Abstract
BACKGROUND & AIMS
Early adverse life events (EALs) are associated with irritable bowel syndrome (IBS). Exposure to EALs as assessed by the Adverse Childhood Experiences (ACE) questionnaire is associated with greater disease prevalence, but ACE has not been studied in gastrointestinal disorders. Study aims were to: 1) Estimate the prevalence of EALs in the IBS patients using the ACE questionnaire; 2) Determine correlations between ACE and Early Trauma Inventory Self Report-Short Form (ETI-SR) scores to confirm its validity in IBS; and 3) Correlate ACE scores with IBS symptom severity.
METHODS
148 IBS (73% women, mean age=31 years) and 154 HCs (59% women, mean age=30 years) completed the ACE and ETI-SR between June 2010 and April 2015. These surveys measured EALs before age 18 in the domains of physical, sexual, emotional abuse, and general trauma. IBS and abdominal pain severity was measured by a 20-point scale (0=none, 20=worst symptoms).
RESULTS
The ACE score increased the odds of having IBS (odds ratio (OR)=2.05 [95% confidence interval (CI): 1.21-3.48], p=0.008). Household mental illness (p<0.001), emotional abuse (p=0.004), and incarcerated household member (p=0.019) were significant predictors of IBS. ACE and ETI-SR scores were strongly correlated (r=0.59, p<0.001). ACE, but not ETI-SR, modestly correlated with IBS severity (r=0.17, p=0.036) and abdominal pain (r=0.20, p=0.015).
CONCLUSION
The ACE questionnaire is a useful instrument to measure EALs in IBS based on its use in large studies, its ability to measure prevalence across different EAL domains, and its correlation with symptom severity.
Keywords: Irritable Bowel Syndrome, Early Life Trauma, Adverse Childhood Experiences, Early Life Trauma Inventory, Abuse
INTRODUCTION
Adverse childhood experiences ACEs, also known as early adverse life events (EALs), include traumas experienced early in life, such as childhood abuse, household mental illness or domestic violence. Patients with irritable bowel syndrome (IBS), a functional gastrointestinal disorder characterized by abdominal pain associated with alterations in stool form and/or frequency,[1] report a higher prevalence of EALs in comparison to healthy controls (HCs). [2-6]
In the last decade, the study of the health and societal impact of EALs has transitioned from a topic of research to a public health imperative. This is largely due to the results of The ACE Study, conducted by investigators from the Centers for Disease Control and Prevention (CDC) and Kaiser Permanente, which demonstrated a dose response effect between the number of EALs as assessed by the ACE questionnaire and a variety of negative health outcomes in a cohort of over 17,000 members of a health maintenance organization.[7]
More recently, data on EALs has been collected in 22 states as part of the CDC's Behavioral Risk Factor Surveillance System, the largest ongoing health survey in the world.[8] Published results show consistent findings in data from 10 states (n=53,998), in which 44.1%, 12.7%, and 2.6% reported 1-3, 4-6 and 7-9 EALs, respectively, and a graded increase in the risk of health conditions including coronary heart disease, diabetes, overall physical and mental health with increasing ACE score.[9]
Knowledge of the negative impact of EALs has led to policy initiatives and law[10] geared toward early intervention and prevention of EALs. Some have questioned whether inquiring about EALs in adults is beneficial. Despite this being a common concern of physicians and researchers, there is little to suggest that asking about EALs is harmful.[11] Studies have demonstrated that IBS patients want to learn about the impact of psychological factors on symptoms,[12] and that psychological treatment such as cognitive behavioral therapy (CBT) reduces IBS symptoms and improves health related quality of life.[13] One goal of CBT is to decrease reliance on negative coping mechanisms,[14] which are common in those with a history of EALs.[15]
In previous studies, we have measured EALs in IBS patients and HCs with the Early Trauma Inventory Self Report- Short Form (ETI-SR),[4, 16] which measures items within the categories of general trauma, physical punishment, emotional abuse and sexual abuse before the age of 18. Emotional abuse was found to be the strongest predictor of IBS.[4] Similar to the ETI-SR, the ACE questionnaire includes items in the domains of physical, emotional, and sexual abuse, and general trauma occurring before the age of 18. However, unlike the ETI-SR, the ACE can provide dichotomous outcome measures for each domain and for the overall survey indicating the presence or absence of EALs (i.e., prevalence). The ACE questionnaire has not been studied in IBS.
