Abstract
Background
Gastric electrical stimulation (GES) is implicated as a potential therapy for difficult-to-treat nausea and vomiting; however, there is a lack of insight into the mechanisms responsible for these effects. The current study tested the relationship between acute GES and emesis in musk shrews, an established emetic model system.
Methods
Urethane-anesthetized shrews were used to record emetic responses (monitoring intra-tracheal pressure and esophageal contractions), respiration rate, heart rate variability, blood pressure, and gastrointestinal electromyograms. We investigated the effects of acute GES pulse duration (0.3, 1, 5, and 10 ms), current amplitude (0.5, 1, and 2 mA), pulse frequency (8, 15, 30, and 60 Hz), and electrode placement (antrum, body, and fundus) on emesis induced by gastric stretch, using a balloon.
Key results
There were four outcomes: (1) GES did not modify the effects of gastric stretch-induced emesis; (2) GES produced emesis, depending on the stimulation parameters, but was less effective than gastric stretch; (3) other physiological changes were closely associated with emesis and could be related to a sub-threshold activation of the emetic system, including suppression of breathing and rise in blood pressure; and (4) a control experiment showed that 8-OH-DPAT, a reported 5-HT1A receptor agonist that acts centrally as an antiemetic, blocked gastric stretch-induced emesis.
Conclusions & Inferences
These results do not support an antiemetic effect of acute GES on gastric distension-induced emesis within the range of conditions tested, but further evaluation should focus on a broader range of emetic stimuli and GES stimulation parameters.
Keywords: Nausea, Emesis, Gastric electrical stimulation, Motility, Suncus murinus
INTRODUCTION
Gastric electrical stimulation (GES) is a potential therapy for difficult-to-treat nausea and vomiting. Several clinical studies suggest that GES reduces nausea and vomiting in patients with gastroparesis, but results are variable (1–8). Experiments using dogs also suggest that GES (and jejunal stimulation) reduces emesis and behavioral signs of nausea following intravenous emetic treatments: vasopressin, and the cancer chemotherapy agent cisplatin (9–15). However, there is considerable lack of understanding of the mechanisms underlying the GES-mediated reduction of nausea and vomiting, which hinders the further development and optimization of this novel therapy for patients.
To fill the gap in knowledge concerning the relationship between GES and emesis, we conducted experiments using an established emetic model with detailed physiological measures of emesis and associated responses (e.g., heart rate variability and blood pressure). We used a small animal model, the musk shrew (Suncus murinus), which, unlike rats and mice, possesses an emetic reflex (16). Musk shrews have also been used in many studies to document antiemetic drug effects after activation of abdominal vagal afferents, area postrema, vestibular inputs, and conditioned emetic responses (17–25). Additionally, the musk shrew’s gastrointestinal physiology is similar to humans with respect to motility responses and a functional motilin system, which is lacking in rodents (26, 27). In the current studies, we used a urethane-anesthetized musk shrew preparation in which physiological responses can be recorded in detail (28–30), including emesis (by monitoring intra-tracheal pressure and esophageal contractions; (31–33), respiration rate, heart rate variability, blood pressure, and gastrointestinal electromyograms. Gastric distention, with a balloon, was used as the emetic stimulus (28–30).
MATERIALS AND METHODS
Animals
One hundred and twenty-six naïve, adult, male musk shrews were used in five studies (Suncus murinus; >35 days of age; 51–88 g). Musk shrews were obtained from a breeding colony at the University of Pittsburgh Cancer Institute and were descendants from animals acquired from the Chinese University of Hong Kong, a Taiwanese strain. Animals were housed in clear plastic cages (28 × 17 × 12 cm), with a filtered air supply, under a 12 h standard light cycle (lights on at 0700 h), in a temperature (~23°C) and humidity (~40%) controlled environment. Food and drinking water were freely available, but food was removed 2 h before surgery. Food consisted of a mixture of 75% Purina Cat Chow Complete Formula and 25% Complete Gro-Fur mink food pellets (34). All animals were randomly assigned to experimental groups. Experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and conducted in compliance with USDA guidelines. Animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care international-accredited animal care facility.
In vivo physiology preparation
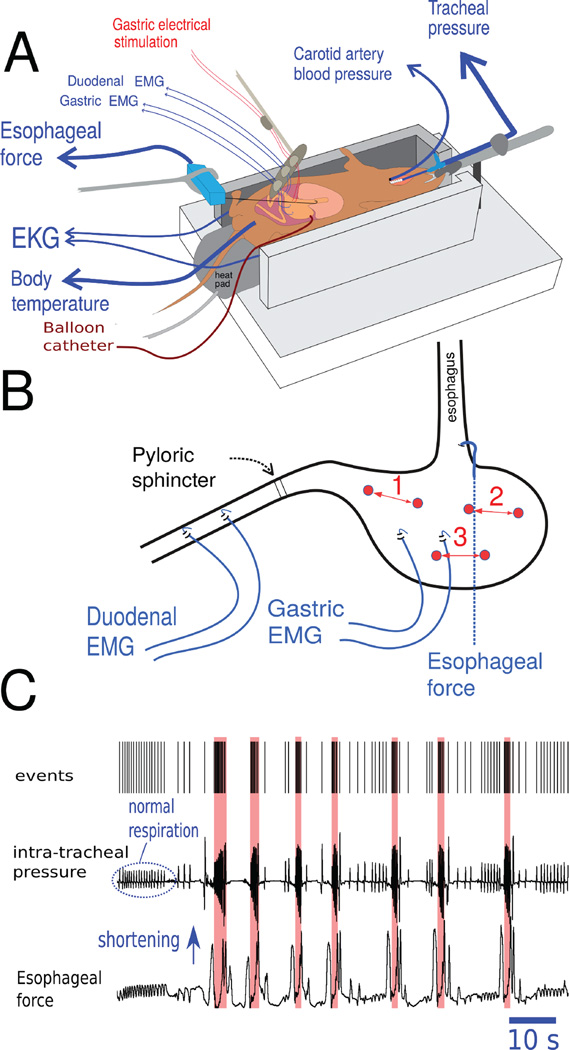
An anesthetized in vivo preparation was used, similar to published reports (28–30) (Fig. 1A). Animals were initially injected with 1 g/kg (ip) urethane to produce a surgical plane of anesthesia, and additional injections were used to maintain general anesthesia (defined as no response to toe pinch). Core temperature was monitored with a rectal probe and regulated to 37°C using a controlled heating pad (CWE; Ardmore, PA, USA). A tracheal tube was installed to monitor intra-tracheal airway pressure (CWE air pressure transducer), which was used to record respiration rate and the occurrence of emetic episodes, i.e., large, high-frequency changes in thoracic pressure indicate retching (28–30). A right carotid artery catheter was used to monitor blood pressure using a fluid filled catheter (Kent Scientific). Animals then underwent laparotomy, with abdominal skin and muscle edges retracted and elevated to form an oval opening, which was filled with 37°C mineral oil. Heart rate (electrocardiogram; EKG) was recorded by placing 16 ga stainless steel needles subcutaneously on the body flanks, connected to a low impedance headstage (Grass Instruments P511 pre-amplifier; 30 Hz and 1 KHz bandpass with 10 KHz sampling rate, 1,000 amplification). Gastric and duodenal electromyogram (EMG) signals were recorded (Grass P511) using a pair of Pt-Ir wires (50.8 µm diameter; A-M Systems, Sequim, WA, USA) with ends, separated by 5 mm, hooked through the serosal muscle layer; gastric leads were placed along the greater curvature of the antrum and duodenal leads were connected to the intestine starting at approximately 1 cm caudal to the pyloric sphincter (Fig. 1B). EMG signals were band-passed at 0.3 Hz to 1 KHz and collected at 10KHz sampling rate, 5,000 amplification. Esophageal contractions were measured using a force transducer attached to a small hook and implanted in the left edge of the lower abdominal esophagus (WPI transbridge).
Figure 1.
Musk shrew in vivo anesthetized preparation. (A) Position of the recording electrodes, gastric electrical stimulation (GES) electrodes, and placement of the gastric balloon catheter for gastric distension. (B) GES electrodes were placed at three sites for the different studies, 1 = antrum, 2 = fundus, and 3 = body. Site 1 was used in Studies 1 to 3 and all three sites were used in Study 4. See Horn et al. (Fig. 7 in reference 16) for an anatomical image of the musk shrew stomach. (C) A representative example of detection of seven emetic episodes using the Kleinberg algorithm for burst detection (35). Detection of emetic episodes (red shading) was based on the sharp rise in frequency of respiration “events” in the top tracing. Apnea will often precede emesis and changes in esophageal force are shown as an additional indication of emesis (e.g., 32, 37).
For electric stimulation of the gastric muscle, bipolar Pt-Ir electrodes were attached to the serosal muscle layer of the antrum (Fig. 1B). Electrodes were separated by 5 mm and attached to a stimulus isolation unit and calibrated stimulator (Model 2100, AM systems). Stimulation trial onset was controlled by the data acquisition device (Spike 2 version 7; CED, Cambridge, UK), which also recorded stimulus events.
For distension of the stomach, a balloon made of latex condom material fixed to PE tubing (4 mm diameter) was placed in the gastric lumen through a 5 mm incision in the far lateral edge of the ventral fundus; the deflated balloon was advanced to rest in the body of the stomach and secured in place by tying a purse-string suture at the incision site. Balloons were filled at a rate of 8 ml/min with 0.15 M NaCl (37°C) connected to a syringe pump (Kent scientific) and operated by the computer interface (Spike 2).
