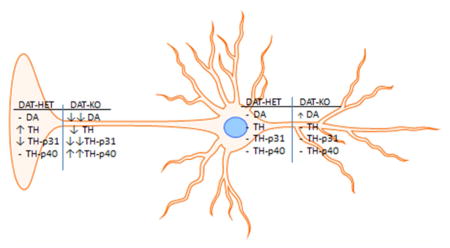
Abstract
Tyrosine hydroxylase (TH) and dopamine transporters (DATs) regulate dopamine (DA) neurotransmission at the biosynthesis and reuptake steps, respectively. Dysfunction or loss of these proteins occurs in impaired locomotor or addictive behavior, but little is known about the influence of DAT expression on TH function. Differences in TH phosphorylation, DA tissue content, L-DOPA biosynthesis, and DA turnover exist between the somatodendritic and terminal field compartments of nigrostriatal and mesoaccumbens pathways. We examined whether differential DAT expression affects these compartmental differences in DA regulation by comparing TH expression and phosphorylation at ser31 and ser40. In heterozygous DAT knockout (KO) (+/−) mice, DA tissue content and DA turnover were unchanged relative to wild-type mice, despite a 40% reduction in DAT protein expression. In DAT KO (−/−) mice, DA turnover increased in all DA compartments, but DA tissue content decreased (90–96%) only in terminal fields. TH protein expression and phosphorylation were differentially affected within DA pathway compartments by relative expression of DAT. TH protein decreased (~74%), though to a significantly lesser extent than DA, in striatum and nucleus accumbens (NAc) in DAT −/− mice, with no decrease in substantia nigra or ventral tegmental area. Striatal ser31 TH phosphorylation and recovery of DA relative to TH protein expression in DAT +/− and DAT −/− mice decreased, whereas ser40 TH phosphorylation increased ~2- to 3-fold in striatum and NAc of DAT −/− mice. These results suggest that DAT expression affects TH expression and phosphorylation largely in DA terminal field compartments, further corroborating evidence for dichotomous regulation of TH between somatodendritic and terminal field compartments of the nigrostriatal and mesoaccumbens pathways.
Keywords: Tyrosine hydroxylase, dopamine transporters, dopamine neurotransmission, L-DOPA
Graphical Abstract
Dopamine (DA) plays a major role in locomotor function and reward-related behavior. In the multistep process of dopaminergic synaptic transmission, the dopamine transporter (DAT) is a vital component of DA signaling in the CNS, serving to transport DA from the synapse back into the presynaptic terminals. In addition to terminating or gating DA signals, this uptake process contributes to the maintenance of readily releasable pools of DA. Much work studying the regulation of DA, including DAT function, has been focused upon terminal field functions in the dorsal striatum (for the nigrostriatal pathway) or the nucleus accumbens (for the mesoaccumbens pathway). However, it is clear that there are significant differences in the efficiency of the DAT for DA clearance between the terminal fields and associated somatodendritic compartments,1–4 suggesting that DAT levels may have a different effect on DA homeostasis in these compartments. For example, DA clearance or uptake occurs at a greater rate in the striatum compared to the substantia nigra (SN).1,2 Therefore, the loss of DAT may bear significantly different consequences for how DA is regulated in the terminal fields or somatodendritic compartments of these two pathways, including TH regulation. In fact, evidence suggests that DA tissue content is more dependent upon TH function in the somatodendritic than the terminal field compartments.5
