Abstract
Most drug-drug interactions involve overlap or competition in drug metabolic pathways. However, there are medications, typically resins, whose function is to bind injurious substances such as bile acids or potassium within the digestive tract. The objective of this article is to review the functions of the stomach and the kinetics of emptying of different food forms or formulations to make recommendations on timing of medication administration in order to avoid intragastric drug interactions. Based on the profiles and kinetics of emptying of liquid nutrients and homogenized solids, a window of 3 hours between administration of a resin drug and another “target” medication would be expected to allow a median of 80% of medications with particle size <1mm to empty from the stomach and, hence, avoid potential interaction such as binding of the “target” medication within the stomach.
Keywords: gastroparesis, resin, solid, liquid, particle, trituration
Introduction
The treatment of gastroparesis entails co-administration of different pharmacological agents that may be metabolized via the same metabolic pathway(s), such as cytochrome P450 enzymes (1). Inhibition or induction of a shared metabolic pathway may lead to changes in systemic levels of prescribed drugs, potentially leading to undesired drug-drug interactions (DDIs). A recent review (1) addressed pharmacokinetic interactions, particularly those related to metabolic interactions. Other potential interactions may occur from differences in absorption, excretion and distribution of the drugs. From a gastroenterology perspective, the potential for interactions in the digestive tract is of significant interest, as it may impact on the optimal timing between the ingestion of medications. There are medications which work by binding intraluminal content in the gastrointestinal tract; examples include resins (e.g. cholestyramine, colesevelam) to bind bile acids in bile acid diarrhea or phosphorus [typically cations such as Al3+, Fe3+, Ca2+, Mg2+ (2)] or potassium [such as patiromer (3)] in chronic renal failure. Hyperkalemia is associated with life-threatening cardiac arrhythmias and sudden death. Although agents inhibiting the renin-angiotensin-aldosterone-system (RAAS) are currently the first-line treatments toward cardio- and nephroprotection in patients with diabetes or heart failure, their administration often leads to hyperkalemia and treatment discontinuation. Two new oral potassium-exchanging compounds, patiromer and sodium zirconium cyclosilicate, were shown in recent randomized controlled trials to normalize elevated serum potassium and maintain potassium homeostasis in the longer term in hyperkalemic patients treated with RAAS blockers (4). Therefore, treatment with such resins is relevant to a potentially life-threatening disease (hyperkalemia) and occurs in a setting that may be associated with gastroparesis, such as diabetes with chronic renal failure.
The objective of this article is to review the kinetics of emptying of different food forms or formulations from the stomach in order to identify optimal timing of medication administration to avoid intragastric drug interactions.
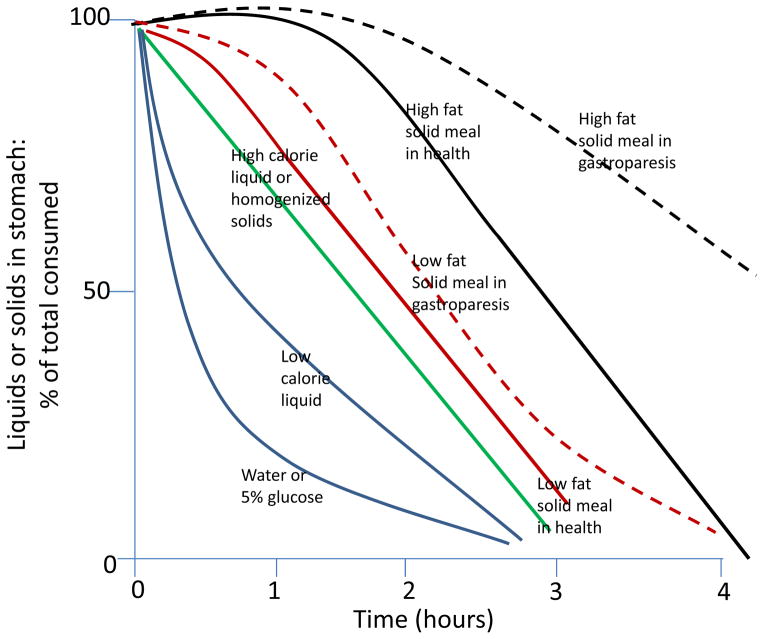
