Abstract
Every four to five days, intestinal epithelial cells (IEC) are terminated as they reach the end of their life. This process ensures that the epithelium is comprised of the fittest cells that maintain an impermeable barrier to luminal contents and the gut microbiota, as well as the most metabolically able cells that conduct functions in nutrient absorption, digestion, and secretion of antimicrobial peptides. IEC are terminated by apical extrusion – or shedding – from the intestinal epithelial monolayer into the gut lumen. Whether death by apoptosis signals extrusion or death follows expulsion by younger IEC has been a matter of debate. Seemingly a minor detail, IEC death before or after apical extrusion bears weight on the potential contribution of apoptotic IEC to intestinal homeostasis as a consequence of their recognition by intestinal lamina propria phagocytes. In inflammatory bowel disease (IBD), excessive death is observed in the ileal and colonic epithelium. The precise mode of IEC death in IBD is not defined. A highly inflammatory milieu within the intestinal lamina propria, rich in the pro-inflammatory cytokine TNF-α, increases IEC shedding and compromises barrier integrity fueling more inflammation. A milestone in the treatment of IBD, anti-TNF-α therapy, may promote mucosal healing by reversing increased and inflammation-associated IEC death. Understanding the biology and consequences of cell death in the intestinal epithelium is critical to the design of new avenues for IBD therapy.
Keywords: apoptosis, intestinal epithelial cell, Tumor necrosis factor, inflammatory bowel disease, intestinal tolerance
Graphical abstract
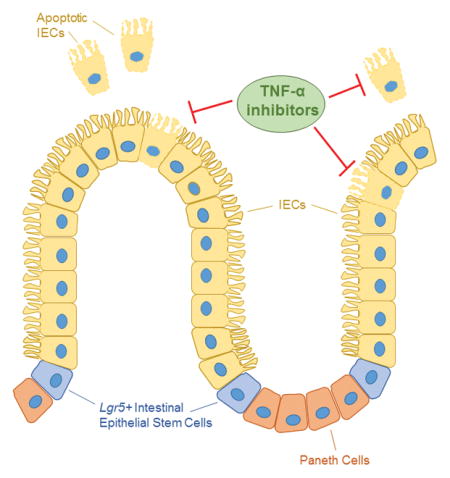
High levels of apoptosis are detected in the intestinal epithelium of inflammatory bowel disease (IBD) patients. This increased level of epithelial intestinal cell (IEC) death disrupts barrier integrity and leads to inflammation, but whether cell death is a symptom or cause of IBD is unknown. Although TNF-α inhibitors help restore barrier function in some patients, additional research on the mechanism of IEC death and how it goes awry in IBD is needed.
The intestinal epithelium
The intestinal epithelium consists of a single layer of intestinal epithelial cells (IEC) shaped into invaginations that form the crypts of Lieberkühn and luminal protrusions that form the characteristic villi of the small intestine. Lgr5+ stem cells reside at the base of the intestinal crypts [1]. These stem cells divide to generate precursors of secretory cells and enterocytes that proliferate and differentiate as they move upwards away from the crypt and towards the villus tip in the small intestine or luminal face of the crypt in the large intestine [1, 2]. Precursors differentiate into various types of specialized cells. Absorptive enterocytes, marked by alkaline phosphatase intestinal (Alpi) gene expression, are specialized in metabolic and digestive functions. Rare Tuft cells are taste-chemosensory epithelial cells that also serve as sentinels of type 2 immunity in response to parasites [3], and specialized microfold (M) cells cover lymphoid aggregates in the ileum called Peyer’s patches and transport luminal antigens to lymphoid cells [2]. Additional specialized lineages of secretory IEC maintain the digestive or barrier functions of the epithelium. These lineages include entero-endocrine cells that secrete hormone regulators of digestive function, goblet cells that secrete mucus (MUC2) into the lumen, and Paneth cells at the crypt bottom that secrete antimicrobial proteins such as the C-type lectin regenerating islet-derived protein IIIγ (REGIIIγ), α-defensins, cathelicidins and lysozyme [4–8]. Besides stem cells, Paneth cells are excluded from the upward migration during their life cycle [9]. The collective action of mucus and antimicrobial proteins establishes a physical and biochemical barrier against the luminal microbiota, precluding their adhesion to the epithelial surface and their translocation into the lamina propria where immune cells reside.
Genetic fate mapping of alkaline phosphate intestinal (Alpi)+ enterocyte progenitors using tamoxifen-inducible Cre recombinase knocked into the Alpi gene showed reporter activity first in the upper crypt followed by the villus domain, higher regions of the villus by day 2, the villus tip by day 3, and finally disappearance by days 4–5 implying shedding into the lumen [10]. This kinetic pattern reflects the 4–5 day life cycle of an IEC [2, 11]. Notably, these studies also revealed that enterocyte precursors could also serve as a reservoir of potential stem cells by dedifferentiating into Lgr5+ stem cells under conditions of injury where rapid crypt regeneration is required [10].
Termination of an intestinal epithelial cell at the end of its life cycle
Besides renewal from stem cells in the crypts, turnover of IEC is also regulated by loss of senescent cells from the epithelial monolayer. In humans, approximately 1010 IEC are shed every day [12, 13]. The rapid nature of the process precludes its visualization unless special handling, slicing and fixation of intestinal tissues are applied [14]. IEC loss occurs predominantly at the villus tip in the small intestine or luminal surface of the colon, and has historically been viewed as passive shedding [11]. Apoptosis as a mechanism of shedding emerged with studies showing the presence of cells positive for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) at the tips of the villi in rodent and human intestinal sections [15–17]. Earlier studies had reported a small level of spontaneous cell death in the crypts with morphology typical of apoptosis [18, 19], but the villus tips contained only rare apoptotic cells [20, 21]. Apoptosis is a form of non-inflammatory cell death that accounts for loss of large numbers of cells during development and tissue remodeling [22]. Other studies in human and guinea pig intestinal epithelium presented transmission and scanning electron microcopy evidence for apoptotic enterocytes in the act of being shed – and interestingly apoptotic IEC appeared surrounded, and in some cases phagocytosed, by subepithelial macrophages [17, 23, 24]. These early studies set the stage for debates to come decades later as to whether apoptosis precedes or follows IEC shedding. Cell death following IEC shedding can occur when live cells are extruded as a consequence of overcrowding due to proliferation and upward migration of new IECs along the crypt-villus axis [25]. Loss of cell-substratum adhesion in this case leads to anoikis [26]. In the human intestinal epithelium, 5.3% of villus sections were reported to contain a shedding cell, and the majority of these cells stained positively for activated caspase-3, a marker for apoptosis, as well as a caspase-cleaved product of cytokeratin 18 [14]. Shedding IEC here were not found to be associated with macrophages [14]. Because late stage apoptotic cells were observed only in the crypt, it was suggested that IEC are shed as soon as they become apoptotic [14]. In healthy subjects with no symptoms or endoscopic findings, colonic sections showed TUNEL+ cells mainly at the surface and not crypt epithelium [27].