Dopaminergic neurotransmission begins at the biosynthesis step, with tyrosine hydroxylase (TH) the rate-limiting enzyme for DA biosynthesis. Physiological regulation of TH phosphorylation in CNS is by site-specific phosphorylation at three sites (ser19, ser31, and ser40).6 Of these sites, historically, much evidence exists for a role for ser40 phosphorylation in regulating TH activity. However, in situ evidence suggests that for increased ser40 phosphorylation to affect L-DOPA biosynthesis, a threshold of phosphorylation reaching 3-fold above basal levels is necessary.7 However, increased L-DOPA synthesis can occur without any increase in ser40 phosphorylation.7,8 Recent work in CNS has revealed a significant role for ser31 phosphorylation in regulation of L-DOPA biosynthesis5 and DA tissue content.9–11 While ser19 phosphorylation does not have a direct impact on TH activity,12 its phosphorylation can covary with ser31 in the somatodendritic compartments or ser40 in the terminal field compartments,5 and covary with glutamatergic neurotransmission in striatum.13
To elucidate how DA biosynthesis can be affected by activity at the other steps of DA neurotransmission in the CNS, experimental modulation of the proteins involved may determine the extent of their influence. For example, DA D2 autoreceptors are well-known to provide feedback inhibition on DA release, firing rates, and synthesis, as well as increasing the activity of the DAT,14 yielding a net effect of decreasing DA signaling. DA D2 autoreceptor regulation of TH phosphorylation has been observed in striatum,10,15–17 as well as in nucleus accumbens, substantia nigra, and ventral tegmental area.18 D2-type DA receptor agonists reduce TH phosphorylation at ser40,16 whereas antagonists (acute) increase TH phosphorylation at ser19, ser31, and ser40,18 and chronic administration decreases TH phosphorylation.17 In the rodent, the relative expression of DAT versus TH protein is much greater in the terminal fields versus the somatodendritic compartments in the nigrostriatal and mesoaccumbens pathways.19 Therefore, it would stand to reason that the loss of DAT may impact TH regulation to a greater extent in the terminal fields, given the evidence that DAT function influences DA homeostasis20–22 and DA-related behavior.23,24 However, DA function in the somatodendritic compartments can affect behavioral outcomes.25–28 Using homozygous (−/−) and heterozygous (+/−) DAT knockout mice with both or only one DAT allele deleted, respectively,23 this study presented the opportunity to determine if TH phosphorylation and DA tissue content in the somatodendritic compartments were affected by the relative abundance of DAT in these areas relative to the terminal field compartments. Here we find that DAT expression has significant effects on TH expression and phosphorylation in terminals (striatum and nucleus accumbens), but comparatively less, if any, effect in somatodendritic (ventral tegmental area (VTA) and substantia nigra (SN)) compartments.
RESULTS
DAT Expression
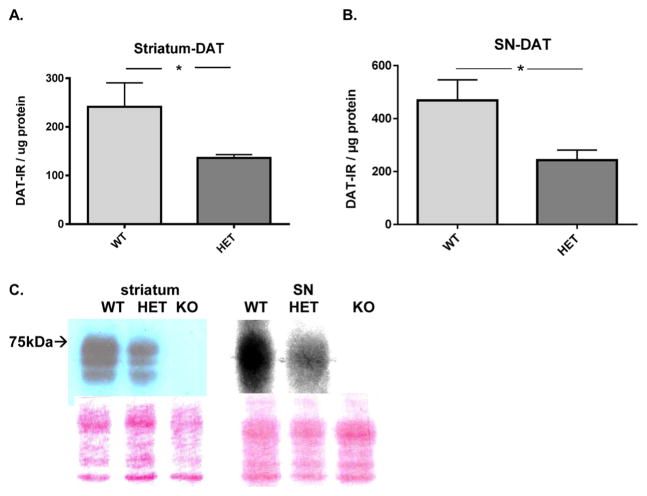
DAT expression was less in the DAT +/− compared to wild-type in both striatum (Figure 1A,C) and SN (Figure 1B,C). The mean reduction of DAT expression in the heterozygote was 44% in the striatum and 50% in the SN. There was no DAT immunoreactivity in the DAT −/− genotype that was above background in either region.
Figure 1.