As shown in Figure 1 (5), different meals are emptied from the stomach at different rates, based on physical consistency (6), fat content and total caloric load. Liquids of low caloric density empty exponentially from the stomach under the pressure gradient between fundic tone and the resistance to flow provided by the pylorus. Higher caloric liquids or homogenized solids empty almost linearly from the stomach (7). Digestible food of more solid consistency requires antral trituration which sets up liquid shearing forces that reduce particle size to <2mm (8). Until particles are reduced by these forces to <2mm, there is a lag before solid emptying can start. After trituration, food empties linearly from the stomach at a rate similar to that of a homogenized solid meal (9,10). It is conceivable, given the diameter of the open human pylorus, that somewhat larger particles may be emptied from the stomach during the linear post-lag gastric emptying phase. Unfortunately, there are no human studies analogous to those performed by Meyer et al. in the dog in which the descending duodenal contents were exteriorized through chronic duodenal fistulas, and the particle size was determined by sieving of the duodenal chyme. Thus, at present, we cannot be sure that digestible particles of >1mm in size can empty from the human stomach. A corollary is that particles <1mm in diameter [such as resin products or patiromer (suspension with particle size <0.1mm)], as well as high caloric liquids [such as nutritional supplements like Ensure® (7)] or homogenized solids do not require trituration and are emptied linearly from the stomach.
Figure 1.
Patterns of gastric emptying of liquids and solids in health and in gastroparesis. Gastric emptying curves for liquids and solids were derived from the published literature. Low fat solid meal is a 2% fat, 255kcal meal; high fat meal is 32% fat, 296kcal meal.
Reproduced with permission from ref. 5, Camilleri M, Shin A. Dig Dis Sci 2013;58:1813–5.
Gastroparesis is associated with significantly delayed gastric emptying in the absence of mechanical obstruction and cardinal symptoms that include early satiety, postprandial fullness, nausea, vomiting, bloating and upper abdominal pain (11). Decreased postprandial antral motility prolongs the gastric emptying time for solids by prolonging the time to complete trituration (lag duration) and slows the rate of post-lag emptying (12); intestinal dysmotility retards the gastric emptying rate, typically without prolonging the lag phase of gastric emptying (12).
Diabetes, postsurgical, idiopathic or postviral gastroparesis are the most common associated conditions; more rarely, gastroparesis is associated with extrinsic neurological disorders including Parkinsonism or myopathic disorders such as scleroderma (11). Whereas upper gastrointestinal symptoms are as frequent in patients with type 1 or 2 diabetes as in nondiabetic community controls (13), the prevalence of objectively delayed gastric emptying of solids among diabetics from the same community is much lower, probably close to 5% (14). Therefore, it is relevant to note that intragastric DDIs, based on gastroparesis, are likely to be relatively infrequent from a community or public health perspective.
Scintigraphy is still widely used in tertiary centers for measurement of gastric emptying. A low fat (2%) EggBeaters® (chicken egg white) radiolabeled meal (15) is regarded as the gold standard due to standardized procedures, methods, and well-established normal values (15). Significant delay is documented by at least 10% retention at 4 hours with the EggBeaters® meal, and >25% retention at 4 hours with the 30% fat meal (16).
In healthy adults, the gastric emptying T1/2 rates of Ensure Plus® (liquid nutrient meal) and of an easily triturated, 2% fat Eggbeaters® meal (egg white sandwich) were 84.6±28.8 (SD) and 91.2±20.9 minutes respectively (7).
