmed the critical role for RIPK1 kinase activity in promoting their expression after treatment with LPS with zVAD (Fig. S1I).
We also determined that siRNA-mediated silencing of RIPK1 (Fig. S1J) attenuated inflammatory mRNA changes in cells treated with LPS with zVAD (Fig. S1K). In contrast, previous reports suggest that necroptosis induced by LPS with zVAD can efficiently proceed through direct TRIF and RIPK3 activation even in the absence of RIPK1 (Dillon et al., 2014; Kaiser et al., 2013) and, consistently, silencing of RIPK1 expression did not attenuate necroptosis in our hands (Fig. S1L). Thus, RIPK1 is indispensable for the regulation of inflammatory gene expression, unlike its function in necroptosis.
Expectedly, BMDMs expressing a kinase-inactive hypomorphic K51A RIPK3 mutant (Ripk3K51A/K51A) were protected from cell death induced by LPS with zVAD (Fig. 1F) (Mandal et al., 2014) and were deficient in accumulation of RIPK1, RIPK3, and pMLKL in the detergent insoluble cellular fractions (Fig. 1G). K51A RIPK3 BMDMs also lacked increased inflammatory gene expression triggered by LPS with zVAD (Fig. 1H and S1M). A similar result was obtained using Ripk3−/− BMDMs (Fig. S1N-P) and a selective RIPK3 kinase inhibitor, GSK872 (Fig. S1Q) (Mandal et al., 2014), confirming the role of RIPK3 kinase activity in promoting inflammatory gene expression.
Catalytic activity of caspase-8 regulates activation of RIPK1 and RIPK3 kinases, necrosome formation and the induction of necroptosis (Moquin et al., 2013; O'Donnell et al., 2011). The requirement for zVAD suggests a similar role for caspase-8 in modulating RIPK1 and RIPK3 kinase-dependent inflammatory response. However, because zVAD is known to inhibit all caspases and additional classes of cysteine proteases (Schotte et al., 1999), we sought to confirm the role of caspase-8. Indeed, Cre-mediated deletion of Casp8 in Casp8flox/flox cells (Fig. S1R) promoted both necroptosis in response to LPS alone (Fig. 1I) and induced cytokine expression that was attenuated by Nec-1s (Fig. 1J and S1S). These observations affirmed a specific role for caspase-8 in blocking LPS-induced RIPK1 and RIPK3 kinase-dependent cytokine synthesis in vitro.
In sum, these data suggested that activation of RIPK1 and RIPK3 kinases in BMDMs promoted enrichment of these kinases in detergent insoluble cellular fractions followed by an increase in the expression of a range of pro-inflammatory molecules. Notably, these events preceded activation of necroptotic cell death.
RIPK1 and RIPK3 selectively potentiate TRIF-dependent LPS-induced inflammation in a cell-autonomous manner
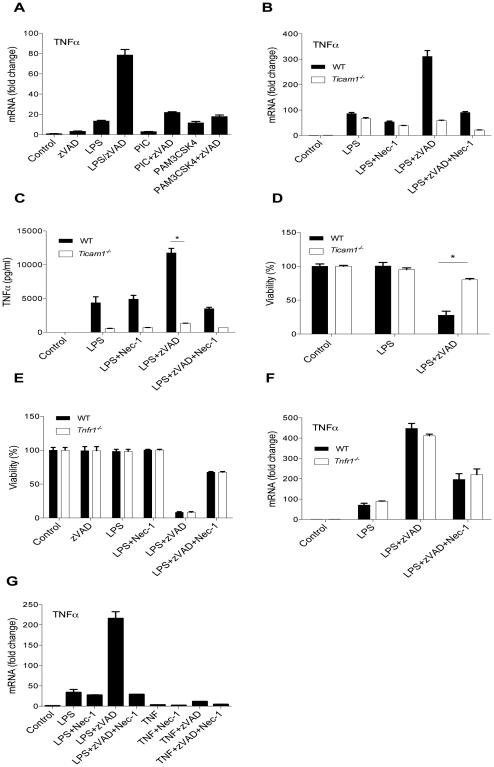
LPS engagement of TLR4 promotes receptor dimerization and engagement of two adapter proteins, MyD88 and TRIF, to initiate downstream signaling events (Yamamoto et al., 2002; Yamamoto et al., 2003). TRIF mediates formation of secondary endosomal complexes, involving additional components including RIPK1 and RIPK3 (Kaiser and Offermann, 2005; Meylan et al., 2004). To examine whether RIPK1 and RIPK3 kinase-dependent inflammatory gene expression similarly required TRIF, we analyzed responses to Pam3CSK4, an agonist of TLR2 which exclusively engages the adapter MyD88, and poly(I:C), an agonist of TLR3 which exclusively engages the adapter TRIF (Kawai and Akira, 2010). Caspase inhibition increased cytokine synthesis induced by LPS and Poly(I:C), but had marginal effect on Pam3CSK4, suggesting that RIPK1 and RIPK3 kinase-dependent cytokine synthesis may specifically require TRIF (Fig. 2A and S2A). To confirm this, we examined cytokine responses induced by LPS with zVAD in MyD88 (Myd88−/−) and TRIF (Ticam1−/−) deficient BMDMs (Fig. S2B,E). Indeed, increased synthesis and release of multiple inflammatory mediators in response to LPS with zVAD was absent in Ticam1−/− BMDMs (Fig. 2B,C and S2C,D), but not affected by the loss of MyD88 (Fig. S2F,G), similar to the specific requirement of TRIF for LPS with zVAD induced cell death (Fig. 2D and S2H). Additionally, LPS with zVAD also potently induced upregulation of IFNβ expression and protein release, which is specifically linked to TRIF, but not MyD88 (van Lieshout et al., 2015), and this was blocked by Nec-1s (Fig. S2I-K). Overall, these data suggested that RIPK1 and RIPK3 specifically potentiate TRIF-dependent inflammatory signaling downstream of TLR4.
Figure 2. RIPK1 and RIPK3 kinase-dependent cytokine synthesis and necroptosis require TRIF and manifest in a cell autonomous manner.
(A) TNF mRNA expression in wild type BMDMs treated for 7 hrs. (B, C) TNF mRNA expression (B) and protein release (C) in wild type and Ticam1−/− BMDMs. (D) Cell viability of wild type and Ticam1−/− BMDMs treated for 24 hrs. (E) Cell viability of BMDMs treated for 24 hrs. (F) TNF mRNA expression in BMDMs treated for 7 hrs. (G) TNF mRNA expression in wild type BMDMs treated with LPS with and without zVAD or TNF with and without zVAD for 7 hrs.