The aims of this study were to: 1) Compare the prevalence of EALs using the ACE score in IBS patients and HCs; 2) Correlate ACE and ETI-SR scores in IBS and HCs to confirm the validity of ACE in IBS, 3) Compare the mean ETI-SR scores in IBS and HCs with and without EALs; and 3) Determine the correlation between ACE with severity of IBS symptoms and abdominal pain.
METHODS
Study Subjects
Male and female participants who were at least 18 years of age were drawn from IBS patients and HCs recruited for studies conducted at our center between June 2010 and April 2015. IBS subjects were predominantly recruited from newspaper or internet community advertisements and from GI clinics who fulfilled Rome III diagnostic criteria. The diagnosis was confirmed by a clinician with expertise in IBS. HC subjects were recruited by advertisement without a history of IBS or other chronic GI or pain conditions, and were not taking psychotropic medication or participating in psychotherapy. Subjects were compensated for completion of a medical history and physical examination and questionnaires.
Questionnaires
Bowel Symptom Questionnaire (BSQ)
This questionnaire measures the presence of IBS and other GI symptoms using Rome III questions and IBS symptom severity and pain severity using a 0 to 20 numeric rating scale over the past week (none to most intense imaginable).
Adverse Childhood Events (ACE) Questionnaire
The ACE questionnaire (Table 1, Supplementary Table 1) assesses the presence of EALs (before age 18) with 18 questions in 8 domains (number of questions) of physical (1), emotional (2), and sexual abuse (4), and includes household substance abuse (2), parental separation or divorce (1), mental illness in household (2), incarcerated household member (1), and parent treated violently (2). ACE score is calculated by assigning 1 point for each domain “Yes”=1 or “No”=0 (ACE score range 0-8= Physical abuse + Emotional Abuse + Sexual Abuse + Household Substance Abuse + Parent Separation/Divorce + Household Mental Illness + Incarcerated Household Member + Parent Treated Violently).[7] ACE can also be scored as presence (ACE total score ≥ 1) or absence (ACE total score = 0) of an EAL.
Table 1.
ACE and ETI-SR Questionnaire Overview
| ACE | ETI-SR |
|---|---|
| • 18 question survey for EALs before age 18 • Domains (number of items): Physical abuse (1), Emotional (2), and Sexual abuse (4), General trauma (8). a) General Trauma Subcategories: Substance abuse (2), Parental separation or divorce (1), Mental illness in household (2), Incarcerated household member (1), and Parent treated violently (2). b) ACE scoring guide uses specific criteria to assign 1point for each domain, “Yes”=1 or “No”=0 c) Total ACE Score (range 0-8)=Physical abuse + Emotional abuse + Sexual abuse + Substance abuse + Parental separation or divorce + Mental illness in household + Incarcerated household member + Parent treated violently |
• 27 question survey for EALs before age 18 • Domains (number of items): General trauma (11), Phy sical abuse (5), Emotional (5), and Sexual abuse (6). • Each of the 27 items was scored as “Yes”=1 or “No”=0 (total score range 0-27). |
ACE, adverse childhood experiences; EALs, early adverse life events; ETI-SR, Early trauma self report short form
Early Trauma Inventory Self Report-Short Form (ETI-SR)
The ETI-SR was administered in conjunction with the ACE in order to help confirm the validity of the ACE in IBS. It inventories the occurrence (before the age of 18) of 27 EAL items in the following domains (number of items): general trauma (11), physical (5), emotional (5), and sexual abuse (6). Each of the 27 items is scored as “Yes”=1 or “No”=0 (total score range 0-27). [16]
Other Psychometric Instruments
Current anxiety and depression symptoms were assessed with the Hospital Anxiety and Depression (HAD) Scale.[17] Somatic symptom severity was measured using the Personal Health Questionnaire (PHQ-15) [18] however, it was modified by removal of three GI symptom items (score 0–24) when comparing IBS with HCs. The Visceral Sensitivity Index (VSI)[19] assessed GI-symptom specific anxiety.
The study was approved by the UCLA Institutional Review Board, and all subjects signed a written informed consent prior to start of study.
