Abstract
Rationale
Maternal prepregnancy obesity has been associated with early wheeze and childhood asthma in their offspring. Some of these studies have been in minority, urban, and disadvantaged populations using parental recall and questionnaires. The association of maternal prepregnancy obesity with bronchodilator dispensing to their offspring, in a primarily insured, non-urban, White population in the United States is unknown.
Objectives and Methods
We conducted a retrospective cohort study using pharmacy dispensing data from the electronic medical records of a large United States health maintenance organization to examine the relationship between maternal prepregnancy body mass index (BMI) and inhaled bronchodilator dispensing in the offspring to four years of age. We included infants ≥ 37 weeks’ gestation with birth weight ≥ 2.5 kg which yielded 6,194 mother-baby pairs. Maternal prepregnancy BMI was categorized as underweight (< 18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), or obese (≥ 30 kg/m2).
Results
In the entire cohort, 27.6% of the offspring received a bronchodilator dispensing. This ranged from 19.2% in the offspring of underweight mothers to 31.3% of those born to obese mothers. In the fully adjusted model using normal BMI as the referent, children of obese mothers had a 22% higher rate of bronchodilator dispensing (adjusted OR = 1.22; 95% CI 1.05–1.41; p=0.008).
Conclusions
In this insured, non-urban, White population, maternal prepregnancy obesity was associated with bronchodilator dispensing in the offspring in early life. These results extend previous data and reaffirm the potential widespread public health impact that prepregnancy obesity may have on subsequent childhood respiratory health.
Keywords: wheeze, maternal obesity, bronchodilator, pediatrics
INTRODUCTION
Respiratory tract symptoms, such as wheeze and cough, are a common pediatric problem, affecting an estimated one half of children under the age of five1. Although all early childhood wheezing phenotypes do not progress to asthma, they represent the largest use of asthma-related health care expenditures2–4. The public health impact of asthma on health care service utilization is important as the prevalence of childhood asthma increased from 7.3% in 2001 to 8.4% in 2010, the highest level ever5. While it is unclear what has led to a rise in asthma diagnoses, the increase has been paralleled by a rise in the prevalence of maternal obesity. Nearly 70% of adults over 20 years old are overweight or obese; in particular 64% of women of reproductive potential are overweight, of which 35% are obese6. Epidemiologic evidence from both large national population databases and studies in minority, urban, and disadvantaged populations using methodologies such as parental recall and questionnaires suggest maternal obesity may be linked to the development of asthma or early childhood wheeze7–13. One hypothesis is that greater maternal adiposity is associated with higher serum levels of pro-inflammatory cytokines. This results in an increased inflammatory response that may affect fetal immunologic or pulmonary development13.
Although asthma is usually a clinical diagnosis in infants and preschool aged children14, there are observed relationships between maternal obesity and respiratory symptoms in their offspring that begin at birth and continue through early childhood. Current evidence shows that children born to obese mothers are more likely to be admitted to the neonatal intensive care for respiratory distress than children born to normal weight mothers15, and suggests that they are at increased risk for early and recurrent wheezing13;16. Despite conflicting evidence of their effect in randomized controlled trials, β2-receptor agonists (short-acting bronchodilators) are widely used in infants and preschool children who wheeze14–17. The goal of our study was to determine whether in an insured, non-urban, primarily White population, children born to mothers with prepregnancy obesity have a higher rate of bronchodilator dispensing to four years of age compared to children born to mothers with a normal body mass index (BMI).
METHODS
Population and study design
Approval for this study was obtained by the authors from the Institutional Review Boards (IRBs) at their respective institutions of Kaiser Permanente Northwest (KPNW) and Oregon Health and Science University. This retrospective cohort study used data from the KPNW electronic medical record (EMR). KPNW is a not-for-profit, prepaid, federally certified, Joint Commission accredited group practice health maintenance organization (HMO) with approximately 480,000 members in northwestern Oregon and southwestern Washington.
We used a validated computer algorithm18 to access multiple KPNW automated data systems to identify singleton pregnancies beginning and ending between January 1, 2000 and December 31, 2005 and resulting in a live birth. We defined maternal baseline (prepregnancy) BMI as underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), or obese (≥ 30 kg/m2) according to the 2009 Institutes of Medicine criteria19. As estimated in prior publications utilizing this dataset20–22, prepregnancy BMI was estimated based on EMR documented weights and heights measured within −6 to +3 months of pregnancy onset (approximated assuming a due date at 280 days). If there was more than one weight measured, the weight closest to pregnancy onset was used. Maternal characteristics included age (years), parity (0,1, 2, 3 or more), race (White, Asian, Black, other), Hispanic ethnicity, marital status (married, single), and the dichotomous (yes/no) variables of tobacco use during pregnancy, Medicaid as primary insurance; maternal chronic hypertension, pregestational and gestational diabetes, maternal history of asthma, maternal atopy, hypertensive disorders of pregnancy, and Cesarean delivery as entered by the providers were all abstracted from the EMR.
We included the records of children born at ≥ 37 weeks of gestation and with birth weights ≥ 2500 grams since premature birth and small for gestational age (SGA) are known to confer an increased risk of childhood respiratory disorders23. Child data included birth weight and length, fetal macrosomia (birth weight> 4000 grams), large for gestational age (LGA, gender and race specific weight for gestational age >90th percentile), weight at 1, 2, and 3 years of age, and age at first bronchodilator dispensing. We excluded women for whom BMI could not be calculated or whose infants were born with major birth defects, died within the first year of life, were in KPNW enrollment for less than one year, or who were missing birth weight (Figure 1).