Values represent Mean ± SD. *p<0.05. BMDMs were treated with LPS=10ng/ml, Poly(I:C)=1µg/mL, Pam3CSK4= 1µg/mL, TNFα=10 ng/ml, zVAD=50 µM, and/or Nec-1s=30 µM.
Also see figure S2.
Autocrine TNFα signaling has been previously implicated in the induction of a RIPK1 and RIPK3 dependent inflammatory profile in macrophages upon inhibition of IAPs (Wong et al., 2014). Treatment of BMDMs with a pan-IAP inhibitor, SM164, induced cell death and secondary inflammatory cytokine synthesis that was absent in BMDMs carrying a genetic deletion of TNFR1 (Tnfr1−/−) and blocked by Nec-1s (Fig. S2L-O). In contrast, cell death and cytokine synthesis induced by LPS with zVAD was not attenuated in Tnfr1−/− macrophages (Fig. 2E,F and S2P). Additionally, we found that the combination of TNFα with zVAD is a weak inducer of cytokine synthesis in primary macrophages compared to LPS with zVAD (Fig. 2G and S2Q). To exclude the possibility that autocrine signals may be responsible for RIPK1 and RIPK3 kinase-dependent cytokine synthesis, inflammatory cytokine mRNA synthesis was evaluated in the presence of protein translation inhibitor, cycloheximide (CHX). Increasing doses of CHX abolished TNFα protein synthesis, but failed to block cytokine mRNAs induced by LPS with zVAD (Figure S2R,S). These data indicated that in contrast to the previously described responses to IAP inhibition, induction of cytokine gene expression by LPS with zVAD occurs in a cell-autonomous manner, independent of new protein synthesis and/or autocrine signaling by TNFα.
RIPK1 and RIPK3 kinase-dependent inflammation proceeds independently of MLKL-dependent necroptosis
As expected, both Ripk3−/− and Mlkl−/− BMDMs were completely protected from LPS with zVAD induced cell death (Fig. 3A). However, in contrast to the deficiency in RIPK3, we still observed induction of cytokine expression, albeit slightly reduced, in Mlkl−/− BMDMs in response to LPS with zVAD (Fig. 3B,C and S3A). Similarly, shRNA silencing of MLKL had no effect on pro-inflammatory signaling, even though it efficiently prevented cell death, in contrast to RIPK3 shRNA, which blocked both responses (Fig. S3B-E). Overall, these data showed that RIPK1 and RIPK3 kinase-dependent induction of cytokine expression still occurred in the cells that did not undergo necroptosis in the absence of MLKL.
Figure 3. MLKL is dispensable for cytokine synthesis and the role of RIPK3 is context-specific.
(A) Cell viability of BMDMs treated for 24 hrs. (B, C) TNF mRNA expression (B) and protein release (C) in wild type, Mlkl−/−, and Ripk3−/− BMDMs treated for 7 hrs. In (C), statistical significance calculated by one way ANOVA. (D) Cell viability of MEFs treated for 24 hrs. (E, F) TNF mRNA expression (E) and protein release (F) in MEFs treated for 4 hr. (G) Cell viability of MEFs treated for 24 hrs. (H, I) TNF mRNA expression (H) and protein release (I) in MEFs treated for 4 hr.
Values represent Mean ± SD. *p<0.05. BMDMs were treated with LPS=10 ng/ml, zVAD=50 µM, and Nec-1s=30 µM. MEFs were treated with TNFα=10 ng/ml, zVAD=25 µM and CHX=1 µg/ml.
Also see figure S3.
To further examine the connection between inflammatory gene expression changes and necroptosis, we evaluated these responses in a different system, using mouse embryonic fibroblasts (MEFs) treated with TNFα, zVAD and a low dose of CHX (Cho et al., 2009; He et al., 2009). As in BMDMs, we found that RIPK1 (Ripk1−/− MEFs) was critical for TNFα and IL6 mRNA induction (Fig. 3E,F and S3F), even though it was dispensable for necroptosis (Fig. 3D). Although deletion of RIPK3 prevented necroptosis (Fig. 3G), it did not attenuate TNFα and IL6 induction (Fig. 3H,I and S3G). Consistently, RIPK3 inhibitor, GSK872, also did not inhibit TNFα mRNA expression in wild type MEFs treated with TNFα, zVAD, and CHX at the concentrations that blocked necroptosis (Fig. S3H,I). These data revealed that RIPK1 is indispensable for the upregulation of inflammatory gene expression, while RIPK3 contributes to this regulation in a context-dependent manner and induction of inflammation still occurs in the absence of MLKL-dependent necroptosis.
Erk1/2 mediate RIPK1 and RIPK3-dependent inflammatory gene expression changes
Examining signaling changes, we observed pronounced phosphorylation of MAPK Erk1/2 (Fig. 4A) and increased catalytic activity of the immunoprecipitated protein (Fig. 4B) upon treatment of BMDMs with LPS with zVAD. This response was RIPK1 and RIPK3 kinase-dependent as it was abolished in genetic models of RIPK1 and RIPK3 kinase inactivation and blocked by Nec-1s (Fig. 4A,B and S4A,B).
Figure 4. Erk-cFos and NFkB pathways mediate RIPK1 and RIPK3 kinase-dependent pro-inflammatory signaling induced by LPS with zVAD.
(A) Western analysis of Erk1/2 phosphorylation in BMDMs treated for 7 hrs. (B) In vitro kinase assay of Erk1/2 protein against Elk1 substrate. Total Erk1/2 was immunoprecipitated from BMDMs treated for 3 hrs. (C-D) TNF mRNA expression (C, 7 hrs) and cell viability (D, 24 hrs) in BMDMs after electroporation with Erk1/2 siRNA. (E) Co-immunoprecipitation of endogenous RIPK1 and Erk1/2 from BMDMs treated for 3 hrs. Western blot analysis for lysate (left panel) and IP (right panel) samples shown. (F) Western analysis in BMDMs treated for 7 hrs. Refer to panel 4A for loading control. (G) Western analysis in wild type BMDMs treated for 7 hrs after electroporation of Erk1/2 siRNA. (H-I) TNF mRNA expression (H, 7 hrs) and cell viability (I, 24 hrs) in BMDMs after electroporation with cFos siRNA. (J) Western analysis in BMDMs treated for 7 hrs. Refer to panel 4A for loading control. (K-L) TNF mRNA expression (K, 7 hrs) and cell viability (L, 24 hrs) in BMDMs after electroporation with p65 siRNA.