Statistical Analysis
Multiple logistic regression was used to predict the odds of IBS status from ACE scores while controlling for age, sex, race and education. Spearman's correlation was used to assess the association between pairs of continuous measures. In a secondary analysis, ACE score was analyzed as a categorical variable similar to a recently published large scale study determining if ACE exposure increases odds of having chronic illness.[9] For this analysis, alcoholism and drug abuse were considered as separate ACE categories rather than as one category of substance abuse; thus the ACE score range was 0-9. A p-value threshold of 0.05 was used to define statistical significance, and a range of ≥ 0.05 to < 0.1 was interpreted as weak evidence for an effect. Regression analysis p-values reported in the manuscript are adjusted for demographics unless otherwise specified. The p-values in the tables represent comparisons with and without controlling for demographics. Psychological symptoms typically correlate with EAL scores, and thus we considered the analyses not adjusted for these variables to be primary. However, in a secondary analysis, we adjusted for both demographics and psychological symptoms. Bivariate analyses were conducted using both Wilcoxon test and χ2 tests. All analyses were done using R version 3.1.1 (http://cran.r-project.org/).
RESULTS
Subject Characteristics
Subjects included 148 Rome III positive IBS patients (73% women) and 154 HCs (59% women). Compared to HCs, IBS patients were comprised of more women, had a lower BMI, and had higher scores for HAD anxiety and depression symptoms, and somatic symptom severity (Table 2). For HAD anxiety, 75% of IBS patients and 100% of HCs were in the normal range (0-10), and for HAD depression, 96% of IBS patients and 99.4% of HCs were in the normal range.
Table 2.
Subject Characteristics
| Traits: Mean (SD) | Missing | HC (n=154) | IBS (n=148) | p value |
|---|---|---|---|---|
| Women (%) | 0 | 91 (59%) | 108 (73%) | 0.015 |
| Age in years (SD) | 0 | 30.26 (10.92) | 31.05 (10.03) | 0.235 |
| BMI (SD) | 5 | 26.56 (6.13) | 24.64 (5.07) | 0.001 |
| Race (%) | 15 | 0.143 | ||
| Asian | 41 (28%) | 26 (19%) | ||
| African American | 18 (12%) | 12 (8%) | ||
| Caucasian | 66 (45%) | 77 (55%) | ||
| Other/Mixed | 22 (15%) | 25 (18%) | ||
| Education (%) | 9 | 0.304 | ||
| High School Graduate or Less | 9 (6%) | 5 (3%) | ||
| Some College | 67 (45%) | 57 (40%) | ||
| College Graduate | 44 (30%) | 42 (29%) | ||
| Any Post Graduate Work | 29 (19%) | 40 (28%) | ||
| HAD Anxiety (0-21) | 0 | 3.92 (2.86) | 7.69 (4.45) | p<0.001 |
| HAD Depression (0-21) | 0 | 1.59 (2.17) | 3.44 (3.19) | p<0.001 |
| VSI Score (0-90) | 2 | 4.25 (6.37) | 39.23 (17.72) | p<0.001 |
| PHQ-12 Score (0-24) | 2 | 2.16 (2.32) | 10.31 (4.64) | p<0.001 |
| GI Symptoms | ||||
| Overall Severity (0-20) | 9.56 (4.39) | |||
| Abdominal Pain (0-20) | 9.26 (4.34) |
SD, standard deviation; HC, healthy control; IBS, irritable bowel syndrome; BMI, body mass index; HAD, hospital anxiety and depression; VSI, visceral sensitivity index; PHQ, personal health questionnaire
Prevalence of EALs in IBS using the ACE Questionnaire
As shown in Table 3, the overall prevalence of EALs was higher in IBS compared to HCs. The presence of EALs (i.e., ACE total score ≥1) increased the odds of having IBS (odds ratio (OR)=2.05 [95% confidence interval (CI): 1.21-3.48], p=0.008). Compared to HCs, IBS subjects had significantly higher ACE total scores (2.07 vs. 1.28, p<0.001) Although the relationship between ACE score and IBS was significant after controlling for demographics, there was only a weak association (p=0.059) after additionally adjusting for HAD anxiety and depression scores.
Table 3.
