Abstract
Background/Aims
We aimed to evaluate the associations between SSc-related systemic manifestations and esophageal function using high-resolution manometry (HRM).
Methods
Patients with SSc that had undergone HRM between 1/2004 and 9/2014 were identified and HRMs were analyzed according to the Chicago Classification. Clinical characteristics were identified via retrospective chart review and compared among motility diagnoses while adjusting for age, gender, race, and SSc-disease duration.
Results
79 patients (85% female, ages 25–77) were included. Clinical characteristics were compared between patients with absent contractility (AC, n = 40), ineffective esophageal motility (IEM; n = 15), and normal motility (n = 19); the 5 remaining patients met criteria for other motility diagnoses. Groups differed in severity of skin involvement measured by the modified Rodnan skin score (0–51): AC (adjusted mean 12.6), IEM (4.4), normal (4.3), p = 0.043. Pulmonary function tests [percent predicted FVC and DLCO) were lower in AC (adjusted mean, FVC: 70.3, DLCO 51.1), than IEM (FVC: 92.0; DLCO: 76.9) and normal motility (FVC: 80.0; DLCO: 67.2), p-values 0.057 (FVC) and 0.007 (DLCO). Groups did not differ by SSc-disease duration, autoantibodies, or reported symptoms of dysphagia or reflux.
Conclusions
In patients with SSc, absent esophageal contractility on HRM was associated with increased skin disease severity and worse lung function. Obtaining HRM to identify SSc patients with more severe esophageal dysfunction could be considered to enable implementation of management strategies in patients potentially at risk for increased morbidity and mortality.
Keywords: systemic sclerosis, high-resolution manometry, esophageal motility, interstitial lung disease, modified Rodnan skin score
Introduction
Systemic sclerosis (SSc) is a chronic and complex connective tissue disease with multisystem manifestations, commonly affecting the esophagus. The incidence of subjective esophageal symptoms (e.g. reflux and/or dysphagia) ranges from 42–80%, and as many as 50–90% of patients with SSc have abnormalities on esophageal motility.(1–3) An esophageal motility pattern consisting of distal aperistalsis and a hypotensive lower esophageal sphincter (LES) is often termed scleroderma esophagus; however, this pattern can be identified in other systemic diseases.(4, 5)
The pathogenesis, risk factors for development, and predictors of progression of SSc esophageal disease are poorly understood. Proposed pathologic mechanisms of esophageal dysfunction in SSc include oxidative stress, collagen deposition, vascular injury and/or vasoconstriction, and immune dysregulation leading to increased autoantibody production, which lead to neurodegeneration, replacement of muscle tissue with fibrosis, and muscle atrophy. (2, 6–10) Given the multisystem involvement of SSc, esophageal dysfunction may share common mechanisms with extra-esophageal clinical features, thus allowing the presence of such disease manifestations to predict esophageal dysfunction, and vice-versa. Potential examples include 1) Raynaud phenomenon (RP)-associated vasospasm that affects esophageal microvasculature causing reduced esophageal muscle blood flow and/or 2) reduced LES tone with peristaltic dysfunction increasing susceptibility to gastroesophageal reflux and micro-aspiration, and subsequent development or progression of interstitial lung disease (ILD).(6, 11–13)
Despite proposed mechanistic connections, previous studies have reported inconsistent associations between esophageal dysfunction (defined subjectively and objectively) and SSc disease manifestations, including SSc-disease subtype (i.e. extent of skin involvement), prevalence of RP and ILD, and presence of autoantibodies.(12, 14–22) However, the majority of prior studies have utilized conventional manometry and varying diagnostic criteria for both esophageal motility patterns and SSc, often 1980 American College of Rheumatology (ACR) criteria or Leroy and Medsger’s 2001 criteria.(12, 14–24) Updated diagnostic criteria and recent advances in esophageal motility tools may allow for improved assessment of associations between esophageal dysfunction and extra-esophageal SSc-disease manifestations. The updated 2013 ACR SSc criteria provide improved sensitivity and specificity for SSc diagnosis over previous classification schemes.(25, 26) Additionally, implementation of high-resolution manometry (HRM) and esophageal pressure topography (EPT) provides improved spatial resolution over conventional manometry, as well as standardized metrics of esophageal motility characteristics and a consensus classification scheme of esophageal motility disorders.(27) Thus, the aim of this study is to evaluate SSc-related systemic manifestations and their associations with esophageal function assessed using HRM/EPT.
Methods
Patients
Patients diagnosed with SSc who had undergone HRM between January 2004 and September 2014 were retrospectively identified by billing codes generated using the Northwestern University Enterprise Database Warehouse, a comprehensive database of electronic health records of Northwestern Memorial Hospital and affiliated clinics. Manometry was obtained at the discretion of patients’ treating physicians, often for evaluation of dysphagia or reflux-related symptoms. Charts of identified SSc patients were reviewed to ensure that subjects fulfilled 2013 American College of Rheumatology (ACR) SSc criteria.(25) Patients with prior upper gastrointestinal surgery and technical limitations of HRM were excluded. The study protocol was approved by the Northwestern University Institutional Review Board. A waiver of informed consent was obtained for the retrospective analysis.
High-resolution manometry
Manometry studies were completed after at least a six-hour fast. The HRM studies were completed using a 4.2-mm outer diameter, solid-state assembly with 36 circumferential pressure sensors spaced 1 cm apart (Medtronic Inc, Shoreview, MN). The HRM assembly was placed transnasally and positioned to record from the hypopharynx to the stomach with about three intragastric pressure sensors. The HRM protocol included a 5-minute baseline recording and ten 5-ml water swallows at 20–30 second intervals in a supine position. If patients underwent more than one HRM evaluation, only the first was analyzed.