Values represent Mean ± SD. *p<0.05. BMDMs were treated with LPS=10 ng/ml, zVAD=50 µM, and Nec-1s=30 µM.
Also see figures S4 and S5.
Importantly, siRNA silencing of Erk1/2 kinases resulted in the pronounced attenuation of multiple gene expression changes and TNFα protein release elicited by LPS with zVAD, without perturbing RIPK1 and RIPK3 kinase-independent responses to LPS alone (Fig. 4C and S4C,D). Furthermore, silencing of Erk1/2 had no effect on cell death induced by LPS with zVAD (Fig. 4D), suggesting that Erk1/2 is a selective transducer of pro-inflammatory signals from RIPK1 and RIPK3. To verify Erk siRNA data, we tested responses in Erk1-deficient (Mapk3−/−) BMDMs, and similarly observed attenuation of inflammatory responses, but not cell death, in cells treated with LPS with zVAD (Fig. S4E,F).
To elucidate this regulation, we analyzed the effects of two small molecule inhibitors of Erk1/2 pathway. While a direct Erk1/2 inhibitor, SCH772984 (Morris et al., 2013), blocked inflammation induced by LPS with zVAD (Fig. S4G), CL-1040, an inhibitor of upstream Erk1/2 MAPKKs, MEK1 and MEK2, did not reproduce this effect (Fig. S4H), indicating that Erk1/2 regulation by RIPK1 and RIPK3 may not follow a canonical growth factor induced MAPK cascade. This was consistent with the absence of MEK1 and MEK2 activation in BMDMs treated by LPS with zVAD (Fig. S4I). To explore interactions between RIPK1 and Erk1/2, we performed immunoprecipitation of RIPK1 from primary BMDMs, and observed that its association with Erk1/2 was induced in LPS with zVAD stimulated cells and blocked by addition of Nec-1s (Fig. 4E). A reciprocal Erk1/2 IP experiments also confirmed association with RIPK1 (Fig. S4J). Additionally, we observed co-enrichment of phosphorylated Erk1/2 with RIPK1 and RIPK3 in NP40-insoluble cellular fraction in response to LPS with zVAD, which was reduced by Nec-1s and in K51A RIPK3 BMDMs (Fig. S4K,L). Notably, we did not observe MEK1 or MEK2 in the NP40-insoluble fraction (Fig. S4M).
To further investigate constituents of the detergent-insoluble fraction, we probed samples for additional players known to participate in the regulation of RIPK1 and RIPK3 and found TRIF, caspase-8 and CYLD to be similarly enriched (Fig. S5A). Finally, because we previously detected p-MLKL increase in detergent-insoluble aggregates (Fig. 1B), we examined whether Erk1/2 was present in the insoluble fraction from Mlkl−/− BMDMs. Indeed, we observed that Erk1/2 co-segregated with RIPK1 and RIPK3 in a MLKL-independent manner (Fig. S5B). These findings suggested that “necrosome”-like detergent insoluble aggregates of RIPK1 and RIPK3 may serve as a signaling platform for engaging Erk1/2 and bypassing MEK1 and MEK2.
Similar to previous data in L929 cells (Christofferson et al., 2012; McNamara et al., 2013), silencing of cJun abrogated inflammatory responses induced by LPS with zVAD in BMDMs (Fig. S5C). However, selective JNK inhibitor VIII did not prevent LPS with zVAD induced gene expression, even though cJun phosphorylation was blocked (Fig. S5D,E), suggesting that cJun phosphorylation by JNK may be co-incidental with RIPK1 and RIPK3 kinase-dependent regulation. The binding partner of cJun in AP1 complex, cFos, is a known direct target of phosphorylation and stabilization by Erk1/2 (Chen et al., 1996; Okazaki and Sagata, 1995). Total and phosphorylated cFos were increased upon stimulation with LPS with zVAD in parallel with Erk1/2 in a RIPK1 and RIPK3 kinase-dependent manner (Fig. 4F and S4A,B,). Furthermore, changes in cFos, but not cJun phosphorylation were blocked by Erk1/2 siRNA (Fig. 4G), correlating with changes in inflammatory gene expression. Silencing of cFos (Fig. S5F) confirmed its requirement for inflammatory gene induction (Fig. 4H and S5G,H). Similar to Erk1/2, cFos only contributed to RIPK1 and RIPK3 kinase-dependent increase after treatment with LPS with zVAD, but was dispensable for LPS induced responses (Fig. 4H) and necroptosis (Fig. 4I), identifying Erk1/2-cFos as a selective axis for inflammatory regulation by RIPK1 and RIPK3.
Along with AP-1, NFκB is established to play a central role in acute inflammatory responses to LPS (Kawai and Akira, 2006). As expected, LPS triggered rapid and transient NFκB activation as evidenced by p65 phosphorylation and IκB degradation. This activation was sustained up to 6-9 hr when RIPK1 and RIPK3 kinases became engaged in the presence of zVAD (Fig. 4J and S5I). Unlike cFos siRNA, siRNA silencing of p65 (Fig. S5J) attenuated pro-inflammatory responses to LPS as well as LPS with zVAD; however, similar to cFos silencing, cell death was unperturbed (Fig. 4K,L and S5K,L), reflecting a contribution of NFκB signaling to TRIF-dependent inflammation (Kawai and Akira, 2006). Importantly, activation of NFκB was inhibited by siRNA silencing and the small molecule inhibitor of Erk1/2 (Fig. 4G and S5M), but not by cFos siRNA (Fig. S5N) marking these two factors as independent targets of the RIPK1 and RIPK3-Erk1/2 axis that may be activated synergistically to achieve optimal potentiation of the inflammatory responses.
Erk, cFos, and NFκB phosphorylation changes induced by LPS with zVAD were blocked in Ripk3−/−, RIPK3 K51A and Ticam1−/− BMDMs (Fig. S4A,B, S5O), all of which were found to be deficient in inflammatory cytokine production induced by LPS with zVAD (Figs. 1, 2). Conversely, these phosphorylation changes were not blocked in Mlkl−/− BMDMs, which retained an increased inflammatory response to LPS with zVAD (Fig. 3 and S5P). As further verification of the specificity of this regulation, we determined that phosphorylation of Erk1/2, cFos, and p65, and degradation of IκB, induced by LPS alone in caspase-8-deficient BMDMs, became sensitive to inhibition by Nec-1s (Fig. S5Q). In sum, these data established the importance of Erk, cFos, and NFκB as mediators of RIPK1 and RIPK3 kinase-dependent inflammatory gene expression.