An alternative scintigraphic approach uses a 30% fat, 320kcal meal; this method is well-validated with normal data from 319 healthy controls and reported performance characteristics (16). With this meal, gastric emptying T1/2 values (16) were 127.7±28.7 (SD) minutes in females (n=214) and 109.9±28.6 minutes in males (n=105). Overall, the proportion of solids emptied from the stomach was 50% (37–64 [median ((IQR))]) at 2 hours and 96% (88–98) at 4 hours (16) in healthy controls, and 35% (24–43 [median ((IQR))]) at 2 hours and 75% (58–80) at 4 hours among 46 patients with diabetes and delayed gastric emptying (17).
In a comparison of 25 unselected, mechanically ventilated patients) and 14 healthy subjects of similar ages,, Chapman et al. demonstrated that gastric emptying of liquid nutrient (radiolabeled Ensure®) was delayed in ~50% of critically ill patients (18) with severe gastroparesis. Delayed emptying by this scintigraphic test was documented by T1/2 of >89.8 minutes and retention at 60, 120 and 180 minutes of >73%, >32% and >13% respectively. Among these patients, the median retention at 3 hours (180 minutes) was 16% (range 0–97; normal <13% retention). Given the results from the comparison of egg white sandwich to liquid nutrient meal emptying (7), it is reasonable to assume that the median retention of a small particle <1mm meal would mimic that of the liquid nutrient and, therefore, <20% median would be retained in the stomach at 3 hours in patients with severe gastroparesis.
The conclusion is that a window of 3 hours would be expected to allow a median of at least 80% of a resin or medication with particle size <1mm to empty from the stomach and, hence, to avoid potential interactions such as binding within the stomach of a target medication. Such separation would potentially avoid DDIs resulting from delayed gastric emptying, as long as the binder drug has a particle size <1mm. Ingestion of food within the three-hour time window after ingestion of the resin would require “re-starting the clock” to the time of ingesting that food. Given the potential for such DDIs, it is recommended that there should be a strong clinical indication for prescription of such resins and no alternative for that clinical indication, e.g. in patients with hyperkalemia due to chronic renal failure or administration of RAAS blockers.
Key Messages.
The objective of this article is to review the functions of the stomach and the kinetics of emptying of different food forms or formulations in order to make recommendations on timing of medication administration to avoid intragastric drug interactions.
The basic methodology of the study was a review of the published data on the emptying of different foods from the stomach.
A window of 3 hours would be expected to allow a median of at least 80% of a resin-like or other medication with particle size <1mm to empty from the stomach and, hence, to avoid potential interaction such as binding within the stomach.
Acknowledgments
Funding support: The author is supported by grant R56-DK67071 from National Institutes of Health.
Footnotes
Disclosures: The author serves on an advisory board for Relypsa.
References
- 1.Youssef AS, Parkman HP, Nagar S. Drug-drug interactions in pharmacologic management of gastroparesis. Neurogastroenterol Motil. 2015;27:1528–41. doi: 10.1111/nmo.12614. [DOI] [PubMed] [Google Scholar]
- 2.Cannata-Andía JB, Martin KJ. The challenge of controlling phosphorus in chronic kidney disease. Nephrol Dial Transplant. 2015 Mar 13; doi: 10.1093/ndt/gfv055. pii: gfv055. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, Wittes J, Christ-Schmidt H, Berman L, Pitt B OPAL-HK Investigators. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372:211–21. doi: 10.1056/NEJMoa1410853. [DOI] [PubMed] [Google Scholar]