Figure 1.
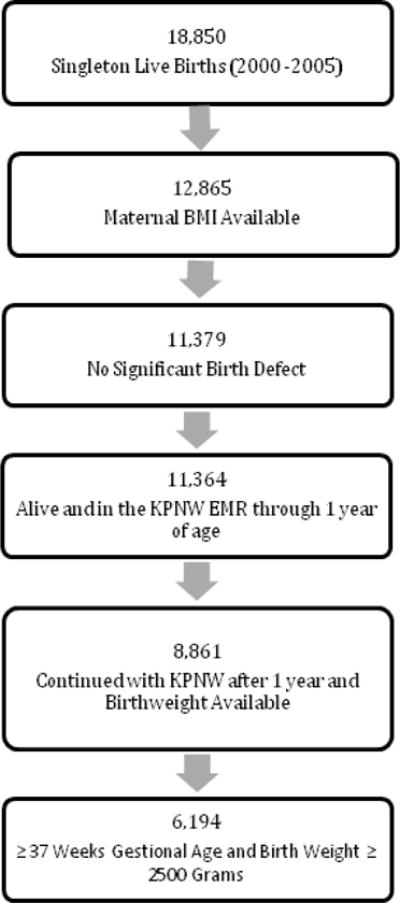
Cohort selection process for pregnancies beginning and ending between 2000 and 2005 (*only first pregnancy episode included for women with more than one pregnancy in the study period)
Study Design
The subjects were stratified into four groups by maternal baseline BMI. We examined the relationship between maternal prepregnancy BMI categories as outlined above and bronchodilator dispensing to their children until their fourth birthday. We first identified the proportion of children in the cohort who had a least one dispensing for an inhaled bronchodilator. Among those with at least one dispensing, we also determined the number of refills to serve as a surrogate of disease severity or recurrence. Lastly, we examined the ICD-9 codes entered at the time of bronchodilator dispensing to evaluate the distribution of diagnoses associated with bronchodilator in this age group.
Statistical Methods
We computed descriptive statistics and examined distributions graphically to identify outliers and/or out of range values, and to guide distributional assumptions. We systematically examined the proportion and pattern of missing values for covariates and found smoking status was missing for <1% of patients and other covariate data was complete. We then compared the four BMI-defined groups on the characteristics of the mothers and their children. For the dichotomous and categorical variables, we present the number of observations and proportions in each group. For the continuous variables, we present means and standard deviations. For each variable we tested the null hypothesis, “no association with maternal BMI category”, via an appropriate test procedure (Kruskal-Wallis, chi-square, or Fisher’s exact test) and tabulated the p value along with descriptive statistics.
Logistic regression was used to examine the unadjusted association (odds ratios [OR] with 95% confidence intervals [95% CI]) between prepregnancy BMI and bronchodilator dispensing up to four years of age (birth to 3.99 years old) (Model 1). Pregravid BMI was entered as a four category variable, with the “normal” category designated as referent. We assessed the contribution of several candidate effect modifying variables, which were added individually and in combination into the logistic regression models along with BMI. These included the maternal demographics of: age at pregnancy onset, race, insurance status, tobacco use, asthma, atopy, chronic diabetes, hypertension, preeclampsia, and gestational diabetes. We also evaluated the gender of baby and Cesarean section delivery as they have been reported to have associations with childhood asthma in previous studies24–30. We utilized a change-in-estimate variable selection strategy - that is, those covariate or covariate combinations that changed the regression coefficients for BMI approximately 10% or more, or were demonstrated in the literature to be important confounders or covariates (e.g. cesarean section deliveries), were retained in the fully adjusted model (Model 2)31. We did not include infant birth weight or infant BMI at time of bronchodilator dispensing in the model since birth weight may be a potential factor in the causal pathway27, and visits that resulted in bronchodilator dispensing often did not have a recorded weight. We evaluated interactions between BMI and each of the retained variables, and retained those that were clinically plausible and had an interaction of p ≤ 0.25 in the model and/or improved the model fit as measured by Akaike information criterion.
In Model 3, our parsimonious final multi-variable model, we assessed the statistical significance of each variable after removing nonsignificant variables from model 2 via Wald tests and the trend across ordinal categories via tests of contrasts. This model included tobacco, BMI, maternal asthma, the gender of the baby, and cesarean section delivery. Additional sensitivity analyses were performed to evaluate the robustness of the results to reasonable perturbations of the choices of statistical methods and assumptions. For example, propensity score matching methods were used to compare (only) the ‘overweight’ group to the ‘normal’ group. The propensity scores were estimated as a function of baseline (at conception) covariates: age, race, insurance status, tobacco use, maternal asthma, maternal atopy, baby gender, and cesarean section delivery. All statistical computations were performed using SAS software, version 9.3. Copyright (C) 2011, SAS Institute Inc., Cary, NC, USA.