DAT protein expression in nigrostriatal pathway. (A) Striatal DAT protein expression. DAT protein expression was on average 44% less in the DAT-HET versus WT (t = 1.97, *p = 0.035, df = 13). (B) Nigral DAT protein expression. DAT protein expression was on average 50% less in the DAT-HET versus WT (t = 2.62, *p < 0.05, df = 14). (C) Representative Western blot of DAT expression; wild-type (WT), heterozygotes (+/−, HET), and knockout (−/−, KO) in striatum (left) and SN (right). Nominal protein loads were 12 μg for striatum and 90 μg for the SN and reflect the difference in DAT expression between the two regions, being greater in the striatum. The Ponceau staining below the image reflects the protein loading among the genotypes from these particular sample preparations.
TH Protein Expression
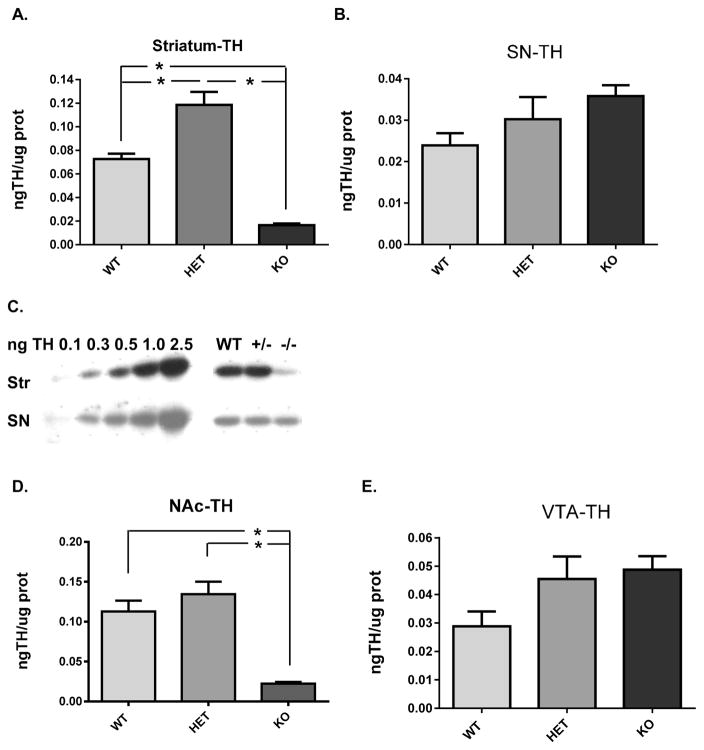
In both the nigrostriatal and mesoaccumbens pathway, there was a stark contrast in relative TH protein expression among the three genotypes between the terminal field and somatodendritic compartments. The expression of TH protein in the DAT −/− in both striatum and nucleus accumbens was greatly diminished as compared to the wild-type, whereas in the cognate somatodendritic compartments of the SN and VTA, TH expression was unaffected in the DAT −/−, although a trend toward an increase was clearly observed (Figure 2C,E). In striatum, TH protein expression increased 63% in the DAT +/− compared to the WT, whereas TH expression decreased 73% in the DAT −/− (Figure 2A). In the SN, no significant differences in TH expression were observed among the three genotypes. However, a trend toward an increase was observed in the DAT −/− genotype (Figure 2B). In the NAc, TH protein expression was decreased 80% in the DAT −/− (Figure 2D), whereas in the VTA, no significant differences in TH expression were observed among the three genotypes. However, as observed in the SN, there was a trend toward an increase in the DAT −/− genotype (Figure 2E).
Figure 2.
