Abstract
Background
5-aminosalicylic acid (5-ASA) is a classic anti-inflammatory drug for the treatment of ulcerative colitis. N-Acetyltransferase (NAT) enzymes convert 5-ASA to its metabolite N-acetyl-5-aminosalicylic acid (Ac-5-ASA) and it is unresolved whether 5-ASA or Ac-5-ASA is the effective therapeutic molecule. We previously demonstrated that colonic production of Ac-5-ASA (NAT activity) is decreased in Dextran Sulfate Sodium (DSS)-induced colitis. Our hypothesis is that 5-ASA is the therapeutic molecule to improve colitis, with the corollary that altered NAT activity affects drug efficacy. Since varying clinical effectiveness of 5-ASA has been reported, we also ask if NAT activity varies with inflammation in pediatric or adult patients.
Method
Acute colonic inflammation was induced in C57BL/6 NAT wild-type (WT) or knockout (KO) mice, using 3.5 % DSS (w/v) concurrent with 5-ASA treatment. Adult and pediatric rectosigmoid biopsies were collected from control or ulcerative colitis patients. Tissue was analyzed for NAT and myeloperoxidase activity.
Results
DSS induced colitis was of similar severity in both NAT WT and KO mice, and NAT activity was significantly decreased in NAT WT mice. In the setting of colitis, 5-ASA significantly restored colon length and decreased myeloperoxidase activity in NAT KO, but not WT mice. Myeloperoxidase activity negatively correlated with NAT activity in pediatric patients, but correlation was not observed in adult patients.
Conclusions
Inflammation decreases NAT activity in colon of mice and human pediatric patients. Decreased NAT activity enhances the therapeutic effect of 5-ASA in mice. A NAT activity assay could be useful to help predict the efficacy of 5-ASA therapy.
Keywords: drug metabolism, inflammatory bowel disease, fluorogenic enzyme assay, human
Introduction
5-aminosalicylic acid (5-ASA) is a classic anti-inflammatory drug for the treatment of ulcerative colitis and Crohn's disease. 5-ASA is metabolized by N-acetyltransferase (NAT) enzymes that catalyze the transfer of an acetyl moiety from acetyl-coenzyme A (AcCoA) to the nitrogen (N-acetylation) of the 5-ASA to form the metabolite N-acetyl-5-ASA (Ac-5-ASA)(1). Although the molecular mechanisms underlying the beneficial effects of 5-ASA are still the topic of active study (2-7), it is known that 5-ASA therapy reduces inflammation of the colonic mucosa and drug efficacy is correlated to mucosal concentration rather than systemic levels (8).
Three NAT isoforms have been identified in mouse and human. Human NAT1 (hNAT1) is the ortholog of mouse NAT2 (mNAT2), and human NAT2 (hNAT2) is the ortholog of mouse NAT1 (mNAT1). Human hNAT3 is a pseudogene (NATP) (9) and murine mNAT3 is relatively inactive (10). We recently established a method to measure NAT enzyme activity and demonstrated that mouse mNAT2 mediates 5-ASA metabolism in homogenates of mouse colonic tissue (1). Additionally, we demonstrated that mNAT2 enzyme function and expression decreased in the inflamed mouse colonic mucosa (1). It remains unknown whether 5-ASA or the mNAT2 metabolite Ac-5-ASA, is the effective molecule for therapy.
5-ASA accounts for 81 % of all ulcerative colitis prescriptions (11) and is the preferred therapy to help both adult and pediatric IBD patients with mild to moderate inflammation stay in remission (12). However, treatments for pediatric patients currently rely on clinical studies derived exclusively from adult patients, and although the drug is well-tolerated the response to 5-ASA is variable in both populations. For example, pediatric patients in remission have a pattern of frequent relapse, and only 40 % of ulcerative colitis pediatric patients followed in a multi-center registry achieved a steroid-free remission with 5-ASA alone 52 weeks after diagnosis(13). Alternatively, under 5-ASA monotherapy 48 % of adult ulcerative colitis patients are in steroid-free remission (14, 15) and about 50% of adult patients with ulcerative colitis will relapse in any year (16, 17). The results hint that the efficiency of 5-ASA may differ between pediatric and adult patients. In this study, we test if NAT enzyme activity is comparable between these human populations, and if NAT activity in the two populations displays similar regulation by inflammatory status.
In this present study, we seek to test if variability in NAT activity has the potential to be an underlying confounder in the unpredictable therapeutic response to 5-ASA. We test the effect of 5-ASA on inflammation induced by Dextran Sulfate Sodium (DSS) in Nat1/2 KO mice to determine whether 5-ASA or its metabolite Ac-5-ASA have the predominant therapeutic effect on inflammation. Additionally, we test the hypothesis in both adult and pediatric tissues that NAT enzyme activity is decreased in the inflamed human colonic mucosa, similar to prior observations in mice.
Materials and Methods
Animals
Experiments used C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME USA) and Nat1/Nat2 double-knockout mice (Nat1/2 KO) on a C57BL/6 background (a generous gift from Denis M. Grant, University of Toronto, Canada) (18). All mice were housed in the Laboratory Animal Medical Services facilities at the University of Cincinnati.
Histopathological Analysis
Swiss rolls of mouse distal colon were fresh frozen in Tissue-Tek Optimal Cutting Temperature Compound (Sakura Finetek USA, Torrance, CA)). Tissues sections (10 μm) were stained with Hematoxylin and Eosin Y (Sigma, St. Louis, MO). Slides viewed at 4x by an Olympus BX60 microscope (Olympus, Center Valley, PA), images captured by QColors 5 Olympus camera using QCapture Pro version 5.1.1.14 Software (QImaging, Surrey, BC Canada).