Results
We identified 18,850 singleton live births. 5,985 maternal infant dyads were excluded due to missing maternal BMI data secondary to missing heights which were not part of standard clinical practice at Kaiser Permanente Northwest locations during this time period. In addition, 2,503 maternal infant dyads were excluded due to missing birth weights or loss of Kaiser insurance in the first year of life. Ultimately, 6,194 mother – baby pairs met our inclusion criteria (Figure 1). Nearly one-quarter of pregnancies in this cohort were affected by maternal obesity (Table 1). As expected there was a difference across BMI categories for the mother’s weight at the onset of pregnancy. In addition there was a difference across BMI categories for maternal age at pregnancy onset, insurance coverage, and maternal parity ≤ 1, delivery by cesarean section, racial background, maternal tobacco use, and maternal asthma (Table 1).
Table 1.
Maternal Demographics by Prepregnancy Body Mass Index Status
| Demographic values by maternal BMI cohorts | Under Weight | Normal Weight | Over Weight | Obese | P values* |
|---|---|---|---|---|---|
| Number of mother baby pairs | 146 | 2939 | 1653 | 1456 | |
| Weight at onset of pregnancy(lb)a | 104.1±12.5 | 131.1±15.4 | 160.1±16.5 | 213.5±37.0 | < 0.01 |
| Age at onset of pregnancy(years)a | 25.6±5.8 | 28.0±5.6 | 28.2±5.6 | 28.4±5.5 | < 0.01 |
| Married | 78.7% | 84.4% | 81.6% | 80.2% | < 0.01 |
| Medicaid insurance | 8.9% | 4.6% | 4.9% | 8.8% | < 0.01 |
| Parity ≤ 1 | 94.0% | 83.3% | 77.5% | 71.8% | < 0.01 |
| C-section delivery | 18.5% | 19.4% | 25.2% | 34.3% | < 0.01 |
| Whiteb | 57.2% | 71.1% | 74.2% | 77.0% | < 0.01 |
| Asianb | 34.0% | 17.0% | 8.3% | 3.3% | |
| Blackb | 2.1% | 2.9% | 4.3% | 5.2% | |
| Hispanic ethnicityb | 6.2% | 8.1% | 12.1% | 12.8% | |
| Maternal tobaccoc | 14.6% | 10.4% | 11.7% | 15.9% | < 0.01 |
| Maternal atopy | 43.8% | 47.8% | 48.4% | 47.7% | 0.76 |
| Maternal asthma | 9.6% | 13.1% | 16.0% | 22.6% | < 0.01 |
Mean±SD or percentage as indicated
Category percentages slightly less than 100% due to small percent of “other”
Data on tobacco use missing for <1% of patients
The null hypothesis “no differences among the four BMI categories” was tested via a chi-square or Fisher’s exact procedure for the binary variables and via a Kruskal-Wallis procedure for the interval-scale variables. Here, Fisher’s procedure is not exact due to the stochastic marginal counts.
There were statistically significant differences among the BMI categories for mean birth weight, ranging from 3363 grams for infants born to the underweight BMI cohort to 3654 grams for infants born to the obese BMI cohort (p <0.01 by Kruskal-Wallis test across all BMI categories)(Table 2). Similarly there were statistically significant differences for prevalence of macrosomia and prevalence of large for gestational age which were lowest in the underweight group (6.8% and 5.5%, respectively) and highest in the obese group (23.8% and 22.0%) (Table 2). In this primarily insured, non-urban, largely White population, 27.6% of the children received a bronchodilator dispensing. The prevalence of bronchodilator dispensing was 19.2 % among offspring born to women who were underweight prepregnancy, 25.7% among offspring born to women with a normal prepregnancy BMI, 28.5% of those born to women with an overweight prepregnancy BMI and to 31.3% among those born to women with prepregnancy obesity (p <0.01 across all four BMI categories) (Table 2). A difference in the mean age (days) at first bronchodilator dispensing (p <0.01) was seen across the BMI categories with the infants born to the women with an underweight BMI receiving their first bronchodilator dispensing at a mean age of 368 days of age and offspring of women with normal prepregnancy BMI receiving their first dispensing at 501 days of age. There was also an increase in the number of bronchodilator refills in relation to maternal BMI with offspring of obese women receiving a higher number of bronchodilator refills (Table 2).
Table 2.
Infant Demographics and Bronchodilator Dispensing by Maternal Prepregnancy Body Mass Index Status
| Demographic values by maternal BMI cohorts | Under Weight | Normal Weight | Over Weight | Obese | P values* |
|---|---|---|---|---|---|
| Number of mother baby pairs | 146 | 2939 | 1653 | 1456 | |
| Male (%)a | 49.0% | 48.9% | 51.7% | 48.8% | NS |
| Birth weight (g)a | 3363±383 | 3471±428 | 3569±452 | 3654±479 | < 0.01 |
| Gestational age (weeks) a | 39.7±1 | 39.7±1 | 39.7±1 | 39.6±1 | NS |
| Birth length (cm)a, b | 51.2±2.3 | 51.5±2.5 | 51.8±2.5 | 51.9±2.4 | < 0.01 |
| Fetal macrosomia | 6.8% | 10.7% | 15.9% | 23.8% | < 0.01 |
| Large for gestational age | 5.5% | 9.2% | 14.6% | 22.0% | < 0.01 |
| Weight at 1 year (lb) n=6121 | 18.9±3.5 | 19.9±3.4 | 20.3±4.6 | 20.6±3.3 | < 0.01 |
| Weight at 2 year (lb) n=5801 | 24.4±2.8 | 25.5±3.4 | 26.3±4.7 | 26.5±4.1 | < 0.01 |
| Weight at 3 year (lb) n=4618 | 28.8±3.8 | 30.3±4.1 | 31.0±6.0 | 31.7±4.8 | < 0.01 |
| Percent of patients receiving a bronchodilator dispensing | 19.2% | 25.7% | 28.5% | 31.3% | < 0.01 |
| Number of bronchodilator refillsa | 0.58±1.9 | 0.58±1.9 | 0.66±1.6 | 0.78±1.9 | < 0.01 |
| Age at first dispensing (days)a | 368±270 | 501±370 | 453±336 | 403±295 | < 0.01 |
Mean±SD or percentage as indicated
NS= Not significant
Birth lengths available for the offspring of 139 underweight, 2710 normal weight, 1537 overweight, and 1315 obese mothers
The null hypothesis “no differences among the four BMI categories” was tested via a chi-square or Fisher’s exact procedure for the binary variables and via a Kruskal-Wallis procedure for the interval-scale variables and count variables. Here, Fisher’s procedure is not exact due to the stochastic marginal counts.