The Prevalence of EALs by ACE Questionnaire
| EAL Domain | HC (%) | IBS (%) | Unadjusted p value | Adjusted p value1 |
|---|---|---|---|---|
| Overall Prevalence | 59.74 | 75.68 | 0.003 | 0.008 |
| General Trauma | 56.49 | 70.95 | 0.012 | 0.022 |
| Household Substance Abuse | 23.38 | 31.76 | 0.122 | 0.262 |
| Parental Separation/Divorce | 38.96 | 43.92 | 0.414 | 0.414 |
| Household Mental Illness | 18.18 | 41.22 | p<0.001 | p<0.001 |
| Incarcerated Household Member2* | 4.55 | 10.14 | 0.077 | 0.019 |
| Parents Treated Violently | 11.69 | 20.95 | 0.042 | 0.077 |
| Physical Abuse | 7.79 | 12.16 | 0.249 | 0.194 |
| Emotional Abuse | 13.64 | 27.03 | 0.004 | 0.004 |
| Sexual Abuse | 9.74 | 19.59 | 0.022 | 0.079 |
p-value adjusting for demographic variables (i.e. age, race, sex, and education)
p-value was significant after adjusting for demographic and psychological (i.e., HAD anxiety and depression scores) variables
p=0.0449
EAL, early adverse life events; ACE, adverse childhood experiences; HC, healthy control; IBS, irritable bowel syndrome
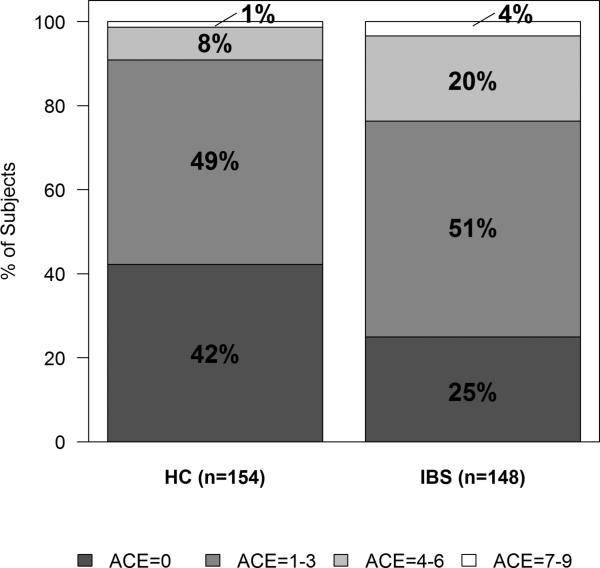
In our secondary analysis, where the ACE score range was 0-9, IBS status again correlated with higher ACE scores in the distributions ranges of 0, 1-3, 4-6, and 7-9 (Figure 1). There was a graded relationship between the number of categories of EAL and IBS status. Compared to an ACE score = 0, the odds of having IBS increased with ACE scores of 1-3 (OR=1.77, 95% CI [1.03, 3.06], p=0.04), 4-6 (OR=3.83, 95% CI [1.66, 8.89], p=0.002), and 7-9 (OR=4.08, 95% CI [0.68, 24.36], p=0.123) after adjusting for age, race, sex, and education.
Figure 1. Distribution of ACE Score in IBS vs. HC.
Distribution of ACE scores within the ranges of 0, 1-3, 4-6, and 7-9 is shown. There was a significant difference between IBS vs. HCs (p=0.007). ACE, adverse childhood experiences; HC, healthy control; IBS, irritable bowel syndrome
General trauma (71% vs. 57%; p=0.022) and emotional abuse (27% vs. 14%; p=0.004) were significantly more prevalent in IBS than HCs. Within the general trauma category IBS was associated with household mental illness (41% of IBS patients vs. 18% of HCs; p<0.001), and an incarcerated household member (10% in IBS vs. 5% in HCs; p=0.019). General trauma was reported in the majority of both IBS patients and HCs, in part due to the substantial rates of parental divorce/separation in both IBS and HCs. While the prevalence of sexual abuse was significantly associated with IBS before adjusting for sex and other demographics (20% vs. 10%, p=0.022), there was only a trend after adjusting for sex and demographics (p=0.079). Physical abuse was not predictive of IBS (Table 3). Incarcerated household member was the only ACE domain that was significantly associated with IBS after additionally adjusting for HAD anxiety and depression scores (p=0.045)
Correlation of ACE score and IBS and non-GI symptoms
In IBS patients, the total ACE score modestly correlated with overall IBS symptom severity (r=0.17, p=0.036) and abdominal pain (r=0.20, p=0.015). ACE score also correlated with overall somatic symptom severity as measured by the PHQ-15 within IBS patients (r=0.27, p<0.001). There was a trend for a positive correlation of ACE score with HAD anxiety (r=0.15 p=0.061), and no correlation with HAD depression (r=0.03, p=0.73) or GI symptom specific anxiety (VSI, r=0.13, p=0.11).