DSS-induced colitis model
Colitis was induced in Nat1/2 WT or Nat1/2 KO mice, with 3.5 % (w/v) Dextran Sulfate Sodium (DSS) (M.W. 40,000, TdB Consultancy, Uppsala, Sweden) by feeding in drinking water for 5 days followed by a two day recovery period of regular water. Control mice received water without DSS. Mice were weighed and health monitored every 24 ± 1 hr for 7 days. 5-ASA (Acros Organics, Geel, Belgium) at a dose of 75 mg/kg (19) dissolved in 0.5 % carboxymethylcellulose sodium (Sigma, St. Louis, MO) was gavaged once per day for 7 days. Mouse colon was collected on day 7 and the length was measured. One third of the distal colon was cut longitudinally, frozen in liquid nitrogen, and stored at −80 °C for the myeloperoxidase (MPO) assay. The remaining distal colon was muscle stripped and either directly frozen in liquid nitrogen as tissue aliquots, or processed for the NAT assay, and then frozen in liquid nitrogen and stored at −80 °C until analyzed.
Human Biopsy Collection
Rectosigmoid biopsies were collected from de-identified pediatric patients, age 6 to 19 (Table1), undergoing colonoscopy for possible inflammatory bowel disease at Cincinnati Children's Hospital Medical Center. Biopsies were obtained from pediatric patients with parental informed consent and patient assent if age 11 or older. Rectosigmoid biopsies were also collected from de-identified adult patients, age 33 to 74 (Table 1), undergoing colonoscopy at the University of Cincinnati Medical Center. Adult patients gave informed consent to obtain extra biopsies for research. Cigarette smoking was an exclusion criterion. Biopsies were immediately frozen in liquid nitrogen and stored at −80 °C until assayed and were handled under BSL2 conditions approved by the University of Cincinnati's Institutional Biosafety Committee. Biopsies were thawed once and assayed for NAT and MPO activity within 30 days of collection.
Table 1.
Table of summarized patient demographics
| Group | Age range | Number of patients | Sex | Ethnicity | Accepted Pathology* (patient ID) |
|---|---|---|---|---|---|
| pediatric control | 6 to 19 | 8 | 2 male 6 female |
Caucasian | reactive colonic mucosa (b), superficial lymphoid follicle (f) |
| pediatric ulcerative colitis | 13 and 16 | 2 | 1 male 1 female |
Caucasian | mild chronic colitis (j), mild glandular architectural distortion (i) |
| adult control | 33 to 74 | 6 | 2 male 4 female |
2 Caucasian 4 African American |
hyperplastic polyp (l, m, p), tubular (k) or serrated (l) adenoma with no high grade dysplasia or malignancy |
| adult ulcerative colitis | 25 and 43 | 2 | 1 male 1 female |
Caucasian | Mild glandular architectural distortion (q), prominent lymphoid aggregates (q), quiescent chronic colitis (q), ulcerative colitis (r) |
N-Acetyltransferase Activity Determinations
Measurement of acetyl-CoA-dependent N-acetylation of 5-ASA was performed as described (1). Briefly, muscle stripped mouse distal colonic mucosa or human rectosigmoid mucosal biopsies were homogenized 30 seconds (Tissue Tearor, Biospec, Bartlesville, OK) in 100 to 200 μL of Sorensen's Buffer (100 μM sodium phosphate, 1 mM DL-dithiothreitol, 1 mM EDTA, pH 7.0). The homogenate was centrifuged at 1500 xg for 3 minutes and supernatant removed and kept on ice until the NAT assay. As described, a fluorometric rate assay was used to directly measure acetylation of 5-ASA by NAT enzyme, compared to a calibration curve of pure Ac-5-ASA (1). Rates were normalized to mg protein and NAT activity presented as nmole Ac-5-ASA/min/mg colon homogenate protein. Protein concentration was determined by the Pierce bicinchoninic acid (BCA) Protein Assay Kit (Thermo Scientific, Waltham, MA).
Myeloperoxidase activity determinations
Biopsy and mouse tissue were prepared by the method of Graff et al (20). In brief, tissue was homogenized 30 seconds in ice-cold 50 mM potassium phosphate buffer, pH 7.4 with 10 mM N-Ethylmaleimide (Alfa Aesar, Ward Hill, MA) and centrifuged at 12,000 xg for 30 min at 4 °C. The pellet was re-suspended in buffer and homogenized again, then the pellet was solubilized into the traditional 50 mM potassium phosphate, pH 6.0, buffer (Sigma, St. Louis, MO and J.T. Baker, Phillipsburg, NJ) with 0.5 % Hexadecyltrimethylammonium bromide (Sigma, St. Louis, MO) and sonicated (W-220F Heat Systems – Ultrasonics, Inc., Plainview, NY) 15 seconds on ice followed by three freeze thaw cycles (liquid nitrogen and 37 °C water bath), followed by centrifugation at 12,000 xg for 30 min at 4 °C. The supernatant was stored on ice until the MPO assay.
MPO activity was assayed colorimetrically at ambient temperature, according to Bradley et al. (21) with slight modifications, at 450 nm absorbance in 50 mM potassium phosphate buffer, pH 6.0, containing 0.165 mg/mL o-dianisidine dihydrochloride (Sigma, St. Louis, MO) and 0.0005 % H2O2 (Fisher Scientific, Pittsburg, PA). Activity rates were obtained from the initial linear absorbance change over time. MPO specific activity was calculated using the molar extinction coefficient of 1.13 × 10 4 M−1·cm−1 of oxidized odianisidine (22). Rates were normalized to protein amount and MPO specific activity was expressed as μmol H2O2/min/mg protein. Protein concentration was determined by the BCA P rotein Assay Kit.
Statistical analysis of data
Descriptive analyses were performed and continuous data were presented as mean ± SEM or mean ± std. Continuous variables were compared with two sample t-test or one-way ANOVA between groups. Pairwise comparison was conducted with Bonferroni's adjustment or Dunnett's. Equal variabilities were checked by Levene's Test. Linear mixed models with random effect were used to assess the correlation between NAT activity and the predictors when considering the correlation within each mouse or human. All analyses were performed with either GraphPad Prism 5 or SAS version 9.3. A P-value of <0.05 was considered statistically significant.