In the unadjusted model (Table 3), children born to mothers who were overweight or obese prepregnancy had a 15% (OR 1.15; 95% CI 1.01–1.32) and 31% higher odds (OR 1.31; 95% CI 1.14–1.51), respectively of a bronchodilator dispensing than children of mothers with a normal prepregnancy BMI. In the fully adjusted model, we examined the effect of maternal age with control of pregnancy onset, race, insurance status, tobacco use, maternal asthma, maternal atopy, gender of baby, cesarean section delivery, and the interaction term of BMI and tobacco as meeting the variable selection strategy for covariates or covariate combinations of the bronchodilator dispensing rate (Table 3, Model 2). In this model there continued to be an association between prepregnancy obesity and bronchodilator dispensing in the offspring.
Table 3.
Models and Associations between Prepregnancy BMI and Bronchodilator Dispensing
| Model 1, Unadjusted a | Model 2, Fully Adjusted b | Model 3, Parsimonious Adjustmentc,d | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% confidence interval | p value | Odds ratio | 95% confidence interval | p value | Odds ratio | 95% confidence interval | p value | |
| Body Mass Index | <0.001 | 0.008 | 0.007 | ||||||
| Underweight(n=146) | 0.69 | 0.45–1.04 | 0.68 | 0.44–1.04 | 0.70 | 0.46–1.07 | |||
| Normal (n= 2939) | Ref | – | Ref | – | Ref | ||||
| Overweight (N=1653) | 1.15 | 1.01–1.32 | 1.12 | 0.97–1.28 | 1.12 | 0.98–1.29 | |||
| Obese(n=1456) | 1.31 | 1.14–1.51 | 1.22 | 1.05–1.41 | 1.22 | 1.06–1.41 | |||
Model 1 includes no adjustments for potential confounders
Model 2 is adjusted for potential confounders of maternal age, race, insurance status, tobacco use, maternal asthma, maternal atopy, gender of baby, cesarean section delivery
Model 3 includes adjustments for statistically significant potential covariates of tobacco use, maternal asthma, and the gender of baby
cesarean section delivery was included but not statistically significant
P values by logistic regression with variables accounted for as outlined above for each model.
From the fully adjusted model, maternal tobacco use, maternal asthma, and the gender of the offspring were found to be statistically significant for the association between prepregnancy BMI and bronchodilator dispensing across all cohorts. These variables were included in model 3 which was the parsimonious adjustment model. Cesarean section delivery was not found to be statistically significant but was also included in model 3 because previous reports suggest it is an important variable in this relationship32. After adjusting for the statistically significant variables as well as for cesarean section delivery, maternal prepregnancy obesity continued to significantly increase the odds of bronchodilator dispensing (OR 1.22; 95% CI 1.06–1.41) (Table 3). In the context of sensitivity analysis, we obtained a nearly identical estimate (OR=1.21) when we applied a competing statistical approach, propensity score matching, to the task of comparing the obese and normal BMI categories.
We also examined individual characteristics and outcomes from the fully adjusted model 2 (see Table 4). Hispanic ethnicity was associated with increased bronchodilator dispensing with an odds ratio of 1.24 (95% CI 1.02–1.50) across all cohorts after adjustment for other covariates. The adjusted OR was similar for Black race, OR = 1.30 (95% CI 0.96–1.77), but the test of association was inconclusive (p>0.05). (The proportion of patients identified as Black was less than 4% in this study). Infants delivered by cesarean section in the adjusted cohort did not have an increased odds for bronchodilator dispensing 0.95 (95% CI 0.83–1.08; p=0.44) (Table 4).
Table 4.
