Abstract
In current practice HIV+ candidates with CD4 >200 cells/mm3 are eligible for kidney transplantation. However, the optimal pre-transplant CD4 count above this threshold remains to be defined. We evaluated clinical outcomes in patients with baseline CD4 >350 and <350 cells/mm3 among 38 anti-thymocyte globulin (ATG)-treated HIV- to HIV+ kidney transplants performed at our center between 2006 and 2013. Median follow-up was 2.6 years. Rates of acute rejection, patient and graft survival were not different between groups. However, occurrence of severe CD4 lymphopenia (<200 cells/mm3) was more common among individuals with a baseline CD4 count 200–349 cells/mm3 compared to those transplanted at higher counts (75% vs 30% at 4 weeks [p=0.04] and 71% vs 5% at 52 weeks [p=0.001] post-transplant, respectively). After adjusting for age, baseline CD4 count of 200–349 cells/mm3 was an independent predictor of severe CD4 lymphopenia at 4 (RR, 2.6; 95% CI, 1.3–5.1) and 52 weeks (RR, 14.3; 95% CI, 2–100.4) post-transplant. Patients with CD4 <200 cells/mm3 at 4 weeks had higher probability of serious infections during first 6 months post-transplant (19% vs. 50%; log-rank 0.05). These findings suggest that ATG must be used with caution in HIV+ kidney allograft recipients with a pre-transplant CD4 count <350 cells/mm3.
INTRODUCTION
End-stage renal disease is an important complication of chronic human immunodeficiency virus (HIV) infection that carries significant morbidity and mortality (1, 2). Kidney transplant has become a viable alternative for individuals with end-stage renal disease since it is associated with better quality of life, fewer medical complications, longer survival than chronic dialysis treatment, and lower cost (3–5). Once considered a contraindication for solid organ transplant, the feasibility of kidney transplantation in HIV-infected (HIV+) individuals in now well established (5–11). Indeed, more than 500 kidney transplants in HIV+ recipients have been performed in the US over the last 15 years with good outcomes and patient/graft survival rates that, in the absence of hepatitis C virus (HCV) co-infection, resemble those of HIV-negative (HIV−) controls (6, 7).
HIV infection is associated with a 2 to 3-fold increase in the risk of rejection following kidney transplant (6, 11, 12). Administration of anti-thymocyte globulin (ATG) reduces the risk of rejection to that of HIV− recipients (12). The biologic half-life of ATG is prolonged (~30 days) (13) and its effects long lasting, with incomplete CD4 reconstitution persisting for several years in some cases (14, 15). This poses a therapeutic dilemma since the salutatory effects of ATG on rejection rates must be balanced against the risk of profound lymphopenia and associated infections in the post-transplant period (11).
In order to be eligible for kidney transplant, HIV+ candidates must have a sustained undetectable viral load and CD4 count >200 cells/mm3 (9). The optimal pre-transplant CD4 count above this threshold, however, remains to be defined. Since immune reconstitution in HIV infection is severely impaired when the CD4 count falls below 350 cells/mm3 (16–18), HIV+ transplant recipients with baseline CD4 between 200–349 cells/mm3 might have a higher risk of prolonged lymphopenia following ATG induction. We conducted a retrospective cohort analysis of ATG-treated HIV+ kidney allograft recipients transplanted at a single center over a 7-year period, with the aim of assessing the impact of the pre-transplant CD4 count on immune reconstitution and post-transplant clinical outcomes.
METHODS
Study subjects
HIV+ adult, first-time kidney transplants performed at the Miami Transplant Institute – Jackson Memorial Hospital between October 2006 and September 2013, with available information on baseline (within 3 months prior to transplant) and post-transplant lymphocyte counts were identified (n=38). All HIV+ recipients had an undetectable viral load at the time of transplant. All patients but one (a kidney-liver recipient) had CD4 count >200 cells/mm3 at the time of transplant. A group of 76 age-matched deceased donor HIV− kidney transplant recipients (median age 51, IQR 45-56) transplanted between 2006 and 2011 who received ATG plus anti-CD25 monoclonal antibody (mAb) induction, served as reference for comparison in selected analyses. The study was approved by the institutional review board (IRB protocol #20150614).
Immunosuppression and antimicrobial prophylaxis protocols
All patients received induction immunosuppression with ATG (1mg/kg IV x 3 doses; two additional doses were given to kidney-pancreas and selected kidney recipients with slow or delayed graft function, DGF) plus anti-CD25 mAb x 2 doses and methylprednisolone (500 mg IV daily x 3 doses). The most common maintenance immunosuppression regimen consisted of mycophenolate (97%), tacrolimus (97%) and prednisone (84%). All patients received 3–6 months of valganciclovir (900 mg po daily or renally-adjusted dose) based on donor/recipient serostatus, and life-long trimethoprim/sulfamethoxazole (80/400 mg daily while Foley catheter in, then three times a week) or dapsone (50-100 mg po daily) if sulfa intolerant for cytomegalovirus (CMV) and Pneumocystis jirovecii pneumonia (PCP) prophylaxis, respectively. Oral nystatin (5mL after meals and bedtime) as antifungal prophylaxis was administered while on steroids. Weekly azithromycin (1,200 mg po) for Mycobacterium avium complex (MAC) prophylaxis was given to patients with CD4 <75 cells/mm3.
Clinical outcomes
The 1- and 3-year outcome measures were: i) patient survival, calculated from the date of transplantation to the date of death or the date of the last follow-up; ii) death-censored graft survival, calculated from the date of transplantation to the date of irreversible graft failure signified by return to long-term dialysis or the date of last follow-up during the period when the transplant was still functioning (in the event of death with a functioning graft, the follow-up period was censored at the date of death); and iii) biopsy-proven acute rejection. We also assessed the proportion of patients with severe CD4 lymphopenia (defined as CD4 count <200 cell/mm3) at 4 and 52 weeks post-transplant. The rate of serious infections (defined as infections requiring admission to the intensive care unit [ICU] during initial transplant hospitalization or readmission to the hospital after discharge) during the first six months post-transplant was estimated for patients with CD4 count <200 cell/mm3 at 4 weeks.