Tyrosine hydroxylase (TH) protein expression in nigrostriatal (A, B, C) and mesoaccumbens (D, E) pathways relative to DAT genotype. (A) Striatum TH protein expression was significantly different among the three genotypes (F2,19 = 48.60; p < 0.0001). Holm–Sidak’s multiple comparisons post hoc: WT vs DAT-HET (+/−) (p < 0.001, t = 4.45); WT vs DAT-KO (−/−) (p < 0.0001, t = 5.24); DAT-HET (+/−) vs DAT-KO (−/−) (p < 0.0001, t = 9.86). (B) Representative Western blot of TH expression in striatum and substantia nigra. TH standard curve (0.10 to 2.5 ng total TH protein load) is shown along with expression levels of TH protein in the WT, HET (+/−), and KO (−/−) groups in representative samples from striatum and SN (12 and 15 μg total protein load, respectively). (C) Substantia nigra TH protein expression was not significantly different among the three genotypes (F2,18 = 2.43; p = 0.117). (D) Nucleus accumbens TH protein expression was significantly different among the three genotypes (F2,21 = 23.95; p < 0.0001). Holm–Sidak’s multiple comparisons post hoc: WT vs DAT-HET (+/−) (ns, t = 1.26); WT vs DAT-KO (−/−) (p < 0.0001, t = 5.26); DAT-HET (+/−) vs DAT-KO (−/−) (p < 0.0001, t = 6.52). (E) Ventral tegmental area TH protein expression was not significantly different among the three genotypes (F2,19 = 2.75; p = 0.089).
TH Phosphorylation at Ser31 and Ser40
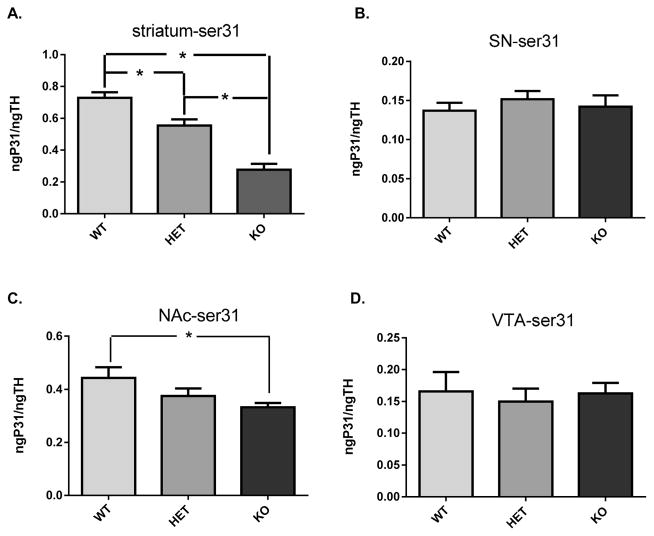
TH phosphorylation at both ser31 and ser40 in the terminal fields differed among the three genotypes, but not in somatodendritic compartments. In the striatum, ser31 was significantly decreased in the DAT +/−, and further decreased in the DAT −/− (Figure 3A). In the SN, no differences in ser31 were observed (Figure 3B). In the NAc, ser31 was significantly decreased in the DAT −/− (Figure 4C), without any differences noted in the VTA (Figure 4D).
Figure 3.
Ser31 TH phosphorylation. (A) Striatum ser31 TH phosphorylation was significantly different among the three genotypes (F2,21 = 37.73; p < 0.0001). Holm–Sidak’s multiple comparisons post hoc: WT vs DAT-HET (+/−) (p < 0.01, t = 3.33); WT vs DAT-KO (−/−) (p < 0.0001, t = 8.61); DAT-HET (+/−) vs DAT-KO (−/−) (p < 0.0001, t = 5.28). (B) Substantia nigra ser31 TH phosphorylation was not significantly different among the three genotypes (F2,21 = 0.38; p = 0.68). (C) Nucleus accumbens ser31 TH phosphorylation was significantly different among the three genotypes (F2,20 = 3.52; p < 0.05). Holm–Sidak’s multiple comparisons post hoc: WT vs DAT-HET (+/−) (ns, t = 1.56); WT vs DAT-KO (−/−) (p < 0.05, t = 2.63); DAT-HET (+/−) vs DAT-KO (−/−) (ns, t = 0.98). (D) Ventral tegmental area ser31 TH phosphorylation was not significantly different among the three genotypes (F2,19 = 0.13; p = 0.88).