All signals were recorded to computer hard disk using the data acquisition system (Spike 2). Animals were euthanized at the conclusion of each experiment by intra-cardiac injection of Beuthanasia-D (0.2 ml, 78 mg sodium pentobarbital).
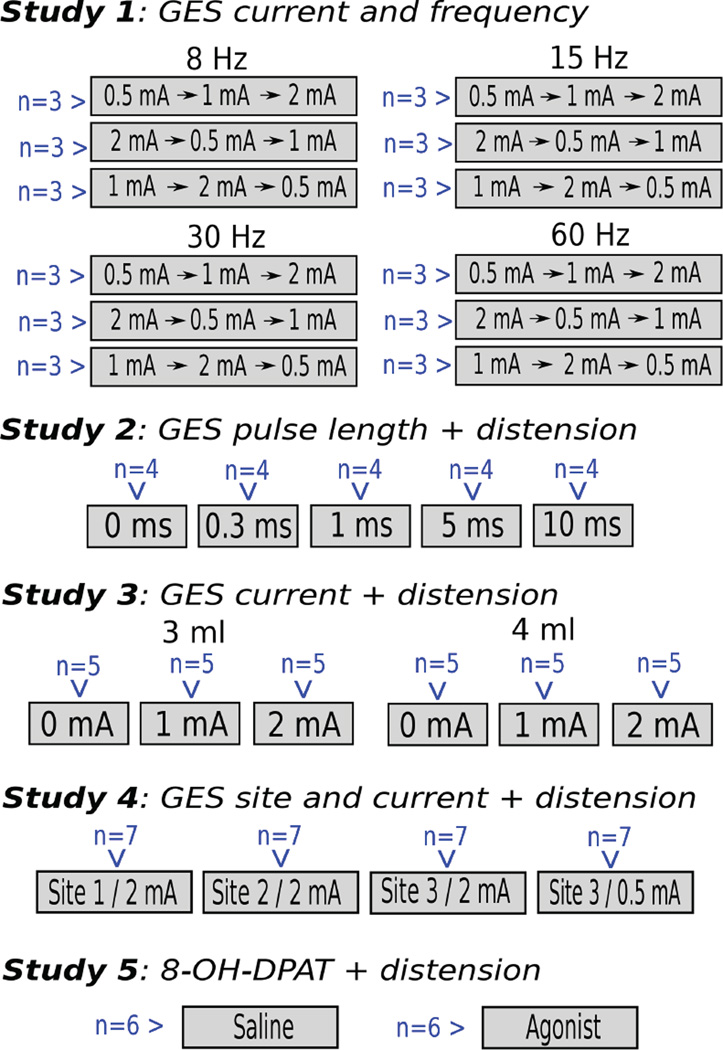
Study 1: Effects of GES current and frequency
Animals (total = 36, n = 9/group; Fig. 2) were used in a repeated measures Latin-square design, with 3 different sequences of electrical current testing; 0.5, 1, and 2 mA (biphasic, 0.3 ms positive and 0.3 ms negative). Different groups of animals also received 8, 15, 30, or 60 Hz frequency of stimulation. GES electrodes were placed on gastric site 1 (Fig. 1) and gastric balloons were not used in this study. Ten minutes after placing the GES electrodes, 10 minutes of baseline were recorded, followed by one of three electrical current levels for another 10 minutes depending on the assigned condition. This sequence of 10 minutes of baseline and 10 minutes of GES was repeated two more times to complete the testing for three current levels in each animal (a Latin-square sequence).
Figure 2.
Experimental groups for each study. Naïve animals were randomly assigned to each condition; conditions are respresented by grey boxes, with the number of animals in blue text. Only in Study 1, a Latin-square design did animals experience more than one GES condition. Note that in Studies 2 to 5 there are within-subject comparisons between before and after gastric distention, which are shown in Figures 3 to 7.
Study 2: Effects of GES pulse length on gastric distension-induced emesis
Shrews (total = 20, n = 4/group; Fig. 2) received GES pulse lengths of 0, 0.3, 1, 5 or 10 ms; pulses were biphasic (15 Hz, 2 mA), for example, 10 ms positive and then 10 ms negative for the 10 ms condition. GES electrodes were placed on site 1 (Fig. 1) and gastric balloons were installed. Ten minutes after placing the GES electrodes, 10 min of baseline were recorded, followed by 10 min of GES stimulation, and then an additional 30 min of GES with gastric distension (4 ml).
Study 3: Effects of GES current on gastric distension-induced emesis
Shrews (total = 30, n = 5/group); Fig. 2) received a GES current level of 0, 1, or 2 mA (15 Hz, biphasic, 0.3 ms positive and 0.3 ms negative) and a gastric distension volume of 3 or 4 ml. GES electrodes were placed on site 1 (Fig. 1) and gastric balloons were installed. Ten min after placing the GES electrodes, 10 min of baseline were recorded, followed by 10 min of GES stimulation, and then an additional 30 min of GES with gastric distension.
Study 4: Effects of GES site and current on gastric distension-induced emesis
Shrews (total = 28, n = 7/group; Fig. 2) received a GES current level of 0.5 or 2 mA (15 Hz, biphasic, 0.3 ms positive and 0.3 ms negative) and were stimulated at gastric sites 1, 2, or 3 (Fig. 1). Gastric balloons were installed. Ten min after placing the GES electrodes, 10 min of baseline were recorded, followed by 10 min of GES stimulation, and then an additional 30 min of GES with gastric distension (4 ml).
Study 5: Effects of 8-OH-DPAT (a 5-HT1A receptor agonist) on gastric distension-induced emesis
Animals (total = 12, n = 6/group; Fig. 2) were subcutaneously injected with saline (0.15 M NaCl) or 8-OH-DPAT (100 ug/kg, sc, intrascapular). Gastric balloons were installed. Ten min after placing the EMG electrodes, 10 min of baseline were recorded, followed by injection and 30 min of recording, and then an additional 10 min of recording with gastric distension.
Data analysis
Raw data files were analyzed on Linux computers running Spike2 (version 7; CED) software, Python 3.4, and R 3.2. Files were initially reduced in size using Spike2 to include only the time of each experiment (i.e., starting 10 min before the stimulation and ending 10 min after the end of stimulation). Signals were preprocessed, filtered, detected for events (e.g., EKG), and processed through routines in Python and R programming languages.
Preprocessing
Channel data from Spike2 files were preprocessed using the following approaches: (1) Tracheal pressure – down-sampled by a factor of 10, smoothed to a time constant of 0.05 s, and filtered for DC signals using a time constant of 1 s; (2) Esophageal force – down-sampled to 100 Hz, smoothed to a time constant of 0.1 s, and filtered to remove DC signals using a time constant of 5 s; (3) EKG – filtered with a low-pass Butterworth 50 Hz, second order filter, with DC removal at a time constant of 0.01 s; (4) blood pressure – smoothed to a time constant of 1 s; and (5) gastric and duodenal EMG – down-sampled to 100 Hz.
Event detection
Times for activation of the syringe pump for inflation of the stomach, intra-tracheal pressure changes (respiration), esophagus events, and EKG events were detected using customized Spike2 scripts. Pump on and off events (inflation and deflation of the stomach) were detected by using a rising or falling threshold. Respiration events were detected with a rising threshold, minimum of 0.1 s, and a level set at 50% of a normal respiration event (using a 10 s period at the start of baseline recording); this resulted in the detection of all respiration and retching events. Because esophagus movements also contain respiration events, we set the threshold at 150% of these movements using the initial 10 s of baseline recording, rising threshold, minimum of 0.1 s; this produced detection of only large esophageal movements associated with emesis. EKG was detected using the “Peak find” function in Spike2, followed by displaying instantaneous frequency to determine and correct outliers.
Analysis of individual data
Text output files from Spike2 were processed using custom Python scripts using SciPy packages (http://www.scipy.org/). Emetic episodes were detected with Pybursts 0.1.1 (https://pypi.python.org/pypi/pybursts/0.1.1), using the Kleinberg algorithm for burst detection(35), with S = 4 (i.e., minimum number of retches in an episode) and gamma = 0.1. EKG was processed to compute heart rate variability (standard deviation of the inter-beat intervals) and time and frequency domain analyses. Time domain analysis consisted of SDNN measurements (standard deviation of the inter-beat intervals). For frequency domain analysis, linear interpolation followed by detrending was used on the RR intervals to obtain evenly sampled data over time (10 Hz samples). These samples were processed through a Fast Fourier Transform in Python (Welch’s method, Hamming window [scipy.signal package]) to obtain measurements of low frequency (0.2 – 1.5 Hz) and high frequency (1.5 – 4 Hz) power spectral density. EMG signals were processed with a second-order Butterworth band-pass filter of 0.03 to 0.5 Hz, down-sampled to 10 Hz, and maximum frequency and power from the power spectral density function were determined in Python (scipy.signal package) using Welch’s method (36).