In vitro studies have elucidated the mechanisms of apoptotic cell extrusion from epithelial monolayers. These studies have shown that apoptotic cells produce the bioactive lipid sphingosine-1-phosphate (S1P), which signals neighboring cells through S1P2 receptor to form and activate Rho-associated kinase (ROCK)-mediated contraction of an actin/myosin ring at the interface between the apoptotic cell and neighboring cells, and leading to apoptotic cell extrusion while maintaining barrier function [25, 28, 29]. In fact, ROCK is cleaved and activated by caspase-3 during apoptosis in order to enable phosphorylation of myosin light chain (MLC), which is necessary for membrane blebbing in apoptotic cells [30, 31]. Over 50% of shedding events observed in the human small intestine were associated with phosphorylated MLC [14]. Also of note, actin/myosin contraction preceded pro-caspase activation or phosphatidylserine exposure [29], an eat-me signal on apoptotic cells [32], making it difficult to accurately assess whether a cell is ‘alive and well’ or already committed to apoptosis prior to extrusion. Finally, the downward contraction of actin and myosin IIA at the basolateral surface along with basolateral targeting of the RHO guanine nucleotide exchange factor 1 (p115RhoGEF) favors the predominant apical as opposed to basal direction of extrusion [33].
Extrusion of live IEC serves as another mechanism of regulating the size of the IEC population. Live IEC extrusion requires stretch activated ion channels that are activated by stress and presumably function upstream of SIP that acts on Rho to mediate extrusion. Extruded cells subsequently undergo anoikis [26], but as mentioned above apoptosis may have already begun prior to extrusion and may have signaled extrusion itself. The aged IEC (live or apoptotic) are shed from the intestinal epithelial monolayer into the lumen, and tight junction protein reorganization beneath the shedding cell ensures that lamellopodia from the neighboring cell come together in a process that was likened to a “zipper” being drawn up, thereby maintaining the epithelial barrier at the shedding site [34].
Basal extrusion is common in the embryonic epithelia of Drosophila unlike in vertebrates [35–37]. Cancer cells exploit basal extrusion to invade and metastasize [38]. Loss or mutation of the tumor suppressor adenomatous polyposis coli (APC) switches cells from apical to basal extrusion [39]. Oncogenic K-Ras enables cells to extrude basally by autophagy-mediated degradation of S1P and disruption of signaling required for apical extrusion [40]. Induction of IEC shedding with a high dose of TNF-α (to mimic pathological conditions) combined with live imaging of mice transgenic for enhanced green fluorescent protein-occludin and red fluorescent protein-1-ZO-1, revealed only apically-oriented IEC shedding that required active basolateral redistribution of these tight junction proteins [41]. Myosin motor activity and microtubules were required to initiate extrusion, and its completion required microfilament remodeling and the activities of ROCK, myosin light chain kinase (MLCK), and dynamin II [41]. The combined activity of these factors maintained epithelial barrier function [41]. Detectable caspase-3 cleavage was reported after tight junction and microtubule protein remodeling in shedding cells [41]. The initiation and progression of IEC extrusion required caspase activity, as it was sensitive to the pan-caspase inhibitor Q-VD-OPH, which did not prevent MLC phosphorylation. Detection of caspase-3 cleavage after microtubule reorganization may reflect S1P production as the first signal from apoptotic epithelial cells that initiates shedding, consistent with the finding discussed above [29]. Collectively, the evidence argues that IEC extrusion from the intestinal epithelium is initiated by signals from cells that begin to undergo apoptosis – while the cells are still anchored – rather than the induction of apoptosis after the cells have been extruded (anoikis).
Evidence from mice deficient for the initiator caspase-8 specifically in IEC has been considered to challenge the concept that apoptosis is essential for intestinal epithelial turnover [42, 43]. Indeed, the inability of IEC to undergo apoptosis in these mice did not lead to obvious histological or morphometric abnormalities in the intestinal epithelium at steady state [42]. Notably, however, the mice developed spontaneous ileitis [42]. On the other hand, spontaneous colitis was observed with 100% penetrance upon IEC-specific deletion of the TNF receptor-associated adaptor FADD (FADDIEC-KO mice) [44], a protein required for death receptor induced apoptosis [45]. Here, 50% of FADDIEC-KO mice died before weaning while surviving mice suffered from intestinal disease [44]. Importantly, in both studies, when apoptosis was impaired either by deletion of caspase-8 or FADD, IEC still underwent cell death albeit through an alternate program of cell death [42, 44]. Instead of apoptosis, RIPK3 and CYLD dependent necroptosis, an inflammatory form of cell death [46], was mobilized and was responsible for triggering colitis in FADDIEC-KO mice [44]. These results show that apoptosis is indeed not essential for intestinal epithelial turnover per se; IEC destined to die will undergo cell death even when key mediators of the apoptotic program of cell death are absent. However, these results strongly suggest that in maintaining a normal size of the IEC population, IEC death specifically by apoptosis may additionally serve a critical role in preserving intestinal homeostasis. Therefore, blockade of apoptosis within senescent IEC switches their death to necroptosis, which by its inflammatory nature leads to the spontaneous development of intestinal disease (either colitis or ileitis upon IEC-specific deletion of FADD or caspase-8, respectively).
Death of intestinal epithelial cells in inflammatory bowel disease
Intestinal epithelial damage is characteristic of inflammatory bowel disease (IBD) [43, 47–52]. IBD is a chronic inflammation of the intestinal tract and comprises Crohn’s disease (CD) and ulcerative colitis (UC) [53–57]. Its pathogenesis involves complex interactions among genetic susceptibility factors, the gut microbiota, and the mucosal immune compartment [53, 58–63]. Intestinal damage in IBD manifests in a notable increase in programmed cell death of IEC [27, 43, 48, 51, 64–66]. High levels of cell death are reported in the epithelium of both patients with UC or CD [27, 43, 49, 64, 65, 67, 68].