- 4.Sarafidis PA, Georgianos PI, Bakris GL. Advances in treatment of hyperkalemia in chronic kidney disease. Exp Opin Pharmacother. 2015;16:2205–15. doi: 10.1517/14656566.2015.1083977. [DOI] [PubMed] [Google Scholar]
- 5.Camilleri M, Shin A. Editorial: Novel and validated approaches for gastric emptying scintigraphy in patients with suspected gastroparesis. Dig Dis Sci. 2013;58:1813–5. doi: 10.1007/s10620-013-2715-9. [DOI] [PubMed] [Google Scholar]
- 6.Kelly KA. Gastric emptying of liquids and solids: roles of proximal and distal stomach. Am J Physiol. 1980;239:G71–6. doi: 10.1152/ajpgi.1980.239.2.G71. [DOI] [PubMed] [Google Scholar]
- 7.Sachdeva P, Kantor S, Knight LC, Maurer AH, Fisher RS, Parkman HP. Use of a high caloric liquid meal as an alternative to a solid meal for gastric emptying scintigraphy. Dig Dis Sci. 2013;58:2001–6. doi: 10.1007/s10620-013-2665-2. [DOI] [PubMed] [Google Scholar]
- 8.Meyer JH, Thomson JB, Cohen MB, Shadchehr A, Mandiola SA. Sieving of solid food by the canine stomach and sieving after gastric surgery. Gastroenterology. 1979;76:804–13. [PubMed] [Google Scholar]
- 9.Camilleri M, Malagelada JR, Brown ML, Becker G, Zinsmeister AR. Relation between antral motility and gastric emptying of solids and liquids in humans. Am J Physiol. 1984;249:G580–5. doi: 10.1152/ajpgi.1985.249.5.G580. [DOI] [PubMed] [Google Scholar]
- 10.Siegel JA, Urbain JL, Adler LP, Charkes ND, Maurer AH, Krevsky B, Knight LC, Fisher RS, Malmud LS. Biphasic nature of gastric emptying. Gut. 1988;29:85–9. doi: 10.1136/gut.29.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L American College of Gastroenterology. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18–37. doi: 10.1038/ajg.2012.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camilleri M, Brown ML, Malagelada JR. Relationship between impaired gastric emptying and abnormal gastrointestinal motility. Gastroenterology. 1986;91:94–9. doi: 10.1016/0016-5085(86)90444-0. [DOI] [PubMed] [Google Scholar]
- 13.Maleki D, Locke GR, III, Camilleri M, Zinsmeister AR, Yawn BP, Leibson C, Melton LJ., III Gastrointestinal symptoms among persons with diabetes in the community. Archives Intern Med. 2000;160:2808–16. doi: 10.1001/archinte.160.18.2808. [DOI] [PubMed] [Google Scholar]
- 14.Jung HK, Choung RS, Locke GR, 3rd, Schleck CD, Zinsmeister AR, Szarka LA, Mullan B, Talley NJ. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225–33. doi: 10.1053/j.gastro.2008.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tougas G, Eaker EY, Abell TL, Abrahamsson H, Boivin M, Chen J, Hocking MP, Quigley EM, Koch KL, Tokayer AZ, Stanghellini V, Chen Y, Huizinga JD, Rydén J, Bourgeois I, McCallum RW. Assessment of gastric emptying using a low-fat meal: establishment of international control values. Am J Gastroenterol. 2000;95:1456–62. doi: 10.1111/j.1572-0241.2000.02076.x. [DOI] [PubMed] [Google Scholar]
- 16.Camilleri M, Iturrino J, Bharucha AE, Burton D, Shin A, Jeong ID, Zinsmeister AR. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterol Motil. 2012;24:1076–e562. doi: 10.1111/j.1365-2982.2012.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bharucha AE, Camilleri M, Forstrom LA, Zinsmeister AR. Relationship between clinical features and gastric emptying disturbances in diabetes mellitus. Clin Endocrinol (Oxf) 2009;70:415–20. doi: 10.1111/j.1365-2265.2008.03351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapman MJ, Besanko LK, Burgstad CM, Fraser RJ, Bellon M, O’Connor S, Russo A, Jones KL, Lange K, Nguyen NQ, Bartholomeusz F, Chatterton B, Horowitz M. Gastric emptying of a liquid nutrient meal in the critically ill: relationship between scintigraphic and carbon breath test measurement. Gut. 2011;60:1336–43. doi: 10.1136/gut.2010.227934. [DOI] [PubMed] [Google Scholar]