The HRM studies were analyzed using Manoview Analysis Software v3.0 (Medtronic). The integrated relaxation pressure (IRP), distal contractile integral (DCI), and distal latency were measured and applied to the Chicago Classification to provide an esophageal motility diagnosis.(27) Basal esophagogastric junction (EGJ) pressure (EGJP) was measured during the baseline recording period by using the isobaric contour at end-expiration (end-expiratory pressure; EGJP); EGJP < 9mmHg was considered hypotensive based on the 25th percentile of asymptomatic controls.(28) The EGJ morphology was described as type I (superimposed LES and crural diaphragm, CD), type II (minimal, but discernible LES-CD separation), and type III (separation of the LES and CD); the distance (cm) of separation between the LES and CD was also measured. (27, 29)
Clinical characteristics/SSc-disease manifestations
Chart review was performed to record demographic information, presence of patient-reported upper gastrointestinal symptoms [dysphagia and reflux (i.e. heartburn and/or regurgitation)], acid-suppression therapy, results of upper endoscopy, esophageal pH testing, and to examine SSc-related clinical characteristics. These included: 1) SSc subtype as defined by physician assessment using Leroy criteria.(30) 2) Modified Rodnan Skin Score (MRSS), a reliable and validated method to measure dermal skin thickness based on 17 body parts, scored 0–51, with higher scores indicating greater skin thickening.(31) 3) Presence of Raynaud phenomenon (RP). 4) SSc disease duration, defined both as 4a) time (months) from the date of onset of RP (RP-disease duration) and 4b) time from the date of onset of non-RP SSc disease manifestation (non-RP disease duration) to the date of HRM. 5) Serologic (i.e. autoantibody) tests, including anti-centromere antibody (ACA), antinuclear antibody (ANA), anti-Scl 70, and anti-RNA polymerase III antibodies. 6) Presence of interstitial lung disease (ILD), defined as radiologic evidence of ILD on high-resolution computed tomography (CT) scan of the thorax. 7) Pulmonary function tests (PFTs) with analysis of the percent predicted forced vital capacity (FVC) and diffusing capacity for carbon monoxide (DLCO).
Statistical analysis
Results were presented as mean (standard deviation, SD), unless otherwise specified. Logistic regression models were used for binary outcomes. Continuous outcome variables of SSc-clinical characteristics were compared using ANOVA and ANCOVA with the Bonferroni correction used for post-hoc analysis. Models were adjusted for patient age, gender, race, and non-RP disease duration; to control for the potential effect of hiatal hernia on pulmonary disease, LES-CD separation (cm) was also included in models assessing ILD, % predicted FVC, and % predicted DLCO. Unadjusted and adjusted odds ratios (ORs) and the associated 95% confidence intervals (CIs) for ILD were reported for each group compared to the reference category group, absent contractility. For MRSS, % predicted FVC and % predicted DLCO, unadjusted and adjusted means and associated 95% CIs were reported. All analyses were performed in SAS version 9.4 (Cary, NC).
Results
Patients
A total of 128 patients met initial search criteria and their charts were reviewed. Forty-nine patients were excluded from analysis: 37 did not meet SSc 2013 ACR disease criteria, 6 had prior upper GI surgery (5 for fundoplication, 1 for lap band surgery), and 6 had technically-limited HRM. Thus, 79 patients (85% female, ages 25 to 77 years) with SSc were retrospectively evaluated.
Esophageal motility characteristics
Esophageal motility diagnoses based on Chicago Classification included 40 patients with absent contractility (defined as 100% failed peristalsis with a normal median IRP); among these patients with absent contractility, hypotensive EGJP was observed in 25/40; thus 25/79 (32% of the total patient cohort) had absent contractility with hypotensive EGJP. 15 patients had ineffective esophageal motility (IEM, defined as 50% ineffective swallows with DCI <450 mmHg-s-cm), and 19 patients had normal esophageal motility. The 74 patients with absent contractility, IEM, and normal motility were included for comparative analysis between motility diagnoses; baseline characteristics for these patients are displayed in Tables 1 and 2. Additionally, 2 patients met EPT criteria for achalasia type I (median IRP > 15 mmHg and 100% absent peristalsis), 2 for EGJ outflow obstruction (median IRP > 15 mmHg and some maintained peristalsis), and 1 for jackhammer esophagus (> 2 swallows with DCI > 8,000 mmHg-s-cm). Of the 4 patients with median IRP > 15 mmHg, 2 had a distal esophageal stricture and 1 had LA-A esophagitis on upper endoscopy.
Table 1.
Baseline characteristics of study subjects.
| N | Mean (SD) or n (%) | |
|---|---|---|
|
| ||
| Age at time of manometry, years | 74 | 50.5 (12.4) |
|
| ||
| Sex, n (% women) | 74 | 62 (84) |
|
| ||
| BMI, kg/m2 | 73 | 25.4 (5.9) |
|
| ||
| Race, n (% white) | 74 | 53 (72) |
|
| ||
| SSc Disease subtype | ||
| n (% diffuse) | 23 (31) | |
| n (% limited) | 74 | 34 (46) |
| n (% SSS) | 6 (8) | |
| n (% other | 10 (14) | |
|
| ||
| Modified Rodnan Skin Score | 52 | 10.4 (11.4) |
|
| ||
| Duration since first Raynaud, months | 67 | 111.4 (119.4) |
|
| ||
| Duration since first non-Raynaud, months | 70 | 81.7 (84.9) |
|
| ||
| On PPI | 74 | 73 (99) |
|
| ||
| Serum autoantibodies, n (% positive) | ||
| Anti-nuclear antibody (ANA) | 67 | 65 (97) |
| Anti-centromere antibody (ACA) | 64 | 13 (20) |
| Scl-70 | 65 | 23 (35) |
| RNA polymerase III | 52 | 5 (10) |
|
| ||
| Pulmonary Function Tests, mean (SD) | ||
| FVC % predicted | 46 | 77.2 (20.5) |
| DLCO % predicted | 46 | 58.1 (20.4) |
|
| ||
| ILD, n (% present) | 63 | 42 (67) |
|
| ||
| Symptoms, n (% positive) | ||
| Dysphagia | 74 | 60 (81) |
| Reflux (heartburn or regurgitation) | 74 | 61 (82) |
Patients with esophageal motility diagnosis of absent contractility, ineffective esophageal motility, and normal esophageal motility are included. BMI – body mass index. PPI – proton pump inhibitor. FVC – forced vital capacity. DLCO – diffusing capacity for carbon monoxide. ILD – interstitial lung disease.