Ethical Considerations
Adult biopsies were obtained from patients who provided informed consent at the University of Cincinnati Medical Center and procedures were approved by the University of Cincinnati Institutional Review Board. Pediatric biopsies were collected with parental consent and patient assent if age 11 or older. Patients were enrolled through protocols approved by the Institutional Review Board at Cincinnati Children's Hospital Medical Center. All animal experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati and were performed following the recommendations in by the National Institutes of Health's Guide for the Care and Use of Laboratory Animals.
RESULTS
NAT enzyme mediates 5-ASA metabolism in the colonic mucosa
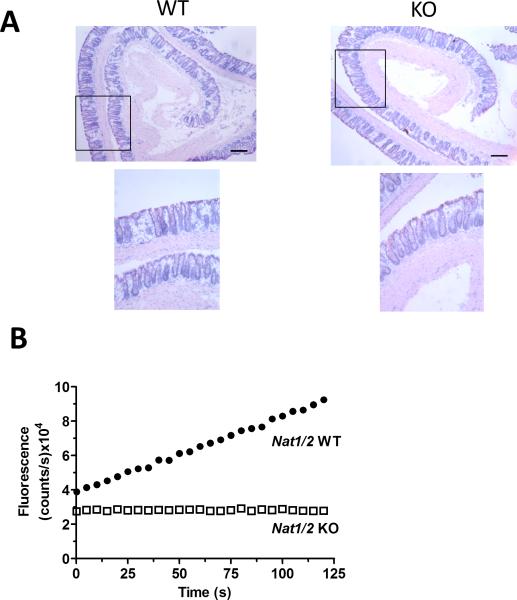
We recently reported that mNAT2 is the main isoform involved in metabolic conversion of 5-ASA by the colon (1). To completely eliminate NAT activity, we used Nat1/2 KO mice, and Sugamori et al (18) confirmed the absence of mNAT1 and mNAT2 gene expression from several tissues including colon via reverse transcription PCR. The Nat1/2 KO mice have a normal phenotype (18) and show no histologic indicators of colonic inflammation (Fig. 1A). To confirm that Nat1/2 WT or KO mice do not have inherent inflammation, we measured MPO activity to report on neutrophil and macrophage accumulation in distal colonic mucosal homogenates (23). This biochemical assay reported no detectable inflammation in distal colon of Nat1/2 KO mice, with a MPO activity range from 0 to 5.98 μmol H2O2/min/mg protein for the WT and 0.069 to 1.42 μmol H2O2/min/mg protein for the KO mice (n = 4, respectively).
FIGURE 1.
Appearance of colonic mucosa and 5-ASA metabolism in Nat1/2 KO mice. (A) Representative images of hematoxylin and eosin stained distal colon in Nat1/2 WT and KO mice. Low resolution (4x) and high resolution (10x) of area indicated by rectangle in low resolution image. Bar = 0.2 mm (B) Representative traces of NAT enzyme activity show fluorescence (Ac-5-ASA: 312 nm excitation/ 437 nm emission) over 120 seconds of Nat1/2 WT (●) or Nat1/2 KO (□) mice distal colon homogenate immediately after the addition of 5-ASA and AcCoA.
When NAT enzyme activity was measured in colonic homogenates, we confirmed previous findings of a linear accumulation of Ac-5-ASA over time in WT mice (1), as detected by direct fluorescence detection of the conversion of 5-ASA to Ac-5-ASA (Fig. 1B). As expected, no production of Ac-5-ASA was observed in the Nat1/2 KO mice, confirming the absence of NAT activity (Fig. 1B). Results confirm that 5-ASA does not convert to Ac-5-ASA in the colonic mucosa of NAT1/2 KO mice.
Effect of inflammation on NAT activity in DSS colitis
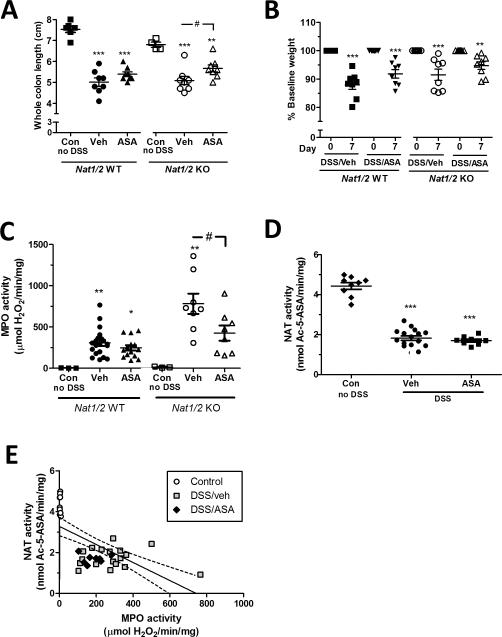
DSS creates acute murine colonic inflammation that mimics some features of human ulcerative colitis. In both WT and Nat1/2 KO mice, DSS treatment (for 5 days followed by a two day recovery period without DSS) caused a significant decrease in whole colon length and animal weight, accompanied by an increase of MPO activity in the colonic mucosa (Fig. 2A-C). Once a day treatment of 5-ASA (75 mg/kg) did not affect any of these measures of DSS-induced colitis in WT mice (Fig. 2A-C). However in the Nat1/2 KO mice 5-ASA significantly recovered colon length and inhibited the increase of MPO activity, with no significant effect on weight loss. Results show that 5-ASA is more therapeutically effective against DSS colitis in the absence of metabolic conversion to Ac-5-ASA.
FIGURE 2.
Effect of 5-ASA on DSS-induced colitis in Nat1/2 WT and KO mice. DSS colitis was induced by adding 3.5% DSS to drinking water for 5 days followed by regular drinking water for two days. During this 7 day DSS and regular water treatment, mice were gavaged daily with vehicle (Veh) or 75 mg/kg/day 5-ASA (ASA). Control (Con) mice did not receive DSS or any treatment. (A) Whole colon lengths of excised mouse colon at day 7. Values are mean ± SEM, **P < 0.01, ***P < 0.001 vs. corresponding control, #P < 0.05 Nat1/2 KO Veh vs. Nat1/2 KO ASA. (B) Percent baseline weight changes at day zero (all mice at 100 %) and day 7. **P < 0.01, ***P < 0.001 vs. corresponding control. (C) MPO activity of distal colon in response to DSS. Values are mean ± SEM. *P < 0.05, **P < 0.01 vs. corresponding control; #P < 0.05 Nat1/2 KO Veh vs. Nat1/2 KO ASA. (D) NAT enzyme activity of distal colon. Values are mean ± SEM. ***P < 0.001 vs. control. (E) Correlation of NAT versus MPO enzyme activity of WT control (○) with no DSS, and vehicle treated ( ), or ASA treated (◆) during DSS. Each point represents one mouse. Linear regression with 95 % confidence levels (dotted line).