Association of ACE and ETI-SR scores in IBS and HCs
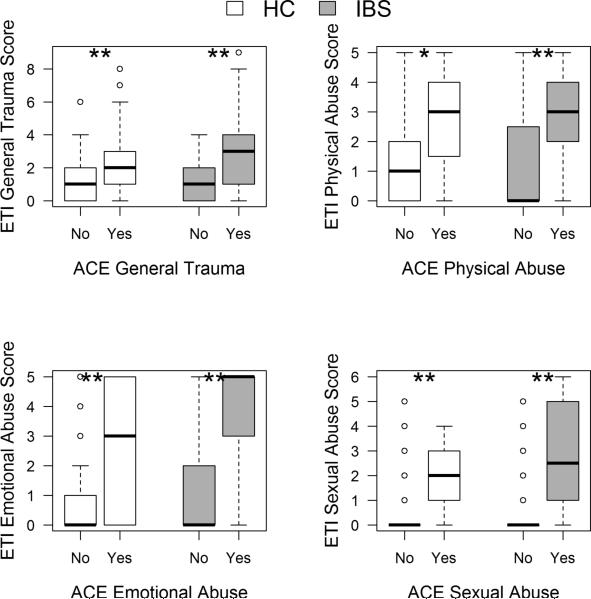
There was a strong positive correlation between the ETI-SR total score and ACE total score in all subjects (r=0.59, p<0.001) as well as within the IBS subjects (r=0.65, p<0.001), and HCs (r=0.51, p<0.001). Using the ACE Questionnaire, participants who were classified as having an EAL in each of the four domains (general trauma, emotional abuse, sexual abuse and physical abuse) had significantly higher ETI-SR scores within that domain (Figure 2, p range: 0.002 - <0.001).
Figure 2. ETI-SR scores according to presence or absence of EALs as defined by the ACE Questionnaire.
ETI-SR score distribution among HC and IBS patients with (“Yes”, ACE score ≥1) and without (“No”, ACE score =0) EALs as defined by ACE in each of the four EAL domains. Box plots show median and interquartile ranges. ETI-SR scores were significantly higher in HC and IBS subjects with an EAL compare to those without an EAL (* p<0.01; **p<0.001). ETI-SR, early trauma inventory self report short form; ACE, adverse childhood experiences; IBS, irritable bowel syndrome
DISCUSSION
EALs have been associated with an increased risk of physical and mental health and behaviors. Large-scale studies have demonstrated a graded relationship between exposure to EALs measured with the ACE questionnaire and prevalence of chronic medical illnesses including coronary artery disease, diabetes, and mental distress; however, the association of ACE scores and GI disorders has not been reported.[9] Here we measured EALs in IBS using the ACE questionnaire and found that ACE score is associated with an increased risk of having IBS. Specific key findings include: 1) IBS status is predicted by a history of emotional abuse and either a mentally ill or incarcerated household member; 2) The advantages of ACE over the ETI-SR include that the ACE provides the prevalence of EALs and the score is positively correlated with overall symptom and abdominal pain severity in IBS, and 3) While ACE and ETI-SR scores were significantly correlated, individuals with an EAL defined by ACE reported a mean of at least 2 items within each ETI-SR domain vs. 0-1 items in those without an ACE defined EAL. The latter finding suggests that using a cutoff score of 1 EAL item in each ETI-SR domain is overly sensitive in determining prevalence of EALs. Even though an association between EALs and IBS prevalence and symptom severity has previously been reported, we believe that the current study provides novel information regarding this relationship: First, the association with IBS was determined among IBS patients with confirmed diagnoses and most IBS patients had normal HAD anxiety and depression symptom scores. Second, we were able to compare the ACE score to another measure of EALs as well as to symptoms.