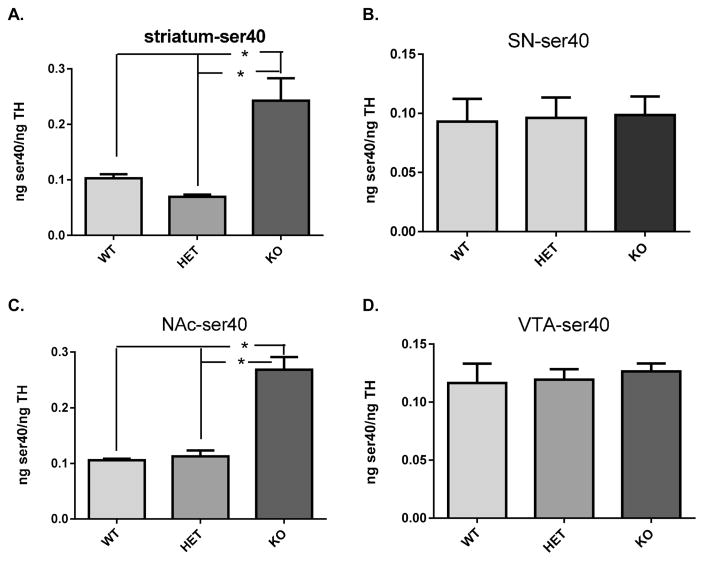
Figure 4.
Ser40 TH phosphorylation. (A) Striatum ser40 TH phosphorylation was significantly different among the three genotypes (F2,20 = 17.01; p < 0.0001). Holm–Sidak’s multiple comparisons post hoc: WT vs DAT-HET (+/−) (ns, t = 1.11); WT vs DAT-KO (−/−) (p < 0.001, t = 4.48); DAT-HET (+/−) vs DAT-KO (−/−) (p < 0.0001, t = 5.55). (B) Substantia nigra ser40 TH phosphorylation was not significantly different among the three genotypes (F2,21 = 0.02; p = 0.98). (C) Nucleus accumbens ser40 TH phosphorylation was significantly different among the three genotypes (F2,16 = 51.09; p < 0.0001). Holm–Sidak’s multiple comparisons post hoc: WT vs DAT-HET (+/−) (ns, t = 0.48); WT vs DAT-KO (−/−) (p < 0.0001, t = 9.28); DAT-HET (+/−) vs DAT-KO (−/−) (p < 0.0001, t = 9.09). (D) Ventral tegmental area ser40 TH phosphorylation was not significantly different among the three genotypes (F2,18 = 0.19; p = 0.83).
TH phosphorylation at ser40 was increased 2–3-fold in striatum and NAc in the DAT −/− mice (Figure 4 A,C). To the contrary, no differences in ser40 were observed in SN or VTA (Figure 4 B,D).