RIPK1 and RIPK3 control LPS-induced inflammatory responses in vivo
Induction of necroptosis is characterized by a well-established dichotomy in activation in vitro, which in most cases requires inhibition of caspase-8, and in vivo, where necroptosis has been observed in a wide range of pathologic injuries in the absence of exogenous caspase inhibition (Duprez et al., 2011; Linkermann and Green, 2014). This has been directly illustrated by the activity of the best characterized inducer of necroptosis, TNFα, which requires caspase inhibition for necroptosis in vitro in most cellular systems described to date, but efficiently and directly induces RIPK1 and RIPK3 kinase-dependent necroptosis in vivo in normal, wild type animals (Duprez et al., 2011). These observations suggest that the strict controls on RIPK1 and RIPK3 kinase activation in vitro may be less efficient in vivo and prompted us to examine whether RIPK1 and RIPK3 kinases may be involved in the control of inflammatory responses to LPS in vivo in the absence of zVAD. To address this question, we injected BALB/c mice intravenously with 30 mg/kg of Nec-1s 15 min prior to intraperitoneal injection with a low dose of LPS (50 µg/kg). Multiple cytokines and chemokines, which were shown to be augmented by zVAD treatment and regulated in a RIPK1 and RIPK3 kinase-dependent manner in vitro (Fig. 1: TNFα, CCL3, CCL4, GM-CSF (CSF-2) and IL6), were induced in the circulation by LPS, 1 hr post-injection, and strikingly reduced ~60-80% by Nec-1s (Fig. 5A and S6A). The one hour time point corresponds to the time of maximal TNFα induction (Fig. S6B). Additionally, we observed that Nec-1s decreased mRNA of these molecules in CD11b+ myeloid precursor cells, isolated from the bone marrow of animals (Fig. 5B and S6C). Analysis of additional genes, which were upregulated by LPS with zVAD in vitro (Fig. 1), showed similar induction by LPS alone in vivo and inhibition by Nec-1s (Table S1). To confirm the role of RIPK1 kinase, we injected wild type and RIPK1 D138N mice with LPS and, similarly, found a robust decrease in circulating inflammatory mediators (Fig. 5C). D138N RIPK1 CD11b+ myeloid precursor cells, isolated from the bone marrow of the LPS-injected animals, also displayed greatly reduced mRNA transcripts of inflammatory molecules (Fig. 5D), supporting control of inflammatory gene expression by RIPK1 kinase activity in vivo.
Figure 5. LPS-induced inflammatory cytokine synthesis requires RIPK1 kinase in vivo.
(A) Circulating TNFα in wild type mice injected with Nec-1s (iv) 15 min prior to LPS (50µg/kg) (ip) by ELISA. n= 8-9 animals per group and *p<0.05. (B) TNF mRNA expression in CD11b+ bone marrow cells isolated from mice post-injection with LPS or LPS/Nec-1s as in (A). n=5-6 animals per group and *p<0.05. (C) Multiplexed ELISA analysis of circulating inflammatory mediators in mice injected with LPS (50µg/kg) intraperitoneally. Serum samples evaluated 1 hr thereafter. n=3-6 animals per group and *p<0.05. (D) mRNA expression of a panel of inflammatory genes in CD11b+ bone marrow cells isolated from LPS-injected mice as in (C). n= 3-6 animals per group and *p<0.05. Values represent Mean ± SD.
Also see figure S6.
To more comprehensively examine the inflammatory profile regulated by RIPK1 kinase, we performed RNA-Seq analysis of the mRNAs from mice treated with vehicle, LPS, and LPS with Nec-1s. Pathway analysis identified multiple innate immune response axes as major pathways induced by LPS in vivo, and notably, the same pathways were identified as key targets of Nec-1s in the LPS-injected animals (Table S2). We also evaluated this regulation in CD11b+ sorted peritoneal macrophages, a model for mature and activated myeloid cells, and found that intravenous administration of Nec-1s, similarly inhibited LPS-induced TNFα, CCL3 and CCL4 mRNA upregulation 1 hr after LPS injection (Fig. S6D). Overall, these data mark the kinase activity of RIPK1 as an unanticipated master regulator of acute inflammatory responses to LPS in vivo.
Our in vitro model also pointed to a crucial role for RIPK3 in regulating RIPK1 kinase-dependent cytokine synthesis. Cytokine production was also diminished in the Ripk3−/− mice confirming an important role for RIPK3 in LPS-induced inflammation in vivo (Fig. 6A). While bone marrow cells provide a limited source for protein analysis, we were able to also observe activation of some of the same signaling pathways in vivo that we have linked to RIPK1 and RIPK3 activation in vitro, including phosphorylation of Erk, cFos and p65 and degradation of IκB. All of these LPS-induced events were inhibited by Nec-1s (Fig. 6B).
Figure 6. LPS-induced inflammatory cytokine synthesis requires RIPK3 but not MLKL in vivo.
(A) Circulating inflammatory mediators in wild type and Ripk3−/− mice by ELISA. n= 4 animals per group, *p<0.05. (B) Western analysis in mouse CD11b+ bone marrow cells from LPS or LPS and Nec-1s injected animals. Samples from 3 animals per group were pooled for the analysis. (C) TNF mRNA expression in CD11b+ bone marrow cells isolated from wild type and Mlkl−/− mice injected with Nec-1 (iv) 15 min prior to LPS (ip) (50µg/kg) or LPS (ip) alone. Cells were collected 1 hr after LPS injection. n=3 animals per group and *p<0.05. (D) Circulating TNFα from wild type and Mlkl−/− mice injected with LPS or LPS and Nec-1 as in (C). n=5 animals per group and *p<0.05.
Also see figure S6.