Statistical analysis
Statistical analysis was performed in R with the packages “ez” for factorial analysis and least significance difference test (LSD-test; https://cran.r-project.org/web/packages/ez/index.html), base functions for Tukey’s, and “survival” for log-rank tests (https://cran.r-project.org/web/packages/survival/index.html). ANOVA was applied to each parametric measure (emetic episodes, esophageal events, etc.), and when interaction or main effects were statistically significant, a planned comparison of means was performed using LSD-tests (emetic episodes and esophageal events). Cardiorespiratory and gastrointestinal measures (EKG, BP, respiration, and EMG) were treated as exploratory with Tukey’s using for mean comparisons; these ANOVAs also included a baseline control (no GES or drug). Exploratory data was analyzed in time blocks of 30 sec (the initial phase of baseline control or stimulation, which was approximately the median latency to the first emetic episode for all animals; median = 29 sec) and 10 min (the full time of baseline control or stimulation for each condition). Electrical recordings from the stomach and intestine typically contained movement artifacts during the baseline period; to remove the effect of these events on the analysis we used only 5 min of data from the baseline and stimulation tests for EMG analyses (in these analyses, it was necessary to exclude some data; refer to degrees of freedom in the ANOVAs; Supplementary Information). Log-rank tests were used to analyze latencies to the first emetic episode. To increase statistical power, we combined data from Studies 2, 3, and 4 for controls (4 ml gastric distension; n = 9) and GES (0.3 ms, 15 Hz, 2 mA, 4 ml gastric distension; n = 16). In all cases, the criterion of p < 0.05 was used to indicate statistical significance.
RESULTS
Results for emetic episodes and esophageal contractions are shown in Figures 3 to 7, with statistical values for ANOVA in the body of the text and figures show the results of Tukey’s HSD-tests. Cardiorespiratory and gastrointestinal data are shown in the Tables S1–S6 (full summaries of means and SEMs) and only those effects with statistically significant Tukey’s test comparisons are indicated in the body of the results section; means and SEMs are also listed in the results section for those effects using collapsed group values (e.g., comparison of main effect levels in an ANOVA); all other means and SEMs can be found in Tables S1–S6. Fig. 8 compares the effects of the antiemetic 8-OH-DPAT to GES (combined data from Studies 2, 3, and 4) using selected cardiorespiratory data (EKG, beats/min, SDNN, blood pressure, and respiration).
Figure 3.
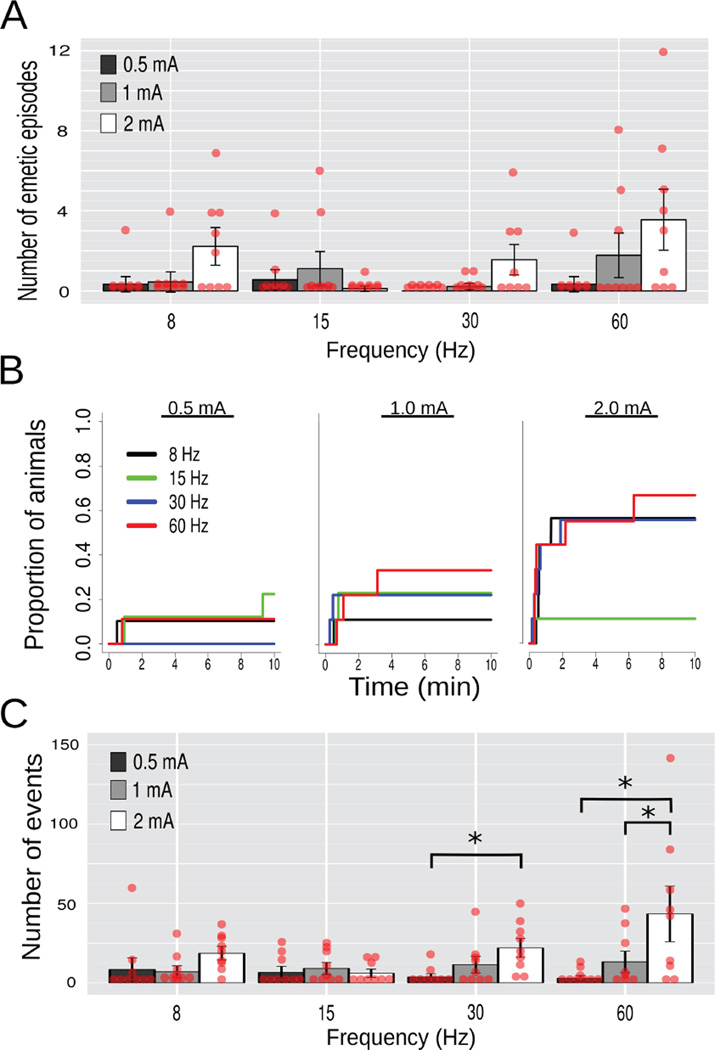
Study 1, Effects of gastric electrical stimulation (GES; 10 min) current (0.5, 1, or 2 mA) and frequency (8, 15, 30, or 60 Hz) at site 1 (Fig. 1) on emesis and esophageal contractions (n = 9/group). (A) Number of emetic episodes. (B) Cumulative proportion of animals showing the latency to the first emetic episode. (C) Number of esophageal contractions. Red dots = scatter plot of raw data; bars = mean ± SEM. * p < 0.05, LSD-test.
Figure 7.
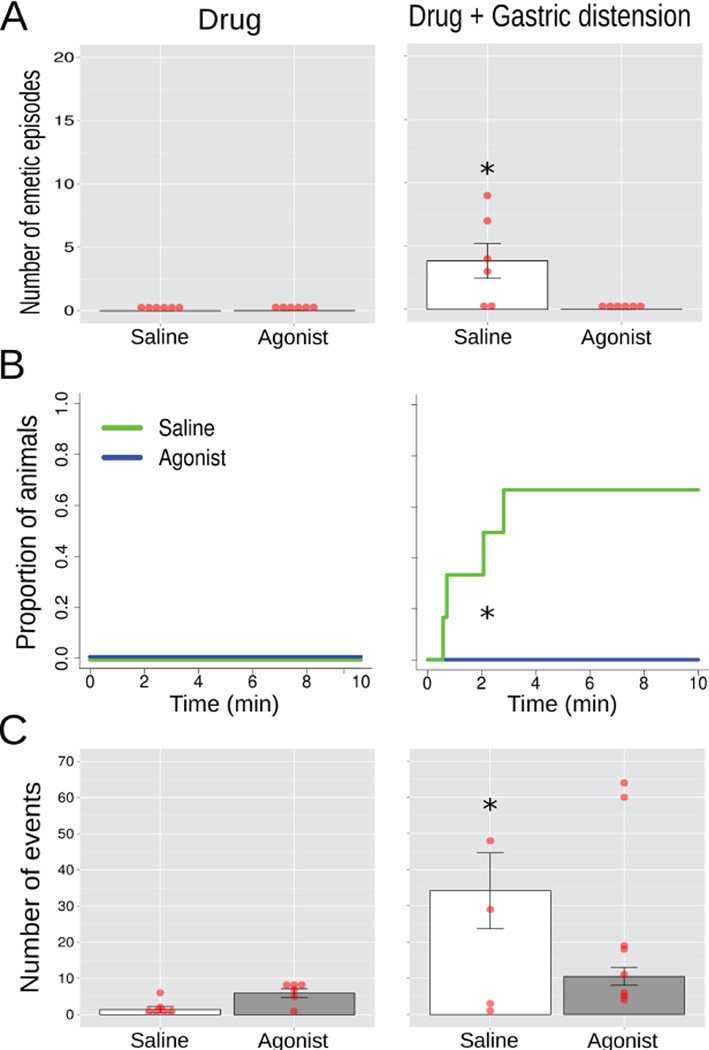
Study 5, the effects of injection of saline or 8-OH-DPAT (100 ug/kg, sc; a 5-HT1A receptor agonist) on emesis and esophageal contractions 30 min after injection (Drug) and after gastric distension (Drug + distension; 10 min of 4 ml of balloon inflation; n = 7/group)(A) Number of emetic episodes. (B) Cumulative proportion of animals showing the latency to the first emetic episode. (C) Number of esophageal contractions. Red dots = scatter plot of raw data; bars = mean ± SEM. * p < 0.05, LSD-test, Fig. 6A and 6C, and Log-rank test, Fig. 6B.
Figure 8.
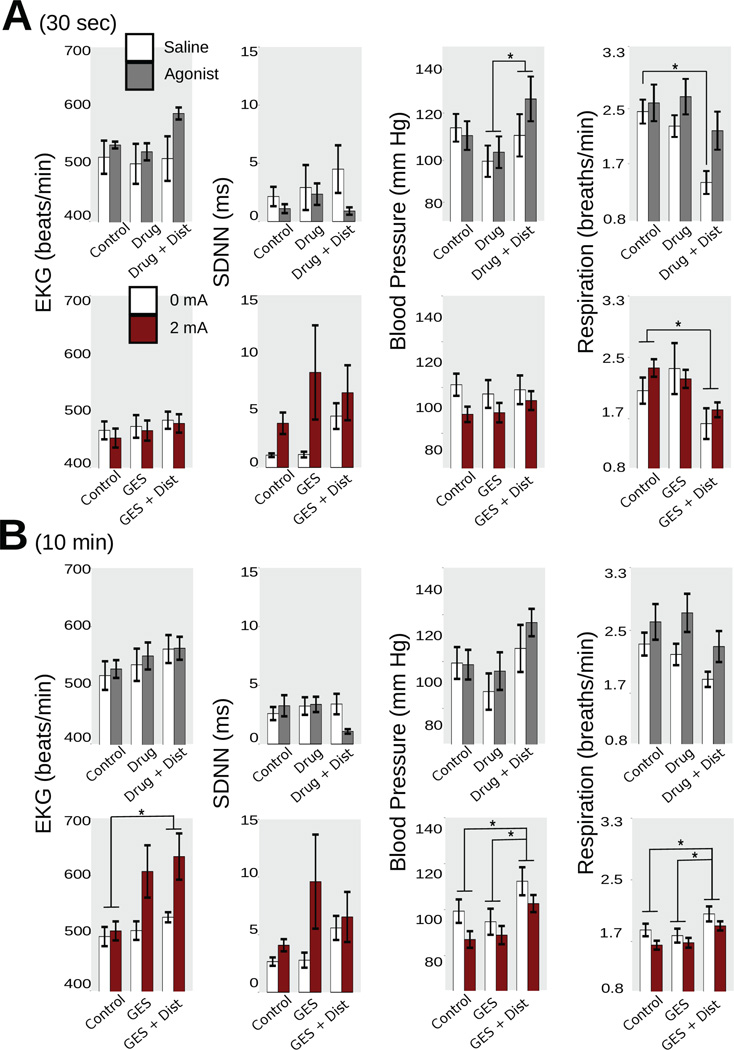
Cardiorespiratory parameters for the combined data, including EKG (beats/min), SDNN (ms; standard deviation of the inter-beat interval), blood pressure (mm Hg), and respiration rate (breaths/min), comparing GES to the effects of 8-OH-DPAT. (A) 30 sec. (B) 10 min. * p < 0.05, Tukey’s HSD-test versus control.