DA Tissue Content and DA Turnover
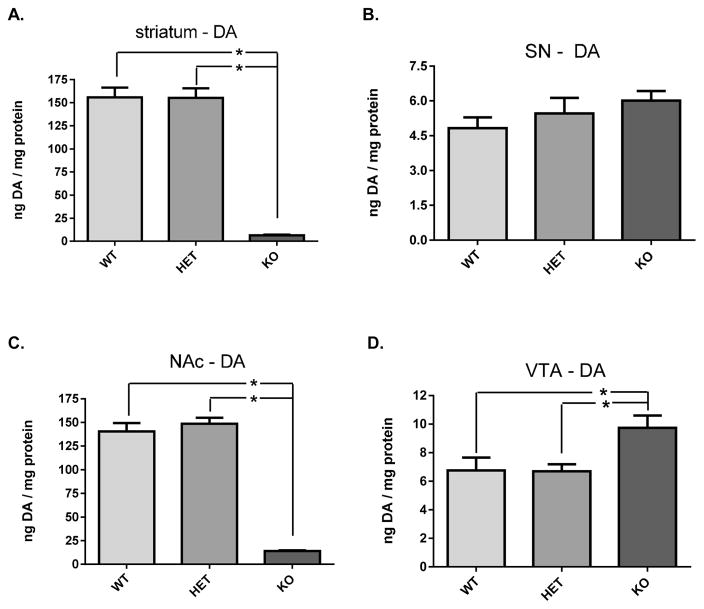
In the terminal field compartments of both the nigrostriatal and mesoaccumbens pathways, DA tissue content (as normalized to protein) was reduced by more than 90% in the DAT −/− mice (Figure 5 A,C). In striatum, DA tissue content decreased an average of 96% in the DAT −/− mice compared to the wild-type, and a similar reduction was observed in the NAc (90%). Despite confirmed reduction of DAT protein in the heterozygote mice (Figure 1 A), DA tissue content did not decrease in striatum (Figure 5A), or the NAc (Figure 5C). Contrary to the major loss of DA tissue content observed in the terminal fields, DA tissue content did not decrease in either the SN or VTA of the DAT −/− mice (Figure 5 B,D). In fact, DA tissue content was significantly increased in the VTA of the DAT −/− (Figure 5D), which was likely due to the 1.6-fold increase in total TH protein expression detected in this group (though not significant).
Figure 5.
DA tissue content in nigrostriatal (A, B) and mesoaccumbens (C, D) pathways relative to DAT genotype. (A) Striatum DA tissue content was significantly different among the three genotypes (F2,21 = 100.4; p < 0.0001). Holm–Sidak’s multiple comparisons post hoc: WT vs DAT-HET (+/−) (ns, t = 0.05); WT vs DAT-KO (−/−) (p < 0.0001, t = 12.30); DAT-HET (+/−) vs DAT-KO (−/−) (p < 0.0001, t = 12.25). (B) Substantia nigra DA tissue content was not significantly different among the three genotypes (F2,18 = 1.25; p = 0.31). (C) Nucleus accumbens DA tissue content was significantly different among the three genotypes (F2,21 = 139.9; p < 0.0001). Holm–Sidak’s multiple comparisons post hoc: WT vs DAT-HET (+/−) (ns, t = 0.88); WT vs DAT-KO (−/−) (p < 0.0001, t = 14.02); DAT-HET (+/−) vs DAT-KO (−/−) (p < 0.0001, t = 14.91). (D) Ventral tegmental area DA tissue content was significantly different among the three genotypes (F2,18 = 5.32; p < 0.05). Holm–Sidak’s multiple comparisons post hoc: WT vs DAT-HET (+/−) (ns, t = 0.04); WT vs DAT-KO (−/−) (p < 0.05, t = 2.69); DAT-HET (+/−) vs DAT-KO (−/−) (p < 0.05, t = 2.85).
Dopamine turnover increased in all four compartments in the DAT −/− mice, being 12- to 40-fold greater than in WT or DAT +/− in the terminal fields, whereas the increase was less (1.3-fold above WT or DAT +/−) in the somatodendritic compartments. DOPAC levels were significantly greater in each region in the DAT −/− mice (Figure 6).
Figure 6.