Flow cytometry
T helper cells (CD3+CD4+), cytotoxic T lymphocytes (CD3+CD8+), natural killer (NK; CD3−CD56+CD16+) and B cells (CD3−CD19+) were measured in peripheral blood samples at baseline, 4, 12, 26 and 52 weeks post-transplant. Surface staining was performed on whole blood using the Lyse/No-Wash protocol. At least 5,000 events were collected on the lymphocyte gate for each sample. Cells were acquired on a BD FACSCalibur™ flow cytometer (BD Systems) and analyzed using the BD Multiset software.
Statistics
The Kaplan-Meier plots with a log-rank test, Chi-square or Fisher exact test, Wilcoxon-Mann-Whitney test, t-tests were used where appropriate. Predictors of severe CD4 lymphopenia were calculated using log-binomial regression. Multivariate model was created using variables with a p value ≤0.05 in the univariate model, and those considered to be clinically relevant. Statistical analyses were performed using SAS 9.2 (Cary, NC).
RESULTS
Patient characteristics
A total of 38 HIV+ adult kidney allograft recipients were studied (Table 1). The median post-transplant follow-up was 2.6 years (IQR, 1–4.3). The median age at the time of transplant was 47 years (range, 30–68). Most patients were males (76%) and African-American (71%). The median duration of HIV diagnosis prior to transplant was 10 years. Median CD4 count at time of transplant was 551 (IQR, 354-686) cells/mm3, with a CD4/CD8 ratio of 0.7 (IQR, 0.6-1). All the patients had sustained HIV viral load suppression (<400 copies/mL) on antiretroviral therapy (ART) post-transplant. Only four subjects had detectable HIV viremia above 50 copies/mL during the first year post-transplant (median peak, 115 [IQR, 107-140] copies/mL). Five (13%) patients were co-infected with HCV. Eleven (29%) patients received allografts from living donors, and three patients underwent dual organ transplantation (kidney-pancreas [n=2] and kidney-liver [n=1]). DGF defined as need for hemodialysis during the first week post-transplant occurred in 16% of cases. All the patients were CMV seropositive at the time of transplantation. Two (5%) patients developed CMV viremia (>500 copies/mL), and three (8%) had BK viremia (>10,000 copies/mL; one of them with biopsy-proven polyomavirus-associated nephropathy) during the first year post-transplant. Other than the pre-transplant CD4 count, there were no differences in the baseline characteristics, immunosuppressive or ART regimens between patients with baseline CD4 count <350 vs those transplanted at CD4 >350 cells/mm3 (Table 1).
Table 1.
Baseline Characteristics of Study Subjects*
| Baseline CD4 | ||||
|---|---|---|---|---|
| Characteristic | Overall n=38 | CD4 >350 n=29 | CD4 <350 n=9 | P value† |
| Demographics | ||||
|
| ||||
| Age, median (IQR) | 47 (42 – 52) | 48 (42 – 52) | 45 (43 – 49) | 0.54 |
|
| ||||
| Male gender | 29 (76.3) | 21 (72.4) | 8 (88.9) | 0.41 |
|
| ||||
| African-American | 27 (71) | 21 (72.4) | 6 (66.7) | 1 |
|
| ||||
| Co-morbidities | ||||
|
| ||||
| Diabetes Mellitus | 6 (15.8) | 3 (10.3) | 3 (33.3) | 0.13 |
|
| ||||
| Hypertension | 22 (57.9) | 16 (55.2) | 6 (66.7) | 0.71 |
|
| ||||
| HIVAN | 26 (68.4) | 21 (72.4) | 5 (62.5) | 0.67 |
|
| ||||
| Hepatitis C | 5 (13.2) | 4 (13.8) | 1 (11.1) | 1 |
|
| ||||
| Overweight (BMI>25) | 20 (52.6) | 15 (51.7) | 5 (55.6) | 1 |
|
| ||||
| Follow up, years, median (IQR) | 2.6 (1 – 4.3) | 3 (1 – 4.8) | 2 (1.1 – 3.5) | 0.54 |
|
| ||||
| Immunosuppression§ | ||||
|
| ||||
| IVIG | 5 (13.2) | 5 (17.2) | 0 | 0.31 |
|
| ||||
| Rituximab | 4 (10.5) | 3 (10.3) | 1 (11.1) | 0.95 |
|
| ||||
| Tacrolimus | 37 (97.4) | 28 (96.5) | 9 (100) | 1 |
|
| ||||
| MMF | 37 (97.4) | 28 (96.5) | 9 (100) | 1 |
|
| ||||
| Prednisone | 32 (84.2) | 23 (79.3) | 9 (100) | 0.30 |
|
| ||||
| Sirolimus | 3 (7.9) | 2 (6.9) | 1 (11.1) | 1 |
|
| ||||
| Cyclosporine | 2 (5.3) | 2 (6.9) | 0 | 1 |
|
| ||||
| Kidney allograft | ||||
|
| ||||
| Living donor | 11 (28.9) | 7 (24.1) | 4 (44.4) | 0.4 |
|
| ||||
| Delayed graft function | 6 (15.8) | 5 (17.2) | 1 (11.1) | 1 |