Others have reported that exposure to EALs in a general population cohort of 53,998 individuals is associated with increased odds of having chronic diseases, including coronary artery disease, myocardial infarction, diabetes, asthma, and frequent mental distress. Our results in a community sample are consistent with these findings, i.e., an increased exposure to EALs is associated with an increased odds of IBS, even after adjusting for demographic variables.[9] In addition, the prevalence of EALs (e.g., any abuse, household substance abuse or mental illness) in our subjects was comparable to that in large community populations of IBS and controls.[20, 21]
The household mental illness EAL significantly differentiated IBS and HCs (41% in IBS vs. 18% in HCs using the ACE). This result is consistent with our findings in a larger sample (28% IBS vs. 13% HCs)[4] and in a recently published population study (37% IBS vs. 22% controls).[20] There are several factors that may explain this association. It is possible that this is due to shared genetic factors as IBS clusters in families[22] and is associated with mental illness.[23] The association may also be due to environmental factors such as the parental response to abdominal pain, which has been suggested as an etiological mechanism in IBS,[24] and may also be affected by mental illness. Instruments measuring EALs in IBS prior to this study have not included questions about incarcerated household members and thus this association with IBS is novel and deserves attention as a topic of future research. The third predictor of IBS, emotional abuse, agrees with findings of studies that used a variety of instruments to assess EALs.[4, 25, 26]
We found that the ACE score positively correlated with severity of overall symptoms and pain in IBS. This association between EALs and symptoms is also consistent with the finding of increased pain to rectal distension among IBS patients with history of abuse in comparison to both IBS patients and HCs without a history of abuse.[27] Results of functional brain imaging studies have shown that abuse in IBS is associated with increased activation of central pain pathways (dorsal cingulate gyrus) and reduced activation of central pain inhibitory pathways,[28] as well as alterations in the salience network,[29] which plays a key role in attention to internal (emotional, cognitive, interoceptive) and external somatosensory stimuli based on personal relevance.[30] Thus, it is not surprising that the relationship between ACE scores and IBS was in part accounted for by current anxiety and depression symptoms. It is possible that EALs are associated with psychological symptoms, which in turn can be associated with a greater risk of developing or reporting IBS symptoms. However, it is also possible that there is a direct relationship of EALs to IBS and the experience of having IBS symptoms is associated with symptom-specific anxiety. To explore this association further, we separately adjusted for VSI and found that the relationship between ACE score and IBS was diminished even more so than with HAD anxiety and depression symptoms. Therefore, the experience of EALs may be associated with an increased risk of having IBS symptoms, which is associated with increased GI symptom-specific anxiety. Based on our previous study assessing the relationship of EALs as measured by ETI-SR and IBS,[4] we believe that there is an association of EALs and IBS independent of current anxiety and depression symptoms. In the current study, the fact that when controlling for anxiety and depression symptoms the effect of ACE and IBS was only weak (p=0.059) may have been in part due to the smaller size. Nonetheless, because our study is cross-sectional, we cannot determine causal effects but this should be established by longitudinal studies in the future.
In this study, the prevalence of sexual abuse as measured by ACE was significantly associated with IBS, but it was not significant after adjusting for demographics including sex. This is consistent with previous findings that the association between sexual abuse and IBS is more predominant in women.[3, 31] Our sexual abuse prevalence in IBS of 20% was consistent with other studies which reported rates of 13-54% depending on the method of assessment and definition of abuse used.[3, 27, 31, 32]
In addition to a potential therapeutic effect of the disclosure of EALs themselves and the resulting insight, determining the presence of EALs in IBS may be helpful in guiding therapy and impacting treatment outcomes. In a survey of 1,242 IBS patients, the majority endorsed a desire to learn about the impact of psychological factors on symptoms.[12] While EALs are not psychological factors per se, they can have psychological consequences. IBS patients also desire empathetic providers,[33] and a high quality provider-patient relationship has been shown to improve patient outcomes.[34] IBS patients with a history of abuse respond better to psychological therapy (pharmacotherapy and CBT) than those without abuse. Psychological therapies may not specifically focus on EALs, but they can improve coping of symptoms, global well-being and disease specific quality of life in IBS patients with abuse.[35, 36] Larger studies have shown that psychological therapies improve GI symptoms independent of their effects on psychological distress in IBS.[37]
Limitations of the present study include a study population predominantly from the West Los Angeles area, the possibility of recall bias given our focus on childhood traumatic events, and lack of information on childhood support and psychological counseling. In addition, the ACE used in this study was an older version that did not include the categories of physical and emotional neglect. Also, while the study was powered to detect a relationship between EALs and IBS status, exploratory analyses conducted within subgroups such as sex were likely underpowered.
In summary, this is the first study to evaluate EALs using the ACE questionnaire in GI disease. In IBS, we found that a history of household mental illness, incarcerated household member and emotional abuse were notable predictors of IBS. The ACE questionnaire is a useful instrument to measure EALs in IBS based on its use in large studies, its ability to measure prevalence across different EAL domains, and its correlation with IBS symptom severity.
Supplementary Material
Key Messages.
This is the first study to evaluate early adverse life events (EALs) using the Adverse Childhood Experiences (ACE) questionnaire in gastrointestinal (GI) disease.
The aims of the study were to estimate the prevalence of EALs in patients with irritable bowel syndrome (IBS) using the ACE questionnaire and determine the correlation of ACE score with GI symptoms.
Male and female IBS and healthy control subjects completed validated questionnaires measuring GI symptoms, EALs (ACE), and current psychological symptoms.
The main results of the study were: 1) A history of EALs (i.e., ACE score ≥1) was associated with significantly higher odds (2-fold) of having IBS, 2) IBS status was predicted by a history of emotional abuse and a mentally ill or incarcerated household member, and 3) ACE score significantly correlates with both overall IBS symptom and abdominal pain severity.