Increased apoptosis has been reported in the epithelium of patients with ulcerative colitis (UC) [27, 68]. Apoptotic indices, representing the ratio of TUNEL+ IEC to total IEC, were significantly higher in UC patients compared to healthy controls but not compared to intestinal sections from patients with infectious colitis [27]. TUNEL+ IEC were frequently distributed at the surface rather than crypt epithelium [27]. Patients with active UC who ultimately require surgery had higher apoptotic indices and CD68 staining, which marks macrophages, than UC patients who were receiving medication [27]. More epithelial cells with markers of apoptosis – including TUNEL positivity, Fas (CD95) and Fas ligand expression as well as DNA laddering and morphological features of apoptosis by electron microscopy – were found in involved and adjacent uninvolved areas of untreated patients with active UC than in the crypts of the normal colon [68]. Increased numbers of TUNEL+ IEC have also been reported in inflamed compared to uninflamed areas of ileal and colonic biopsy specimens from CD patients [64]. Electron microscopy on rectal biopsies of patients with CD and UC compared with normal controls showed morphology characterized as patchy necrosis in four out of seven of the CD patients [65]. RIPK3, associated with necroptosis, is notably expressed at high levels in the terminal ileum of patients with CD [42]. IEC necroptosis is thought to underlie the microerosions and epithelial gaps observed in mice and humans by in vivo imaging [69, 70].
An association between apoptosis and IBD has also been found in epithelial cell proteome studies in both IBD patients and IBD mouse models [71, 72]. A combination of 2-dimensional SDS-PAGE, MALDI-TOF mass spectrometry and Western blots was used to analyze protein expression profiles of primary IEC isolated from inflamed versus uninflamed areas of ileal or colonic tissues from patients with CD and UC, as well as patients with colorectal carcinoma (the latter served as healthy controls) [72]. Proteomic profiles revealed 21 proteins with at least 2-fold changes compared to control samples. Of these, 9 proteins achieved statistical significance of which Rho-GDP dissociation inhibitor α was common to both UC and CD samples [72]. Increased expression of this protein was associated with IEC damage. When comparing inflamed versus uninflamed IEC samples, 40 proteins with significantly altered expression levels were identified of which programmed cell death protein 8 (also known as apoptosis inducing factor AIF – a mitochondrial pro-apoptotic factor [73]) and Annexin A2 (a possible therapeutic target for refractory UC [73]) were notable and showed the highest fold changes [72]. There were also significant changes in proteins with functions in signal transduction, stress response, and energy metabolism especially changes in glycolysis and Tricarboxylic acid (TCA) cycle [72]. A similar approach was used on IEC isolated from Il10−/− mice that had been monocolonized with the colitogenic Enterococcus faecalis [71]. Il10−/− mice are susceptible to chronic intestinal inflammation [74]. 76 target proteins were identified that had to do with endoplasmic reticulum stress, energy metabolism and apoptosis [71]. Combined, these studies paint a stressed and dying picture of IEC in IBD and under conditions of chronic inflammation.
The exact nature and role of IEC death in the pathogenesis of IBD has not been clarified to date. Whether it is causative or secondary to the chronic inflammation is not known. The consequences of IEC death in IBD patients would be predicted to be two-fold. First, IEC death would disrupt barrier integrity leading to alterations in the quality and quantity of epithelium-derived factors and anti-microbial peptides, as well as translocation of the commensal microbiota into the intestinal lamina propria. Second, it leads to inflammation, and increased levels of inflammatory cytokines such as tumor-necrosis factor-α (TNF-α) [60].
Increased systemic and intestinal tissue levels of TNF-α in IBD patients [60, 75] along with IBD risk alleles associated with TNF signaling (RELA, NFKB1, TNFAIP3) that have been identified through genome wide association studies (GWAS), point to a critical role for TNF-α in IBD [76]. Deletion of the TNF-α AU-rich elements (ARE) in mice results in increased steady state levels of TNF-α mRNA and chronic overproduction of TNF-α [77]. TNFΔARE mice develop chronic inflammatory arthritis, but also inflammatory changes resembling human CD with alterations localized primarily to the terminal ileum and on occasion the proximal colon [77]. The impact of TNF-α on the intestinal epithelial response is complex and depends on its levels and poorly understood secondary signals. TNF-α has many roles in supporting survival and inflammation [78]. TNF-α enhances expression of the polymeric immunoglobulin receptor (pIgR) that transports secretory IgA across IEC [79]. Homeostatic patterns of bacterial secretory IgA coating are altered with the emergence of highly secretory IgA coated bacteria, which marks the most colitogenic species [80].
TNF-α can also induce extrinsic caspase-8 and executioner caspase-3 dependent apoptosis as well as RIPK3-dependent necroptosis under conditions where the activities of caspase-8 or TNFAIP3 (A20, a ubiquitin editing enzyme) are impaired [42, 81–85]. To date, no involvement has been reported for necroptosis related genes as IBD susceptibility loci. However, these proteins could be post-transcriptionally regulated. IEC shedding can also be induced by TNF-α [60, 75, 78, 86]. Unlike homeostatic IEC shedding where barrier integrity is maintained by rapid basolateral tight-junction protein redistribution and zipper-like replacement by neighboring cells [34, 41], TNF-α-induced shedding has been reported to be accompanied by necroptosis where multiple adjacent IEC lose contact [70, 75]. Collectively, increased IEC death and loss of barrier integrity would drive further inflammation, more damage to the intestinal epithelium and perhaps even dysbiosis, making it difficult to distinguish cause from effect.
Potential impact of anti-TNF-α therapy on death of intestinal epithelial cells
TNF-α levels in IBD patients directly correlate with clinical manifestations and disease severity. Antibodies targeting TNF-α represent the most successful clinical treatment for IBD to date inducing intestinal healing and improving long-term patient outcomes [87–94]. In SAMP1/YitFc mice, an animal model of spontaneous CD-like ileitis, anti-TNF-α normalizes the increased inflammatory IEC death in these mice [95]. Anti-TNF-α has also been reported to downregulate IEC death and restore the epithelium barrier in a subset of CD patients undergoing anti-TNF-α therapy [96, 97]. In the clinic, a subgroup of patients does not respond to anti-TNF-α therapy within 4–12 weeks after initiation of therapy [98–100]. These patients show little or no changes of clinical symptoms and no macroscopic mucosal healing upon anti-TNF-α therapy but are potentially exposed to side effects of this type of therapy, such as infections, reactivation of tuberculosis, allergic reactions, skin or demyelinating disorders, and lupus-like autoimmunity [101]. Anti-TNF-α therapy may fail due to TNF-α-independent gut inflammation, insufficient dosing [102], or development of anti-drug antibodies in 37–61% of patients that ultimately lead to suboptimal serum levels of the biotherapeutic [103, 104]. The precise mechanisms of action of anti-TNF-α in IBD are not well defined. Apoptosis of lamina propria T cells that mediate intestinal inflammation is proposed to be the main mechanism [105–109] blocking the interaction between membrane-bound TNF-α on macrophages and TNF-α receptor TNFR2 on T cells. Besides T cells, IEC express both TNF-α receptors, TNFR1 and TNFR2, and can also serve as a target of TNF-α. The anti-TNF-α benefits that have been reported in some responder patients include fistula closure in CD [90, 110] and mucosal healing in both CD and UC [89, 111]. Mucosal healing has emerged as a new therapeutic goal and an important correlate of long periods of clinical remission [87–94]. In that respect, how anti-TNF-α therapy impacts IEC death and healing of the intestinal epithelium has not been studied extensively. In patients undergoing anti-TNF-α therapy, a significant decrease in the levels of IEC apoptosis has been noted suggesting that TNF neutralization may rescue IEC from premature death [64, 96]. Gaining a better understanding of the mechanisms for how anti-TNF-α therapy works and why it fails is essential.