|
| ||||
| ABC-PRA, <5% (n=36) | 34 (94.4) | 27 (96.4) | 7 (87.5) | 0.40 |
|
| ||||
| DR-PRA, <5% (n=36) | 33 (91.7) | 26 (92.9) | 7 (87.5) | 0.54 |
|
| ||||
| #HLA-ABDR mismatches, >5 | 14 (37) | 12 (41) | 2 (22.2) | 0.44 |
|
| ||||
| Cold ischemia time, >36 hours (n=30) | 7 (23.3) | 7 (29.1) | 0 | 0.29 |
|
| ||||
| Donor age, years median (IQR), n=34 | 37.5 (28 – 48) | 37 (26 – 48) | 40 (33 – 49) | 0.65 |
|
| ||||
| CMV viremia (n=35) | 2 (5.7) | 2 (7.4) | 0 | 1 |
|
| ||||
| BK viremia (n=31) | 3 (9.7) | 3 (12.5) | 0 | 1 |
|
| ||||
| HIV infection | ||||
|
| ||||
| Time from HIV diagnosis, years, median (IQR) | 10 (5 – 15) | 11 (6 – 15) | 8 (3 – 13.5) | 0.32 |
|
| ||||
| Pre-transplant CD4 count, median (IQR) | 551 (354 – 686) | 636 (502 – 724) | 258 (244 – 315) | <0.0001 |
|
| ||||
| CD4/CD8 ratio, median (IQR) | 0.7 (0.6 – 1) | 0.7 (0.6 – 1.1) | 0.4 (0.3 – 0.7) | 0.07 |
|
| ||||
| %CD3+HLA-DR+, Median (IQR) | 6.1 (4.5 – 11.2) | 6 (4.7 – 9.9) | 6.8 (4.4 – 16) | 0.77 |
|
| ||||
| Antiretroviral therapy^ | ||||
|
| ||||
| NRTI | 35 (92) | 27 (93) | 8 (89) | 1 |
|
| ||||
| NNRTI | 9 (23.7) | 7 (24.1) | 2 (22.2) | 1 |
|
| ||||
| INSTI | 10 (26.3) | 8 (27.6) | 2 (22.2) | 1 |
|
| ||||
| PI | 26 (68.4) | 19 (65.5) | 7 (77.8) | 0.69 |
|
| ||||
| Drug level monitoring | ||||
|
| ||||
| Tacrolimus level at 4 weeks, median (IQR), ng/mL | 6.6 (4.4 – 9.2) | 6.45 (4.1 – 10.4) | 6.6 (5 – 8.5) | 1 |
|
| ||||
| Tacrolimus level at 12 weeks, median (IQR), ng/mL | 5.9 (4.8 – 7.4) | 7.4 (6 – 11.4) | 5.3 (2.1 – 7.7) | 0.36 |
|
| ||||
| Tacrolimus level at 26 weeks, median (IQR), ng/mL | 9 (6.25 – 10) | 6.2 (4.8 – 8.3) | 6.7 (2.3 – 9.5) | 0.77 |
|
| ||||
| Tacrolimus level at 52 weeks, median (IQR), ng/mL | 6.2 (4.4 – 8.3) | 6 (4.3 – 7.6) | 7.7 (5.3 – 10.9) | 0.11 |
ATG, anti-thymocyte globulin; BMI, body mass index; MMF, mycophenolate mofetil; NRTI, nucleoside reverse transcriptase inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitors; INSTI, integrase strand transfer inhibitors; PI, protease inhibitors. HIVAN, HIV-associated nephropathy; CMV, cytomegalovirus; PRA, panel reactive antibody; IVIG, Intravenous immunoglobulin
Data presented as absolute number (%), unless specified otherwise.
P value corresponds to comparison of CD4<350 vs CD4>350 groups by using the Chi-square of Fisher exact test as appropriate. Wilcoxon–Mann–Whitney test was used for variables presented as median and inter-quartile range (IQR).
All the patients received ATG, Basiliximab and Methylprednisolone for induction. Maintenance immunosuppression: Tacrolimus is started soon after transplant, typically on post-operative day 1 or 2. Target level in our center: 6–8 ng/mL during the first three months and 5-7 ng/mL after three months post-transplant. Higher levels are targeted for highly sensitized patients. Mycophenolate (Cellcept® 1000 mg twice a day or Myfortic® 720 mg twice a day) is started from day of the transplant. Sirolimus: initial dose 1 to 5 mg po daily. Goal 24-hour through level 6–8ng/mL. Steroids: For slow or delayed graft function prednisone 20mg po daily. Once tacrolimus within therapeutic range, quick taper over 10 days. For highly sensitized patients, prednisone 40mg po twice a day followed by slow taper over next 5–6 weeks to a maintenance dose of 5 mg po daily.
Refers to ART regimen post-transplant (defined as the regimen the patient was discharged home after transplantation). In 11 patients the ART regimen was changed early during admission to minimize drug-drug interactions.
Dynamics of lymphocyte count following transplant by HIV status
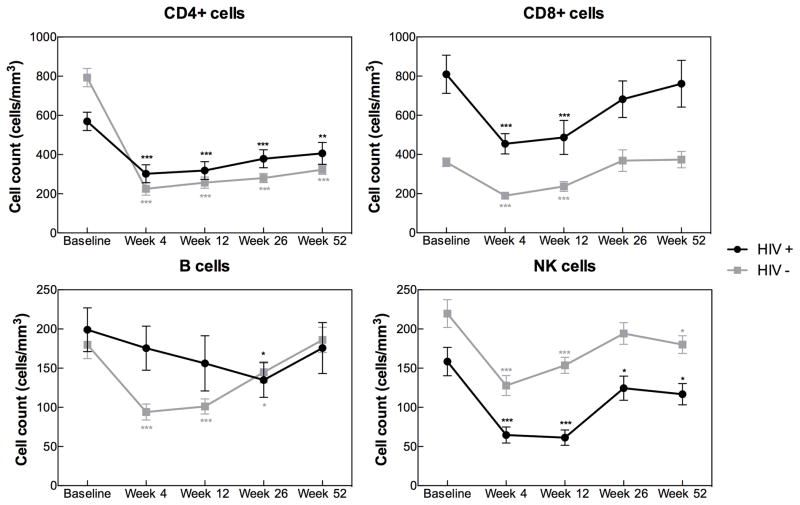
Among HIV+ recipients, CD4, CD8, and NK cells were all significantly depleted at week 4 and 12 post-transplant whereas B cell counts remained relatively stable (Figure 1). CD8 cell recovery occurred by week 26. In contrast, CD4 and NK cell counts failed to return to baseline after 52 weeks (CD4 mean [SD]: 570 [289] vs. 407 [291] cells/mm3, p=0.002; NK mean [SD], 157 [114] vs. 117 [70], p=0.03).