Individual Characteristics and Outcomes in the Fully Adjusted Model (Model 2)
| OR | 95% CI | p value | |
|---|---|---|---|
| Maternal Demographics | 0.22 | ||
| Age at pregnancy onset | 0.993 | 0.98–1.00 | |
| Race | 0.09 | ||
| White | Ref | – | |
| Asian | 1.08 | 0.89–1.32 | |
| Black | 1.30 | 0.96–1.77 | |
| Hispanic Ethnicity | 1.24 | 1.02–1.50 | |
| Native American | 0.52 | 0.23–1.22 | |
| Other | 1.15 | 0.68–1.93 | |
| Unknown | 0.93 | 0.74–1.16 | |
| Insurance Status | 0.21 | ||
| Private | Ref | – | |
| Medicaid | 1.17 | 0.92–1.49 | |
| Maternal Health Characteristics | |||
| Tobacco Use | 1.33 | 1.12–1.58 | <0.01 |
| Asthma | 1.96 | 1.70–2.28 | <0.01 |
| Atopy | 1.11 | 0.99–1.23 | 0.07 |
| Hypertension | 0.98 | 0.54–1.82 | 0.97 |
| Preeclampsia | 0.85 | 0.70–1.04 | 0.12 |
| Gestational Diabetes | 0.97 | 0.71–1.33 | 0.85 |
| Diabetes | 0.93 | 0.71–1.21 | 0.57 |
| Pregnancy Characteristics | |||
| C-section Delivery | 0.95 | 0.83–1.09 | 0.44 |
| Gender (F vs. M) | 0.66 | 0.59–0.74 | <0.01 |
The null hypothesis of no association was tested via logistic regression with the response variable of bronchodilator dispensing and the displayed variables in the table as independent variables to obtain the displayed estimates.
Maternal smoking is a known risk factor for childhood lung health and was associated with bronchodilator dispensing in this study. We assessed the synergistic effects of tobacco use and BMI on bronchodilator dispensing. Interestingly we found an increase for bronchodilator dispensing in nonsmokers (p <0.05), but not in smokers (p = 0.50) in relation to BMI. Children born to non-smoking obese mothers had an increased odds for bronchodilator dispensing, OR=1.30 (95% CI 1.12–1.52, p<0.05), compared to children of non-smoking normal BMI mothers. Among smokers, the same hypothesis test was inconclusive, OR=1.12 (95% CI 0.97–1.30, p=0.50); the confidence interval suggests that substantial association, and no association, are both plausible.
ICD-9Diagnostic Codes
ICD-9 coding was recorded for 79% of the visits (1357 of 1710) that were associated with bronchodilator dispensing across all cohorts. Thirty nine unique diagnostic codes were entered. ICD-9 code 786 or “symptoms including the respiratory system” was the most frequently cited at 28%. Fifteen total sub-codes from 786 were used, with 786.2 (cough) being the largest at 14%. “Diseases of the respiratory system” (ICD-9 code 464.4) was cited for 22% of the visits. Asthma (ICD-9 493) was entered for 14% of the visits, with 493.9 (intrinsic asthma) the largest sub-code at 11%. The codes for pneumonia and bronchitis completed the top five ICD-9 codes associated with bronchodilator dispensing.
Discussion
The novel finding of this study is a statistically significant association in the objective outcome of EMR documented bronchodilator dispensing to offspring/children of women with prepregnancy obesity in an insured, nonurban largely White population in the United States. Compared to studies examining bronchodilator prescriptions and links to childhood asthma33;34, our population was limited to the first four years of life, utilized EMR documented bronchodilator dispensing rates and was not linked to a diagnosis of asthma. In contrast to our study, previous studies in the United States of maternal obesity and offspring lung health in similar aged patients (approximately three years) utilized parental recall, questionnaires, and chart review to establish a diagnosis of asthma or recurrent wheezing episodes as indicators of lung health8;10. These investigations included large urban, disadvantaged, or minority populations, groups known to have higher rates of asthma35;36. Reichman et al10 and Kumar et al8 independently found prepregnancy obesity (BMI > 30 kg/m2) was associated with increased risk for asthma diagnosis or recurrent wheezing. Our adjusted odds ratio of 1.22 for dispensed bronchodilators in the children of women with prepregnancy obesity was lower than the odds ratio of 3.0 reported by Kumar et al8 for recurrent wheezing. This difference is likely due our use of EMR documented bronchodilator dispensing versus the use of parental reported wheezing episodes or survey responses with the potential for recall bias and systematic error37;38. The difference in the odds ratios for treatment is also likely due to our unique population from which we excluded children with known risk factors for wheezing including multiple gestation, prematurity and small for gestational age.
Strengths of our study include our unique United States population which has not previously been reported, strict exclusion criteria for maternal factors known to increase childhood wheeze, and EMR documented bronchodilator dispensing. Lowe et al7 using a similar marker examined purchase rates for inhaled steroids and montelukast in Sweden as a surrogate for asthma diagnosis and demonstrated an association with maternal BMI. Kumar et al8 also found maternal overweight (BMI >25 but < 30) influenced recurrent wheezing rates, which parallels the increased dispensing rate found in a similar BMI group in our report. The severity of recurrent wheeze or asthma has not been addressed in previous studies of maternal BMI and early lung health. We examined the refill rate of wheezing medications as a surrogate for severity. Meter dose inhalers are the most common form of bronchodilators prescribed and provide upwards of 80 treatments (200 puffs) per canister at standard doses and use guidelines. Thus an increase in refill rate suggests either frequent use or returning visits for a similar complaint and prescription redispensing. In our study, we report an increase in the number of bronchodilator refills (Table 2) as maternal prepregnancy BMI increases. Maternal smoking during pregnancy is well known to be associated with early childhood wheeze and subsequent asthma, and to affect lung development as evidenced by altered pulmonary function tests before the onset of viral infections39;40. Women who are unable to quit smoking during pregnancy are highly likely to continue to smoke post-delivery and postnatal second hand smoke exposure has also been associated with increased wheezing in the offspring41.