Implications of intestinal epithelial cell death for immune-mediated intestinal homeostasis
Despite the fact that apoptosis is a normal component of the physiology of the intestine, its impact on the immune mechanisms of gut homeostasis is poorly understood. Perhaps a major reason for this is the paradigm that IEC are merely shed into the gut lumen as part of natural intestinal epithelial turnover and with no consequence on immune homeostasis. However, as discussed here, IEC begin to signal their extrusion, for example by producing S1P, at very early stages of apoptosis and before many of the characteristic markers of apoptosis become detectable. Even if the majority of IEC are extruded live (and undergo apoptosis post shedding), and only a small fraction of IEC initiate apoptosis as a prelude to shedding, the sheer number of IEC in the order of 1010 that are shed daily makes this small fraction quite substantial. Intestinal homeostasis is maintained through innate and adaptive functions of intestinal lamina propria dendritic cells and macrophages [58], modulation by CD4 T helper 17 and regulatory T cell (TREG) cytokines such as IL-17, IL-22, IL-10 and TGF-β [112–120], and innate lymphoid cells (ILC), particularly IL-22-producing ILC3, which maintain epithelial barrier integrity and mediate an early repair response to the damaged epithelium [121, 122]. How IEC apoptosis under healthy steady state conditions impacts gut homeostasis is an important question that has been little explored. Clearance of apoptotic cells by professional phagocytes is known to induce immune tolerance [123, 124]. While most studies have focused on intestinal phagocyte sampling of commensals and pathogens [125–130], only one study in rats has examined phagocyte sampling of apoptotic IEC [131]. Interestingly, disruption of apoptotic cell uptake in mice deficient for the phagocytic receptor BAI1 made these mice more susceptible to colitis and conversely, mice overexpressing BAI1 had attenuated disease [132]. Surprisingly, BAI1 mediated effects did not appear to be dependent on phagocytes such as macrophages, but rather BAI1 expression in colonic epithelial cells, which engulf apoptotic cells within the colon, was sufficient to dampen colitis [132]. A different study has shown that phosphatidylserine on apoptotic IEC, acting through the inhibitory receptor CD300, suppresses commensal driven IFN-β production by intestinal lamina propria CD11c+CD11b+CX3CR1+CD103− dendritic cells, and consequently IFN-β dependent TREG cell proliferation [133]. Here, CD300a+CD11c+ cells were found in proximity to apoptotic IEC [133]. Further investigations are a must in gaining a full understanding of the mechanisms by which apoptotic IEC contribute to intestinal tolerance and homeostasis. The knowledge we gain will provide additional much needed avenues for therapeutic intervention and prevention in IBD.
Acknowledgments
The studies cited in this review are not inclusive of the vast literature on cell death in the intestinal epithelium. Readers are encouraged to also refer to the review articles cited here and references therein in order to gain a full appreciation of different and additional aspects of the biology not discussed here. J.M.B. and her laboratory are supported by NIH grants DK072201, AI095245 and AI123284, the Burroughs Wellcome Fund, and a Leukemia and Lymphoma Society Scholar Award.
Abbreviations
- IEC
intestinal epithelial cells
- IBD
inflammatory bowel disease
- TNFa (a in symbol as alpha)
Tumor necrosis factor alpha
- M cells
microfold cells
- MUC2
mucus 2
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- S1P
sphingosine 1 phosphate
- MLC
myosin light chain
- ROCK
Rho-associated kinase
- p115 RhoGEF
RHO guanine nucleotide exchange factor 1
- APC
adenomatous polyposis coli
- MLCK
myosin light chain kinase
- FADD
Fas-associated via death domain
- RIPK3
receptor interacting protein kinase 3
- CYLD
Cylindromatosis (turban tumor syndrome)
- CD
Crohn’s disease
- UC
ulcerative colitis
- TCA cycle
Tricarboxylic acid (TCA) cycle
- GWAS
genome wide association studies
- ARE
AU-rich elements
- pIgR
polymeric immunoglobulin receptor
- TNFAIP3
Tumor necrosis factor, alpha-induced protein 3, also known as A20
- TNFR2
TNF-a (symbol alpha) receptor 2
References
- 1.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–84. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 2.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annual review of physiology. 2009;71:241–60. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 3.Howitt MR, Lavoie S, Michaud M, Blum AM, Tran SV, Weinstock JV, Gallini CA, Redding K, Margolskee RF, Osborne LC, Artis D, Garrett WS. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science. 2016;351:1329–33. doi: 10.1126/science.aaf1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gutzeit C, Magri G, Cerutti A. Intestinal IgA production and its role in host-microbe interaction. Immunol Rev. 2014;260:76–85. doi: 10.1111/imr.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fung TC, Artis D, Sonnenberg GF. Anatomical localization of commensal bacteria in immune cell homeostasis and disease. Immunol Rev. 2014;260:35–49. doi: 10.1111/imr.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–53. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 7.Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. 2012;12:503–16. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Current gastroenterology reports. 2010;12:319–30. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–8. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tetteh PW, Basak O, Farin HF, Wiebrands K, Kretzschmar K, Begthel H, van den Born M, Korving J, de Sauvage F, van Es JH, van Oudenaarden A, Clevers H. Replacement of Lost Lgr5-Positive Stem Cells through Plasticity of Their Enterocyte-Lineage Daughters. Cell stem cell. 2016;18:203–13. doi: 10.1016/j.stem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Mayhew TM, Myklebust R, Whybrow A, Jenkins R. Epithelial integrity, cell death and cell loss in mammalian small intestine. Histology and histopathology. 1999;14:257–67. doi: 10.14670/HH-14.257. [DOI] [PubMed] [Google Scholar]
- 12.Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–20. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 13.Watson AJ. Apoptosis and colorectal cancer. Gut. 2004;53:1701–9. doi: 10.1136/gut.2004.052704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bullen TF, Forrest S, Campbell F, Dodson AR, Hershman MJ, Pritchard DM, Turner JR, Montrose MH, Watson AJ. Characterization of epithelial cell shedding from human small intestine. Laboratory investigation; a journal of technical methods and pathology. 2006;86:1052–63. doi: 10.1038/labinvest.3700464. [DOI] [PubMed] [Google Scholar]