Study 1: Effects of GES current and frequency
Emesis and esophageal contractions
Emetic episodes were defined as four or more closely spaced retches (with or without expulsion of gastric contents) using the Kleinberg algorithm for burst detection (35)(see Methods). A three-way ANOVA of Order of stimulation (ABC, BCA, or CBA Latin-square) x Frequency (8, 15, 30, or 60 Hz) x Current level (0.5, 1, or 2 mA) was conducted on the number of emetic episodes or esophageal contractions, but all effects that included Order were p > 0.05 and, therefore, only Frequency and Current are plotted (Fig. 3). There was a main effect of Current on the number of emetic episodes [F(2,48) = 6.7, p = 0.003]; 1 and 2 mA (0.89 ± 0.33 and 1.86 ± 0.46 emetic episodes, respectively) produced more emesis than 0.5 mA (0.31 ± 0.16) (LSD-tests, p < 0.05). A log-rank test including all 12 curves (Fig. 3B) produced X2 (11) = 27.7, p < 0.004, but additional tests at each current revealed ps > 0.05 (log-rank) but comparisons of the three 30 Hz and 60 Hz curves were X2 (2) = 6.9, p < 0.04 and X2 (2) = 7.0, p < 0.04, respectively. There was an interaction effect of Current x Frequency on esophageal contractions [F(6,48) = 3.0, p = 0.02]; only 30 and 60 produced mean comparisons of p < 0.05 (LSD-tests; Fig. 3C).
Cardiorespiratory
In the 30 sec data (ANOVAs; Table 1a), blood pressure was decreased using 2 mA GES (93.7 ± 2.3 vs. control, 102.3 ± 1.8 mmHg; Tukey’s test).
Gastrointestinal
In the 30 sec data (ANOVAs; Table 1a), 0.5 mA GES produced a lower gastric EMG peak (1.39 ± 0.16 mV2/Hz) and higher power (127.7 ± 43.3 µV RMS) compared to control values (2.09 ± 0.14 and 22.6 ± 3.4; Tukey’s tests); 0.5 and 2 mA GES generated greater intestinal EMG power (92.8 ± 17.2 and 95.8 ± 25.7 µV RMS) compared to control (16.8 ± 3.3; Tukey’s tests). In the 5 min data (ANOVA, Table 2b), 2 mA plus 60 Hz GES produced a greater intestinal EMG peak compared to control (Tukey’s test); 0.5 and 1 mA GES generated an increased intestinal EMG power (80.1 ± 13.3 and 62.3 ± 13.2 µV RMS) versus control (14.3 ± 3.6; Tukey’s test).
Summary
An increase in GES current produced an increase in emesis and lowering of blood pressure.
Study 2: Effects of GES pulse length on gastric distension-induced emesis
Emesis and esophageal contractions
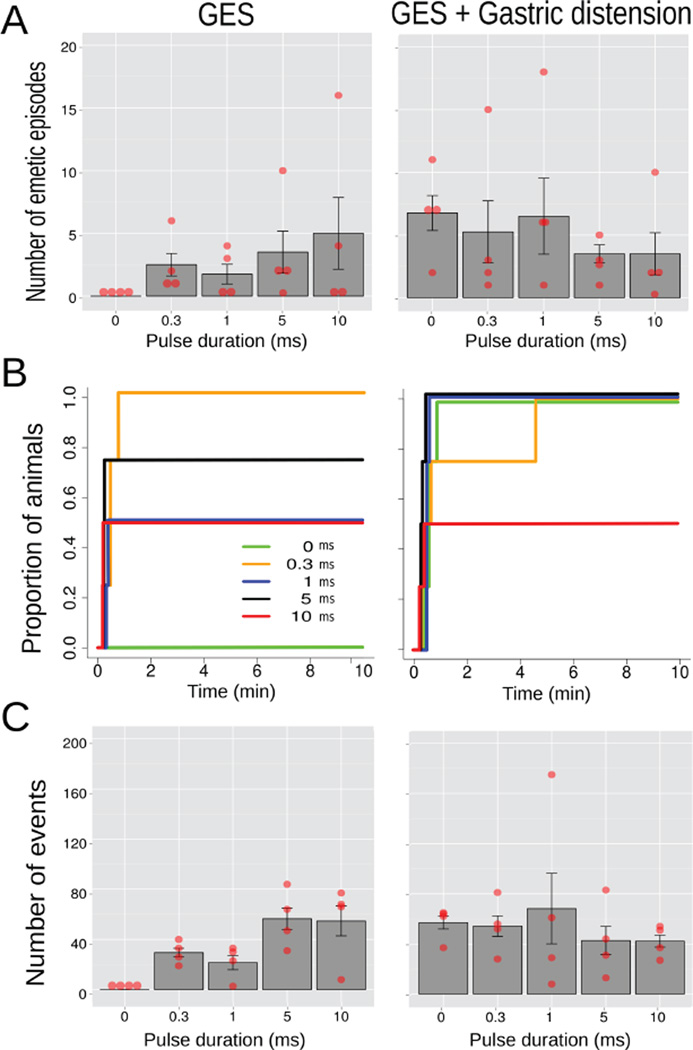
An ANOVA of Condition (GES only or GES plus gastric distension) x Pulse duration (0, 0.3, 1, 5, or 10 ms) produced no effects on the number of emetic episodes (ps > 0.05; Fig. 4); but there was a main effect of Condition on esophageal contractions [F(1,15) = 5.3, p = 0.04], with more contractions during GES plus gastric distension compared to GES alone (52.9 ± 7.9 vs. 32.5 ± 6.2; Fig. 4C). Fig. 4B shows the cumulative latency to the first emetic episode but a Log-rank test of all 10 curves was p = 0.1.
Figure 4.
Study 2, the effects of GES pulse length (0, 0.3, 1, 5, and 10 ms; 10 min; site 1, Fig. 1) on emesis and esophageal contractions alone or combined with gastric distension (10 min of 4 ml of balloon inflation; n=4/group). (A) Number of emetic episodes. (B) Cumulative proportion of animals showing the latency to the first emetic episode for different pulse lengths. (C) Number of esophageal contractions. Red dots = scatter plot of raw data; bars = mean ± SEM.
Cardiorespiratory
In the 30 sec data (ANOVAs, Table 2a), gastric distension increased blood pressure (121.7 ± 3.5 vs. control, 111.3 ± 3.1 mmHg; Tukey’s test); 0.3 ms pulse duration GES decreased (98.3 ± 3.2 mmHg) and 10 ms pulse duration increased (128.4 ± 3.0) blood pressure compared to control (111.3 ± 3.1; Tukey’s tests). In the 10 min data (ANOVAs, Table 2b), blood pressure was increased during gastric distension plus GES (122.5 ± 2.7 mmHg) compared to both control (107.4 ± 3.1) and GES alone (111.5 ± 3.6; Tukey’s tests); 0.3 ms duration GES lowered blood pressure (98.4 ± 3.8 vs. control, 120.4 ± 4.1; Tukey’s test); gastric distension decreased respiration (1.72 ± 0.92 vs. control, 2.18 ± 0.12 breaths/min; Tukey’s test).
Gastrointestinal
In the 30 sec data (ANOVAs, Table 2a), 1, 5 and 10 ms GES pulse duration decreased gastric peak EMG (Tukey’s tests); gastric distension plus GES increased gastric EMG power (547.4 ± 99.8 mV2/Hz) compared to GES alone (234.1 ± 39.6) and control (26.7 ± 4.7; Tukey’s tests); and, distension plus GES also produced an increase in intestinal EMG power (526.1 ± 160.0 vs. control, 10.5 ± 2.3 mV2/Hz; Tukey’s test). In the 5 min data (ANOVA, Table 2b), gastric distension plus GES (0.78 ± 0.11 mV2/Hz and 313.3 ± 51.6 µV RMS) and GES alone (1.08 ± 0.16 and 149.4 ± 25.7) conditions lowered gastric EMG peak and increased power compared to control values (2.01 ± 0.19 and 22.2 ± 4.9; Tukey’s test); GES increased intestinal EMG peak (1.27 ± 0.15 vs. control, 0.76 ± 0.13 mV2/Hz), and distension plus GES increased intestinal EMG power (446.7 ± 77.8 vs. control, 19.7 ± 9.7 µV RMS; Tukey’s tests).