In this dataset, we found an increased risk of bronchodilator dispensing for offspring of the smokers, adjusted OR=1.33 95% CI [1.12, 1.58], compared to those not smoking during pregnancy. In subgroup analyses comparing obese BMI to normal BMI categories on bronchodilator dispensing, the estimated odds ratio in non-smokers was OR=1.30 95% CI [1.12, 1.52]) and the odds ratio in smokers was OR=1.12 95% CI [0.97, 1.30].This could be due to the relatively small percentage (about 12%) of the total cohort being categorized as a smoker and to potential underreporting of smoking status in this insured population. A 2012 United States report showed that 21.8 percent of pregnant White women aged 15 to 44 had smoked a cigarette within 30 days of being interviewed42. Smoking status in this dataset was from self-report, which has historically been shown to underestimate the true prevalence of smoking in pregnancy43, and no objective biomarker validation of smoking status was performed in this study. Alternatively, this finding may suggest that the effect of obesity on subsequent offspring respiratory health becomes less important/impactful in the face of maternal prepregnancy obesity. Further evaluation of the interaction between maternal BMI, smoking during pregnancy, and subsequent bronchodilator dispensing in the offspring may require prospective evaluations with biomarker confirmation of smoking status.
Other maternal factors such as socioeconomic status, race and ethnicity, cesarean section delivery, and maternal asthma have been associated with the development of childhood respiratory disease. After adjustments for significant individual covariates, the odds ratio of bronchodilator dispensing across the BMI cohorts decreased, but remained statistically significant for the obese cohort. Although Cesarean section delivery was not a significant covariate in our analysis, a large Norweigan cohort32 (sample size of 1,000,000 persons) with a patient population similar to our study demonstrated a statistically significant association of Cesarean section delivery and infant wheezing so we retained this variable in all of our models. Our parsimonious model (Model 3) confirmed the association of maternal asthma and male gender with early life wheezing.
Our study has limitations. Lower tract respiratory infections are most likely to be associated with wheeze and cough in the age group studied, thus having more clinical data at the time of dispensing could have established a better link to causation. Although we documented yearly childhood weights through age 3, more detailed weight data at the time of bronchodilator dispensing could have allowed better evaluation of the possible association between early childhood obesity and early bronchodilator use. Additional analysis of individual birthweight by maternal BMI cohort could have strengthened the study because of the known associations of low birthweight and childhood lung health. A large analysis of obese pregnancy and outcomes showed most infants were carried to term and had normal to large birthweights44. Thus, our approach of restricting birthweight limited the influence of the low-birth weight infant, but did not control for large birth weight infants. Subject selection and/or missing data may have affected the generalizability of our results. The majority of missing data was due to missing maternal heights, missing newborn birth weights, and loss of Kaiser insurance in the first year of life. These data should be missing at random and not be related to maternal BMI so this missing data is unlikely to have affected the generalizability of the results. It is also plausible, although unclear, that maternal variables such as gestational diabetes, preeclampsia, macrosomia and others could be in the causal pathway from BMI to bronchodilator dispensing. Maternal weight at the onset of pregnancy was an approximation based on EMR entry through the first trimester in the original dataset. Because the average weight gain in pregnant women through that time point is only between 0.4 and 2.0 kg, and well within the individual ranges for the four maternal BMI categories, the risk for mis-classification of maternal BMI is very low.
Other environmental and lifestyle factors we did not have data to evaluate include breast feeding, infant nutrition, attendance at daycare, secondhand smoke exposure, respiratory illness in other family members, socio-economic factors, factors related to physical activity, life-style and occupation, and other interactions not represented in the model-based analysis. These unmeasured potential confounders may have introduced bias into the data analysis and may have substantially biased our estimates of association between prepregnancy BMI and bronchodilator dispensings.
In conclusion, in this study we observed a statistically significant association between electronic medical record documented bronchodilator dispensing to offspring of women with prepregnancy obesity in an insured, nonurban largely White population in the United States. Although unmeasured potential variables may have biased our results, this study appears to extend similar findings documented in urban, disadvantaged or minority populations and emphasizes the impact that obesity may have on health outcomes. It also supports the continuation of rigorous prospective investigations in the role of prepregnancy obesity and its potential association with early respiratory symptoms of the offspring. Lastly, our study adds to the growing body of evidence that preconception weight management could lead to improved childhood health.
Acknowledgments
Work was supported by National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR000128. National Institutes of Health/NIHCD HD057588 02 (KDM) Parker B Francis Fellowship (KDM). NIH, National Heart Lung Blood Institute, K23 HL080231 and R01 HL105447 with cofunding from the Office of Dietary Supplement (CTM). The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Footnotes
The authors all report no conflicts of interest to disclose.