), or ASA treated (◆) during DSS. Each point represents one mouse. Linear regression with 95 % confidence levels (dotted line).
Colonic NAT activity in WT animals (4.43 ± 0.50 nmol Ac-5-ASA/min/mg protein) was reduced 59% by DSS-colitis (1.82 ± 0.45 nmol Ac-5-ASA/min/mg protein) (Fig. 2D), which is consistent with previous findings (1). We therefore tested if administration of 5-ASA affects NAT activity in the distal colon, in the setting of DSS colitis. We found no significant difference in NAT activity between gavage with 5-ASA versus vehicle in WT mice treated with DSS (Fig. 2D). In order to determine if 5-ASA influenced NAT activity in the absence of inflammation, WT mice were gavaged with 5-ASA or vehicle (no DSS) for 7 days. No significant difference in NAT activity was observed between 5-ASA (n = 8) or vehicle gavaged mice (n = 7). These data suggest that 5-ASA does not directly affect NAT enzyme expression activity over a 1 week time course, either in the presence or absence of inflammation.
Figure 2E shows the correlation between NAT activity and MPO activity in WT mice, aggregating all information from control and DSS colitis conditions in the absence or presence of 5-ASA. Using linear regression a significant decrease in NAT activity is observed with increasing MPO activity (Fig. 2E). These data suggest that in WT mice colonic mucosal inflammation lowers NAT activity and is not recovered by 5-ASA treatment.
5-ASA metabolism in adult and pediatric rectosigmoid biopsies
In order to test the hypothesis that NAT activity is varied among patients and is decreased in the inflamed human colonic mucosa, we obtain de-identified biopsies. This pilot study examined biopsies from 10 pediatric (8 controls and 2 ulcerative colitis, age 6 to 19) and 8 adult (6 controls and 2 ulcerative colitis, age 25 to 74) patients. The pediatric control group were patients receiving a diagnostic colonoscopy in response to a health concern. The adult control group were patients receiving a colonoscopy either for a diagnostic purpose or for a routine colorectal cancer screening. Table 1 lists the clinical evaluation of colonic pathology for all biopsies. Both male and females were used with ethnicities of Caucasian and African American (Table 1). Patients who smoked were excluded. The pediatric ulcerative colitis patients received 5-ASA mono-therapy, whereas the adult ulcerative colitis patients received 5-ASA and prednisone treatment. Biopsies varied in size with total protein content ranging from 0.19 to 0.50 mg protein.
We measure NAT and MPO enzyme activity in rectosigmoid mucosal biopsies. MPO and NAT enzyme assays were either accessed in the same biopsy cut in two pieces, with one half processed for each assay, or accessed from two biopsies from the same colonic region of a single patient. Two to four biopsies were collected per patient.
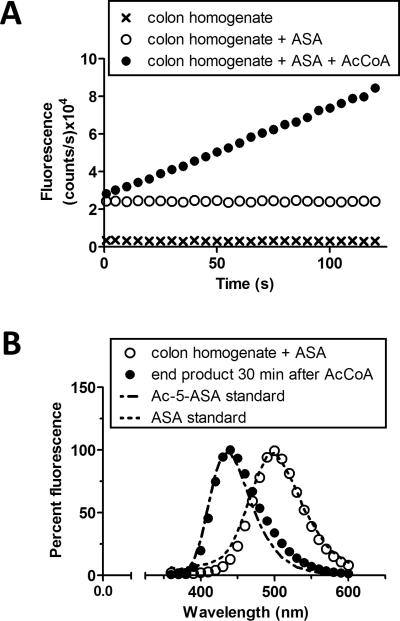
We first determined if the NAT assay that we established in mice is also valid with human biopsies. We validated the human NAT assay to be AcCoA dependent. No NAT activity was observed without the addition of the NAT cofactor AcCoA (Fig. 3A). The end product observed 30 minutes after the addition of AcCoA matched the intrinsic fluorescent spectrum of Ac-5-ASA, whereas the initial reaction mixture containing biopsy homogenate and 5-ASA in buffer matched the intrinsic fluorescent spectrum of 5-ASA (Fig. 3B).
FIGURE 3.
Measurement of human NAT activity. (A) Representative traces of NAT enzyme activity at 312 nm excitation and 437 nm emission. Fluorescence of colon homogenate (x) was recorded over 120 seconds, then 5-ASA (ASA) was added sequentially to the same homogenate (o), then thirdly, AcCoA was added to start the reaction (●). After 5-ASA or AcCoA addition, representative traces were reset to time zero for comparison. (B) Colon homogenate with 5-ASA (○) compared to the spectra of 5-ASA standard (dotted line). Same colon homogenate plus 5-ASA 30 minutes after addition of AcCoA (●), to initiate the reaction, compared to the spectra of Ac-5-ASA standard (dot-dash-dot line).
Our primary goal was to understand the variability in patient NAT activity, with a secondary goal to ask whether NAT activity correlated with severity of colonic inflammation. We evaluated the variability of NAT or MPO activity within multiple biopsies from the same patient, evaluating pediatric and adult groups separately. We used Levene's test, which assessed the equality of variances of NAT and MPO values within the same patient (from multiple biopsies). Levene's test shows adult patient variability of NAT and MPO activity are not equal, with P-values < 0.0001 (NAT) and 0.035 (MPO), respectively. In contrast, Levene's test applied to the pediatric patients shows that variability of NAT and MPO values do not differ with P-values 0.063 and 0.636, respectively. Therefore, since the homogeneity assumption does not hold for adults, adults cannot be used to compare with the pediatric group. Therefore, we subsequently analyzed the pediatric and adult patient analysis separately.