Figure 1.
Lymphocyte recovery in kidney transplant recipients by HIV status. Post-transplant CD4, CD8, B and NK cell trajectories are presented as mean (SE) cell count at baseline (HIV+, n=38; HIV−, n=76), week 4 (HIV+, n=35; HIV−, n=76), week 12 (HIV+, n=30; HIV−, n=75), week 26 (HIV+, n=27; HIV−, n=72), and week 52 (HIV+, n=27; HIV−, n=75) post-transplant. *p<0.05, **p<0.01, ***p<0.001 for comparison between a given time point post-transplant and the baseline cell count using the paired Student’s t-test.
This pattern of lymphocyte recovery in HIV+ recipients mimicked that of a 2:1 age-matched, ATG plus anti-CD25-treated HIV− kidney transplant recipient control group (Figure 1) with prolonged CD4 and NK cell lymphopenia during the first year post-transplant and CD8 cell recovery by week 26 regardless of HIV status. Even more, although not surprisingly the baseline mean CD4 count and CD4/CD8 ratio were significantly lower in HIV+ individuals than in HIV− controls (570 vs. 793 cells/mm3, p=0.001; and 0.9 vs. 2.6, p=1.2 x 10−9, respectively), the CD4 count at 4, 12, 26, and 52 weeks post-transplant were not different between these two groups (Figure 1), and the rates of CD4 lymphopenia <200 cells/mm3 at 52 weeks post-transplant were similar in HIV+ and HIV− patients (22 vs 27%, p=0.79).
Baseline CD4 count in HIV+ recipients and risk of lymphopenia post-transplant
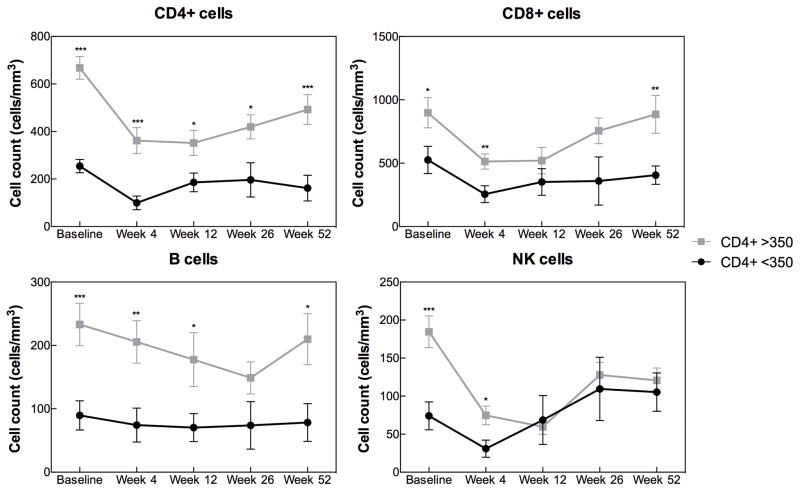
The trends for the CD4 counts in the post-transplant period differed according to the baseline CD4 count. The overall CD4 count within the group of HIV+ recipients with baseline CD4<350 cells/mm3 declined to ~100 cells/mm3 at 4 weeks post-transplant and remained <200 cells/mm3 thereafter (Figure 2). Among the group of patients transplanted at CD4 >350 cells/mm3, a rapid decline in CD4 count was also observed at 4 weeks but CD4 gain was evident by week 26 and CD4 counts continued to rise by week 52. Notably, in this group with pre-transplant CD4 >350 cells/mm3, the overall CD4 count remained ~150–300 cells above 200 cells/mm3 during the entire first year post-transplant (Figure 2).
Figure 2.
Post-transplant lymphocyte (CD4, CD8, B and NK cell) trajectories by baseline CD4 count among 38 HIV+ kidney transplant recipients. Data presented as mean (SE) cell count at the specified time points for patients with CD4<350 (n=9) and CD4>350 (n=29) at the time of transplantation. *p<0.05, **p<0.01, ***p<0.001 for comparison between CD4<350 and CD4>350 groups using the unpaired Student’s t-test.
The risk of severe CD4 lymphopenia (CD4 <200 cells/mm3) was highest among HIV+ individuals with a baseline CD4 <350 cells/mm3, even after excluding one patient who had baseline CD4 count below 200 cell/mm3. Rates of severe CD4 lymphopenia at 4 and 52 weeks were 75% and 71% for patients with baseline CD4 of 200–349 cells/mm3 compared with 30% and 5% of patients with pre-transplant CD4 >350 cells/mm3 (p=0.04 and p=0.001 for 4 and 52 weeks, respectively).
We next examined whether the association between baseline CD4 count and risk of severe CD4 lymphopenia post-transplant was independent of other factors known to influence CD4 recovery including age 40 or older (15, 19), male gender (20) and African-American ethnicity (21). In univariate analysis, baseline CD4 count was the only factor that influenced risk of severe CD4 lymphopenia in this cohort. After adjusting for age in a multivariate analysis, a baseline CD4 of 200–349 cells/mm3 remained an independent predictor of severe CD4 lymphopenia at 4 (RR, 2.6; 95% CI, 1.3–5.1) and 52 weeks (RR, 14.3; 95% CI, 2–100.4) post-transplant (Table 2).
Table 2.