Our in vitro data strongly suggested a critical role for RIPK1 and RIPK3 kinases as inducers of cytokine synthesis independent of cell death. Next, we sought to evaluate whether this is also the case in vivo. Notably, our studies used low dose of LPS, which is ≤10% of what is used to trigger systemic toxicity. Accordingly, we did not observe cell death in CD11b+ cells that we used for mRNA analysis using AQUA LIVE/DEAD staining (Fig. S6E). Similarly, we did not see any increase in the circulating markers of injury, such as ALT, at either 1 hr or at 7 hrs after LPS injection (Fig. S6F). These data support the notion that RIPK1 regulation of inflammation is not associated with cell death in vivo. To examine RIPK1 and RIPK3 kinase-dependent cytokine synthesis in the absence of necroptosis, we assessed the efficacy of Nec-1s in regulating LPS-induced cytokine production in Mlkl−/− mice. Consistent with our hypothesis, loss of MLKL did not impair LPS-induced serum cytokines and mRNA expression in CD11b+ cells, and importantly, this induction was still blocked by Nec-1s (Figure 6C,D and S6G,H). Altogether, these data indicate the cell death independent control of inflammation by kinase activity of RIPK1 and RIPK3 in vivo, analogous to our data in vitro.
We also considered whether TNFα upregulation due to the LPS stimulation might be a contributing factor to the acute RIPK1 and RIPK3 kinase-dependent inflammatory responses. However, administration of TNFα only led to a relatively weak inflammatory response, compared to LPS, and this response was not inhibited by Nec-1s (Fig. S6I). This observation was not surprising as previously published data showed that circulating concentrations of inflammatory mediators, such as IL6, were elevated only several hours after injection of TNFα and this inflammatory response was clearly secondary to the induction of cell death induced by the molecule (Duprez et al., 2011).
Factors that may allow activation of RIPK1 and RIPK3 kinase-dependent injury without perturbation of caspase-8 activity in a variety of mouse models in vivo have not yet been addressed comprehensively. One possibility may be lower efficiency of caspase-8 activation in vivo. We found that Caspase-Glo caspase-8 activity assay provided a sensitive method to detect caspase-8 activation by LPS alone in vitro, and this was inhibited by zVAD (Fig. S6J). Notably, using the same assay, we did not observe caspase-8 activation in bone marrow cells in response to LPS challenge in vivo. Only a much higher, toxic dose of LPS was required to elicit robust caspase-8 activation (Fig. S6K). Thus, while development of better and more sensitive tools will be useful for the evaluation of caspase-8 activation, our data suggests the possibility that limitations on caspase-8 activity may explain the direct activation of RIPK1 and RIPK3 signaling by LPS in vivo.
Ultimately, these observations suggest that RIPK1 and RIPK3, which are controlled in vitro by caspase-8, play a major role in acute inflammatory responses to LPS in vivo. Our work revealed a mechanism for this regulation, which is mediated by Erk1/2 in a cell death-independent and cell-autonomous manner.
Discussion
Our observations suggest that kinase activity of RIPK1 is a critical component in negotiating acute inflammatory cytokine expression, unlike the role of this kinase in necroptosis, which can be by-passed by direct engagement of RIPK3 (Dannappel et al., 2014; Dillon et al., 2014; Kaiser et al., 2014; Kearney et al., 2015; Rickard et al., 2014; Takahashi et al., 2014; Upton et al., 2010, 2012). In the absence of kinase activity of RIPK1, LPS and LPS with zVAD-induced acute inflammatory changes were attenuated both in vivo and in vitro, respectively. Similarly, Ripk3−/− mice and BMDMs also displayed a significant defect in cytokine production induced by LPS in vivo or LPS with zVAD in vitro. However, RIPK3, but not RIPK1, was dispensable for inflammatory gene expression changes in TNFα-treated MEFs. These observations distinguish kinase functions of RIPK1 from RIPK3 and suggest that while RIPK3 serves as the requisite cell executioner, RIPK1 may serve as an essential driver of de novo cytokine synthesis. Consistent with this framework, recent works from other groups have elucidated additional RIPK1 kinase-dependent inflammatory changes that do not require RIPK3. For example, RIPK1 kinase, but not RIPK3 was responsible for inflammation observed in Ptpn6spin mice and caspase-8 deficient dendritic cells (Cuda et al., 2014; Lukens et al., 2013). In another example, work by Yatim et al. showed that chemically enforced RIPK3 oligomerization is insufficient to drive NFκB-dependent cytokine synthesis without RIPK1 (Yatim et al., 2015). Together, our work and that of others suggest an important role for RIPK1 catalytic activity in the regulation of multiple inflammatory pathways.
The precise molecular details of inflammatory signaling by the RIPK1 and RIPK3 necrosome will be important to further understand. Previous work established that TRIF can directly engage RIPK3 (Kaiser et al., 2013) and oligomerization of just RIPK3 is sufficient to induce necroptosis (Cook et al., 2014; Orozco et al., 2014; Wu et al., 2014). In contrast, our data show that RIPK1 kinase, also a direct binding partner of TRIF (Meylan et al., 2004), is indispensable for TRIF-dependent cytokine synthesis. Evidence also suggests that RIPK1 and RIPK3 form detergent insoluble amyloid-like “necrosome” aggregates (Li et al., 2012) which serve as a signaling platform for MLKL activation by RIPK3. Analogous RIPK1-containing “ripoptosome” complexes were described as a platform for RIPK1-dependent caspase-8 activation (Feoktistova et al., 2011; Tenev et al., 2011). Our data suggest that similar detergent-insoluble RIPK1 and RIPK3 “necrosome”-like aggregates may mediate pro-inflammatory signaling through Erk1/2, bypassing requirement for MEK1 and/or MEK2. However, it remains to be determined whether a singular RIPK1 and RIPK3 complex is formed to carry out distinct pro-inflammatory and pro-death functions or, alternatively, physically and/or compositionally distinct “necrosome”-like complexes are formed to differentially regulate necroptosis and pro-inflammatory gene expression. A comprehensive proteomics analysis of molecular interactions associated with RIPK1 and RIPK3 aggregate formation in BMDMs treated with LPS with zVAD will be needed to further reveal mechanistic details of this regulation.
RIPK1 and RIPK3 have attracted major interest as therapeutic targets for a wide range of pathologies, including ischemia-reperfusion injuries, atherosclerosis, pancreatitis, retinal damage, multiple sclerosis and many others (Linkermann and Green, 2014; Ofengeim et al., 2015). While inflammation is often viewed as a consequence of necroptosis (Pasparakis and Vandenabeele, 2015; Silke et al., 2015), our findings raise the possibility that the contributions of RIPK1 and RIPK3 to inflammatory pathologies may also reflect cell death-independent regulation of inflammation. Accordingly, it will be critical to consider whether inflammatory pathologies, in which roles for RIPK1 and RIPK3 may not have been closely examined due to the limited influence of cell death, may involve these proteins in a cell death independent capacity.