- 15.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. The Journal of cell biology. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107(Pt 12):3569–77. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 17.Shibahara T, Sato N, Waguri S, Iwanaga T, Nakahara A, Fukutomi H, Uchiyama Y. The fate of effete epithelial cells at the villus tips of the human small intestine. Archives of histology and cytology. 1995;58:205–19. doi: 10.1679/aohc.58.205. [DOI] [PubMed] [Google Scholar]
- 18.Potten CS. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature. 1977;269:518–21. doi: 10.1038/269518a0. [DOI] [PubMed] [Google Scholar]
- 19.Potten CS. The significance of spontaneous and induced apoptosis in the gastrointestinal tract of mice. Cancer metastasis reviews. 1992;11:179–95. doi: 10.1007/BF00048063. [DOI] [PubMed] [Google Scholar]
- 20.Potten CS, Booth C, Pritchard DM. The intestinal epithelial stem cell: the mucosal governor. International journal of experimental pathology. 1997;78:219–43. doi: 10.1046/j.1365-2613.1997.280362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potten CS, Allen TD. Ultrastructure of cell loss in intestinal mucosa. Journal of ultrastructure research. 1977;60:272–7. doi: 10.1016/s0022-5320(77)80071-3. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Garijo A, Steller H. Spreading the word: non-autonomous effects of apoptosis during development, regeneration and disease. Development. 2015;142:3253–62. doi: 10.1242/dev.127878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han H, Iwanaga T, Uchiyama Y, Fujita T. Aggregation of macrophages in the tips of intestinal villi in guinea pigs: their possible role in the phagocytosis of effete epithelial cells. Cell Tissue Res. 1993;271:407–16. doi: 10.1007/BF02913723. [DOI] [PubMed] [Google Scholar]
- 24.Iwanaga T, Han H, Adachi K, Fujita T. A novel mechanism for disposing of effete epithelial cells in the small intestine of guinea pigs. Gastroenterology. 1993;105:1089–97. doi: 10.1016/0016-5085(93)90953-a. [DOI] [PubMed] [Google Scholar]
- 25.Eisenhoffer GT, Loftus PD, Yoshigi M, Otsuna H, Chien CB, Morcos PA, Rosenblatt J. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature. 2012;484:546–9. doi: 10.1038/nature10999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frisch SM, Screaton RA. Anoikis mechanisms. Current opinion in cell biology. 2001;13:555–62. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 27.Hagiwara C, Tanaka M, Kudo H. Increase in colorectal epithelial apoptotic cells in patients with ulcerative colitis ultimately requiring surgery. Journal of gastroenterology and hepatology. 2002;17:758–64. doi: 10.1046/j.1440-1746.2002.02791.x. [DOI] [PubMed] [Google Scholar]
- 28.Gu Y, Forostyan T, Sabbadini R, Rosenblatt J. Epithelial cell extrusion requires the sphingosine-1-phosphate receptor 2 pathway. The Journal of cell biology. 2011;193:667–76. doi: 10.1083/jcb.201010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenblatt J, Raff MC, Cramer LP. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Current biology: CB. 2001;11:1847–57. doi: 10.1016/s0960-9822(01)00587-5. [DOI] [PubMed] [Google Scholar]
- 30.Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nature cell biology. 2001;3:339–45. doi: 10.1038/35070009. [DOI] [PubMed] [Google Scholar]
- 31.Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nature cell biology. 2001;3:346–52. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- 32.Penberthy KK, Ravichandran KS. Apoptotic cell recognition receptors and scavenger receptors. Immunol Rev. 2016;269:44–59. doi: 10.1111/imr.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slattum G, McGee KM, Rosenblatt J. P115 RhoGEF and microtubules decide the direction apoptotic cells extrude from an epithelium. The Journal of cell biology. 2009;186:693–702. doi: 10.1083/jcb.200903079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madara JL. Maintenance of the macromolecular barrier at cell extrusion sites in intestinal epithelium: physiological rearrangement of tight junctions. The Journal of membrane biology. 1990;116:177–84. doi: 10.1007/BF01868675. [DOI] [PubMed] [Google Scholar]
- 35.Vidal M, Larson DE, Cagan RL. Csk-deficient boundary cells are eliminated from normal Drosophila epithelia by exclusion, migration, and apoptosis. Dev Cell. 2006;10:33–44. doi: 10.1016/j.devcel.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Gibson MC, Perrimon N. Extrusion and death of DPP/BMP-compromised epithelial cells in the developing Drosophila wing. Science. 2005;307:1785–9. doi: 10.1126/science.1104751. [DOI] [PubMed] [Google Scholar]
- 37.Ninov N, Chiarelli DA, Martin-Blanco E. Extrinsic and intrinsic mechanisms directing epithelial cell sheet replacement during Drosophila metamorphosis. Development. 2007;134:367–79. doi: 10.1242/dev.02728. [DOI] [PubMed] [Google Scholar]
- 38.Slattum GM, Rosenblatt J. Tumour cell invasion: an emerging role for basal epithelial cell extrusion. Nature reviews Cancer. 2014;14:495–501. doi: 10.1038/nrc3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marshall TW, Lloyd IE, Delalande JM, Nathke I, Rosenblatt J. The tumor suppressor adenomatous polyposis coli controls the direction in which a cell extrudes from an epithelium. Molecular biology of the cell. 2011;22:3962–70. doi: 10.1091/mbc.E11-05-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slattum G, Gu Y, Sabbadini R, Rosenblatt J. Autophagy in oncogenic K-Ras promotes basal extrusion of epithelial cells by degrading S1P. Current biology: CB. 2014;24:19–28. doi: 10.1016/j.cub.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marchiando AM, Shen L, Graham WV, Edelblum KL, Duckworth CA, Guan Y, Montrose MH, Turner JR, Watson AJ. The epithelial barrier is maintained by in vivo tight junction expansion during pathologic intestinal epithelial shedding. Gastroenterology. 2011;140:1208–1218. e1–2. doi: 10.1053/j.gastro.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gunther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF, Becker C. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–9. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gunther C, Neumann H, Neurath MF, Becker C. Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut. 2013;62:1062–71. doi: 10.1136/gutjnl-2011-301364. [DOI] [PubMed] [Google Scholar]
- 44.Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernandez-Majada V, Ermolaeva M, Kirsch P, Sterner-Kock A, van Loo G, Pasparakis M. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477:330–4. doi: 10.1038/nature10273. [DOI] [PubMed] [Google Scholar]