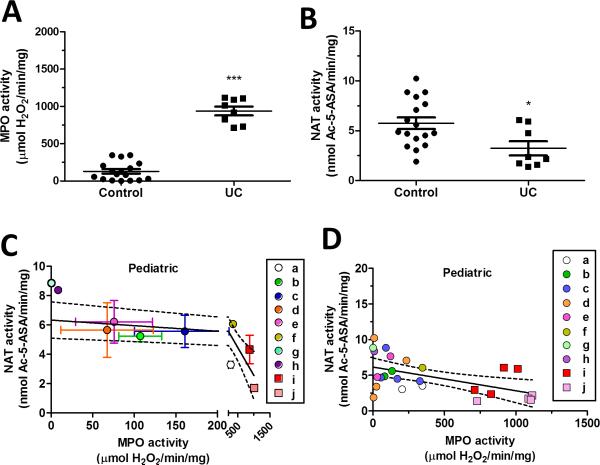
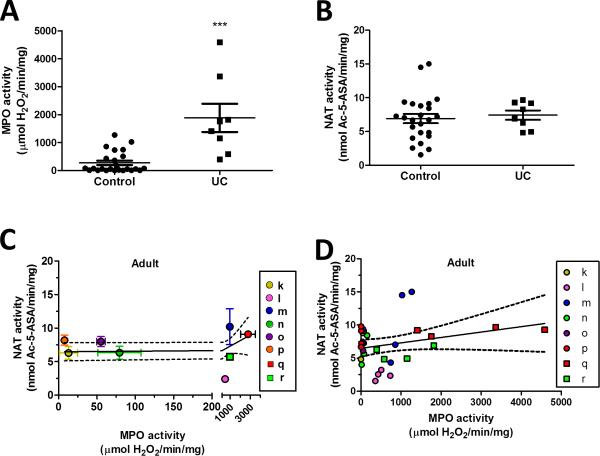
In pediatric and adult patients, as expected, we observed a significant increase in MPO activity in ulcerative colitis (UC) patients. Pediatric MPO activity is 128 ± 30 (control) versus 938 ± 60 (UC) and adult is 273 ± 78 (control) versus 1880 ± 500 (UC) μmol H2O2/min/mg protein (Fig. 4A and 5A) where data points are shown for each biopsy). In contrast, NAT activity in the same colitis patients was significantly decreased in pediatric patients but not in adult patients. Pediatric NAT activity was 5.74 ± 0.58 (control) versus 3.24 ± 0.71 (UC) and adults is 6.91 ± 0.69 (control) versus 7.42 ± 0.69 (UC) (Fig. 4B and 5B).
FIGURE 4.
Effect of inflammation on 5-ASA metabolism in pediatric rectosigmoid biopsies. (A) MPO enzyme activity (B) and NAT enzyme activity was measured in rectosigmoid biopsies of de-identified pediatric control and ulcerative colitis (UC) patients. Values are mean ± SEM. *P < 0.05 and ***P < 0.0001 vs. corresponding control. Pediatric control patients are assigned the letters #a, through h, solid colored circles; ulcerative colitis patients are #i and j, solid colored squares. Graphs (C and D) show the correlation of NAT to MPO enzyme activity and are analyzed by linear regression with 95 % confidence levels (dotted line). (C) Each point represents one patient. Values are mean ± SEM for MPO and NAT activity. The region between 0 and 200 μmol H2O2/min/mg MPO activity is expanded to better show the SEM for each patient. (D) Each point represents one biopsy and each color (solid colored symbol) represents one patient.
FIGURE 5.
Effect of inflammation on 5-ASA metabolism in adult rectosigmoid biopsies. (A) MPO enzyme activity (B) and NAT enzyme activity was measured in rectosigmoid biopsies of de-identified adult control and ulcerative colitis (UC) patients. Values are mean ± SEM. ***P < 0.0001, vs. corresponding control. NAT activity between control and UC biopsies are not significant. Adult control patients are assigned the letters #k, through p, solid colored circles; ulcerative colitis patients are #q and r, solid colored squares. Graphs (C and D) show the correlation of NAT enzyme to MPO enzyme activity and are analyzed by linear regression with 95 % confidence levels (dotted line). (C) Each point represents one patient. Values are mean ± SEM for MPO and NAT activity. The region between 0 and 200 μmol H2O2/min/mg MPO activity is expanded to better show the SEM for each patient. (D) Each point represents one biopsy and each color (solid colored symbol) represents one patient.
We then examined all outcomes regardless of diagnosis, separately in both adult and pediatric samples, correlating rectosigmoid mucosal NAT and MPO activity at the level of patient outcomes (Fig. 4C and 5C) or at the level of individual biopsies (Fig. 4D and 5D). In Figure 4C, linear regression of the pediatric patient outcomes, with a 95 % confidence level (dotted line), shows a decreasing slope significantly different from zero, which shows that NAT activity significantly changes with increasing inflammation, where P = 0.0022 with a Y-intercept of 6.33 ± 0.60 nmol Ac-5-ASA/min/mg . In Figure 5C, linear regression of the adult patients had a Y-intercept of 6.48 ± 0.63 nmol Ac-5-ASA/min/mg with a slope not significantly different from zero showing NAT activity does not change linearly with increasing inflammation in the adult patients.
When similar linear regression was performed on results of individual pediatric biopsies (Fig. 4D), results show a decreasing slope significantly different from zero. A linear mixed model with random effect showed for the pediatric group, whose MPO ≥ 150 μmol H2O2/min/mg protein, a NAT activity that is significantly (2.5 nmol Ac-5-ASA/min/mg protein) less than those with MPO values below 150 μmol H2O2/min/mg protein (Table 2A). In contrast, analysis of the adult biopsies with a linear mixed model with random effect showed (Table 2B) no significant change in NAT activity with increasing inflammation and the slope of the linear regression was not significantly different from zero (Fig. 5D).