DA turnover (as DOPAC) in nigrostriatal (A, B) and mesoaccumbens (C, D) pathways relative to DAT genotype. DA turnover was quantified by dividing the value of DOPAC by DA inherent to each tissue sample. (A) Striatum DOPAC was significantly different among the three genotypes (F2,21 = 13.7; p = 0.0002). Holm–Sidak’s multiple comparisons post hoc: WT vs DAT-HET (+/−) (ns, t = 1.55); WT vs DAT-KO (−/−) (p < 0.001, t = 3.56); DAT-HET (+/−) vs DAT-KO (−/−) (p < 0.0001, t = 5.11). (B) Substantia nigra DOPAC was significantly different among the three genotypes (F2,20 = 5.35; p = 0.014). Holm–Sidak’s multiple comparisons post hoc: WT vs DAT-HET (+/−) (ns, t = 0.33); WT vs DAT-KO (−/−) (p < 0.05, t = 2.69); DAT-HET (+/−) vs DAT-KO (−/−) (p < 0.05, t = 2.93). (C) Nucleus accumbens DA tissue content was significantly different among the three genotypes (F2,20 = 8.60; p = 0.002). Holm–Sidak’s multiple comparisons post hoc: WT vs DAT-HET (+/−) (ns, t = 1.01); WT vs DAT-KO (−/−) (p < 0.05, t = 3.03); DAT-HET (+/−) vs DAT-KO (−/−) (p < 0.01, t = 3.94). (D) Ventral tegmental area DA tissue content was significantly different among the three genotypes (F2,20 = 15.8; p < 0.0001). Holm–Sidak’s multiple comparisons post hoc: WT vs DAT-HET (+/−) (ns, t = 1.84); WT vs DAT-KO (−/−) (p < 0.001, t = 5.00); DAT-HET (+/−) vs DAT-KO (−/−) (p < 0.001, t = 4.67).
Relationship of DA Tissue Content and TH Phosphorylation
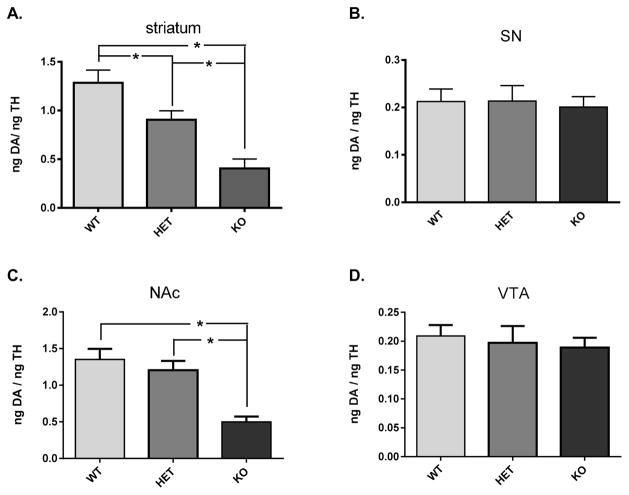
Given the differences in TH protein expression found in the terminal fields, DA tissue content in each sample was normalized to the amount of TH protein recovered in each tissue sample. DA tissue content, as normalized to TH protein, was decreased in striatum in the DAT +/− group and even further decreased in the DAT −/− (Figure 7A). In the NAc, there was also a decrease in the DAT −/−, but not DAT +/−(Figure 7B). In somatodendritic compartments, no loss of DA was observed in the DAT −/− mice in either SN or VTA.
Figure 7.
DA tissue content normalized to TH content in nigrostriatal (A, B) and mesoaccumbens (C, D) pathways relative to DAT genotype. (A) Striatum DA tissue content normalized to inherent TH protein was significantly different among the three genotypes (F2,20 = 15.98; p < 0.0001). Holm–Sidak’s multiple comparisons post hoc: WT vs DAT-HET (+/−) (p < 0.05, t = 2.53); WT vs DAT-KO (−/−) (p < 0.0001, t = 5.65); DAT-HET (+/−) vs DAT-KO (−/−) (p < 0.01, t = 3.21). (B) Substantia nigra DA tissue content normalized to inherent TH protein was not significantly different among the three genotypes (F2,19 = 0.07; p = 0.93). (C) Nucleus accumbens DA tissue content normalized to inherent TH protein was significantly different among the three genotypes (F2,21 = 14.72; p = 0.0001). Holm–Sidak’s multiple comparisons post hoc: WT vs DAT-HET (+/−) (ns, t = 0.87); WT vs DAT-KO (−/−) (p < 0.001, t = 5.07); DAT-HET (+/−) vs DAT-KO (−/−) (p < 0.001, t = 4.20). (D) Ventral tegmental area DA tissue content normalized to inherent TH protein was not significantly different among the three genotypes (F2,21 = 0.21; p = 0.81).