References
- 1.Bush A. Diagnosis of asthma in children under five. Prim Care Respir J. 2007;16(1):7–15. doi: 10.3132/pcrj.2007.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282(15):1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 3.Akinbami LJ, Schoendorf KC. Trends in childhood asthma: prevalence, health care utilization, and mortality. Pediatrics. 2002;110(2):315–322. doi: 10.1542/peds.110.2.315. [DOI] [PubMed] [Google Scholar]
- 4.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123:S131–S145. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 5.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, Xiang L. Trends in Asthma Prevalence, Health Care Use, and Mortality in the United States, 2001–2010. NCHS Data Brief. 2012;(94) [PubMed] [Google Scholar]
- 6.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 7.Lowe A, Braback I, Ekeus C, Hjern A, Forsberg B. Maternal obesity during pregnancy as a risk for early-life asthma. J Allergy Clin Immunol. 2011;128(5):1107–1109 e1–2. doi: 10.1016/j.jaci.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 8.Kumar R, Story RE, Pongracic JA, Hong X, Arguelles L, Wang G, Kuptsova-Clarkson N, Pearson C, Ortiz K, Bonzagni A, et al. Maternal pre-pregnancy obesity and recurrent wheezing in early childhood. Pediatr Allergy Immunol Pulmonol. 2010;23(3):183–190. doi: 10.1089/ped.2010.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leermakers ET, Sonnenschein-van der Voort AM, Gaillard R, Hofman A, de Jongste JC, Jaddoe VW, Duijts L. Maternal weight, gestational weight gain and preschool wheezing. The Generation R Study. Eur Respir J. 2013;42:1234–1243. doi: 10.1183/09031936.00148212. [DOI] [PubMed] [Google Scholar]
- 10.Reichman NE, Nepomnyaschy L. Maternal pre-pregnancy obesity and diagnosis of asthma in offspring at age 3 years. Matern Child Health J. 2008;12(6):725–33. doi: 10.1007/s10995-007-0292-2. [DOI] [PubMed] [Google Scholar]
- 11.Scholtens S, Wijga AH, Brunekreef B, Kerkhof M, Postma DS, Oldenwening M, de Jongest JC, Smit HA. Maternal overweight before pregnancy and asthma in offspring followed for 8 years. Int J Obes. 2010;34(4):606–613. doi: 10.1038/ijo.2009.194. [DOI] [PubMed] [Google Scholar]
- 12.Haberg SE, Stigum H, London SJ, Nystad W, Nafstad P. Maternal obesity in pregnancy and respiratory health in early childhood. Paediatr Perinat Epidemiol. 2009;23(4):352–362. doi: 10.1111/j.1365-3016.2009.01034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pike KC, Inskip HM, Robinson SM, Cooper C, Godfrey KM, Roberts G, Lucas JS. The relationship between maternal adiposity and infant weight gain, and childhood wheeze and atopy. Thorax. 2013;68(4):372–379. doi: 10.1136/thoraxjnl-2012-202556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boehmer AL, Merkus PJ. Asthma therapy for children under 5 years of age. Curr Opin Pulm Med. 2006;12(1):34–41. doi: 10.1097/01.mcp.0000199810.42011.8e. [DOI] [PubMed] [Google Scholar]
- 15.Vasudevan C, Renfrew M, McGuire W. Fetal and perinatal consequences of maternal obesity. Archives of Disease in Childhood - Fetal and Neonatal Edition. 2011;96(5):F378–F382. doi: 10.1136/adc.2009.170928. [DOI] [PubMed] [Google Scholar]
- 16.Guerra S, Sartini C, Mendez M, Morales E, Guxens M, Basterrechea M, Arranz L, Sunyer J. Maternal prepregnancy obesity is an independent risk factor for frequent wheezing in infants by age 14 months. Paediatr Perinat Epidemiol. 2013;27(1):100–108. doi: 10.1111/ppe.12013. [DOI] [PubMed] [Google Scholar]
- 17.Bush A. Practice Imperfect - Treatment for Wheezing in Preschoolers. N Engl J Med. 2009;360(4):409–410. doi: 10.1056/NEJMe0808951. [DOI] [PubMed] [Google Scholar]
- 18.Hornbrook MC, Whitlock Ep, Berg CJ, Callaghan WM, Backman DJ, Gold R, Bruce FC, Deitz PM, Williams SB. Development of an algorithm to identify pregnancy episodes in an integrated health care delivery system. Health Serv Res. 2007;42(2):908–927. doi: 10.1111/j.1475-6773.2006.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasmussen KM, Yaktine AL. Weight gain during pregnancy: reexamining the guidelines. The National Academies Press. 20 [PubMed] [Google Scholar]
- 20.Sharma AJ, Vesco KK, Bulkley J, et al. Associations of Gestational Weight Gain with Preterm Birth among Underweight and Normal Weight Women. Matern Child Health J. 2015;19:2066–2073. doi: 10.1007/s10995-015-1719-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vesco KK, Dietz PM, Rizzo J, et al. Excessive gestational weight gain and postpartum weight retention among obese women. Obstet Gynecol. 2009;114:1069–1075. doi: 10.1097/AOG.0b013e3181baeacf. [DOI] [PubMed] [Google Scholar]
- 22.Vesco KK, Sharma AJ, Dietz PM, et al. Newborn size among obese women with weight gain outside the 2009 Institute of Medicine recommendation. Obstet Gynecol. 2011;117:812–818. doi: 10.1097/AOG.0b013e3182113ae4. [DOI] [PubMed] [Google Scholar]