Table 2.
| A. Solution for Fixed Effects: Pediatric | |||||
|---|---|---|---|---|---|
| Effect | Estimate | Standard Error | Degree of Freedom | t Value | P Value |
| Intercept | 6.2900 | 0.6805 | 9 | 9.24 | < 0.0001 |
| MPO > 150 | −2.5386 | 0.9094 | 14 | −2.79 | −2.79 |
| B. Solution for Fixed Effects: Adults | |||||
|---|---|---|---|---|---|
| Effect | Estimate | Standard Error | Degree of Freedom | t Value | P Value |
| Intercept | 6.6868 | 1.2355 | 7 | 5.41 | 0.0010 |
| MPO > 150 | 0.6560 | 1.5596 | 23 | 0.42 | 0.6780 |
MPO, myeloperoxidase
We also evaluated the variability of NAT and MPO enzyme activity between patients. We focused on the control patients as the ulcerative colitis patients were in various stages of the disease. The pediatric group had no significant variability in NAT or MPO activity between patients, whereas adult patients showed more variability in NAT and MPO activity with the analysis of variance being significant for adults with P = 0.0120 and P < 0.0001, respectively.
Taken together, these data suggest that adult, but not pediatric, control patients have highly variable NAT and MPO activity between multiple biopsies from the same patient and are highly variable between different patients. In contrast, pediatric, but not adult, patients show a significant reduction in NAT activity with increasing colonic mucosal inflammation which may affect 5-ASA drug efficacy. Results suggest that the regulation of NAT activity in the inflamed gut is dissimilar between pediatric and adult patients.
Discussion
5-ASA is a proven medication to reduce inflammation caused by ulcerative colitis in pediatric and adult patients. Cytosolic NAT enzymes metabolize 5-ASA to Ac-5-ASA in a wide range of tissues including the gut, liver, placenta, lungs, kidney and bladder. In normal colon tissue, 5-ASA enters the colonocyte (24-26) and is either rapidly metabolized to Ac-5-ASA (27) or the 5-ASA is excreted basolaterally into the blood so that no significant amount accumulates in the tissue (26). The acetylated form of the drug is more stable (and/or membrane-impermeable) than 5-ASA and collects within the cell and is then exclusively excreted apically back into the lumen (26). We recently established a method to measure NAT enzymatic conversion of 5-ASA in mouse tissue and demonstrated that mNAT2 is the main colonic enzyme to metabolize 5-ASA (1), although recent studies show that both human and mouse orthologs of NAT1 and NAT2 are competent to metabolize 5-ASA (10, 28).
Kumar et al (19) demonstrated that administration of 75 mg/kg/day p.o. 5-ASA to mice for 7 days during 5 % (w/v) DSS in drinking water significantly suppressed MPO activity in colonic tissue. In our mice, we used a milder DSS treatment model of 3.5 % (w/v) DSS in drinking water followed by a two day recovery period of regular drinking water concurrent with administration of 75 mg/kg/day p.o. 5-ASA for 7 days. We chose a milder DSS colitis model to produce modest damage. In the present study, treatment with 5-ASA in our WT mice did not improve inflammation possibly due to healing of the mucosa during the two day recovery period, whereas we did observe a significant improvement in inflammation and whole colon length in the Nat1/2 KO mice. Although one double-blind controlled therapeutic trial showed a beneficial effect of Ac-5-ASA enemas (29), our data suggest that 5-ASA, not Ac-5-ASA, is the predominant anti-inflammatory drug.
Interestingly, the Nat1/2 KO mice on DSS show higher inflammation activity (MPO activity) than the WT. Sim et al. (30) suggested that NAT enzymes have an endogenous role(s) but are as yet unidentified and Minchin et al. (31) suggested that an appropriate stress, such as DSS, may be required before an endogenous role for the xenobiotic metabolizing NAT enzyme is found. Because the Nat1/2 KO mice show no obvious phenotype (18), yet show greater DSS induced inflammation in the absence of NAT activity, these suggest that NAT enzymes are involved in regulation of mucosal homeostasis, yet further study will be needed.
We previously showed (1) that reduced NAT activity was not due to loss of epithelial cells after DSS exposure and results suggested that DSS reduced colonic 5-ASA metabolism by decreasing mouse mNAT2 protein translation or stability and may decrease enzymatic turnover number. We observed that inflammation significantly impaired distal colonic 5-ASA metabolism in 9 to 13 week old WT mice. These ages of mice correspond to human teenage years to about 20 years old (32, 33), which we can compare to our pediatric human group. Indeed, we found a significant correlation of lower 5-ASA metabolism with increasing inflammation in mice (Fig. 2E) that is similar to pediatric patients (Fig. 4D). Furthermore, the pediatric group (both control and ulcerative colitis patients) shows no significant mean variance between control patients in 5-ASA metabolism or inflammation levels. Further investigation, to see if NAT activity is recovered in disease remission may be useful. These data suggest that 5-ASA metabolism, in the pediatric patient, may be influenced by the disease condition itself by reducing NAT activity. The mechanism by which 5-ASA induces its anti-inflammatory effect within the colonocyte is not completely understood and multiple mechanisms have been proposed (2, 3, 5, 7, 34-36) but a higher concentration of 5-ASA within the colonocyte may be beneficial. We demonstrated in mouse experiments that 5-ASA is the therapeutic molecule, therefore reducing NAT acetylation of 5-ASA inside the colonocyte may significantly increase the 5-ASA concentration within the cell before the 5-ASA is excreted basolaterally into the blood. Based on these results, we speculate that 5-ASA will gain efficacy with increasing inflammation in pediatric patients with mild to moderate colitis.
In contrast, the adult group shows no correlation between their 5-ASA metabolism and inflammation levels (Fig. 5D), in rectosigmoid mucosal biopsies. The adult control population has significant variability in 5-ASA metabolism and inflammation levels between different adult patients and between biopsies from the same patient. Adults are a widely variable group possibly due to gut aging (our controls were age 33 to 74) and/or a plethora of medications with up to 17 medications per adult patient (list not shown) that may affect 5-ASA metabolism, whereas the pediatric patients were on a maximum of 7 medications. Based on these results, we speculate that 5-ASA may be more effective for treatment of colitis in pediatric patients than in adults.