Association between pre-transplant CD4 count and risk of CD4 lymphopenia <200 cells/mm3 following kidney transplant
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| RR (CI) | P value | Adjusted RR (CI) | P value | |
| CD4<200 at 4 weeks | ||||
| Age (<40 years) | 1.8 (0.5–6.3) | 0.37 | 1.8 (0.5–6.1) | 0.32 |
| Male gender | 1.3 (0.4–3.5) | 0.64 | ||
| African-American | 0.9 (0.4–2.1) | 0.75 | ||
| Baseline CD4 <350 | 2.5 (1.2–5.1) | 0.01 | 2.6 (1.3–5.1) | 0.01 |
| HCV co-infection | 1.3 (0.4–3.8) | 0.64 | ||
| CD4<200 at 52 weeks | ||||
| Age (<40 years) | 0.8 (0.2–3.7) | 0.82 | 0.8 (0.2–3) | 0.75 |
| Male gender | 4.9 (0.3–78.1) | 0.26 | ||
| African-American | 1.7 (0.2– 12.5) | 0.58 | ||
| Baseline CD4 <350 | 14.3 (2–102.1) | 0.01 | 14.3 (2–100.4) | 0.01 |
| HCV co-infection | 0.5 (0.03–7) | 0.59 | ||
CI, confidence interval; RR, relative risk.
Baseline CD4 count and transplant outcomes in HIV+ recipients
The 1- and 3-year patient and death-censored graft survival for the entire cohort were 86.6 and 82.7%, and 91.2 and 82.4%, respectively. There were no differences in patient or death-censored graft survival by baseline CD4 count (data not shown).
Nine (23.6%) patients had a first episode of biopsy-proven acute rejection. Cellular, antibody-mediated and mixed histological pattern accounted for 44.4, 11.1 and 44.4% of the cases, respectively. Overall, the cumulative incidence of acute rejection at 1 and 3 years was 15.1% and 26.5%, respectively. The baseline CD4 count did not affect rates of acute rejection (14.3 vs. 15.7% at 1 year [log rank 0.96] and 12.5% vs. 30.1 at 3 years [log rank 0.451] for baseline CD4<350 and >350 cells/mm3, respectively).
Risk of infection in HIV+ recipients with severe CD4 lymphopenia
Since profound lymphopenia has been associated with infectious complications following solid organ transplant (11, 22, 23), and most serious infections in HIV+ kidney recipients occur within the first six months after transplant (11), we compared the rate of serious infections within the first six months post-transplant by CD4 strata.
Overall, twelve (32%) patients developed serious non-opportunistic infections (OI) within six months of transplant (Table 3). The incidence of serious infections was higher in HIV/HCV co-infected than HIV-monoinfected patients (80 vs. 25%, p=0.03), and lower among patients who experienced acute rejection compared to those who did not (0 vs 41.4%, p=0.04). The median time from transplant to infection was 49 days (range, 11-132). The median CD4 count at the time of serious infection was 129 cells/mm3 (range, 0–390). Bacterial infections were the most common (Table 3). Notably, in the two cases of death attributed to infection, both patients had a CD4 count of zero immediately prior to the date of index infection.
Table 3.
Infections requiring ICU or hospital re-admission within six months post-transplant among 38 HIV+ kidney allograft recipients
| Age at time of transplant | HCV | Pre- transplant BMI | Pre- transplant CD4 cells/mm3 | CD4 cells/mm3 at 4 weeks | CD4 cells/mm3 at time of infection | Tacrolimus level (ng/mL) at time of infection | Site of infection | Etiological agent | Time from transplant to index infection, days | Outcome† |
|---|---|---|---|---|---|---|---|---|---|---|
| 44 | − | 22.1 | 244 | 95 | 116 | 11.7 | PNA | Unknown^ | 132 | Recovered |
| 54 | + | 26.6 | 258 | 61 | 160 | 9.2 | BSI, PNA | Acinetobacter baumannii | 56 | Recovered |
| 45 | − | 31.1 | 228 | 50 | 4 | 4.4 | UTI, pelvic abscess | Escherichia coli | 15 | Recovered |
| 56 | + | 27.2 | 483 | − | 0 | 10.4 | UTI, BSI BSI |
E coli* A. baumannii |
11 | Died |
| 51 | − | 20.3 | 1036 | 251 | 301 | 9.3 | SSTI Esophagitis |
MRSA*
Candida |
115 | Recovered |
| 49 | + | 22.4 | 1448 | 591 | 157 | 6.7 | SSTI | MRSA | 94 | Recovered |
| 45 | − | 32.5 | 619 | 166 | 101 | 3.8 | BSI PNA |
Serratia marcescens*
Aspergillus terreus |
43 | Recovered |
| 30 | − | 19.8 | 628 | 660 | 222 | 26.6 | UTI | CoNS | 15 | Recovered |
| 48 | − | 22.8 | 382 | 0 | 0 | n/aΔ | BSI, peritonitis PNA |
VRE Aspergillus spp |
54 | Died |
| 45 | + | 22.9 | 815 | 59 | 390 | 9.8 | BSI PNA |
MSSA*
Aspergillus spp |
85 | Recovered |
| 62 | − | 32.7 | 666 | 202 | 202 | 6.6 | UTI | E. coli | 35 | Recovered |
| 62 | − | 32.3 | 742 | 148 | 7 | 3.4 | BSI | C. glabrata | 15 | Recovered |
MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus faecium; CoNS, coagulase-negative Staphylococcus. BSI, bloodstream infection; PNA, pneumonia; UTI, urinary tract infection; SSTI, skin and soft tissue infection.
Five of these patients died during study follow-up; outcome here is for the admission due to serious infection.
Bronchoalveolar lavage and blood cultures failed to reveal the etiological agent in this patient with presumptive bacterial pneumonia who responded well to antibiotic therapy.
Index infection for patients with concurrent infections.