- 45.Wilson NS, Dixit V, Ashkenazi A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nat Immunol. 2009;10:348–55. doi: 10.1038/ni.1714. [DOI] [PubMed] [Google Scholar]
- 46.Oberst A. Death in the fast lane: what’s next for necroptosis? The FEBS journal. 2015 doi: 10.1111/febs.13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Negroni A, Cucchiara S, Stronati L. Apoptosis, Necrosis, and Necroptosis in the Gut and Intestinal Homeostasis. Mediators of inflammation. 2015;2015:250762. doi: 10.1155/2015/250762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nunes T, Bernardazzi C, de Souza HS. Cell death and inflammatory bowel diseases: apoptosis, necrosis, and autophagy in the intestinal epithelium. Bio Med research international. 2014;2014:218493. doi: 10.1155/2014/218493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watson AJ. Necrosis and apoptosis in the gastrointestinal tract. Gut. 1995;37:165–7. doi: 10.1136/gut.37.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edelblum KL, Yan F, Yamaoka T, Polk DB. Regulation of apoptosis during homeostasis and disease in the intestinal epithelium. Inflamm Bowel Dis. 2006;12:413–24. doi: 10.1097/01.MIB.0000217334.30689.3e. [DOI] [PubMed] [Google Scholar]
- 51.Ramachandran A, Madesh M, Balasubramanian KA. Apoptosis in the intestinal epithelium: its relevance in normal and pathophysiological conditions. Journal of gastroenterology and hepatology. 2000;15:109–20. doi: 10.1046/j.1440-1746.2000.02059.x. [DOI] [PubMed] [Google Scholar]
- 52.Renehan AG, Bach SP, Potten CS. The relevance of apoptosis for cellular homeostasis and tumorigenesis in the intestine, Canadian journal of gastroenterology = Journal canadien de gastroenterologie. 2001;15:166–76. doi: 10.1155/2001/164727. [DOI] [PubMed] [Google Scholar]
- 53.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–78. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590–605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 55.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713–25. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 56.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–17. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 58.Gross M, Salame TM, Jung S. Guardians of the Gut - Murine Intestinal Macrophages and Dendritic Cells. Frontiers in immunology. 2015;6:254. doi: 10.3389/fimmu.2015.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGovern DP, Kugathasan S, Cho JH. Genetics of Inflammatory Bowel Diseases. Gastroenterology. 2015;149:1163–1176. e2. doi: 10.1053/j.gastro.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–42. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 61.Peters CP, Mjosberg JM, Bernink JH, Spits H. Innate lymphoid cells in inflammatory bowel diseases. Immunology letters. 2015 doi: 10.1016/j.imlet.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 62.Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 63.Sartor RB, Muehlbauer M. Microbial host interactions in IBD: implications for pathogenesis and therapy. Current gastroenterology reports. 2007;9:497–507. doi: 10.1007/s11894-007-0066-4. [DOI] [PubMed] [Google Scholar]
- 64.Di Sabatino A, Ciccocioppo R, Luinetti O, Ricevuti L, Morera R, Cifone MG, Solcia E, Corazza GR. Increased enterocyte apoptosis in inflamed areas of Crohn’s disease. Diseases of the colon and rectum. 2003;46:1498–507. doi: 10.1007/s10350-004-6802-z. [DOI] [PubMed] [Google Scholar]
- 65.Dourmashkin RR, Davies H, Wells C, Shah D, Price A, O’Morain C, Levi J. Epithelial patchy necrosis in Crohn’s disease. Human pathology. 1983;14:643–8. doi: 10.1016/s0046-8177(83)80207-x. [DOI] [PubMed] [Google Scholar]
- 66.Chen L, Park SM, Turner JR, Peter ME. Cell death in the colonic epithelium during inflammatory bowel diseases: CD95/Fas and beyond. Inflamm Bowel Dis. 2010;16:1071–6. doi: 10.1002/ibd.21191. [DOI] [PubMed] [Google Scholar]
- 67.Souza HS, Tortori CJ, Castelo-Branco MT, Carvalho AT, Margallo VS, Delgado CF, Dines I, Elia CC. Apoptosis in the intestinal mucosa of patients with inflammatory bowel disease: evidence of altered expression of FasL and perforin cytotoxic pathways. International journal of colorectal disease. 2005;20:277–86. doi: 10.1007/s00384-004-0639-8. [DOI] [PubMed] [Google Scholar]
- 68.Iwamoto M, Koji T, Makiyama K, Kobayashi N, Nakane PK. Apoptosis of crypt epithelial cells in ulcerative colitis. The Journal of pathology. 1996;180:152–9. doi: 10.1002/(SICI)1096-9896(199610)180:2<152::AID-PATH649>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 69.Kiesslich R, Goetz M, Angus EM, Hu Q, Guan Y, Potten C, Allen T, Neurath MF, Shroyer NF, Montrose MH, Watson AJ. Identification of epithelial gaps in human small and large intestine by confocal endomicroscopy. Gastroenterology. 2007;133:1769–78. doi: 10.1053/j.gastro.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 70.Watson AJ, Hughes KR. TNF-alpha-induced intestinal epithelial cell shedding: implications for intestinal barrier function. Ann N Y Acad Sci. 2012;1258:1–8. doi: 10.1111/j.1749-6632.2012.06523.x. [DOI] [PubMed] [Google Scholar]
- 71.Werner T, Shkoda A, Haller D. Intestinal epithelial cell proteome in IL-10 deficient mice and IL-10 receptor reconstituted epithelial cells: impact on chronic inflammation. Journal of proteome research. 2007;6:3691–704. doi: 10.1021/pr070222x. [DOI] [PubMed] [Google Scholar]
- 72.Shkoda A, Werner T, Daniel H, Gunckel M, Rogler G, Haller D. Differential protein expression profile in the intestinal epithelium from patients with inflammatory bowel disease. Journal of proteome research. 2007;6:1114–25. doi: 10.1021/pr060433m. [DOI] [PubMed] [Google Scholar]
- 73.Cande C, Cecconi F, Dessen P, Kroemer G. Apoptosis-inducing factor (AIF): key to the conserved caspase-independent pathways of cell death? J Cell Sci. 2002;115:4727–34. doi: 10.1242/jcs.00210. [DOI] [PubMed] [Google Scholar]
- 74.Rennick DM, Fort MM, Davidson NJ. Studies with IL-10−/− mice: an overview. J Leukoc Biol. 1997;61:389–96. doi: 10.1002/jlb.61.4.389. [DOI] [PubMed] [Google Scholar]
- 75.Leppkes M, Roulis M, Neurath MF, Kollias G, Becker C. Pleiotropic functions of TNF-alpha in the regulation of the intestinal epithelial response to inflammation. Int Immunol. 2014;26:509–15. doi: 10.1093/intimm/dxu051. [DOI] [PubMed] [Google Scholar]
- 76.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Buning C, Cohain A, Cichon S, D’Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H, Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, et al. IBDGC International. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–24. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–98. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 78.Brenner D, Blaser H, Mak TW. Regulation of tumour necrosis factor signalling: live or let die. Nat Rev Immunol. 2015;15:362–74. doi: 10.1038/nri3834. [DOI] [PubMed] [Google Scholar]