Another factor effecting 5-ASA metabolism in human populations may be due to NAT gene polymorphisms that produce rapid or slow acetylation. So far, it has been difficult to link 5-ASA therapy outcomes to NAT acetylation types (37-39). These data suggest that rapid NAT acetylation may reduce 5-ASA effectiveness.
In conclusion, our study shows 5-ASA, and not its metabolite Ac-5-ASA is the effective moiety in mouse gut anti-inflammation therapy. Results show that in young adult mice and pediatric patients, colonic mucosal inflammation affects 5-ASA metabolism by lowering NAT activity, whereas adult 5-ASA metabolism did not correlate to inflammation levels. Most humans have gut NAT activity, therefore blocking colonic NAT activity with inhibitors to increase 5-ASA efficacy may be an area for further study. Our data suggests that increasing inflammation may regulate the efficiency of 5-ASA therapy causing the therapy outcome to be more variable in pediatric populations. Thus, the determination of the rates of colonic 5-ASA metabolism in a patient may be useful as a new tool to help predict the efficacy of 5-ASA therapy and to keep the disease in remission.
Acknowledgements
We thank Ramona Bezold, RN, BSN and Kathleen Lake, MSW, LISW, CCRC for their assistance in collecting pediatric biopsies, Veronica Ramirez-Alcantara for technical assistance with the NAT and MPO assays, and Chet Closson for technical assistance with the fluorometer and microscopes.
Funding: The project described was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number UL1TR000077. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
This project was supported in part by NIH P30 DK078392 (Bioinformatics Core of the Digestive Disease Research Core Center in Cincinnati)
Footnotes
Disclosures: LAD: Advisory board for Avaxia Biologics, Grant funding from Janssen Pharmaceuticals. BRY: Advisory board for Seres Health, Inc., Consultant to Procter & Gamble Pharmaceuticals, Speaker's Bureau for UCB, Janssen Biotech, Inc., Shire Pharmaceuticals, and Actavis Pharmaceuticals, Grant funding from Merck/Cubist Pharmaceuticals. The remaining authors declare no conflict of interest.
References
- 1.Ramirez-Alcantara V, Montrose MH. Acute murine colitis reduces colonic 5-aminosalicylic acid metabolism by regulation of N-acetyltransferase-2. Am J Physiol Gastrointest Liver Physiol. 2014;306:G1002–1010. doi: 10.1152/ajpgi.00389.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egan LJ, Mays DC, Huntoon CJ, et al. Inhibition of interleukin-1-stimulated NF-kappaB RelA/p65 phosphorylation by mesalamine is accompanied by decreased transcriptional activity. J Biol Chem. 1999;274:26448–26453. doi: 10.1074/jbc.274.37.26448. [DOI] [PubMed] [Google Scholar]
- 3.Galvez J, Garrido M, Rodriguez-Cabezas ME, et al. The intestinal anti-inflammatory activity of UR-12746S on reactivated experimental colitis is mediated through downregulation of cytokine production. Inflamm Bowel Dis. 2003;9:363–371. doi: 10.1097/00054725-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Grisham MB, Granger DN. Neutrophil-mediated mucosal injury. Role of reactive oxygen metabolites. Dig Dis Sci. 1988;33:6S–15S. doi: 10.1007/BF01538126. [DOI] [PubMed] [Google Scholar]
- 5.Horvath K, Varga C, Berko A, et al. The involvement of heme oxygenase-1 activity in the therapeutic actions of 5-aminosalicylic acid in rat colitis. Eur J Pharmacol. 2008;581:315–323. doi: 10.1016/j.ejphar.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Reifen R, Nissenkorn A, Matas Z, et al. 5-ASA and lycopene decrease the oxidative stress and inflammation induced by iron in rats with colitis. J Gastroenterol. 2004;39:514–519. doi: 10.1007/s00535-003-1336-z. [DOI] [PubMed] [Google Scholar]
- 7.Rousseaux C, Lefebvre B, Dubuquoy L, et al. Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-gamma. J Exp Med. 2005;201:1205–1215. doi: 10.1084/jem.20041948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Vos M, Verdievel H, Schoonjans R, et al. Concentrations of 5-ASA and Ac-5-ASA in human ileocolonic biopsy homogenates after oral 5-ASA preparations. Gut. 1992;33:1338–1342. doi: 10.1136/gut.33.10.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blum M, Grant DM, McBride W, et al. Human arylamine N-acetyltransferase genes: isolation, chromosomal localization, and functional expression. DNA Cell Biol. 1990;9:193–203. doi: 10.1089/dna.1990.9.193. [DOI] [PubMed] [Google Scholar]
- 10.Estrada-Rodgers L, Levy GN, Weber WW. Substrate selectivity of mouse N-acetyltransferases 1, 2, and 3 expressed in COS-1 cells. Drug Metab Dispos. 1998;26:502–505. [PubMed] [Google Scholar]
- 11.Je Everhart. The burden of digestive diseases in the United States. US Government Printing Office; Washington, DC: 2008. pp. 97–106. NIH Publication No. 09-6443. [Google Scholar]
- 12.van Bodegraven AA, van Everdingen JJ, Dijkstra G, et al. [Guideline 'Diagnosis and treatment of inflammatory bowel disease in adults'. I. Diagnosis and treatment]. Ned Tijdschr Geneeskd. 2010;154:A1899. [PubMed] [Google Scholar]
- 13.Zeisler B, Lerer T, Markowitz J, et al. Outcome following aminosalicylate therapy in children newly diagnosed as having ulcerative colitis. J Pediatr Gastroenterol Nutr. 2013;56:12–18. doi: 10.1097/MPG.0b013e31826ac41a. [DOI] [PubMed] [Google Scholar]
- 14.The Facts About Inflammatory Bowel Diseases. Crohn's & Colitis Foundation of America; 733 Third Ave Suite 510 New York, NY 10017: 2014. [1/7/2016]. Available at: www.ccfa.org. [Google Scholar]
- 15.Langholz E, Munkholm P, Davidsen M, et al. Course of ulcerative colitis: analysis of changes in disease activity over years. Gastroenterology. 1994;107:3–11. doi: 10.1016/0016-5085(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 16.Carter MJ, Lobo AJ, Travis SP. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53(Suppl 5):V1–16. doi: 10.1136/gut.2004.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howell H. Ulcerative Colitis: Achieving and Maintaining Remission. US Pharmacist. 2008;33:30–38. [Google Scholar]
- 18.Sugamori KS, Wong S, Gaedigk A, et al. Generation and functional characterization of arylamine N-acetyltransferase Nat1/Nat2 double-knockout mice. Mol Pharmacol. 2003;64:170–179. doi: 10.1124/mol.64.1.170. [DOI] [PubMed] [Google Scholar]
- 19.Kumar GK, Dhamotharan R, Kulkarni NM, et al. Embelin ameliorates dextran sodium sulfate-induced colitis in mice. Int Immunopharmacol. 2011;11:724–731. doi: 10.1016/j.intimp.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Graff G, Gamache DA, Brady MT, et al. Improved myeloperoxidase assay for quantitation of neutrophil influx in a rat model of endotoxin-induced uveitis. J Pharmacol Toxicol Methods. 1998;39:169–178. doi: 10.1016/s1056-8719(98)00023-9. [DOI] [PubMed] [Google Scholar]
- 21.Bradley PP, Priebat DA, Christensen RD, et al. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 22.Worthington K, Worthington V. Worthington Enzyme Manual. [3/29/2012];Worthington Biochemical Corporation. 2011 Available at: http://www.worthington-biochem.com/HPO/default.html.