Table 2.
Comparison of clinical characteristics by esophageal motility diagnosis.
| Disease characteristics | Absent Contractility | IEM | Normal |
|---|---|---|---|
| Age (years) | 50 (12) | 51 (11) | 51 (14) |
| Female/male | 31/9 | 12/3 | 15/0 |
| Race (% white) | 65 | 73 | 84 |
| BMI (kg/m2) | 24 (5) | 26 (5) | 28 (7) |
| RP - Disease duration (months) Median (IQR) |
78 (36 – 151) | 73 (28 – 228) | 73 (28 – 228) |
| Non-RP Disease duration (months) Median (IQR) |
73 (35 – 116) | 46 (10 -- 57) | 60 (14 – 84) |
| Autoantibodies | |||
| Anti-centromere | 5/33 (15%) | 4/15 (27%) | 4/16 (25%) |
| Anti-RNA Polymerase III | 2/29 (7%) | 2/12 (17%) | 1/11 (9%) |
| Anti-Scl 70 | 12/34 (35%) | 4/14 (29%) | 7/17 (41%) |
Values are presented as mean (standard deviation) or [N present/N assessed, % positive], unless otherwise specified as appropriate. Low basal EGJ pressure (EGJP) was considered at < 9 mmHg normal EGJP ≥ 9 mmHg. Statistical differences were not detected between motility diagnoses in any of the displayed measures. IEM – ineffective esophageal motility. BMI – body mass index. RP – Raynaud’s phenomenon. IQR – interquartile range.
Among the total cohort of 79 patients, EGJ morphology included 55 patients with type I EGJ, six with type II, and 18 type III; the median (range) LES-CD separation of the patients with type III EGJ morphology was 2.9 cm (2 – 6).
Upper endoscopy reports were available for 69 of the 74 patients with absent contractility, IEM, or normal motility. The interval between endoscopy and HRM was < 3 months in 55 patients, 3–12 months for six patients, and > 1 year for eight patients. Patients were treated with proton pump inhibitor (PPI) therapy prior to and at the time of endoscopy in all but three patients. Eight patients had endoscopically-identified esophagitis (5, 2, 1, and 0 with LA-grades A, B, C, and D, respectively): all eight patients with endoscopically-identified esophagitis had absent contractility 7 of whom were on acid-suppression therapy (six with PPI, one with histamine-2 receptor antagonist) during endoscopic evaluation. Six of those 7 patients had hypotensive EGJ pressures and two had hiatal hernia identified on HRM (type III EGJ, both with LES-CD separation of 2.9 cm). Endoscopy was performed within one month of HRM 7/8 patients with esophagitis. Non-dysplastic Barrett’s esophagus was present in 4 patients (3 with absent contractility, 1 with IEM) and candida esophagitis was present in 3 patients (all 3 with absent contractility). 13 patients underwent esophageal pH-testing (11 with 24-hour pH-impedance tests, 2 with wireless pH-sensors (one 48 hours, one 96 hours), all completed while on PPI therapy. Abnormal esophageal acid exposure (> 5.5% time with pH > 4) was detected in four patients: 3 of 8 patients with absent contractility, 1 of 2 patients with IEM, and 0 of 3 patients with normal motility.
The proportions of patients reporting dysphagia and reflux symptoms did not differ between absent contractility (73% dysphagia; 88% reflux), IEM (87% dysphagia; 67% reflux), and normal motility (95% dysphagia, 84% reflux) (dysphagia p-value 0.15; reflux p-value 0.21).
Association of esophageal motility and SSc-Disease Characteristics
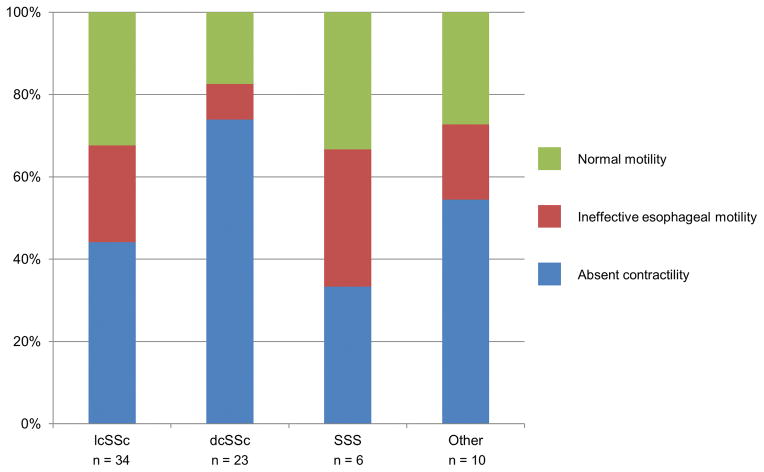
Patients with absent contractility, IEM, and normal motility did not differ in terms of age, gender, race, or body mass index (Table 2). There was no difference in proportions of absent contractility, IEM, and normal esophageal motility among SSc subtypes (p = 0.34; Figure 1). Comparative analysis of only patients with lcSSc (n = 34) and dcSSc (n = 23) demonstrated a trend (p = 0.08) towards a smaller percentage of patients with absent contractility and a greater percentage with normal motility among lcSSc than dcSSc patients (Figure 1).
Figure 1. Systemic sclerosis (SSc) subtype by esophageal motility diagnosis.
The percentage of esophageal motility diagnosis among each SSc subtype is displayed. Other subtypes included SSc overlap, localized SSc, mixed connective tissue disease, and undifferentiated connective tissue disease. lcSSc – limited cutaneous SSc; dcSSc – diffuse cutaneous SSc; SSS – SSc sine scleroderma. EGJP – esophagogastric junction pressure.