- 79.Bruno ME, Frantz AL, Rogier EW, Johansen FE, Kaetzel CS. Regulation of the polymeric immunoglobulin receptor by the classical and alternative NF-kappaB pathways in intestinal epithelial cells. Mucosal immunology. 2011;4:468–78. doi: 10.1038/mi.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, Degnan PH, Hu J, Peter I, Zhang W, Ruggiero E, Cho JH, Goodman AL, Flavell RA. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158:1000–10. doi: 10.1016/j.cell.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gurung P, Man SM, Kanneganti TD. A20 is a regulator of necroptosis. Nat Immunol. 2015;16:596–7. doi: 10.1038/ni.3174. [DOI] [PubMed] [Google Scholar]
- 82.Onizawa M, Oshima S, Schulze-Topphoff U, Oses-Prieto JA, Lu T, Tavares R, Prodhomme T, Duong B, Whang MI, Advincula R, Agelidis A, Barrera J, Wu H, Burlingame A, Malynn BA, Zamvil SS, Ma A. The ubiquitin-modifying enzyme A20 restricts ubiquitination of the kinase RIPK3 and protects cells from necroptosis. Nat Immunol. 2015;16:618–27. doi: 10.1038/ni.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vereecke L, Sze M, Mc Guire C, Rogiers B, Chu Y, Schmidt-Supprian M, Pasparakis M, Beyaert R, van Loo G. Enterocyte-specific A20 deficiency sensitizes to tumor necrosis factor-induced toxicity and experimental colitis. J Exp Med. 2010;207:1513–23. doi: 10.1084/jem.20092474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Blander JM. A long-awaited merger of the pathways mediating host defence and programmed cell death. Nat Rev Immunol. 2014;14:601–18. doi: 10.1038/nri3720. [DOI] [PubMed] [Google Scholar]
- 85.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–72. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kiesslich R, Duckworth CA, Moussata D, Gloeckner A, Lim LG, Goetz M, Pritchard DM, Galle PR, Neurath MF, Watson AJ. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut. 2012;61:1146–53. doi: 10.1136/gutjnl-2011-300695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Armuzzi A, Van Assche G, Reinisch W, Pineton de Chambrun G, Griffiths A, Sladek M, Preiss JC, Lukas M, D’Haens G. Results of the 2nd scientific workshop of the ECCO (IV): therapeutic strategies to enhance intestinal healing in inflammatory bowel disease. Journal of Crohn’s & colitis. 2012;6:492–502. doi: 10.1016/j.crohns.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 88.Beigel F, Deml M, Schnitzler F, Breiteneicher S, Goke B, Ochsenkuhn T, Brand S. Rate and predictors of mucosal healing in patients with inflammatory bowel disease treated with anti-TNF-alpha antibodies. PloS one. 2014;9:e99293. doi: 10.1371/journal.pone.0099293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Colombel JF, Rutgeerts P, Reinisch W, Esser D, Wang Y, Lang Y, Marano CW, Strauss R, Oddens BJ, Feagan BG, Hanauer SB, Lichtenstein GR, Present D, Sands BE, Sandborn WJ. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194–201. doi: 10.1053/j.gastro.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 90.Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, Schreiber S, Byczkowski D, Li J, Kent JD, Pollack PF. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 91.Peyrin-Biroulet L, Ferrante M, Magro F, Campbell S, Franchimont D, Fidder H, Strid H, Ardizzone S, Veereman-Wauters G, Chevaux JB, Allez M, Danese S, Sturm A, Colitis O Cs. Scientific Committee of the European. Results from the 2nd Scientific Workshop of the ECCO. I: Impact of mucosal healing on the course of inflammatory bowel disease. Journal of Crohn’s & colitis. 2011;5:477–83. doi: 10.1016/j.crohns.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 92.Rieder F, Karrasch T, Ben-Horin S, Schirbel A, Ehehalt R, Wehkamp J, de Haar C, Velin D, Latella G, Scaldaferri F, Rogler G, Higgins P, Sans M. Results of the 2nd scientific workshop of the ECCO (III): basic mechanisms of intestinal healing. Journal of Crohn’s & colitis. 2012;6:373–85. doi: 10.1016/j.crohns.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 93.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–76. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 94.Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, Van Assche G, Hoffman I, Van Steen K, Vermeire S, Rutgeerts P. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis. 2009;15:1295–301. doi: 10.1002/ibd.20927. [DOI] [PubMed] [Google Scholar]
- 95.Marini M, Bamias G, Rivera-Nieves J, Moskaluk CA, Hoang SB, Ross WG, Pizarro TT, Cominelli F. TNF-alpha neutralization ameliorates the severity of murine Crohn’s-like ileitis by abrogation of intestinal epithelial cell apoptosis. Proc Natl Acad Sci U S A. 2003;100:8366–71. doi: 10.1073/pnas.1432897100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zeissig S, Bojarski C, Buergel N, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Downregulation of epithelial apoptosis and barrier repair in active Crohn’s disease by tumour necrosis factor alpha antibody treatment. Gut. 2004;53:1295–302. doi: 10.1136/gut.2003.036632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Suenaert P, Bulteel V, Lemmens L, Noman M, Geypens B, Van Assche G, Geboes K, Ceuppens JL, Rutgeerts P. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn’s disease. The American journal of gastroenterology. 2002;97:2000–4. doi: 10.1111/j.1572-0241.2002.05914.x. [DOI] [PubMed] [Google Scholar]
- 98.Danese S, Colombel JF, Peyrin-Biroulet L, Rutgeerts P, Reinisch W. Review article: the role of anti-TNF in the management of ulcerative colitis -- past, present and future. Alimentary pharmacology & therapeutics. 2013;37:855–66. doi: 10.1111/apt.12284. [DOI] [PubMed] [Google Scholar]
- 99.Danese S, Fiorino G, Reinisch W. Review article: Causative factors and the clinical management of patients with Crohn’s disease who lose response to anti-TNF-alpha therapy. Alimentary pharmacology & therapeutics. 2011;34:1–10. doi: 10.1111/j.1365-2036.2011.04679.x. [DOI] [PubMed] [Google Scholar]
- 100.Sandborn WJ, Hanauer SB. Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safety. Inflamm Bowel Dis. 1999;5:119–33. doi: 10.1097/00054725-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 101.Colombel JF, Sandborn WJ, Panaccione R, Robinson AM, Lau W, Li J, Cardoso AT. Adalimumab safety in global clinical trials of patients with Crohn’s disease. Inflamm Bowel Dis. 2009;15:1308–19. doi: 10.1002/ibd.20956. [DOI] [PubMed] [Google Scholar]
- 102.Eser A, Primas C, Reinisch W. Drug monitoring of biologics in inflammatory bowel disease. Curr Opin Gastroenterol. 2013;29:391–6. doi: 10.1097/MOG.0b013e328361f7f6. [DOI] [PubMed] [Google Scholar]