- 23.Loria V, Dato I, Graziani F, et al. Myeloperoxidase: a new biomarker of inflammation in ischemic heart disease and acute coronary syndromes. Mediators Inflamm. 2008;2008:135625. doi: 10.1155/2008/135625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brogden RN, Sorkin EM. Mesalazine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in chronic inflammatory bowel disease. Drugs. 1989;38:500–523. doi: 10.2165/00003495-198938040-00003. [DOI] [PubMed] [Google Scholar]
- 25.Greenfield SM, Punchard NA, Teare JP, et al. Review article: the mode of action of the aminosalicylates in inflammatory bowel disease. Aliment Pharmacol Ther. 1993;7:369–383. doi: 10.1111/j.1365-2036.1993.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhou SY, Fleisher D, Pao LH, et al. Intestinal metabolism and transport of 5-aminosalicylate. Drug Metab Dispos. 1999;27:479–485. [PubMed] [Google Scholar]
- 27.Ireland A, Priddle JD, Jewell DP. Comparison of 5-aminosalicylic acid and N-acetylaminosalicylic acid uptake by the isolated human colonic epithelial cell. Gut. 1992;33:1343–1347. doi: 10.1136/gut.33.10.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawamura A, Graham J, Mushtaq A, et al. Eukaryotic arylamine N-acetyltransferase. Investigation of substrate specificity by high-throughput screening. Biochem Pharmacol. 2005;69:347–359. doi: 10.1016/j.bcp.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Willoughby CP, Piris J, Truelove SC. The effect of topical N-acetyl-5-aminosalicylic acid in ulcerative colitis. Scand J Gastroenterol. 1980;15:715–719. doi: 10.3109/00365528009181520. [DOI] [PubMed] [Google Scholar]
- 30.Sim E, Lack N, Wang CJ, et al. Arylamine N-acetyltransferases: structural and functional implications of polymorphisms. Toxicology. 2008;254:170–183. doi: 10.1016/j.tox.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 31.Minchin RF, Hanna PE, Dupret JM, et al. Arylamine N-acetyltransferase I. Int J Biochem Cell Biol. 2007;39:1999–2005. doi: 10.1016/j.biocel.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Fox JG. The mouse in biomedical research. 2nd ed. Elsevier, AP; Amsterdam ; Boston: 2007. [Google Scholar]
- 33.Harrison D. Life span as a biomarker: in Gerontology: mechanisms of aging. 2008 Available. [Google Scholar]
- 34.Hawkey CJ, Boughton-Smith NK, Whittle BJ. Modulation of human colonic arachidonic acid metabolism by sulfasalazine. Dig Dis Sci. 1985;30:1161–1165. doi: 10.1007/BF01314051. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen OH VH, Elmgreen J. Inhibition of intestinal macrophage chemotaxis to leukotriene B4 by sulphasalazine, olsalazine, and 5-aminosalicylic acid. Alimen Pharmacol Ther. 1988;2:203–211. doi: 10.1111/j.1365-2036.1988.tb00689.x. [DOI] [PubMed] [Google Scholar]
- 36.Simmonds NJ, Millar AD, Blake DR, et al. Antioxidant effects of aminosalicylates and potential new drugs for inflammatory bowel disease: assessment in cell-free systems and inflamed human colorectal biopsies. Aliment Pharmacol Ther. 1999;13:363–372. doi: 10.1046/j.1365-2036.1999.00484.x. [DOI] [PubMed] [Google Scholar]
- 37.Hausmann M, Paul G, Menzel K, et al. NAT1 genotypes do not predict response to mesalamine in patients with ulcerative colitis. Z Gastroenterol. 2008;46:259–265. doi: 10.1055/s-2007-963673. [DOI] [PubMed] [Google Scholar]
- 38.Hein DW, Doll MA, Fretland AJ, et al. Molecular genetics and epidemiology of the NAT1 and NAT2 acetylation polymorphisms. Cancer Epidemiol Biomarkers Prev. 2000;9:29–42. [PubMed] [Google Scholar]
- 39.Ricart E, Taylor WR, Loftus EV, et al. N-acetyltransferase 1 and 2 genotypes do not predict response or toxicity to treatment with mesalamine and sulfasalazine in patients with ulcerative colitis. Am J Gastroenterol. 2002;97:1763–1768. doi: 10.1111/j.1572-0241.2002.05838.x. [DOI] [PubMed] [Google Scholar]