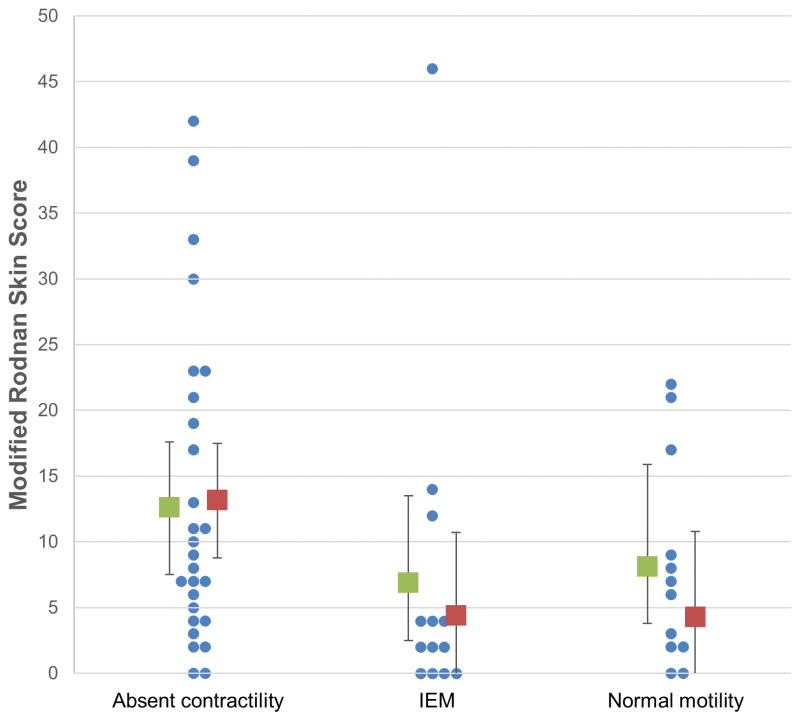
Of patients with reported MRSS (52/74, 70%), groups did not differ in severity and extent of skin involvement measured by the MRSS in unadjusted analysis (p = 0.19), but a difference between motility diagnoses was detected on adjusted analysis (p = 0.043). However, adjusted comparison did not detect significant pairwise differences between groups (Figure 2).
Figure 2. Modified Rodnan-skin score (MRSS) among patients with absent contractility, ineffective esophageal motility (IEM), and normal esophageal motility.
Each patients’ MRSS is represent by blue dots; the unadjusted (green) and adjusted (red) means (boxes) and 95% confidence intervals (error bars) are displayed for each motility diagnosis.
There were no differences in RP-disease duration (p = 0.76) or non-RP disease duration (p = 0.13) between motility diagnoses (Table 2). Autoantibody presence (ACA, anti-RNA polymerase III, and anti-Scl70) also did not differ among esophageal motility (p-values = 0.32 – 0.77; Table 2). Because ANA was positive in all but two patients in the cohort, ANA presence was not subjected to additional comparative testing.
High-resolution CT-scans and PFTs were available in 35 and 24 patients with absent contractility, 12 and 9 with IEM, and 15 and 13 with normal motility, respectively. Time interval [median (IQR) in months] between HRM and CT-scan and HRM and PFTs, respectively, were in 4 (2 – 12) and 3 (1 – 5) for absent contractility, 7.5 (4 – 21) and 2 (1 – 4) for IEM, and 11 (3 – 23) and 2.5 (2 – 4) for normal motility; neither interval significantly differed between groups (p-values = 0.267 and 0.807). There appeared to be decreased odds of ILD presence in IEM (OR, 95% CI 0.19, 0.05 – 0.73), but not normal motility (0.59, 0.16 – 2.25; p = 0.054), compared to absent contractility; however on adjusted analysis, odds of having ILD were similar among groups (IEM: 0.10, 0.03 – 1.12; normal: 1.37, 0.30 – 6.33; p = 0.108). When evaluating the available PFT parameters among motility diagnoses (n = 46; Table 3), the unadjusted and FVC means differed by motility group such that absent contractility had reduced FVC compared with IEM (p = 0.024), but not normal motility (p = 0.26). On adjusted analysis, there was a statistical trend towards a difference in FVC means among motility groups. DLCO differed among motility diagnoses (Table 3) in both adjusted and unadjusted analysis such that absent contractility had a lower DLCO than IEM (unadjusted p-value = 0.007; adjusted p-value = 0.010) and a statistical trend was detected towards lower DLCO in absent contractility than normal motility (unadjusted p-value = 0.066; adjusted p-value = 0.075).
Table 3.
Unadjusted and adjusted results of pulmonary function test results among esophageal motility diagnoses.
| Pulmonary Function Test | Absent Contractility | IEM | Normal | p-value |
|---|---|---|---|---|
|
| ||||
| FVC % predicted | ||||
| Unadjusted | 70.0 (62.1 – 77.9) | 91.8 (78.1 – 105.4) | 81.3 (71.0 – 91.6) | 0.019 |
| Adjusted* | 70.3 (60 – 80.7) | 92.0 (74.4 –109.7) | 80.0 (64.9 – 95.0) | 0.057 |
|
| ||||
| DLCO % predicted | ||||
| Unadjusted | 49.3 (41.8 – 56.9) | 72.4 (60.1– 84.8) | 64.4 (54.1 – 74.7) | 0.004 |
| Adjusted* | 51.1 (41.4 – 60.8) | 76.9 (60.6 – 93.2) | 67.2 (52.9 – 81.5) | 0.007 |
Values displayed are mean (95% confidence intervals).
Adjusted for age, gender, race, non-Raynaud’s SSc-disease duration, and lower-esophageal sphincter-crural diaphragm separation (cm). IEM – ineffective esophageal motility. FVC – forces vital capacity. DLCO – diffusing capacity for carbon monoxide.
Discussion
By retrospectively evaluating patients diagnosed with SSc by 2013 ACR criteria utilizing HRM/EPT, we identified heterogeneous esophageal motility diagnoses among SSc patients and found that skin score (assessed using MRSS) and PFT parameters were associated with esophageal motility disorders assigned using the Chicago classification. Though we report the results of the first study to utilize the 2013 ACR diagnostic criteria for SSc combined with the Chicago Classification of esophageal motility diagnoses, our results share some similarities with previous studies of esophageal motility in SSc.