- 103.Neurath MF. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal immunology. 2014;7:6–19. doi: 10.1038/mi.2013.73. [DOI] [PubMed] [Google Scholar]
- 104.Roda G, Jharap B, Neeraj N, Colombel JF. Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clinical and translational gastroenterology. 2016;7:e135. doi: 10.1038/ctg.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Atreya R, Zimmer M, Bartsch B, Waldner MJ, Atreya I, Neumann H, Hildner K, Hoffman A, Kiesslich R, Rink AD, Rau TT, Rose-John S, Kessler H, Schmidt J, Neurath MF. Antibodies against tumor necrosis factor (TNF) induce T-cell apoptosis in patients with inflammatory bowel diseases via TNF receptor 2 and intestinal CD14(+) macrophages. Gastroenterology. 2011;141:2026–38. doi: 10.1053/j.gastro.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 106.Mitoma H, Horiuchi T, Hatta N, Tsukamoto H, Harashima S, Kikuchi Y, Otsuka J, Okamura S, Fujita S, Harada M. Infliximab induces potent anti-inflammatory responses by outside-to-inside signals through transmembrane TNF-alpha. Gastroenterology. 2005;128:376–92. doi: 10.1053/j.gastro.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 107.Van den Brande JM, Braat H, van den Brink GR, Versteeg HH, Bauer CA, Hoedemaeker I, van Montfrans C, Hommes DW, Peppelenbosch MP, van Deventer SJ. Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn’s disease. Gastroenterology. 2003;124:1774–85. doi: 10.1016/s0016-5085(03)00382-2. [DOI] [PubMed] [Google Scholar]
- 108.Van den Brande JM, Koehler TC, Zelinkova Z, Bennink RJ, te Velde AA, ten Cate FJ, van Deventer SJ, Peppelenbosch MP, Hommes DW. Prediction of antitumour necrosis factor clinical efficacy by real-time visualisation of apoptosis in patients with Crohn’s disease. Gut. 2007;56:509–17. doi: 10.1136/gut.2006.105379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Di Sabatino A, Ciccocioppo R, Cinque B, Millimaggi D, Morera R, Ricevuti L, Cifone MG, Corazza GR. Defective mucosal T cell death is sustainably reverted by infliximab in a caspase dependent pathway in Crohn’s disease. Gut. 2004;53:70–7. doi: 10.1136/gut.53.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Present DH. Review article: the efficacy of infliximab in Crohn’s disease--healing of fistulae. Alimentary pharmacology & therapeutics. 1999;13(Suppl 4):23–8. doi: 10.1046/j.1365-2036.1999.00026.x. discussion 38. [DOI] [PubMed] [Google Scholar]
- 111.Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, Rutgeerts P, Group AIS. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–9. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 112.Cherrier M, Ohnmacht C, Cording S, Eberl G. Development and function of intestinal innate lymphoid cells. Curr Opin Immunol. 2012 doi: 10.1016/j.coi.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 113.Molloy MJ, Bouladoux N, Belkaid Y. Intestinal microbiota: shaping local and systemic immune responses. Semin Immunol. 2012;24:58–66. doi: 10.1016/j.smim.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Santaolalla R, Abreu MT. Innate immunity in the small intestine. Curr Opin Gastroenterol. 2012;28:124–9. doi: 10.1097/MOG.0b013e3283506559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ivanov, Littman DR. Modulation of immune homeostasis by commensal bacteria. Curr Opin Microbiol. 2011;14:106–14. doi: 10.1016/j.mib.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chow J, Lee SM, Shen Y, Khosravi A, Mazmanian SK. Host-bacterial symbiosis in health and disease. Adv Immunol. 2010;107:243–74. doi: 10.1016/B978-0-12-381300-8.00008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Macpherson AJ, Geuking MB, McCoy KD. Homeland security: IgA immunity at the frontiers of the body. Trends Immunol. 2012;33:160–7. doi: 10.1016/j.it.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 118.Elson CO, Cong Y. Host-microbiota interactions in inflammatory bowel disease. Gut Microbes. 2012:3. doi: 10.4161/gmic.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maldonado-Contreras AL, McCormick BA. Intestinal epithelial cells and their role in innate mucosal immunity. Cell Tissue Res. 2011;343:5–12. doi: 10.1007/s00441-010-1082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Young VB. The intestinal microbiota in health and disease. Curr Opin Gastroenterol. 2012;28:63–9. doi: 10.1097/MOG.0b013e32834d61e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cortez VS, Robinette ML, Colonna M. Innate lymphoid cells: new insights into function and development. Curr Opin Immunol. 2015;32:71–7. doi: 10.1016/j.coi.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goldberg R, Prescott N, Lord GM, MacDonald TT, Powell N. The unusual suspects-innate lymphoid cells as novel therapeutic targets in IBD. Nature reviews Gastroenterology & hepatology. 2015;12:271–283. doi: 10.1038/nrgastro.2015.52. [DOI] [PubMed] [Google Scholar]
- 123.Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–63. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Griffith TS, Ferguson TA. Cell death in the maintenance and abrogation of tolerance: the five Ws of dying cells. Immunity. 2011;35:456–66. doi: 10.1016/j.immuni.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, Stanley ER, Nussenzweig M, Lira SA, Randolph GJ, Merad M. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–25. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–52. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–8. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 128.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–7. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 129.Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101–14. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, Fehling HJ, Hardt WD, Shakhar G, Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–12. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 131.Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, MacPherson GG. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191:435–44. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee CS, Penberthy KK, Wheeler KM, Juncadella IJ, Vandenabeele P, Lysiak JJ, Ravichandran KS. Boosting Apoptotic Cell Clearance by Colonic Epithelial Cells Attenuates Inflammation In Vivo. Immunity. 2016;44:807–20. doi: 10.1016/j.immuni.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nakahashi-Oda C, Udayanga KG, Nakamura Y, Nakazawa Y, Totsuka N, Miki H, Iino S, Tahara-Hanaoka S, Honda S, Shibuya K, Shibuya A. Apoptotic epithelial cells control the abundance of Treg cells at barrier surfaces. Nat Immunol. 2016;17:441–50. doi: 10.1038/ni.3345. [DOI] [PubMed] [Google Scholar]