Patient was on tacrolimus-sparing protocol due to delayed graft function.
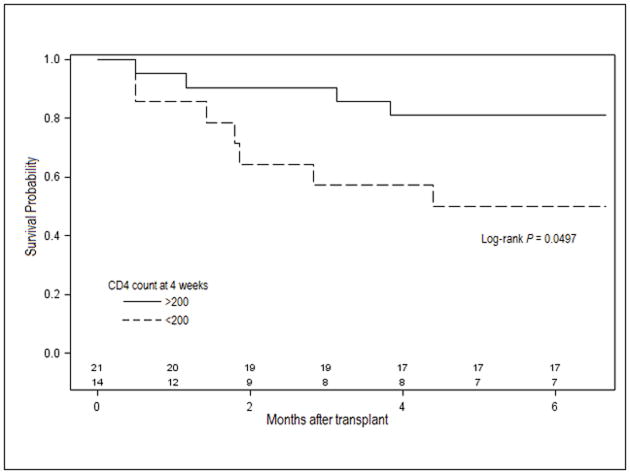
A survival analysis indicated that severe CD4 lymphopenia at 4 weeks post-transplant was associated with higher probability of serious infections during first 6 months post-transplant (19% vs. 50%; log-rank 0.0497; Figure 3).
Figure 3.
Infection-free survival by CD4 strata at 4 weeks. Solid lines represent the group of patients with CD4 >200 cells/mm3 at 4 weeks post-transplant. Dashed lines represent the group with CD4 <200 cells/mm3 at 4 weeks post-transplant. Number of patients in each group is shown in the bottom.
DISCUSSION
The incidence and severity of infectious complications following transplant are largely dictated by the recipient’s capacity for immune reconstitution. Our results indicate that: 1) ATG-induced CD4 lymphopenia can be prolonged, and even one year after transplant a substantial proportion of patients have CD4 counts below 200 cells/mm3. 2) The risk of severe CD4 lymphopenia is highest among HIV+ recipients with a baseline CD4 <350 cells/mm3; and 3) individuals with CD4 <200 cells/mm3 at 4 weeks have increased risk of serious infections within the first 6 months post-transplant. The baseline CD4 count did not influence the risk of death, graft loss or acute rejection. These findings suggest that although in current practice HIV+ candidates with pre-transplant CD4 counts between 200 and 349 cells/mm3 are eligible for kidney transplantation [9], and likely to have similar outcomes to those with higher counts, this group of patients carries a substantial risk for lymphopenia and associated infections following ATG induction.
Profound CD4 lymphopenia during the first year post-transplant is typically observed in ATG-treated solid organ transplant recipients regardless of HIV status (11, 14, 15, 22, 24, 25). In the present study, the pattern of lymphocyte recovery was similar between HIV+ and HIV− recipients; only three (8%) HIV+ patients developed AIDS-defining conditions during the study follow-up including one case of biopsy-proven cutaneous Kaposi's sarcoma, one CMV esophagitis and one Candida esophagitis; two of these patients had a baseline CD4 <350 cells/mm3. These observations, along with the fact that all the HIV+ recipients attained sustained viral load suppression while on ART, confirm the notion that post-transplant CD4 lymphopenia in HIV+ recipients is primarily driven by ATG induction (11, 25) and does not represent HIV disease progression per se.
Consistent with the well-known increased risk of allograft rejection in HIV+ recipients (6, 11, 12), the cumulative incidence of acute rejection among seropositive individuals was higher than that previously reported by our group (26) in ATG-treated HIV− kidney recipients (27% versus 14% at 36 months, respectively). Although ATG reduces the risk of rejection (12), the optimal immunosuppressive regimen for HIV+ solid organ transplant recipients has not been defined. More than half of our patients experienced infection or rejection events, underscoring the need to optimize immunosuppression protocols in HIV+ recipients. One could argue that based on the high incidence of severe CD4 lymphopenia we observed in seropositive kidney recipients with pre-transplant CD4 <350 cells/mm3, and associated infections among those with CD4 <200 cells/mm3, administration of ATG should be restricted to patients at very high immunologic risk for rejection (11). Basiliximab induction is a potential alternative since it results in fewer infections than ATG (27), and has been used in HIV+ recipients with acceptable outcomes and post-transplant CD4 counts in excess of 400 cells/mm3 for up to 2 years (5).
Our findings have “real-life” implications because up to 30% of HIV+ patients can have a muted recovery of CD4 cell counts despite ART-induced viral load suppression (28), and consequently, a significant proportion of HIV+ candidates listed on the UNOS (United Network for Organ Sharing) national transplant waiting list can be expected to have marginal CD4 counts. Since profound ATG-induced lymphopenia is associated with higher incidence of infections and cancer, as well as increased risk of death following kidney transplant (11, 23, 24, 25), we propose that a reduced intensity induction protocol (e.g., ATG-free or ultra-low dose ATG, 1-1.5mg/kg total) should be considered in selected HIV+ candidates with CD4 count of 200–349 cells/mm3 and otherwise relatively “low” immunological risk for rejection (e.g., older recipients, living donor, not highly sensitized, few HLA mismatches, etc). Of note, half of the patients with non-OIs had above target levels of tacrolimus at the time of infection, which could further impact the net state of immunosuppression in an already lymphodepleted host. Thus, in an effort to minimize infectious complications, drug-drug interactions should be avoided; in particular, the use of protease inhibitors and cobicistat-containing ART regimens can lead to supratherapeutic levels of calcineurin (and mTOR) inhibitors.