Acknowledgments
Grant support: NIH/NIDDK P50 DK064539, P30 DK 41301, T32 DK07180, L30 DK106759
Abbreviations
- IBS
irritable bowel syndrome
- EALs
early adverse life events
- ACE
adverse childhood experiences
- ETI-SR
Early Trauma Inventory Self Report-Short Form
- VSI
visceral sensitivity index
- HAD
Hospital anxiety and depression scale
- PHQ
Personal Health Questionnaire
- VSI
Visceral Sensitivity Index
- CDC
Centers for Disease Control and Prevention
- BSQ
Bowel Symptom Questionnaire
- OR
odds ratio
Footnotes
Disclosures: No potential conflicts of interest.
- Sarah H. Park: study concept and design, analysis and interpretation of data, drafting of the manuscript; critical revision of the manuscript for important intellectual content
- Elizabeth Videlock: drafting of the manuscript, critical revision and editing of the manuscript for important intellectual content
- Wendy Shih: acquisition of data, analysis and interpretation of data, statistical analysis, critical review and editing of manuscript
- Angela P. Presson: analysis and interpretation of data, critical review and editing of the manuscript
- Emeran Mayer: funding, critical revision of the manuscript for important intellectual content,
- Lin Chang: study concept and design, analysis and interpretation of data, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, funding, administrative and material support, study supervision
REFERENCES
- 1.Longstreth GF, et al. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Chitkara DK, et al. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol. 2008;103(3):765–74. doi: 10.1111/j.1572-0241.2007.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drossman DA, et al. Sexual and physical abuse in women with functional or organic gastrointestinal disorders. Ann Intern Med. 1990;113(11):828–33. doi: 10.7326/0003-4819-113-11-828. [DOI] [PubMed] [Google Scholar]
- 4.Bradford K, et al. Association between early adverse life events and irritable bowel syndrome. Clin Gastroenterol Hepatol. 2012;10(4):385–90. e1–3. doi: 10.1016/j.cgh.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talley NJ, et al. Gastrointestinal tract symptoms and self-reported abuse: a population-based study. Gastroenterology. 1994;107(4):1040–1049. doi: 10.1016/0016-5085(94)90228-3. [DOI] [PubMed] [Google Scholar]
- 6.Halland M, et al. A case-control study of childhood trauma in the development of irritable bowel syndrome. Neurogastroenterol Motil. 2014;26(7):990–8. doi: 10.1111/nmo.12353. [DOI] [PubMed] [Google Scholar]
- 7.Felitti VJ, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–58. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention [2015 June, 17];Behavioral Risk Factor Surveillance System. Available from: http://www.cdc.gov/brfss/index.html.
- 9.Gilbert LK, et al. Childhood adversity and adult chronic disease: an update from ten states and the District of Columbia, 2010. Am J Prev Med. 2015;48(3):345–9. doi: 10.1016/j.amepre.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Kagi R, Regala D. Translating the Adverse Childhood Experiences (ACE) Study into public policy: progress and possibility in Washington State. J Prev Interv Community. 2012;40(4):271–7. doi: 10.1080/10852352.2012.707442. [DOI] [PubMed] [Google Scholar]
- 11.Becker-Blease KA, Freyd JJ. Research participants telling the truth about their lives: the ethics of asking and not asking about abuse. Am Psychol. 2006;61(3):218–26. doi: 10.1037/0003-066X.61.3.218. [DOI] [PubMed] [Google Scholar]
- 12.Halpert A, et al. What patients know about irritable bowel syndrome (IBS) and what they would like to know. National Survey on Patient Educational Needs in IBS and development and validation of the Patient Educational Needs Questionnaire (PEQ). Am J Gastroenterol. 2007;102(9):1972–82. doi: 10.1111/j.1572-0241.2007.01254.x. [DOI] [PubMed] [Google Scholar]