Previous studies have reported variable associations between the extent of skin involvement and esophageal function. Three studies that analyzed SSc patients who fulfilled 1980 ACR SSc criteria or 2001 Leroy-Medsger criteria and used conventional manometry reported that absent peristalsis was observed more frequently in patients with dcSSc than lcSSc.(14, 19, 20) However, two studies of SSc patients fulfilling 1980 ACR criteria and one using Leroy-Medsger criteria did not find any significant difference between skin involvement and esophageal dysfunction as assessed by conventional manometry.(12, 21, 32). Additionally, a study by Roman et al utilizing HRM reported more frequent esophageal dysmotility among patients with dcSSc versus lcSSc.(16) Similarly, we found a higher prevalence of absent contractility among patients with dcSSc than lcSSc. However, when we analyzed our entire cohort, which included six patients with SSS, a group not typically included in previous studies that had an equal distribution of esophageal motility disorders in our study, the difference in motility disorder by SSc subtype was no longer present. Previous studies that have found more severe esophageal function in dcSSc have hypothesized that this SSc subtype involves more internal organ involvement (dcSSc is often associated with renal, pulmonary, and cardiac involvement).(33, 34) On the other hand, lcSSc has been associated with the CREST syndrome, which by definition involves esophageal dysmotility.(7, 33) Thus, there is controversy regarding which SSc patients are at highest risk for esophageal dysmotility.
To our knowledge, this is the first study to evaluate the relationship between esophageal dysfunction based on manometry and skin assessment with the MRSS—a validated measure to assess skin fibrosis and severity based on assessment of both skin thickness and extent of skin involvement. Akin to our finding of an association with more severe skin disease assessed by MRSS and more pronounced esophageal dysmotility (i.e. absent contractility), Vischio et al found a correlation between MRSS and abnormal esophageal transit scintigraphy.(35) Dermal skin thickness, which is caused by increased collagen deposition, is thought to correlate with more severe internal organ involvement, morbidity, and mortality.(34, 36–38). A study utilizing endoscopic ultrasound identified thickened (compared with normal controls) esophageal walls, mainly in the submucosa and muscularis, in 14 patients with dcSSc and 11 lcSSc, which also correlated with the presence of dysphagia.(39) Prior studies report variable associations between esophageal dysfunction and esophageal symptoms in patients with SSc, including reports of asymptomatic individuals with severe esophageal disease.(1, 3, 32) Similar to the Roman et al. study utilizing HRM in SSc, we did not observe an association with symptoms (however, in our study HRM was obtained in most patients because of symptoms, which were assessed retrospectively in a subjective and non-quantifiable manner).(16) However, the association between esophageal symptoms and manometric findings is also inconsistent in patients without SSc and although a possibility of esophageal hyposensitivity in SSc has been raised, clinical studies testing this theory have yielded mixed results. (40–43) While a shared pathologic mechanism between skin and esophageal wall thickening could provide insight into the method of esophageal dysfunction in SSc, the presence and composition of esophageal thickening has not been identified on SSc-autopsy studies.(9, 10) Therefore, additional study of well-characterized esophageal function and histology, skin involvement, and symptoms is required to clarify these associations.
Proposed pathologic mechanisms and associations of esophageal neuromuscular degeneration in SSc have included vasoconstriction and ischemia (possibly relating to RP), progressive collagen deposition and fibrosis (possibly relating to disease duration), and/or auto-antibody mediated inflammation (related to serologic tests).(6, 9–11) However, studies evaluating these associations have yielded varying results.(12, 16, 17, 21, 32) Though all of the patients in our cohort demonstrated RP, which is similar to reported RP-prevalence rates (that range from 94–100% of SSc patients), we still detected heterogeneous motility diagnoses that did not differ with regards to the duration since RP onset.(7) Furthermore, we did not find an association between esophageal motility diagnosis and non-RP disease duration nor autoantibody presence. Thus, our study does not support a progressive ischemic, infiltrative, or autoantibody (at least among those measured) mediated process as a potential cause of SSc esophageal dysfunction, which is supported by autopsy evaluations of SSc esophagi that primarily observed smooth muscle atrophy without evidence of ischemia or fibrosis.(9, 10) Furthermore, prior evaluation of progression of esophageal disease in SSc is limited, but one small study of 15 lcSSc and 2 dcSSc patients did not observe manometric progression of esophageal dysfunction over the course of 9 – 111 months.(44) Others have suggested that severe organ involvement occurs earlier in the disease course and is not as progressive as previously thought.(34) It has also been proposed that once skin thickening peaks, which occurs in the transition from early to late dcSSc, fibrotic changes of internal organs can progress but it is uncommon for new visceral involvement to occur.(45)
Similar to prior reports, we observed a potential increase in the prevalence of radiographically-evident ILD and reduced lung function as assessed by FVC and DLCO in patients with absent esophageal contractility.(3, 12, 13, 19) The association of absent contractility and reduced PFTs remained when adjusting for other variables, including SSc-disease duration, however, the association of esophageal motility disorder and ILD prevalence was no longer present. Further, we did not identify an apparent gradient of esophageal dysmotility effect with increased prevalence of ILD nor greater reduction in PFTs progressing from absent contractility to IEM to normal motility. Despite multiple studies indicating an association between esophageal dysmotility and pulmonary disease, the current understanding of causality is limited. (12, 46, 47) Proposed mechanistic links include esophageal dysmotility-induced susceptibility to gastroesophageal reflux disease (GERD), subsequent micro-aspiration, and chemical lung damage that may contribute to ILD development or progression. Further, altered lung compliance in ILD may actually contribute to GERD or esophageal dysmotility through exaggerated respiration-associated intrathoracic pressure changes that may be transmitted to the esophagus.(46) Thus, it remains unclear if a causal relationship between lung disease and esophageal dysfunction in SSc exists or if contemporaneous involvement of these organ systems are both expressions of advanced disease. Given that lung disease is the leading cause of death in SSc, and there is a potential association of esophageal dysmotility with ILD and worse lung function, there may be utility in performing HRM to identify patients with absent esophageal contractility.(48) Absent contractility was also frequently present in the patients identified with esophagitis or abnormal reflux monitoring despite PPI use. Particularly because other clinical characteristics, including esophageal symptoms, were not associated with esophageal motility, our study suggests that patients with dcSSc or an elevated MRSS may warrant consideration of evaluation with HRM in order to identify patients with esophageal dysmotility who may be susceptible to esophageal peptic injury and/or more severe pulmonary disease.