Lastly, the dichotomy in RIPK1 and RIPK3 activation in vivo and in vitro remains to be fully understood. In vitro, LPS-induced RIPK1 and RIPK3 kinase-dependent inflammatory cytokine production requires inactivation of caspase-8. However, in vivo, LPS-induced RIPK1 and RIPK3 kinase-dependent inflammation proceeds without exogenous manipulation of caspase-8 function. This paradigm is analogous to many other in vivo observations, such as the requirement of caspase-8 inhibition in vitro, but not in vivo for necroptosis activation by TNFα (Duprez et al., 2011). One explanation for these differences may be a discrepancy in the activity of the regulators of RIPKs, such as caspase-8, CYLD, FLIPL, TAK1, and/or A20, resulting in less stringent controls on RIPK activation in vivo. Along these lines, while LPS induced caspase-8 activity in vitro in our experiments, this was not the case in vivo, consistent with the possibility of differences in caspase-8 activation by LPS in vitro and in vivo. Notably, recent report by Ofengeim et al. similarly suggested deficient activation of caspase-8 in white matter lesions of multiple sclerosis patients are associated with changes in the regulation of RIPK1 and RIPK3 signaling (Ofengeim et al., 2015). Nevertheless, more sensitive and specific methods, especially to assess caspase-8 activity in necroptosis-inhibiting caspase-8 and FLIPL heterodimeric complex (Oberst et al., 2011), are needed to further address this question in LPS-induced and other in vivo models of RIPK1 and RIPK3 kinase regulation.
Experimental Procedures
Animals
Female Balb/c (Charles River Labs) or C57BL/6 mice 6-8 weeks of age were used for LPS experiments. Ripk3−/− (on C57BL/6 background) and matched controls were previously described (Newton et al., 2004) and provided to us by Dr. Vishva Dixit (Genentech). Ticam1−/− (C57BL/6J-Ticam1Lps2/J) mice, Myd88−/− (B6.129-Myd88tm1.1Defr/J), Mapk3−/− (B6.129-Mapk3tm1Gela/J) mice and corresponding control mice (C57BL/6J, B6.129) were purchased from Jackson labs. Tnfr1−/− mice (C56BL/6-Tnfrsf1tm1lmx/J) on a C57BL/6 background were obtained from Jackson labs. Floxed caspase-8 (Casp8fl/fl) mice were generated on FVB/N background and were a generous gift of Dr. Stan Krajewski (Burnham institute) (Krajewska et al., 2011). RIPK1 D138N and RIPK3 K51A (Ripk1D138N/D138N and Ripk3K51A/K51A) mice were previously described (Moriwaki et al., 2015; Polykratis et al., 2014). All use of animals was approved by the Tufts University, UMASS and Fox Chase Cancer Center Institutional Animal Care and Use Committees. Mice were maintained in animal facilities in cages with light and dark cycle and experiments were performed according to the protocol with all efforts to minimize the number and suffering of the animals.
Cells
Bone marrow derived macrophages (BMDMs) were prepared from bone marrow cells collected from femurs and tibias of mice. Bone marrow cells were differentiated over 7 days in the presence of conditioned media from L929 cells (30% L929 conditioned media, 20% FBS and 1% antibiotics in RPMI1640) in petri dishes. Media was replenished on day 3. On day 7, adherent cells were collected and reseeded in tissue-culture treated plates for experimentation. After reseeding, cells were maintained in media containing 10% L929 conditioned media, 20% FBS, and 1% antibiotics for 48 hours prior to carrying out experiments. For mRNA and Western blot experiments, 2X106 BMDMs were seeded into 35 mm2 dishes. For cell viability experiments, 50,000 cells per well were seeded in 96-well plates. WT, RIPK1−/−, and RIPK3−/− MEFs (Cho et al., 2009; Kelliher et al., 1998) were maintained in DMEM, 10% FBS and 1% antibiotics. Cells were seeded in a similar format to BMDMs for experiments. Further details can be found in the Supplement.
Cell viability, qRT-PCR and ELISA
For qRT-PCR analysis, total RNA was isolated using ZR RNA mini kit (Zymo research), cDNAs were prepared using iScript cDNA Synthesis Kit (Bio Rad) and reactions were performed using Veriquest 2XSYBR master mix (Affymetrix) in a Roche480 thermocycler. Data for one representative experiment out of at least three independent repeats are shown. Cell viability was assessed using CellTiter-Glo assay (Promega). ELISA analysis was performed using Meso Scale Discovery’s (MSD) 96-Well MULTI-SPOT mouse multiplex assay (TNFα, CCL3, CCL4, GMCSF, IL6 and p-RIPK1) or colorimetric ELISA assay (TNFα and IFNβ). For cell viability and ELISA, combined data for three independent experiments are shown unless otherwise stated. Further details can be found in the Supplement.
Gene expression analysis
Affymetrix gene chip analysis of BMDM samples was performed using GeneChip Mouse Gene 1.0 ST Array. Functional annotation clustering of the genes, increased ≥ 1.5-fold in LPS with zVAD vs. LPS treated cells, was performed using NIH DAVID.
http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE72797
For RNA-Seq analysis, CD11b+ bone marrow cells were isolated by FACS, RNA samples were converted to cDNA and analyzed using Illumina HiSeq 2500 using SBS V3 chemistry. Gene expression profiles were analyzed using Ingenuity pathway analysis (IPA) software to identify pathways regulated by LPS and Nec-1.
http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE72797
Further details can be found in the Supplement.
In vivo LPS challenge and Nec-1 treatment
Mice were injected intravenously via tail vein with 100µL of 30 mg/kg optimized Nec-1 (7-Cl-O-Nec-1), (Degterev et al., 2013) 15 min prior to intraperitoneal injection of 50 μg/kg LPS (Sigma) dissolved in 500µL PBS. Nec-1 was dissolved in PBS containing 25% Polyethylene Glycol 400 by water sonication for 15 min. Blood was collected 1 hour after LPS injection and cytokines were measured using multiplexed ELISA assay (Meso Scale Discovery) or colorimetric ELISA assay. Bone marrow cells were flushed from femurs and tibias with PBS, centrifuged at 430g, resuspended and blocked with 1 μM EDTA and 2% BSA in PBS for 45 min at 4°C, and stained using anti-CD11b+-PE antibody (Biolegend, clone M1/70, 1:500) for 60 min at 4°C. CD11b+ cells were sorted by fluorescence-activated cell sorting (FACS) using MoFlo sorter.