- 13.Palsson OS, Whitehead WE. Psychological treatments in functional gastrointestinal disorders: a primer for the gastroenterologist. Clin Gastroenterol Hepatol. 2013;11(3):208–16. doi: 10.1016/j.cgh.2012.10.031. quiz e22-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craske MG, et al. A cognitive-behavioral treatment for irritable bowel syndrome using interoceptive exposure to visceral sensations. Behav Res Ther. 2011;49(6-7):413–21. doi: 10.1016/j.brat.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sikkema KJ, et al. Reductions in traumatic stress following a coping intervention were mediated by decreases in avoidant coping for people living with HIV/AIDS and childhood sexual abuse. J Consult Clin Psychol. 2013;81(2):274–83. doi: 10.1037/a0030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bremner JD, Bolus R, Mayer EA. Psychometric properties of the Early Trauma Inventory-Self Report. J Nerv Ment Dis. 2007;195:211–8. doi: 10.1097/01.nmd.0000243824.84651.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 18.Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64(2):258–66. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Labus JS, et al. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther. 2004;20:89–97. doi: 10.1111/j.1365-2036.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 20.Knight JR, et al. Family history of mental illness or alcohol abuse and the irritable bowel syndrome. J Psychosom Res. 2015;78(3):237–41. doi: 10.1016/j.jpsychores.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koloski NA, Talley NJ, Boyce PM. A history of abuse in community subjects with irritable bowel syndrome and functional dyspepsia: the role of other psychosocial variables. Digestion. 2005;72(2-3):86–96. doi: 10.1159/000087722. [DOI] [PubMed] [Google Scholar]
- 22.Saito YA, et al. Familial Aggregation of Irritable Bowel Syndrome: A Family Case- Control Study. Am J Gastroenterol. 2010;105(4):833–841. doi: 10.1038/ajg.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker EA, et al. Comorbidity of gastrointestinal complaints, depression, and anxiety in the Epidemiologic Catchment Area (ECA) Study. Am J Med. 1992;92(1A):26S–30S. doi: 10.1016/0002-9343(92)90133-v. [DOI] [PubMed] [Google Scholar]
- 24.Levy RL. Exploring the intergenerational transmission of illness behavior: from observations to experimental intervention. Ann Behav Med. 2011;41(2):174–82. doi: 10.1007/s12160-010-9254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali A, et al. Emotional abuse, self-blame, and self-silencing in women with irritable bowel syndrome. Psychosom Med. 2000;62(1):76–82. doi: 10.1097/00006842-200001000-00011. [DOI] [PubMed] [Google Scholar]
- 26.White DL, et al. Trauma history and risk of the irritable bowel syndrome in women veterans. Aliment Pharmacol Ther. 2010;32(4):551–61. doi: 10.1111/j.1365-2036.2010.04387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ringel Y, et al. Effect of abuse history on pain reports and brain responses to aversive visceral stimulation: An fMRI study. Gastroenterology. 2008;134(2):396–404. doi: 10.1053/j.gastro.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Ringel Y, et al. Regional brain activation in response to rectal distension in patients with irritable bowel syndrome and the effect of a history of abuse. Dig Dis Sci. 2003;48(9):1774–1781. doi: 10.1023/a:1025455330704. [DOI] [PubMed] [Google Scholar]
- 29.Gupta A, et al. Early adverse life events and resting state neural networks in patients with chronic abdominal pain: evidence for sex differences. Psychosom Med. 2014;76(6):404–12. doi: 10.1097/PSY.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talley NJ, Boyce PM, Jones M. Is the association between irritable bowel syndrome and abuse explained by neuroticism? A population based study. Gut. 1998;42(1):47–53. doi: 10.1136/gut.42.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Talley NJ, et al. Gastrointestinal tract symptoms and self-reported abuse: a population-based study. Gastroenterology. 1994;107(4):1040–9. doi: 10.1016/0016-5085(94)90228-3. [DOI] [PubMed] [Google Scholar]
- 33.Halpert A, Godena E. Irritable bowel syndrome patients' perspectives on their relationships with healthcare providers. Scand J Gastroenterol. 2011;46(7-8):823–30. doi: 10.3109/00365521.2011.574729. [DOI] [PubMed] [Google Scholar]
- 34.Stewart M, et al. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49(9):796–804. [PubMed] [Google Scholar]
- 35.Creed F, et al. Reported sexual abuse predicts impaired functioning but a good response to psychological treatments in patients with severe irritable bowel syndrome. Psychosom Med. 2005;67(3):490–499. doi: 10.1097/01.psy.0000163457.32382.ac. [DOI] [PubMed] [Google Scholar]
- 36.Drossman DA, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125(1):19–31. doi: 10.1016/s0016-5085(03)00669-3. [DOI] [PubMed] [Google Scholar]
- 37.Lackner JM, et al. How does cognitive behavior therapy for irritable bowel syndrome work? A mediational analysis of a randomized clinical trial. Gastroenterology. 2007;133(2):433–444. doi: 10.1053/j.gastro.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.