This study does have limitations, particularly due to its retrospective design. Patients’ HRMs, as well as other diagnostic tests (e.g. high resolution CT, PFTs) were performed at the discretion of their treatment physicians and not according to a research protocol; thus, there may be some inherent bias in the cohort inclusion of additional factors that prompted particular evaluation. Additionally, as some measures were not applied to the entire cohort, some of our group comparisons represented small sample sizes and thus may not have sufficient power to detect group differences.
In conclusion, our retrospective analysis of a well-characterized cohort of patients with SSc with previously obtained HRM found that more extensive skin disease, assessed with the MRSS and possibly dcSSc subtype, and reduction in PFTs, were associated with absent esophageal contractility. As SSc-disease duration did not appear to be related to esophageal motility, clinical phenotypes of SSc, instead of a progressive process, appear related to esophageal disease of SSc. Though continuing study to identify methods to phenotype SSc patients is needed, obtaining HRM in patients with more extensive skin disease may be considered to identify patients with more severe esophageal dysfunction. In doing so, escalating gastroesophageal reflux treatment may reduce the risk of esophagitis and furthermore, management strategies could be implemented to potentially reduce micro-aspiration and subsequent reduction in lung function. However, prospective study remains necessary to clarify SSc-disease associations and mechanisms of esophageal disease, as well as to demonstrate improved clinical outcomes related to HRM/EPT use in SSc.
Key messages.
Obtaining high-resolution manometry (HRM) in systemic sclerosis (SSc) patients with more severe skin disease may be considered as a strategy to identify patients with esophageal dysfunction, which is associated with more severe lung disease.
The aim of this study was to evaluate the associations between SSc-related systemic manifestations and esophageal motility diagnoses using HRM and assigned according to the Chicago Classification.
79 patients with SSc and previously obtained HRM were retrospectively reviewed and SSc-subtypes, skin severity (measured using the Modified Rodnan Skin Score), SSc-disease duration, and presence of Raynaud’s phenomenon, autoantibodies, and interstitial lung disease, and pulmonary function test results, were compared among patients with absent contractility, ineffective esophageal motility, and normal esophageal motility.
More severe skin disease and worse pulmonary function were associated with absent esophageal contractility. SSc-disease duration and measured autoantibody presence did not differ among motility diagnoses.
Acknowledgments
Funding: This work was supported by T32 DK101363 (JEP), R01 DK079992 (JEP), K23 AR059763 and funding from the Scleroderma Research Foundation (MH), P60AR064464 (JL), and UL1TR000159 (Northwestern University Clinical and Translational Sciences Institute, Enterprise Data Warehouse) from the Public Health service.
Footnotes
Conflict of interest:
John E. Pandolfino: Given Imaging (Consultant, Grant, Speaking), Sandhill Scientific (Consulting, Speaking), Takeda (Speaking), Astra Zeneca (Speaking)
Jessica N. Kimmel, Dustin A. Carlson, Monique Hinchcliff, Mary A. Carns, MA, Kathleen A Aren, Jungwha Lee: None
Author contributions: JNK and DAC contributed to study concept and design, data acquisition, data analysis, interpretation of data, and drafting of the manuscript. MH contributed to study concept, data analysis and interpretation, and revised the manuscript critically. MAC and KAA contributed to data acquisition. JL provided statistical support. JEP contributed to study concept, interpretation of the data, and revised the manuscript critically. All authors approved the final version.
References
- 1.Abu-Shakra M, Guillemin F, Lee P. Gastrointestinal manifestations of systemic sclerosis. Seminars in arthritis and rheumatism. 1994;24(1):29–39. doi: 10.1016/0049-0172(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 2.Sjogren RW. Gastrointestinal motility disorders in scleroderma. Arthritis and rheumatism. 1994;37(9):1265–82. doi: 10.1002/art.1780370902. [DOI] [PubMed] [Google Scholar]
- 3.Lock G, Holstege A, Lang B, Scholmerich J. Gastrointestinal manifestations of progressive systemic sclerosis. The American journal of gastroenterology. 1997;92(5):763–71. [PubMed] [Google Scholar]
- 4.Mukhopadhyay AK, Graham DY. Esophageal motor dysfunction in systemic diseases. Archives of internal medicine. 1976;136(5):583–8. [PubMed] [Google Scholar]
- 5.Smout A, Fox M. Weak and absent peristalsis. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2012;24(Suppl 1):40–7. doi: 10.1111/j.1365-2982.2011.01831.x. [DOI] [PubMed] [Google Scholar]
- 6.Ebert EC. Esophageal disease in scleroderma. Journal of clinical gastroenterology. 2006;40(9):769–75. doi: 10.1097/01.mcg.0000225549.19127.90. [DOI] [PubMed] [Google Scholar]
- 7.Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. The New England journal of medicine. 2009;360(19):1989–2003. doi: 10.1056/NEJMra0806188. [DOI] [PubMed] [Google Scholar]
- 8.Gilliam AC. Scleroderma. Current directions in autoimmunity. 2008;10:258–79. doi: 10.1159/000131502. [DOI] [PubMed] [Google Scholar]
- 9.Treacy WL, Baggenstoss AH, Slocumb CH, Code CF. Scleroderma of the Esophagus. A Correlation of Histologic and Physiologic Findings Annals of internal medicine. 1963;59:351–6. doi: 10.7326/0003-4819-59-3-351. [DOI] [PubMed] [Google Scholar]