- 23.Been JV, Lugtenberg MJ, Smets E, van Schayck CP, Kramer BW, Mommers M, Sheikh A. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med. 2014;11(1):e1001596. doi: 10.1371/journal.pmed.1001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abbott S, Becker P, Green RJ. The relationship between maternal atopy and childhood asthma in Pretoria, South Africa. ISRN Allergy. 2013;2013:4. doi: 10.1155/2013/164063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brew BK, Marks GB. Perinatal factors and respiratory health in children. Clin Exp Allergy. 2012;42(11):1621–1629. doi: 10.1111/j.1365-2222.2012.04056.x. [DOI] [PubMed] [Google Scholar]
- 26.Burke W, Fesinmeyer M, Reed K, Hampson L, Carlsten C. Family history as a predictor of asthma risk. Am J Prev Med. 2003;24(2):160–169. doi: 10.1016/s0749-3797(02)00589-5. [DOI] [PubMed] [Google Scholar]
- 27.Caudri D, Wijga A, Gehring U, Smit HA, Brunekreef B, Kerkhof M, Hoekstra M, Gerritsen J, de Jongste JC. Respiratory symptoms in the first 7 years of life and birth weight at term: the PIAMA Birth Cohort. Am J Respir Crit Care Med. 2007;175(10):1078–1085. doi: 10.1164/rccm.200610-1441OC. [DOI] [PubMed] [Google Scholar]
- 28.Crespo NC, Ayala GX, Vercammen-Grandjean CD, Slymen DJ, Elder JP. Socio-demographic disparities of childhood asthma. J Child Health Care. 2011;15(4):358–369. doi: 10.1177/1367493510397680. [DOI] [PubMed] [Google Scholar]
- 29.Gilliland FD, Li YF, Peters JM. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am J respir Crit Care Med. 2001;163(2):429–436. doi: 10.1164/ajrccm.163.2.2006009. [DOI] [PubMed] [Google Scholar]
- 30.Roduit C, Scholtens S, de Jongste JC, Wijga AH, Gerritsen J, Postma DS, Brunekreef B, Hoekstra MO, Aalberse R, Smit HA. Asthma at 8 years of age in children born by caesarean section. Thorax. 2009;64:107–113. doi: 10.1136/thx.2008.100875. [DOI] [PubMed] [Google Scholar]
- 31.Walter S, Tiemeier H. Variable selection: current practice in epidemiological studies. Eur J Epidemiol. 2009;24(12):733–736. doi: 10.1007/s10654-009-9411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magnus MC, Haberg Se, Stigum H, Nafstad P, London Sj, Vangen S, Nystad W. Delivery by cesarean section and early childhood respiratory symptoms and disorders: The Norwegian mother and child cohort study. Am J Epidemiol. 2011;174(11):1275–1285. doi: 10.1093/aje/kwr242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furu K, et al. Use of anti-asthmatic medications as a proxy for prevalence of asthma in children and adolescents in Norway: a nationwide prescription database analysis. Eur J Clin Pharmacol. 2007;63(7):693–698. doi: 10.1007/s00228-007-0301-9. [DOI] [PubMed] [Google Scholar]
- 34.Moth G, Vedsted P, Schiotz P. Identification of asthmatic children using prescription data and diagnosis. Eur J Clin Pharmacol. 2007;63(6):605–611. doi: 10.1007/s00228-007-0286-4. [DOI] [PubMed] [Google Scholar]
- 35.Evans R., III Asthma among minority children: A growing problem. Chest. 1992;101:368S–371S. [PubMed] [Google Scholar]
- 36.Flores G, Snowden-Bridon C, Torres S, Perz R, Walter T, Brotanek J, Lin H, Tomany-Korman S. Urban minority children with asthma: substantial morbidity, compromised quality and access to specialists, and the importance of poverty and specialty care. J Asthma. 2009;46(4):392–398. doi: 10.1080/02770900802712971. [DOI] [PubMed] [Google Scholar]
- 37.Magnus P, Jaakkola JJ. Secular trend in the occurrence of asthma among children and young adults: critical appraisal of repeated cross sectional surveys. BMJ. 1997;314(7097):1795. doi: 10.1136/bmj.314.7097.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smeeton NC, Rona RJ, Oyarzun M, Diaz PV. Agreement between responses to a standardized asthma questionnaire and a questionnaire following a demonstration of asthma symptoms in adults. Am J Epidemiol. 2006;163(4):384–391. doi: 10.1093/aje/kwj052. [DOI] [PubMed] [Google Scholar]
- 39.Tager IB, Weiss St, Munoz A, Rosner B, Speizer FE. Longitudinal study of the effects of maternal smoking on pulmonary function in children. N Engl J Med. 1983;309(12):699–703. doi: 10.1056/NEJM198309223091204. [DOI] [PubMed] [Google Scholar]
- 40.McEvoy CT, Schilling D, Clay N, Jackson K, Go MD, Spitale P, Bunten C, Leiva M, Gonzales D, Hollister-Smith J, et al. Vitamin C supplementation for pregnant smoking somen and pulmonary function in their newborn infants a randomized clinical trial. JAMA. 2014;311:2074–2082. doi: 10.1001/jama.2014.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burke H, Leonardi-Bee J, hashim A, Pine-Abata H, Chen Y, Cook DG, Britton JR, McKeever TM. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129(4):735–744. doi: 10.1542/peds.2011-2196. [DOI] [PubMed] [Google Scholar]
- 42.Substance Abuse and Mental Health Services Administration. Substance Use during pregnancy varies by race and ethnicity. Data Spotlight. 2012;1 [Google Scholar]
- 43.Cnattingius S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tobacco Res. 2004;6:S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 44.Sebire NJ, Jolly M, Harris JP, et al. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord. 2001;25:1175–1182. doi: 10.1038/sj.ijo.0801670. [DOI] [PubMed] [Google Scholar]