Summary
Combining gastric distension with GES, compared to GES alone, increased esophageal contractions and blood pressure and decreased respiration.
Study 3: Effects of GES current on gastric distension-induced emesis
Emesis and esophageal contractions
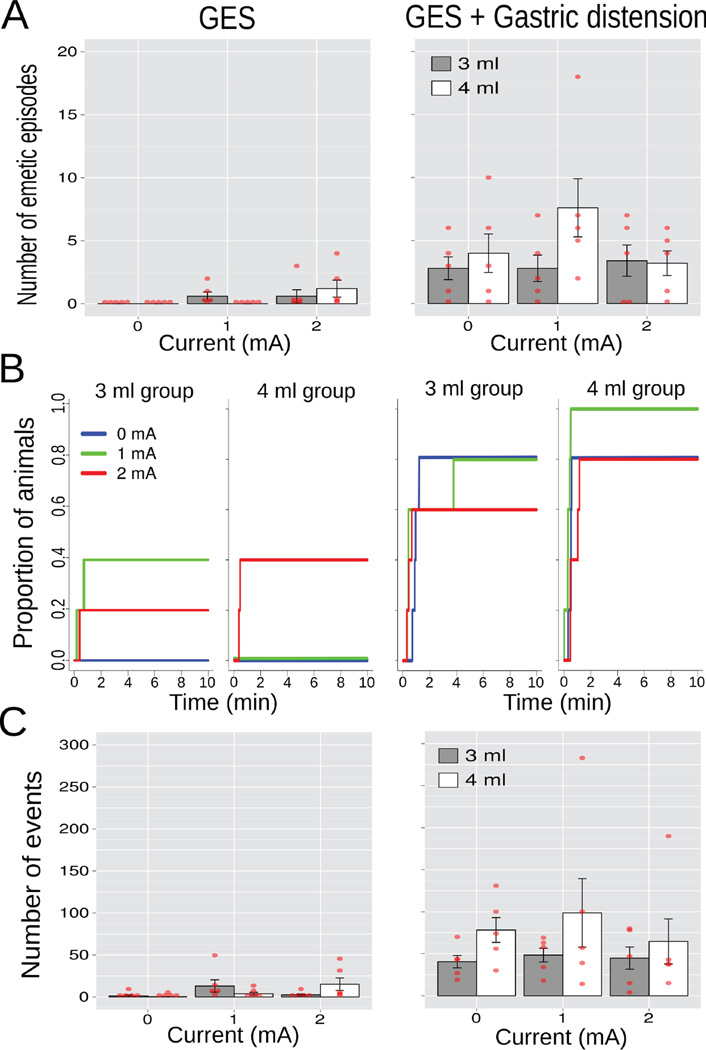
A three-way ANOVA of Condition (GES only or GES plus gastric distension) x Current (0, 1, or 2 mA) x Volume (3 or 4 ml) produced a main effect of Condition on the number of emetic episodes [F(1,24) = 26.5, p = 0.00003; Fig. 5A, the left-hand column shows the 3 and 4 ml groups before distension); GES plus distension increased the number of emetic episodes compared to GES alone (4.0 ± 0.7 vs. 0.4 ± 0.2; LSD-test, p < 0.05). Similarly there was a main effect of Time on esophagus contractions [F(1,24) = 24.9, p = 0.00005; Fig. 5C], with more contractions during GES plus gastric distension compared to GES alone (62.5 ± 10.4 vs. 6.1 ± 2.2; LSD-test, p < 0.05). A log-rank test including all 12 curves (Fig. 5B) produced X2 (11) = 48.6, p < 0.000002, but additional tests at each Volume revealed a p = 0.2 (log-rank) for 3 ml (6 curves) and a X2 (5) = 32.3, p < 0.000006 for 4 ml (6 curves).
Figure 5.
Study 3, the effects of GES current (0, 1, or 2 mA; 10 min; site 1; Fig. 1) on emesis and esophageal contractions alone or combined with gastric distension (10 min of 3 or 4 ml of balloon inflation; n=5/group). The left column shows the 3 and 4 ml groups prior to distension. (A) Number of emetic episodes. (B) Cumulative proportion of animals showing the latency to the first emetic episode. (C) Number of esophageal contractions. Red dots = scatter plot of raw data; bars = mean ± SEM.
Cardiorespiratory
In the 30 sec data (ANOVAs, Table 3a), gastric distension affected EKG low frequency, high frequency, and ratio responses (0.41 ± 0.04, 0.59 ± 0.04, and 0.89 ± 0.14) compared to control values (0.31 ± 0.03, 0.69 ± 0.03, and 0.55 ± 0.08; Tukey’s tests); gastric distension also reduced respiration (1.94 ± 0.09 vs. control, 2.41 ± 0.08; Tukey’s test). In the 10 min data (ANOVAs, Table 3b), gastric distension changed EKG rate, blood pressure, and respiration rate (526.2 ± 7.4, 118 ± 3, and 2.04 ± 0.07) compared to control (488.8 ± 11.7 beats/min, 109 ± 2 mmHg, and 2.41 ± 0.08 breaths/min; Tukey’s tests).
Gastrointestinal
In the 30 sec data (ANOVAs, Table 3a), gastric distension affected gastric EMG peak and power and intestinal EMG power (0.74 ± 0.07 mV2/Hz, 408.5 ± 71.9 µV RMS, and 246.7 ± 52.4 µV RMS) compared to controls (2.21 ± 0.13, 18.6 ± 2.4, and 13.5 ± 2.3; Tukey’s tests); gastric distension alone (without GES) produced a decrease in intestinal EMG peak (0.67 ± 0.01 vs. control, 1.07 ± 0.03 mV2/Hz; Tukey’s test); and, 4 ml of distension plus 2 mA GES decreased intestinal EMG peak (0.71 ± 0.04 vs. control, 1.47 ± 0.26 mV2/Hz; Tukey’s test). In the 5 min data, gastric distension changed gastric and intestinal EMG peaks and intestinal EMG power (0.66 ± 0.10 mV2/Hz, 0.67 ± 0.11 mV2/Hz, and 213.2 ± 33.5 µV RMS) compared to controls (2.05 ± 0.17, 0.57 ± 0.09, and 13.4 ± 3.5; Tukey’s tests); 4 ml of distension plus 2 mA GES increased gastric EMG power (251.1 ± 56.0 vs. control, 13.0 ± 1.9 µV RMS; Tukey’s test).
Summary
Combining gastric distension with GES, compared to GES alone, increased emesis, esophageal contractions, heart rate, and blood pressure and decreased respiration.
Study 4: Effects of GES site and current on gastric distension-induced emesis
Emesis and esophageal contractions
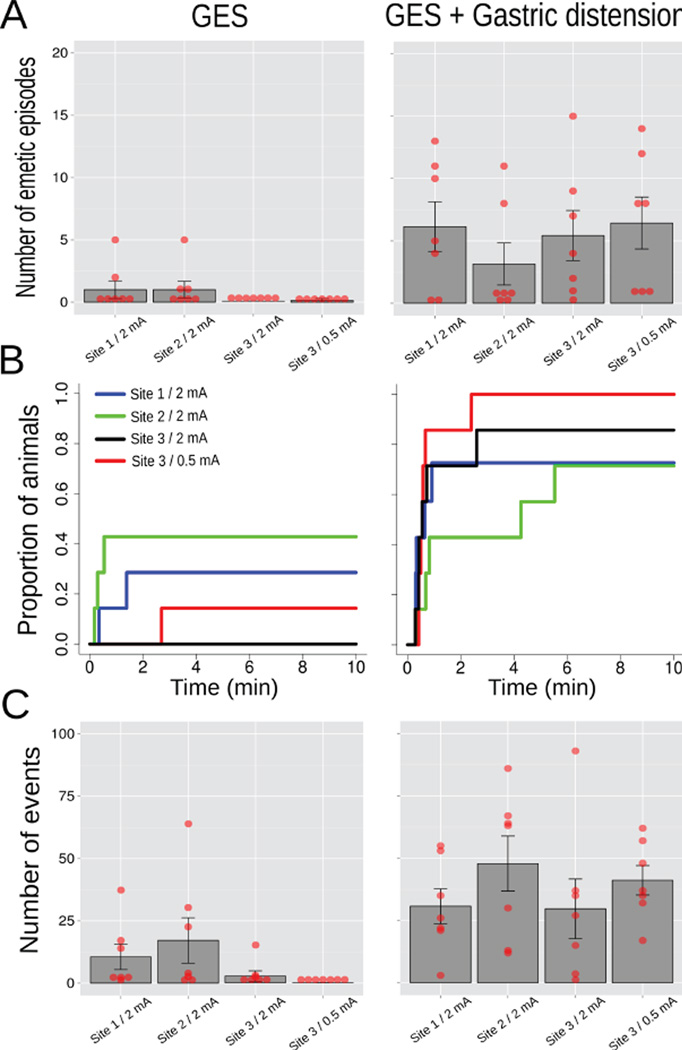
A two-way ANOVA of Condition (GES only or GES plus gastric distension) x Parameters (site 1 plus 2 mA, site 2 plus 2 mA, site 3 plus 2 mA, or site 3 plus 0.5 mA) produced a main effect of Condition on the number of emetic episodes [F(1,24) = 20.7, p = 0.0002; Fig. 6A; GES plus distension increased the number of emetic episodes compared to GES alone, 5.0 ± 1.0 vs. 0.5 ± 0.3, respectively, LSD-test, p < 0.05] and the number of esophageal contractions [F(1,24) = 22.6, p = 0.00008, Fig. 6C; GES plus distension increased the number of esophageal contractions compared to GES alone, 37.4 ± 4.6 vs. 7.6 ± 2.8, respectively, LSD-test, p < 0.05]. Log-rank tests including all 8 curves (Fig. 6B) produced X2 (7) = 27.1, p < 0.0004, a p = 0.2 for GES alone (4 curves), a p = 0.3 for GES plus gastric distension (4 curves). Comparing GES alone to GES plus distension yielded (2 curves each): a p = 0.08 for Site 1 plus 2 mA, a p = 0.6 for Site 2 plus 2 mA, a X2 (1) = 10.3, p < 0.002 for Site 3 plus 2 mA, and a X2 (1) = 14.5, p < 0.0002 for Site 3 plus 0.5 mA.