- 10.Roberts CG, Hummers LK, Ravich WJ, Wigley FM, Hutchins GM. A case-control study of the pathology of oesophageal disease in systemic sclerosis (scleroderma) Gut. 2006;55(12):1697–703. doi: 10.1136/gut.2005.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belch JJ, Land D, Park RH, McKillop JH, MacKenzie JF. Decreased oesophageal blood flow in patients with Raynaud’s phenomenon. British journal of rheumatology. 1988;27(6):426–30. doi: 10.1093/rheumatology/27.6.426. [DOI] [PubMed] [Google Scholar]
- 12.Marie I, Dominique S, Levesque H, Ducrotte P, Denis P, Hellot MF, Courtois H. Esophageal involvement and pulmonary manifestations in systemic sclerosis. Arthritis and rheumatism. 2001;45(4):346–54. doi: 10.1002/1529-0131(200108)45:4<346::AID-ART347>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 13.Christmann RB, Wells AU, Capelozzi VL, Silver RM. Gastroesophageal reflux incites interstitial lung disease in systemic sclerosis: clinical, radiologic, histopathologic, and treatment evidence. Seminars in arthritis and rheumatism. 2010;40(3):241–9. doi: 10.1016/j.semarthrit.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Bassotti G, Battaglia E, Debernardi V, Germani U, Quiriconi F, Dughera L, Buonafede G, Puiatti P, et al. Esophageal dysfunction in scleroderma: relationship with disease subsets. Arthritis and rheumatism. 1997;40(12):2252–9. doi: 10.1002/art.1780401222. [DOI] [PubMed] [Google Scholar]
- 15.Lahcene M, Oumnia N, Matougui N, Boudjella M, Tebaibia A, Touchene B. Esophageal dysmotility in scleroderma: a prospective study of 183 cases. Gastroenterologie clinique et biologique. 2009;33(6–7):466–9. doi: 10.1016/j.gcb.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Roman S, Hot A, Fabien N, Cordier JF, Miossec P, Ninet J, Mion F Reseau Sclerodermie des Hospices Civils de L. Esophageal dysmotility associated with systemic sclerosis: a high-resolution manometry study. Diseases of the esophagus: official journal of the International Society for Diseases of the Esophagus/ISDE. 2010 doi: 10.1111/j.1442-2050.2010.01150.x. [DOI] [PubMed] [Google Scholar]
- 17.Hurwitz AL, Duranceau A, Postlethwait RW. Esophageal dysfunction and Raynaud’s phenomenon in patients with scleroderma. The American journal of digestive diseases. 1976;21(8):601–6. doi: 10.1007/BF01071951. [DOI] [PubMed] [Google Scholar]
- 18.Tang DM, Pathikonda M, Harrison M, Fisher RS, Friedenberg FK, Parkman HP. Symptoms and esophageal motility based on phenotypic findings of scleroderma. Diseases of the esophagus: official journal of the International Society for Diseases of the Esophagus/ISDE. 2013;26(2):197–203. doi: 10.1111/j.1442-2050.2012.01349.x. [DOI] [PubMed] [Google Scholar]
- 19.Airo P, Della Casa D, Danieli E, Missale G, Cattaneo R, Cestari R. Oesophageal manometry in early and definite systemic sclerosis. Clinical rheumatology. 2005;24(4):370–6. doi: 10.1007/s10067-004-1049-6. [DOI] [PubMed] [Google Scholar]
- 20.Lock G, Pfeifer M, Straub RH, Zeuner M, Lang B, Scholmerich J, Holstege A. Association of esophageal dysfunction and pulmonary function impairment in systemic sclerosis. The American journal of gastroenterology. 1998;93(3):341–5. doi: 10.1111/j.1572-0241.1998.00341.x. [DOI] [PubMed] [Google Scholar]
- 21.Savarino E, Mei F, Parodi A, Ghio M, Furnari M, Gentile A, Berdini M, Di Sario A, et al. Gastrointestinal motility disorder assessment in systemic sclerosis. Rheumatology (Oxford) 2013;52(6):1095–100. doi: 10.1093/rheumatology/kes429. [DOI] [PubMed] [Google Scholar]
- 22.Skare TL, Fonseca AE, Luciano AC, Azevedo PM. Autoantibodies in scleroderma and their association with the clinical profile of the disease. A study of 66 patients from southern Brazil. Anais brasileiros de dermatologia. 2011;86(6):1075–81. doi: 10.1590/s0365-05962011000600003. [DOI] [PubMed] [Google Scholar]
- 23.Preliminary criteria for the classification of systemic sclerosis (scleroderma) Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis and rheumatism. 1980;23(5):581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 24.LeRoy EC, Medsger TA., Jr Criteria for the classification of early systemic sclerosis. The Journal of rheumatology. 2001;28(7):1573–6. [PubMed] [Google Scholar]
- 25.van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, Matucci-Cerinic M, Naden RP, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis and rheumatism. 2013;65(11):2737–47. doi: 10.1002/art.38098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann-Vold AM, Gunnarsson R, Garen T, Midtvedt O, Molberg O. Performance of the 2013 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Systemic Sclerosis (SSc) in large, well-defined cohorts of SSc and mixed connective tissue disease. The Journal of rheumatology. 2015;42(1):60–3. doi: 10.3899/jrheum.140047. [DOI] [PubMed] [Google Scholar]
- 27.Kahrilas PJ, Bredenoord AJ, Fox M, Gyawali CP, Roman S, Smout AJ, Pandolfino JE International High Resolution Manometry Working G. The Chicago Classification of esophageal motility disorders, v3. 0. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2015;27(2):160–74. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicodeme F, Pipa-Muniz M, Khanna K, Kahrilas PJ, Pandolfino JE. Quantifying esophagogastric junction contractility with a novel HRM topographic metric, the EGJ-Contractile Integral: normative values and preliminary evaluation in PPI non-responders. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2014;26(3):353–60. doi: 10.1111/nmo.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandolfino JE, Kim H, Ghosh SK, Clarke JO, Zhang Q, Kahrilas PJ. High-resolution manometry of the EGJ: an analysis of crural diaphragm function in GERD. The American journal of gastroenterology. 2007;102(5):1056–63. doi: 10.1111/j.1572-0241.2007.01138.x. [DOI] [PubMed] [Google Scholar]