Only two patients developed CMV reactivation, and there were no cases of PCP, toxoplasma or MAC suggesting that despite profound lymphopenia in ATG-treated HIV+ recipients, OI prophylaxis is highly effective. We observed, however, an increased risk of serious non-OIs requiring ICU or hospital admission within 6 months post-transplant among patients with CD4 <200 cells/mm3 at 4 weeks. These results complement previous observations by others. Trullas et al reported three HIV+ kidney transplant recipients (baseline CD4: 615 cells/mm3) who received ATG induction (8.75mg/kg total); two of them developed CD4 lymphopenia <200 cells/mm3 at 4 weeks and bacterial infections post-transplant (29). Carter et al reported a high incidence of profound lymphopenia (from 475±192 to 9±10 cells/mm3), delayed immune reconstitution (up to 2 years) and high rate of serious non-OIs requiring hospitalization (55%) following administration of ATG (mean, 7.0 mg/kg) for the treatment of acute rejection or slow graft function in eleven HIV+ kidney transplant recipients (23). In the NIH multicenter trial that included 150 HIV+ recipients followed for up to 3 years, 38% of the patients developed infections requiring hospitalization (11). Notably, patients who received ATG induction had twice as many serious infections as patients who did not (11). Similar to our findings, the rate of non-OIs infections was higher among those co-infected with HCV (11). It is estimated that the risk of serious infections requiring hospitalization is ten times higher in HIV+ recipients with CD4 <200 cells/mm3 (23). Consistent with this, we observed that 67% of our patients who developed serious infections had a preceding CD4 <200 cells/mm3.
One limitation of our study is that the ATG plus anti-CD25 mAb induction regimen used at our institution for kidney transplant recipients is not common across transplant centers limiting the applicability of our observations. However, unlike ATG, anti-CD25 mAb primarily targets activated (not resting) T-cells (27) and when used as a single agent for induction therapy, anti-CD25 mAb, does not result in statistically significant alterations in the absolute number of T cells (5, 15, 30); neither potentiates the T-cell depleting activity of ATG when used in combination (30). Thus, we feel that our observations might still be applicable to centers using ATG-based induction.
Naturally, given the uniqueness of the patient population in this study, small size is another limitation and our findings need to be validated at the multicenter cohort level. Despite these limitations, our observations and those by others (11, 23, 25, 29), suggest that given the narrow therapeutic window of ATG among HIV+ recipients, particularly those with low pre-transplant CD4 count (200–349 cells/mm3), this agent must be used with caution and close monitoring for infectious complications until CD4 recovery occurs.
Acknowledgments
We thank Analucía Schneégans and Alyajahan Bhimji for technical assistance. We are indebted to all the patients that participated in the present study. Because of space constraints, we regret our inability to cite other excellent work relevant to the present article. This work was supported in part by a Miami Center for AIDS research (CFAR) pilot award to J.F.C., funded by a grant (P30AI073961) from the National Institutes of Health (NIH).
ABBREVIATIONS
- AIDS
acquired immune deficiency syndrome
- ART
antiretroviral therapy
- ATG
anti-thymocyte globulin
- BMI
body mass index
- CI
confidence interval
- CMV
cytomegalovirus
- DGF
delayed graft function
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- HIVAN
HIV-associated nephropathy
- INSTI
integrase strand transfer inhibitor
- ICU
intensive care unit
- IQR
inter-quartile range
- IRB
institutional review board
- IVIG
intravenous immunoglobulin
- PCP
Pneumocystis jirovecii pneumonia
- PRA
panel reactive antibody
- PI
protease inhibitor
- MAC
Mycobacterium avium complex
- mAb
monoclonal antibody
- MMF
mycophenolate mofetil
- NK
natural killer
- NRTI
nucleoside reverse transcriptase inhibitor
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- OI
opportunistic infection
- UNOS
United Network for Organ Sharing
Footnotes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure. The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Abraham AG, Althoff KN, Jing Y, Estrella MM, Kitahata MM, Wester CW, et al. End-stage renal disease among HIV-infected adults in North America. Clin Infect Dis. 2015;60:941–949. doi: 10.1093/cid/ciu919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucas GM, Ross MJ, Stock PG, Shlipak MG, Wyatt CM, Gupta SK, et al. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e96–138. doi: 10.1093/cid/ciu617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA. 1993;270:1339–1343. [PubMed] [Google Scholar]
- 4.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 5.Kumar MS, Sierka DR, Damask AM, Fyfe B, McAlack RF, Heifets M, et al. Safety and success of kidney transplantation and concomitant immunosuppression in HIV-positive patients. Kidney Int. 2005;67:1622–1629. doi: 10.1111/j.1523-1755.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 6.Locke JE, Mehta S, Reed RD, MacLennan P, Massie A, Nellore A, et al. A National Study of Outcomes among HIV-Infected Kidney Transplant Recipients. J Am Soc Nephrol. 2015;26:2222–2229. doi: 10.1681/ASN.2014070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawinski D, Forde KA, Eddinger K, Troxel AB, Blumberg E, Tebas P, et al. Superior outcomes in HIV-positive kidney transplant patients compared with HCV-infected or HIV/HCV-coinfected recipients. Kidney Int. 2015;88:341–349. doi: 10.1038/ki.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waheed S, Sakr A, Chheda ND, Lucas GM, Estrella M, Fine DM, et al. Outcomes of Renal Transplantation in HIV-1 Associated Nephropathy. PLoS One. 2015;10:e0129702. doi: 10.1371/journal.pone.0129702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blumberg EA, Rogers CC Practice ASTIDCo. Human immunodeficiency virus in solid organ transplantation. Am J Transplant. 2013;13:169–178. doi: 10.1111/ajt.12109. [DOI] [PubMed] [Google Scholar]