Figure 6.
Study 4, the effects of GES site (sites 1, 2, and 3, Fig. 1; 10 min) and current (2 or 0.5 mA) on emesis and esophageal contractions alone or combined with gastric distension (10 min of 4 ml of balloon inflation; n=7/group)(A) Number of emetic episodes. (B) Cumulative proportion of animals showing Lthe latency to the first emetic episode. (C) Number of esophageal contractions. Red dots = scatter plot of raw data; bars = mean ± SEM.
Cardiorespiratory
In the 30 sec data (ANOVAs, Table 4a), gastric distension decreased respiration (1.85 ± 0.14 vs. control, 2.41 ± 0.13 breaths/min; Tukey’s test). In the 10 min data, gastric distension changed EKG rate, low frequency, high frequency, and blood pressure (534.8 ± 15.7 beats/min, 0.41 ± 0.04, 0.59 ± 0.04, and 116.3 ± 3.2 mmHg) compared to control values (464.3 ± 13.5, 0.28 ± 0.03, 0.72 ± 0.03, and 102.0 ± 2.4; Tukey’s tests).
Gastrointestinal
In the 30 sec data (ANOVAs, Table 4a), both 0.5 mA GES alone and with gastric distension at Site 3 elicited a decrease in gastric EMG peak compared to control values; gastric distension with 2 or 0.5 mA GES produced an increase in gastric power compared to controls; and, gastric distension generated an increased intestinal power (277.2 ± 94.1 vs. control, 28.8 ± 11.2 µV RMS; Tukey’s test). In the 5 min data (ANOVAs, Table 4b), gastric distension affected gastric EMG peak and power and intestinal EMG peak and power (0.59 ± 0.08 mV2/Hz, 222.0 ± 34.1 µV RMS, 0.47 ± 0.04 mV2/Hz, and 249.5 ± 31.5 µV RMS) compared to controls (1.62 ± 0.20, 26.1 ± 7.4, 0.69 ± 0.15, and 18.6 ± 7.4; Tukey’s tests).
Summary
Combining gastric distension with GES, compared to GES alone, increased emesis and esophageal contractions and decreased respiration.
Study 5: Effects of 8-OH-DPAT (a 5-HT1A receptor agonist) on gastric distension-induced emesis
Emesis and esophageal contractions
A two-way ANOVA of Condition (agonist only or agonist plus gastric distension) x Injection (saline or agonist) produced a Condition by Injection interaction effect on the number of emetic episodes [F(1,10) = 6.6, p = 0.03; agonist injection reduced the number of emetic episodes compared to saline injection, LSD-test, p < 0.05, Fig. 7A] and the number of esophagus contractions [F(1,10) = 6.7, p = 0.03, agonist injection reduced the number of esophagus contractions compared to saline injection, LSD-test, p < 0.05, Fig. 7C]. A log-rank test including all 4 curves (Fig. 7B) produced X2 (3) = 16.4, p < 0.001, and a X2 (1) = 5.6, p < 0.02 for the GES plus gastric distension condition (Fig. 7B).
Cardiorespiratory
In the 30 sec data (ANOVAs, Table 5a), gastric distension in the saline condition decreased respiration breaths/min compared to control (Tukey’s test; Fig. 8A). In the 10 min data (ANOVAs, Table 5b), gastric distension increased blood pressure (121.1 ± 5.8 vs. control, 109.0 ± 4.4 mmHg; Tukey’s test).
Gastrointestinal
In the 30 sec data (ANOVAs, Table 5a), gastric distension changed gastric EMG peak and power (0.67 ± 0.01 mV2/Hz and 268.5 ± 61.1 µV RMS) compared to control (1.89 ± 0.23 and 20.2 ± 3.0; Tukey’s tests). Similarly, in the 5 min data (ANOVAs, Table 5b), gastric distension changed gastric EMG peak and power (0.38 ± 0.07 mV2/Hz and 92.6 ± 19.9 µV RMS) compared to control (2.04 ± 0.22 and 18.9 ± 3.5; Tukey’s tests).
Summary
The 5-HT1A agonist treatment inhibited gastric distension-induced emesis and esophageal contractions.
Combined GES analysis: GES (2 mA, 0.3 ms pulse width, 15 Hz) compared to controls
Tables 6a and 6b in the Supporting Information show the results of ANOVAs for combining GES analysis using 2 mA of GES (Studies 2, 3, and 4). Figure 7 shows the effects of these combined data compared to the antiemetic 8-OH-DPAT (Study 5) on EKG beats/min, SDNN, blood pressure, and respiration.
Cardiorespiratory
In the 30 sec data (ANOVAs, Table 6a), gastric distension decreased respiration (1.72 ± 0.10 vs. control, 2.25 ± 0.10 breaths/min; Tukey’s test; Fig. 8A). In the 10 min data (ANOVAs, Table 6b), gastric distension changed EKG rate, blood pressure, and respiration (595.4 ± 27.6 beats/min, 116.1 ± 3.3 mmHg, and 1.83 ± 0.10 breaths/min) compared to controls (500.6 ±12.0, 101.5 ± 3.1, and 2.27 ± 0.11; Tukey’s tests).
Gastrointestinal
In the 30 sec data (ANOVAs, Table 6a), gastric distension decreased the gastric EMG peak for both 0 and 2 mA conditions compared to controls (Tukey’s tests); gastric distension increased gastric and intestinal EMG power (357.7 ± 73.4 and 307.0 ± 83.5) compared to controls (23.7 ± 5.6 µV RMS and 9.4 ± 2.0 µV RMS; Tukey’s test). In the 5 min data (ANOVAs, Table 6b), gastric distension decreased gastric EMG peak (0.39 ± 0.04 vs. control, 1.32 ± 0.25 mV2/Hz; Tukey’s test).
Summary
Gastric distension increased heart rate and blood pressure but decreased respiration.
DISCUSSION
The primary goal of this project was to assess the detailed physiological effects of GES on gastric distension-induced emesis using an established emetic test system: an in vivo musk shrew preparation, (28–30, 37). We tested the effects of GES pulse duration (0.3, 1, 5, and 10 ms), current amplitude (0.5, 1, and 2 mA), pulse frequency (8, 15, 30, and 60 Hz), and electrode placement (antrum, body, and fundus). There were four primary results: (1) GES did not modify the effects of gastric stretch-induced emesis; (2) GES produced emesis, but was less effective than gastric stretch; (3) other physiological changes were closely associated with emesis and could be related to a sub-threshold activation of the emetic system, including suppression of breathing, esophageal contractions, and rise in blood pressure; and, (4) a control experiment showed that the centrally acting 5HT1A receptor agonist, 8-OH-DPAT (38, 39), blocked gastric stretch-induced emesis.
The lack of effect of GES in reducing emesis appears to be at odds with both animal and human studies (e.g., 4, 13). Is the musk shrew unique in its response to GES? Unfortunately, it is not possible to answer this question because of the limited amount of data, both from the current studies and published reports. In terms of published literature, the effects of GES on emesis have not been evaluated in detail; by example, the antiemetic effects of 5-HT3 and NK1 receptor antagonists have been assessed in numerous studies from academia and industry in several countries, including established emetic tests using intra-gastric copper sulfate (CuSO4), apomorphine, several cytotoxic chemotherapy agents, and provocative motion in dogs, ferrets, cats, pigs, and musk shrews (see reviews 40, 41).
Based on our knowledge, only dogs were used in prior GES studies and these investigations have two problems that limit interpretation. First, they used non-standard measures of emesis, often reporting a total “symptom score” and not absolute, objectively quantifiable numbers of retches or vomits (9, 11–15, 42, 43). The exception is one paper listing total emetic episodes; however, this report also has a potential confound by using repeated cisplatin chemotherapy injections in the same animals, with only the first test session as the control condition (10). Although not specifically established for the vagus, it is known that cisplatin can produce peripheral neuropathy (44, 45). The reported symptom scores were often combined with other measures, sometimes including licking tongue, closing eyes, yawning, belching, murmuring, rapid breathing, defecating (13, 46), and in other reports vomiting was scored 3, 4 or 5 if the symptoms occurred 1, 2 or 3 times and scored as “0” if not present (9–12, 14, 43). We believe that these reports are largely not interpretable and difficult (if not impossible) to fit into the larger literature on emesis and evaluation of antiemetic therapies. Secondly, only vasopressin, duodenal distension, and cisplatin chemotherapy were used in these prior studies and additional standard emetic tests, such as apomorphine, motion, or CuSO4, have not been used. Finally, there are reports in dog (although with different stimulation parameters) that GES can produce emesis (47).