- 30.LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, Jr, Rowell N, Wollheim F. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. The Journal of rheumatology. 1988;15(2):202–5. [PubMed] [Google Scholar]
- 31.Clements P, Lachenbruch P, Siebold J, White B, Weiner S, Martin R, Weinstein A, Weisman M, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. The Journal of rheumatology. 1995;22(7):1281–5. [PubMed] [Google Scholar]
- 32.Lahcene M, Oumnia N, Matougui N, Boudjella M, Tebaibia A, Touchene B. Esophageal involvement in scleroderma: clinical, endoscopic, and manometric features. ISRN rheumatology. 2011;2011:325826. doi: 10.5402/2011/325826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hachulla E, Launay D. Diagnosis and classification of systemic sclerosis. Clinical reviews in allergy & immunology. 2011;40(2):78–83. doi: 10.1007/s12016-010-8198-y. [DOI] [PubMed] [Google Scholar]
- 34.Steen VD, Medsger TA., Jr Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis and rheumatism. 2000;43(11):2437–44. doi: 10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 35.Vischio J, Saeed F, Karimeddini M, Mubashir A, Feinn R, Caldito G, Striegel K, Rothfield N. Progression of esophageal dysmotility in systemic sclerosis. The Journal of rheumatology. 2012;39(5):986–91. doi: 10.3899/jrheum.110923. [DOI] [PubMed] [Google Scholar]
- 36.Verrecchia F, Laboureau J, Verola O, Roos N, Porcher R, Bruneval P, Ertault M, Tiev K, et al. Skin involvement in scleroderma--where histological and clinical scores meet. Rheumatology (Oxford) 2007;46(5):833–41. doi: 10.1093/rheumatology/kel451. [DOI] [PubMed] [Google Scholar]
- 37.Clements PJ, Hurwitz EL, Wong WK, Seibold JR, Mayes M, White B, Wigley F, Weisman M, et al. Skin thickness score as a predictor and correlate of outcome in systemic sclerosis: high-dose versus low-dose penicillamine trial. Arthritis and rheumatism. 2000;43(11):2445–54. doi: 10.1002/1529-0131(200011)43:11<2445::AID-ANR11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 38.Shand L, Lunt M, Nihtyanova S, Hoseini M, Silman A, Black CM, Denton CP. Relationship between change in skin score and disease outcome in diffuse cutaneous systemic sclerosis: application of a latent linear trajectory model. Arthritis and rheumatism. 2007;56(7):2422–31. doi: 10.1002/art.22721. [DOI] [PubMed] [Google Scholar]
- 39.Zuber-Jerger I, Muller A, Kullmann F, Gelbmann CM, Endlicher E, Muller-Ladner U, Fleck M. Gastrointestinal manifestation of systemic sclerosis--thickening of the upper gastrointestinal wall detected by endoscopic ultrasound is a valid sign. Rheumatology (Oxford) 2010;49(2):368–72. doi: 10.1093/rheumatology/kep381. [DOI] [PubMed] [Google Scholar]
- 40.Xiao Y, Kahrilas PJ, Kwasny MJ, Roman S, Lin Z, Nicodeme F, Lu C, Pandolfino JE. High-Resolution Manometry Correlates of Ineffective Esophageal Motility. The American journal of gastroenterology. 2012 doi: 10.1038/ajg.2012.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lazarescu A, Karamanolis G, Aprile L, De Oliveira RB, Dantas R, Sifrim D. Perception of dysphagia: lack of correlation with objective measurements of esophageal function. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2010;22(12):1292–7. e336–7. doi: 10.1111/j.1365-2982.2010.01578.x. [DOI] [PubMed] [Google Scholar]
- 42.Basilisco G, Barbera R, Molgora M, Vanoli M, Bianchi P. Acid clearance and oesophageal sensitivity in patients with progressive systemic sclerosis. Gut. 1993;34(11):1487–91. doi: 10.1136/gut.34.11.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orringer MB, Dabich L, Zarafonetis CJ, Sloan H. Gastroesophageal reflux in esophageal scleroderma: diagnosis and implications. The Annals of thoracic surgery. 1976;22(2):120–30. doi: 10.1016/s0003-4975(10)63972-0. [DOI] [PubMed] [Google Scholar]
- 44.Dantas RO, Meneghelli UG, Oliveira RB, Villanova MG. Esophageal dysfunction does not always worsen in systemic sclerosis. Journal of clinical gastroenterology. 1993;17(4):281–5. doi: 10.1097/00004836-199312000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Medsger TA., Jr Natural history of systemic sclerosis and the assessment of disease activity, severity, functional status, and psychologic well-being. Rheumatic diseases clinics of North America. 2003;29(2):255–73. vi. doi: 10.1016/s0889-857x(03)00023-1. [DOI] [PubMed] [Google Scholar]
- 46.Hershcovici T, Jha LK, Johnson T, Gerson L, Stave C, Malo J, Knox KS, Quan S, et al. Systematic review: the relationship between interstitial lung diseases and gastro-oesophageal reflux disease. Alimentary pharmacology & therapeutics. 2011;34(11–12):1295–305. doi: 10.1111/j.1365-2036.2011.04870.x. [DOI] [PubMed] [Google Scholar]
- 47.Gasper WJ, Sweet MP, Golden JA, Hoopes C, Leard LE, Kleinhenz ME, Hays SR, Patti MG. Lung transplantation in patients with connective tissue disorders and esophageal dysmotility. Diseases of the esophagus: official journal of the International Society for Diseases of the Esophagus/ISDE. 2008;21(7):650–5. doi: 10.1111/j.1442-2050.2008.00828.x. [DOI] [PubMed] [Google Scholar]
- 48.Rubio-Rivas M, Royo C, Simeon CP, Corbella X, Fonollosa V. Mortality and survival in systemic sclerosis: Systematic review and meta-analysis. Seminars in arthritis and rheumatism. 2014 doi: 10.1016/j.semarthrit.2014.05.010. [DOI] [PubMed] [Google Scholar]