Supplementary Material
Highlights.
RIPK1 and RIPK3 kinases promote TRIF-dependent cytokine production by LPS in vitro.
RIPK1 and RIPK3 kinase-dependent inflammation is independent of cell death.
Erk1/2 mediates RIPK1 and RIPK3 kinase dependent inflammation.
RIPK1 kinase and RIPK3 are mediators of LPS-induced cytokine production in vivo.
eTOC Blurb.
Kinase activities of RIPK1 and RIPK3 are critical for necroptotic cell death. Degterev and colleagues demonstrate that RIPK1 and RIPK3 kinases also direct inflammatory gene expression induced by Toll-like receptor 4 ligand, lipopolysaccharide, in vitro and in vivo. This regulation is independent of necroptosis and requires Erk1/2, cFos and NFκB.
Acknowledgments
This work was supported in part by grants from NIH to A.D. (R01GM080356 and R01GM084205), to S.B. and A.D. (R01CA190542), to M.K. (R01AI07118), and to M.W. (R01NS047447). We thank Drs. Vishva Dixit, Stan Krajewski, Katherine Fitzgerald and Sergei Nedospasov for providing the reagents. P.J.G., J.B and J.N.F. are employees of GlaxoSmithKline. A.D. is a consultant for Denali Therapeutics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
M.N., D.S., M.Z., S.N., A.T., J.F. and S.S. performed the experiments. A.P. and M.P. developed RIPK1 D138N mice. M.N., D.S., J.B., P.G., M.W., M.K., S.B., M.P., A.P. and A.D. wrote and revised the manuscript.
References
- Berger SB, Kasparcova V, Hoffman S, Swift B, Dare L, Schaeffer M, Capriotti C, Cook M, Finger J, Hughes-Earle A, et al. Cutting Edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J Immunol. 2014;192:5476–5480. doi: 10.4049/jimmunol.1400499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RH, Juo PC, Curran T, Blenis J. Phosphorylation of c-Fos at the C-terminus enhances its transforming activity. Oncogene. 1996;12:1493–1502. [PubMed] [Google Scholar]
- Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofferson DE, Li Y, Hitomi J, Zhou W, Upperman C, Zhu H, Gerber SA, Gygi S, Yuan J. A novel role for RIP1 kinase in mediating TNFalpha production. Cell Death Dis. 2012;3:e320. doi: 10.1038/cddis.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofferson DE, Yuan J. Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol. 2010;22:263–268. doi: 10.1016/j.ceb.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook WD, Moujalled DM, Ralph TJ, Lock P, Young SN, Murphy JM, Vaux DL. RIPK1- and RIPK3-induced cell death mode is determined by target availability. Cell Death Differ. 2014;21:1600–1612. doi: 10.1038/cdd.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuda CM, Misharin AV, Gierut AK, Saber R, Haines GK, 3rd, Hutcheson J, Hedrick SM, Mohan C, Budinger GS, Stehlik C, et al. Caspase-8 acts as a molecular rheostat to limit RIPK1- and MyD88-mediated dendritic cell activation. J Immunol. 2014;192:5548–5560. doi: 10.4049/jimmunol.1400122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusson-Hermance N, Khurana S, Lee TH, Fitzgerald KA, Kelliher MA. Rip1 mediates the Trif-dependent toll-like receptor 3- and 4-induced NF-{kappa}B activation but does not contribute to interferon regulatory factor 3 activation. J Biol Chem. 2005;280:36560–36566. doi: 10.1074/jbc.M506831200. [DOI] [PubMed] [Google Scholar]
- Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, Wachsmuth L, Eftychi C, Lin J, Corona T, Hermance N, et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature. 2014;513:90–94. doi: 10.1038/nature13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- Degterev A, Maki JL, Yuan J. Activity and specificity of necrostatin-1, small-molecule inhibitor of RIP1 kinase. Cell Death Differ. 2013;20:366. doi: 10.1038/cdd.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, Verbist KC, Brewer TL, Llambi F, Gong YN, et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–1202. doi: 10.1016/j.cell.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprez L, Takahashi N, Van Hauwermeiren F, Vandendriessche B, Goossens V, Vanden Berghe T, Declercq W, Libert C, Cauwels A, Vandenabeele P. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity. 2011;35:908–918. doi: 10.1016/j.immuni.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, Cain K, MacFarlane M, Hacker G, Leverkus M. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Liang Y, Shao F, Wang X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3- mediated pathway. Proc Natl Acad Sci U S A. 2011;108:20054–20059. doi: 10.1073/pnas.1116302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Kaiser WJ, Daley-Bauer LP, Thapa RJ, Mandal P, Berger SB, Huang C, Sundararajan A, Guo H, Roback L, Speck SH, et al. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc Natl Acad Sci U S A. 2014;111:7753–7758. doi: 10.1073/pnas.1401857111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WJ, Offermann MK. Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J Immunol. 2005;174:4942–4952. doi: 10.4049/jimmunol.174.8.4942. [DOI] [PubMed] [Google Scholar]
- Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kearney CJ, Cullen SP, Tynan GA, Henry CM, Clancy D, Lavelle EC, Martin SJ. Necroptosis suppresses inflammation via termination of TNF- or LPS-induced cytokine and chemokine production. Cell Death Differ. 2015;22:1313–1327. doi: 10.1038/cdd.2014.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- Krajewska M, You Z, Rong J, Kress C, Huang X, Yang J, Kyoda T, Leyva R, Banares S, Hu Y, et al. Neuronal deletion of caspase 8 protects against brain injury in mouse models of controlled cortical impact and kainic acid-induced excitotoxicity. PLoS One. 2011;6:e24341. doi: 10.1371/journal.pone.0024341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao YS, Damko E, Moquin D, Walz T, McDermott A, et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012;150:339–350. doi: 10.1016/j.cell.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkermann A, Green DR. Necroptosis. N Engl J Med. 2014;370:455–465. doi: 10.1056/NEJMra1310050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukens JR, Vogel P, Johnson GR, Kelliher MA, Iwakura Y, Lamkanfi M, Kanneganti TD. RIP1-driven autoinflammation targets IL-1alpha independently of inflammasomes and RIP3. Nature. 