In relation to the clinical literature, the analysis is tempered by the existence of few controlled studies, which show slight or no effects of GES on emesis (or nausea) when comparing “on” versus “off” stimulation conditions (4, 48–51). The variability of effect of GES on nausea and vomiting in these studies, combined with the absence of proper controls (e.g. sham or no-stimulation), make it difficult to directly compare the patient-reported symptom improvement with the objectively-measured physiological responses quantified in this study. However, we are confident the results from the present study are reliable and valid with specific regard to gastric distension-induced emesis, but cannot address the effect of GES on emesis induced in this model by other mechanisms including gastroparesis. In summary, the published data from human and preclinical reports provide only weak support for GES as an antiemetic therapy and it is too early to know if musk shrew (or dog) will be representative of the translational potential of GES therapy to humans.
In the present study, emesis-inducing gastric distension resulted in an increase in blood pressure, a decrease in respiration, decreases in gastric and intestinal EMG peaks, and increases in gastric and intestinal EMG power. These physiological responses may constitute an emesis prodromal signal that could be used to predict and/or detect emetic episodes. Furthermore, GES (2 mA in the combined study) did not elicit any physiological response similar to responses elicited by the antiemetic drug 8-OH-DPAT (Fig. 8); specifically, GES did not inhibit changes in respiration that are indicative of emetic stimulation. In contrast, GES also increases heart rate which may also be a component of the emesis prodromal signal. These data indicate that it is unlikely that GES (using the current parameters) is having any impact on the physiological signals of emesis, even more subtle effects, that could produce an inhibition of the emetic response to gastric distension.
While the lack of effect of GES on gastric distension-induced emesis in this report is unequivocal, there are several caveats to consider when interpreting the current results. First, we used a limited range of stimulation parameters, with inclusion of only 0.5 to 2 mA, 8 to 60 Hz, and 0.3 to 10 ms pulse duration. Second, we used only one emetic stimulus, vagal (29), and there are a least three additional routes for emetic stimulation: vestibular, area postrema, and forebrain (review 52). Excessive gastric distension can also provide nociceptive input to the spinal cord (53, 54); and thus, is not a purely vagal stimulus. Furthermore, the number of emetic episodes following gastric stretch is less than with other emetic stimuli, such as subcutaneous nicotine or intra-gastric CuSO4 (24, 55, 56), and our unpublished data (CH and DR) indicate that gastric stretch is a rapidly adapting emetic stimulus; these observations suggest that gastric distension could be relatively insensitive to small anti-emetic effects. Third, the experiments were of very short duration. The current studies were up to 2 h and this time frame does not match the clinical literature in which GES has been applied for many weeks or months for the assessment of anti-nausea and anti-emetic effects (2, 4, 7).
In summary, the current results do not support an anti-emetic effect of acute GES. Further evaluation is needed using a broader range of emetic stimuli (both peripheral and central) and GES parameters. It would also be useful to conduct long-term, free-moving animal studies to assess the effects of chronic GES on emesis using standard metrics with reports of total emetic episodes; indeed, the GES-induced emesis observed in the current work might be an acute response that will transition to suppression of emesis over a longer time frame.
Supplementary Material
Key Messages.
Gastric electrical stimulation (GES) did not reduce gastric distension-induced emesis; however, GES was moderately emetogenic. These results do not support an antiemetic effect of GES, but more testing is warranted including the use of a broader range of emetic stimuli and GES parameters.
The goal of this project was to assess the effects of acute GES on emesis in a well-established physiological model of emesis using the musk shrew and detailed in vivo electrophysiology measures.
The current study focused on the use of an in vivo electrophysiology preparation (under urethane anesthesia) to document the effects of acute GES and emetic stimulation (gastric distension) on changes in emesis, blood pressure, electrocardiogram, and gastrointestinal electromyogram.
Acknowledgments
We thank (1) Leanna Travis for help with part of the data analysis, and (2) the University of Pittsburgh, Division of Laboratory Animal Research, especially Dawn Everard, Katie Leschak, Megan Lambert, and Dr. Joseph Newsome for excellent care of the musk shrew colony at the University of Pittsburgh Cancer Institute (UPCI).
FUNDING
This research was supported by a sponsored contract to the University of Pittsburgh from Medtronic Inc. and an NIH grant to support the animal facility, P30 CA047904 (Cancer Center Support Grant; CCSG). The authors had complete access to the data generated by this project.
Footnotes
CONFLICTS OF INTEREST
Dr. Zirpel is a scientist employed by Medtronic. Dr. Horn was supported for this research project by Medtronic. All other authors declare no competing interests.
AUTHOR CONTRIBUTION
CCH designed the study, performed the experiments, and wrote the manuscript. LZ designed the studies and edited the manuscript. MS and DR analyzed data and edited the manuscript. None of the data reported in this manuscript has been altered or censored by the funding source.
REFERENCES
- 1.Chu H, Lin Z, Zhong L, McCallum RW, Hou X. Treatment of high-frequency gastric electrical stimulation for gastroparesis. J Gastroenterol Hepatol. 2012;27:1017–1026. doi: 10.1111/j.1440-1746.2011.06999.x. [DOI] [PubMed] [Google Scholar]
- 2.Gourcerol G, Huet E, Vandaele N, et al. Long term efficacy of gastric electrical stimulation in intractable nausea and vomiting. Dig Liver Dis. 2012;44:563–568. doi: 10.1016/j.dld.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Lu PL, Teich S, Di Lorenzo C, Skaggs B, Alhajj M, Mousa HM. Improvement of quality of life and symptoms after gastric electrical stimulation in children with functional dyspepsia. Neurogastroenterol Motil. 2013;25:567–e456. doi: 10.1111/nmo.12104. [DOI] [PubMed] [Google Scholar]
- 4.McCallum RW, Sarosiek I, Parkman HP, et al. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil. 2013;25:815–e636. doi: 10.1111/nmo.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison NS, Williams PA, Walker MR, et al. Evaluation and treatment of gastric stimulator failure in patients with gastroparesis. Surg Innov. 2014;21:244–249. doi: 10.1177/1553350613503735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jayanthi NV, Dexter SP, Sarela AI. Gastric electrical stimulation for treatment of clinically severe gastroparesis. J Minim Access Surg. 2013;9:163–167. doi: 10.4103/0972-9941.118833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–428. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 8.Abell TL, Van Cutsem E, Abrahamsson H, et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion. 2002;66:204–212. doi: 10.1159/000068359. [DOI] [PubMed] [Google Scholar]
- 9.Song GQ, Hou X, Yang B, Sun Y, Qian W, Chen JD. A novel method of 2-channel dual-pulse gastric electrical stimulation improves solid gastric emptying in dogs. Surgery. 2008;143:72–78. doi: 10.1016/j.surg.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song J, Zhong DX, Qian W, Hou XH, Chen JD. Short pulse gastric electrical stimulation for cisplatin-induced emesis in dogs. Neurogastroenterol Motil. 2011;23:468–474. doi: 10.1111/j.1365-2982.2011.01684.x. e178. [DOI] [PubMed] [Google Scholar]
- 11.Yu X, Yang J, Hou X, Zhang K, Qian W, Chen JD. Cisplatin-induced gastric dysrhythmia and emesis in dogs and possible role of gastric electrical stimulation. Dig Dis Sci. 2009;54:922–927. doi: 10.1007/s10620-008-0470-0. [DOI] [PubMed] [Google Scholar]
- 12.Song G, Hou X, Yang B, et al. Efficacy and efficiency of gastric electrical stimulation with short pulses in the treatment of vasopressin-induced emetic responses in dogs. Neurogastroenterol Motil. 2006;18:385–391. doi: 10.1111/j.1365-2982.2006.00758.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen JD, Qian L, Ouyang H, Yin J. Gastric electrical stimulation with short pulses reduces vomiting but not dysrhythmias in dogs. Gastroenterology. 2003;124:401–409. doi: 10.1053/gast.2003.50048. [DOI] [PubMed] [Google Scholar]