- 10.Locke JE, Reed RD, Mehta SG, Durand C, Mannon RB, MacLennan P, et al. Center-Level Experience and Kidney Transplant Outcomes in HIV-Infected Recipients. Am J Transplant. 2015;15:2096–2104. doi: 10.1111/ajt.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stock PG, Barin B, Murphy B, Hanto D, Diego JM, Light J, et al. Outcomes of Kidney Transplantation in HIV-Infected Recipients. New Engl J Med. 2010;363:2004–2014. doi: 10.1056/NEJMoa1001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Locke JE, James NT, Mannon RB, Mehta SG, Pappas PG, Baddley JW, et al. Immunosuppression regimen and the risk of acute rejection in HIV-infected kidney transplant recipients. Transplantation. 2014;97:446–450. doi: 10.1097/01.TP.0000436905.54640.8c. [DOI] [PubMed] [Google Scholar]
- 13.Bunn D, Lea CK, Bevan DJ, Higgins RM, Hendry BM. The pharmacokinetics of anti-thymocyte globulin (ATG) following intravenous infusion in man. Clin Nephrol. 1996;45:29–32. [PubMed] [Google Scholar]
- 14.Muller TF, Grebe SO, Neumann MC, Heymanns J, Radsak K, Sprenger H, et al. Persistent long-term changes in lymphocyte subsets induced by polyclonal antibodies. Transplantation. 1997;64:1432–1437. doi: 10.1097/00007890-199711270-00010. [DOI] [PubMed] [Google Scholar]
- 15.Longuet H, Sautenet B, Gatault P, Thibault G, Barbet C, Marliere JF, et al. Risk factors for impaired CD4+ T-cell reconstitution following rabbit antithymocyte globulin treatment in kidney transplantation. Transpl Int. 2014;27:271–279. doi: 10.1111/tri.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badri M, Lawn SD, Wood R. Short-term risk of AIDS or death in people infected with HIV-1 before antiretroviral therapy in South Africa: a longitudinal study. Lancet. 2006;368:1254–1259. doi: 10.1016/S0140-6736(06)69117-4. [DOI] [PubMed] [Google Scholar]
- 17.Moore RD, Keruly JC. CD4(+) cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 2007;44:441–446. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan JE, Hanson DL, Cohn DL, Karon J, Buskin S, Thompson M, et al. When to begin highly active antiretroviral therapy? Evidence supporting initiation of therapy at CD4+ lymphocyte counts <350 cells/microL. Clin Infect Dis. 2003;37:951–958. doi: 10.1086/377606. [DOI] [PubMed] [Google Scholar]
- 19.Hakim FT, Memon SA, Cepeda R, Jones EC, Chow CK, Kasten-Sportes C, et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J Clin Invest. 2005;115:930–939. doi: 10.1172/JCI22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corbeau P, Reynes J. Immune reconstitution under antiretroviral therapy: the new challenge in HIV-1 infection. Blood. 2011;117:5582–5590. doi: 10.1182/blood-2010-12-322453. [DOI] [PubMed] [Google Scholar]
- 21.Smith CJ, Sabin CA, Youle MS, Kinloch-de Loes S, Lampe FC, Madge S, et al. Factors influencing increases in CD4 cell counts of HIV-positive persons receiving long-term highly active antiretroviral therapy. J Infect Dis. 2004;190:1860–1868. doi: 10.1086/425075. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Ruiz M, Kumar D, Humar A. Clinical immune-monitoring strategies for predicting infection risk in solid organ transplantation. Clin Transl Immunology. 2014;3:e12. doi: 10.1038/cti.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carter JT, Melcher ML, Carlson LL, Roland ME, Stock PG. Thymoglobulin-associated Cd4+ T-cell depletion and infection risk in HIV-infected renal transplant recipients. Am J Transplant. 2006;6:753–760. doi: 10.1111/j.1600-6143.2006.01238.x. [DOI] [PubMed] [Google Scholar]
- 24.Ducloux D, Courivaud C, Bamoulid J, Vivet B, Chabroux A, Deschamps M, et al. Prolonged CD4 T cell lymphopenia increases morbidity and mortality after renal transplantation. J Am Soc Nephrol. 2010;21:868–875. doi: 10.1681/ASN.2009090976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gasser O, Bihl F, Sanghavi S, Rinaldo C, Rowe D, Hess C, et al. Treatment-dependent loss of polyfunctional CD8+ T-cell responses in HIV-infected kidney transplant recipients is associated with herpesvirus reactivation. Am J Transplant. 2009;9:794–803. doi: 10.1111/j.1600-6143.2008.02539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciancio G, Gaynor JJ, Guerra G, Sageshima J, Chen L, Mattiazzi A, et al. Randomized trial of three induction antibodies in kidney transplantation: long-term results. Transplantation. 2014;97:1128–1138. doi: 10.1097/01.TP.0000441089.39840.66. [DOI] [PubMed] [Google Scholar]
- 27.Issa NC, Fishman JA. Infectious complications of antilymphocyte therapies in solid organ transplantation. Clin Infect Dis. 2009;48:772–786. doi: 10.1086/597089. [DOI] [PubMed] [Google Scholar]
- 28.Camargo JF, Kulkarni H, Agan BK, Gaitan AA, Beachy LA, Srinivas S, et al. Responsiveness of T cells to interleukin-7 is associated with higher CD4+ T cell counts in HIV-1-positive individuals with highly active antiretroviral therapy-induced viral load suppression. J Infect Dis. 2009;199:1872–1882. doi: 10.1086/598858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trullas JC, Cofan F, Cocchi S, Cervera C, Linares L, Aguero F, et al. Effect of thymoglobulin induction on HIV-infected renal transplant recipients: differences between HIV-positive and HIV-negative patients. AIDS Res Hum Retroviruses. 2007;23:1161–1165. doi: 10.1089/aid.2007.0015. [DOI] [PubMed] [Google Scholar]
- 30.Sageshima J, Ciancio G, Gaynor JJ, Chen L, Guerra G, Kupin W, et al. Addition of anti-CD25 to thymoglobulin for induction therapy: delayed return of peripheral blood CD25-positive population. Clin Transplant. 2011;25:E132–135. doi: 10.1111/j.1399-0012.2010.01360.x. [DOI] [PubMed] [Google Scholar]