2013;498:224–227. doi: 10.1038/nature12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H, Lich JD, Finger J, Kasparcova V, Votta B, et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell. 2014;56:481–495. doi: 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara CR, Ahuja R, Osafo-Addo AD, Barrows D, Kettenbach A, Skidan I, Teng X, Cuny GD, Gerber S, Degterev A. Akt Regulates TNFalpha synthesis downstream of RIP1 kinase activation during necroptosis. PLoS One. 2013;8:e56576. doi: 10.1371/journal.pone.0056576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M, Tschopp J. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- Moquin DM, McQuade T, Chan FK. CYLD deubiquitinates RIP1 in the TNFalpha-induced necrosome to facilitate kinase activation and programmed necrosis. PLoS One 8, e76841. 2013 doi: 10.1371/journal.pone.0076841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki K, Bertin J, Gough PJ, Chan FK. A RIPK3-caspase 8 complex mediates atypical pro-IL-1beta processing. J Immunol. 2015;194:1938–1944. doi: 10.4049/jimmunol.1402167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris EJ, Jha S, Restaino CR, Dayananth P, Zhu H, Cooper A, Carr D, Deng Y, Jin W, Black S, et al. Discovery of a novel ERK inhibitor with activity in models of acquired resistance to BRAF and MEK inhibitors. Cancer Discov. 2013;3:742–750. doi: 10.1158/2159-8290.CD-13-0070. [DOI] [PubMed] [Google Scholar]
- Newton K, Sun X, Dixit VM. Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol. 2004;24:1464–1469. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell MA, Perez-Jimenez E, Oberst A, Ng A, Massoumi R, Xavier R, Green DR, Ting AT. Caspase 8 inhibits programmed necrosis by processing CYLD. Nature cell biology. 2011;13:1437–1442. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofengeim D, Ito Y, Najafov A, Zhang Y, Shan B, DeWitt JP, Ye J, Zhang X, Chang A, Vakifahmetoglu-Norberg H, et al. Activation of necroptosis in multiple sclerosis. Cell Rep. 2015;10:1836–1849. doi: 10.1016/j.celrep.2015.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki K, Sagata N. The Mos/MAP kinase pathway stabilizes c-Fos by phosphorylation and augments its transforming activity in NIH 3T3 cells. EMBO J. 1995;14:5048–5059. doi: 10.1002/j.1460-2075.1995.tb00187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco S, Yatim N, Werner MR, Tran H, Gunja SY, Tait SW, Albert ML, Green DR, Oberst A. RIPK1 both positively and negatively regulates RIPK3 oligomerization and necroptosis. Cell Death Differ. 2014;21:1511–1521. doi: 10.1038/cdd.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- Polykratis A, Hermance N, Zelic M, Roderick J, Kim C, Van TM, Lee TH, Chan FK, Pasparakis M, Kelliher MA. Cutting edge: RIPK1 Kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J Immunol. 2014;193:1539–1543. doi: 10.4049/jimmunol.1400590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard JA, O'Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers T, Vince JE, Lawlor KE, Ninnis RL, Anderton H, et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell. 2014;157:1175–1188. doi: 10.1016/j.cell.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Schotte P, Declercq W, Van Huffel S, Vandenabeele P, Beyaert R. Non-specific effects of methyl ketone peptide inhibitors of caspases. FEBS Lett. 1999;442:117–121. doi: 10.1016/s0014-5793(98)01640-8. [DOI] [PubMed] [Google Scholar]
- Schworer SA, Smirnova II, Kurbatova I, Bagina U, Churova M, Fowler T, Roy AL, Degterev A, Poltorak A. Toll-like receptor-mediated down-regulation of the deubiquitinase cylindromatosis (CYLD) protects macrophages from necroptosis in wild-derived mice. J Biol Chem. 2014;289:14422–14433. doi: 10.1074/jbc.M114.547547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silke J, Rickard JA, Gerlic M. The diverse role of RIP kinases in necroptosis and inflammation. Nat Immunol. 2015;16:689–697. doi: 10.1038/ni.3206. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Vereecke L, Bertrand MJ, Duprez L, Berger SB, Divert T, Goncalves A, Sze M, Gilbert B, Kourula S, et al. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature. 2014;513:95–99. doi: 10.1038/nature13706. [DOI] [PubMed] [Google Scholar]
- Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, Zachariou A, Lopez J, MacFarlane M, Cain K, et al. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell. 2011;43:432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, Andrake M, Rall GF, Degterev A, Balachandran S. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci U S A. 2013;110:E3109–3118. doi: 10.1073/pnas.1301218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7:302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11:290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lieshout MH, Florquin S, Van't Veer C, de Vos AF, van der Poll T. TIR-Domain-Containing Adaptor-Inducing Interferon-beta (TRIF) Mediates Antibacterial Defense during Gram-Negative Pneumonia by Inducing Interferon-gamma. Journal of innate immunity. 2015 doi: 10.1159/000430913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivarelli MS, McDonald D, Miller M, Cusson N, Kelliher M, Geha RS. RIP links TLR4 to Akt and is essential for cell survival in response to LPS stimulation. J Exp Med. 2004;200:399–404. doi: 10.1084/jem.20040446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, Wang FS, Wang X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54:133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Wong WW, Vince JE, Lalaoui N, Lawlor KE, Chau D, Bankovacki A, Anderton H, Metcalf D, O'Reilly L, Jost PJ, et al. cIAPs and XIAP regulate myelopoiesis through cytokine production in an RIPK1- and RIPK3-dependent manner. Blood. 2014;123:2562–2572. doi: 10.1182/blood-2013-06-510743. [DOI] [PubMed] [Google Scholar]
- Wu XN, Yang ZH, Wang XK, Zhang Y, Wan H, Song Y, Chen X, Shao J, Han J. Distinct roles of RIP1-RIP3 hetero- and RIP3-RIP3 homo-interaction in mediating necroptosis. Cell Death Differ. 2014;21:1709–1720. doi: 10.1038/cdd.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi M, Fujita T, et al. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420:324–329. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, Akira S. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol. 2003;4:1144–1150. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- Yatim N, Jusforgues-Saklani H, Orozco S, Schulz O, Barreira da Silva R, Reis ESC, Green DR, Oberst A, Albert ML. RIPK1 and NF-kappaB signaling in dying cells determines cross-priming of CD8+ T cells. Science. 2015 doi: 10.1126/science.aad0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.