- 14.Xu J, Chen JD. Intestinal electrical stimulation improves delayed gastric emptying and vomiting induced by duodenal distension in dogs. Neurogastroenterol Motil. 2008;20:236–242. doi: 10.1111/j.1365-2982.2007.00995.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Qiao X, Chen JD. Therapeutic potentials of a novel method of dual-pulse gastric electrical stimulation for gastric dysrhythmia and symptoms of nausea and vomiting. Am J Surg. 2006;191:255–261. doi: 10.1016/j.amjsurg.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Horn CC, Kimball BA, Wang H, et al. Why can’t rodents vomit? A comparative behavioral, anatomical, and physiological study. PLoS One. 2013;8:e60537. doi: 10.1371/journal.pone.0060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horn CC, Henry S, Meyers K, Magnusson MS. Behavioral patterns associated with chemotherapy-induced emesis: a potential signature for nausea in musk shrews. Front Neurosci. 2011;5:88. doi: 10.3389/fnins.2011.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horn CC, Meyers K, Pak D, Nagy A, Apfel CC, Williams BA. Post-anesthesia vomiting: impact of isoflurane and morphine on ferrets and musk shrews. Physiol Behav. 2012;106:562–568. doi: 10.1016/j.physbeh.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horn CC, Still L, Fitzgerald C, Friedman MI. Food restriction, refeeding, and gastric fill fail to affect emesis in musk shrews. Am J Physiol Gastrointest Liver Physiol. 2010;298:G25–G30. doi: 10.1152/ajpgi.00366.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith JE, Friedman MI, Andrews PL. Conditioned food aversion in Suncus murinus (house musk shrew) - a new model for the study of nausea in a species with an emetic reflex. Physiol Behav. 2001;73:593–598. doi: 10.1016/s0031-9384(01)00538-8. [DOI] [PubMed] [Google Scholar]
- 21.Percie du Sert N, Chu KM, Wai MK, Rudd JA, Andrews PL. Telemetry in a motion-sickness model implicates the abdominal vagus in motion-induced gastric dysrhythmia. Exp Physiol. 2010;95:768–773. doi: 10.1113/expphysiol.2009.052001. [DOI] [PubMed] [Google Scholar]
- 22.Hu DL, Zhu G, Mori F, et al. Staphylococcal enterotoxin induces emesis through increasing serotonin release in intestine and it is downregulated by cannabinoid receptor 1. Cell Microbiol. 2007;9:2267–2277. doi: 10.1111/j.1462-5822.2007.00957.x. [DOI] [PubMed] [Google Scholar]
- 23.Ueno S, Matsuki N, Saito H. Suncus murinus: a new experimental model in emesis research. Life Sci. 1987;41:513–518. doi: 10.1016/0024-3205(87)90229-3. [DOI] [PubMed] [Google Scholar]
- 24.Chan SW, Rudd JA, Lin G, Li P. Action of anti-tussive drugs on the emetic reflex of Suncus murinus (house musk shrew) Eur J Pharmacol. 2007;559:196–201. doi: 10.1016/j.ejphar.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Rock EM, Parker LA. Synergy between cannabidiol, cannabidiolic acid, and Delta(9)-tetrahydrocannabinol in the regulation of emesis in the Suncus murinus (house musk shrew) Behav Neurosci. 2015;129:368–370. doi: 10.1037/bne0000057. [DOI] [PubMed] [Google Scholar]
- 26.Tsutsui C, Kajihara K, Yanaka T, et al. House musk shrew (Suncus murinus, order: Insectivora) as a new model animal for motilin study. Peptides. 2009;30:318–329. doi: 10.1016/j.peptides.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Sanger GJ, Holbrook JD, Andrews PL. The translational value of rodent gastrointestinal functions: a cautionary tale. Trends Pharmacol Sci. 2011;32:402–409. doi: 10.1016/j.tips.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Uchino M, Ishii K, Kuwahara M, Ebukuro S, Tsubone H. Role of the autonomic nervous system in emetic and cardiovascular responses in Suncus murinus. Auton Neurosci. 2002;100:32–40. doi: 10.1016/s1566-0702(02)00141-8. [DOI] [PubMed] [Google Scholar]
- 29.Uchino M, Ito K, Kuwahara M, Ebukuro S, Tsubone H. Interactions of carotid sinus or aortic input with emetic signals from gastric afferents and vestibular system. Auton Neurosci. 2008;144:36–42. doi: 10.1016/j.autneu.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Uchino M, Kuwahara M, Ebukuro S, Tsubone H. Modulation of emetic response by carotid baro- and chemoreceptor activations. Auton Neurosci. 2006;128:25–36. doi: 10.1016/j.autneu.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Brizzee KR. Mechanics of vomiting: a minireview. Can J Physiol Pharmacol. 1990;68:221–229. doi: 10.1139/y90-035. [DOI] [PubMed] [Google Scholar]
- 32.Smith JE, Paton JF, Andrews PL. An arterially perfused decerebrate preparation of Suncus murinus (house musk shrew) for the study of emesis and swallowing. Exp Physiol. 2002;87:563–574. doi: 10.1113/eph8702424. [DOI] [PubMed] [Google Scholar]
- 33.Miller AD. Respiratory muscle control during vomiting. Can J Physiol Pharmacol. 1990;68:237–241. doi: 10.1139/y90-037. [DOI] [PubMed] [Google Scholar]
- 34.Temple JL. The musk shrew (Suncus murinus): a model species for studies of nutritional regulation of reproduction. Ilar j. 2004;45:25–34. doi: 10.1093/ilar.45.1.25. [DOI] [PubMed] [Google Scholar]
- 35.Kleinberg J. Bursty and hierarchical structure in streams. Data Mining and Knowledge Discovery. 2003;7:373–397. [Google Scholar]
- 36.Welch PD. The use of fast fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Transactions on Signal Processing. 1967;15:70–73. [Google Scholar]
- 37.Andrews P, Torii Y, Saito H, Matsuki N. The pharmacology of the emetic response to upper gastrointestinal tract stimulation in Suncus murinus. European journal of pharmacology. 1996;307:305–313. doi: 10.1016/0014-2999(96)00275-0. [DOI] [PubMed] [Google Scholar]
- 38.Javid FA, Naylor RJ. The effect of the 5-HT1A receptor agonist, 8-OH-DPAT, on motion-induced emesis in Suncus murinus. Pharmacol Biochem Behav. 2006;85:820–826. doi: 10.1016/j.pbb.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 39.Andrews PL, Okada F, Woods AJ, et al. The emetic and anti-emetic effects of the capsaicin analogue resiniferatoxin in Suncus murinus, the house musk shrew. Br J Pharmacol. 2000;130:1247–1254. doi: 10.1038/sj.bjp.0703428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andrews PLR, Rudd JA. The Role of Tachykinins and the Tachykinin NK1 Receptor in Nausea and Emesis. In: Holzer P, editor. Tachykinins. Berlin Heidelberg: Springer; 2004. pp. 359–440. [Google Scholar]
- 41.Reynolds DJPLA. Serotonin and the Scientific Basis of Anti-Emetic Therapy. British Journal of Cancer - BRIT J CANCER. 1995;75:282. [Google Scholar]
- 42.Xu X, Qian L, Chen JD. Anti-dysrhythmic effects of long-pulse gastric electrical stimulation in dogs. Digestion. 2004;69:63–70. doi: 10.1159/000077390. [DOI] [PubMed] [Google Scholar]
- 43.Yu X, Tu L, Lei P, Song J, Xu H, Hou X. Antiemesis effect and brain fMRI response of gastric electrical stimulation with different parameters in dogs. Neurogastroenterol Motil. 2014;26:1049–1056. doi: 10.1111/nmo.12362. [DOI] [PubMed] [Google Scholar]
- 44.Han FY, Wyse BD, Smith MT. Optimization and pharmacological characterization of a refined cisplatin-induced rat model of peripheral neuropathic pain. Behav Pharmacol. 2014;25:732–740. doi: 10.1097/FBP.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 45.Erken HA, Koc ER, Yazici H, Yay A, Onder GO, Sarici SF. Selenium partially prevents cisplatin-induced neurotoxicity: a preliminary study. Neurotoxicology. 2014;42:71–75. doi: 10.1016/j.neuro.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Xu X, Brining DL, Chen JD. Effects of vasopressin and long pulse-low frequency gastric electrical stimulation on gastric emptying, gastric and intestinal myoelectrical activity and symptoms in dogs. Neurogastroenterol Motil. 2005;17:236–244. doi: 10.1111/j.1365-2982.2004.00616.x. [DOI] [PubMed] [Google Scholar]
- 47.Ueno T, Chen JD. Vomiting and gastric electrical dysrhythmia in dogs. Scand J Gastroenterol. 2004;39:344–352. doi: 10.1080/00365520310008601. [DOI] [PubMed] [Google Scholar]
- 48.O’Grady G, Egbuji JU, Du P, Cheng LK, Pullan AJ, Windsor JA. High-frequency gastric electrical stimulation for the treatment of gastroparesis: a meta-analysis. World journal of surgery. 2009;33:1693–1701. doi: 10.1007/s00268-009-0096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andersson S, Elfvin A, Ringstrom G, Lonroth H, Abrahamsson H, Simren M. A slow caloric satiety drinking test in patients with temporary and permanent gastric electrical stimulation. European journal of gastroenterology & hepatology. 2010;22:926–932. doi: 10.1097/MEG.0b013e3283365642. [DOI] [PubMed] [Google Scholar]
- 50.McCallum RW, Snape W, Brody F, Wo J, Parkman HP, Nowak T. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2010;8:947–954. doi: 10.1016/j.cgh.2010.05.020. quiz e116. [DOI] [PubMed] [Google Scholar]
- 51.Abell TL, Johnson WD, Kedar A, et al. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointestinal endoscopy. 2011;74:496–503.e493. doi: 10.1016/j.gie.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horn CC. Why is the neurobiology of nausea and vomiting so important? Appetite. 2008;50:430–434. doi: 10.1016/j.appet.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozaki N, Gebhart GF. Characterization of mechanosensitive splanchnic nerve afferent fibers innervating the rat stomach. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1449–G1459. doi: 10.1152/ajpgi.2001.281.6.G1449. [DOI] [PubMed] [Google Scholar]
- 54.Traub RJ, Sengupta JN, Gebhart GF. Differential c-fos expression in the nucleus of the solitary tract and spinal cord following noxious gastric distention in the rat. Neuroscience. 1996;74:873–884. doi: 10.1016/0306-4522(96)00173-x. [DOI] [PubMed] [Google Scholar]
- 55.Horn CC, Meyers K, Lim A, et al. Delineation of vagal emetic pathways: intragastric copper sulfate-induced emesis and viral tract tracing in musk shrews. Am J Physiol Regul Integr Comp Physiol. 2014;306:R341–R351. doi: 10.1152/ajpregu.00413.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horn CC, Meyers K, Oberlies N. Musk shrews selectively bred for motion sickness display increased anesthesia-induced vomiting. Physiol Behav. 2014;124:129–137. doi: 10.1016/j.physbeh.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.