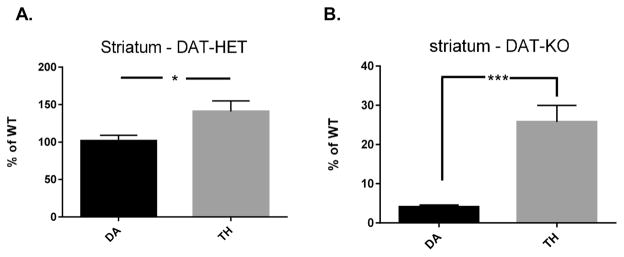
The relative differences in DA recovered against TH expression were reflected by ser31 phosphorylation differences seen in Figure 3. In comparing striatal DA tissue content against TH protein expression in the DAT +/− and DAT −/− genotypes, in both cases DA tissue content was significantly less than TH expression (Figure 8A,B), suggesting that DA biosynthesis was reduced in both genotypes.
Figure 8.
Striatal DA tissue content versus TH protein expression and relationship of TH phosphorylation with DA tissue content. (A) DAT-HET genotype. There was significantly less DA tissue content versus TH protein expression in the DAT-HET (+/−) genotype compared to WT (t = 2.54, *p < 0.05, df = 13, unpaired two-tailed t test). (B) DAT-KO genotype. There was significantly less DA tissue content versus TH protein expression in the DAT-KO (−/−) genotype compared to WT (t = 2.54, ***p = 0.0002, df = 14, unpaired two-tailed t test).
DISCUSSION
Despite the fact that the somatodendritic and terminal field compartments comprise the same neuron populations, much evidence supports that there is a dichotomy in regulation of DA neurotransmission between these two compartments in both the nigrostriatal and mesoaccumbens pathways, including TH expression and phosphorylation at the biosynthesis step,5,11,18 DA uptake,2–4 and DAT expression.19 DAT expression is much less in SN and VTA compared with striatum and nucleus accumbens in rodent,19 and the present results provide additional evidence that DA regulation is less influenced by DAT in the somatodendritic compartments. The loss of DAT was associated with decreased DA tissue content and TH protein, but only in the terminal fields of striatum and nucleus accumbens. The major loss of DA and TH in dorsal striatum of DAT −/− mice has been previously reported.20,22 This study adds that this loss of DAT has comparatively much less impact upon DA tissue content, TH protein expression, or phosphorylation in the somatodendritic DA compartments. Previous work indicates that the DAT −/− genotype produces a loss (though comparatively small) of TH-positive neurons in the midbrain.22 We did not observe evidence of a decrease in TH protein expression in either SN or VTA, and, if anything, a nonsignificant increase was observed in these areas. This disconnect between numbers of TH-positive neurons and TH expression has been previously reported29 and may be related to differences in neuronal activity, as affected by differences in extracellular DA, among the three genotypes.
DAT deficiency affected TH phosphorylation at ser31 and ser40 only in the terminal field compartments, with phosphorylation at ser31 decreasing while increasing at ser40. Acute noncontingent cocaine also blocks DAT function, and some similarity of our results is seen with this previous work, with effects on TH phosphorylation at both ser31 and ser40 in terminal field regions, but not in the somatodendritic compartments.30 This study reported decreased L-DOPA synthesis in conjunction with decreased TH phosphorylation at both sites. However, in our study, ser31 decreased and ser40 increased in the DAT −/− genotype, and the decrease in DA normalized to remaining TH protein matched ser31 phosphorylation differences. However, the large increase in DA turnover in the terminal fields revealed in the DAT −/− genotype confounds a direct interpretation of the impact of these disparate changes in phosphorylation, as an increase in DA turnover would presumably diminish DA bioavailability. Increased DA biosynthesis has been observed in the DAT −/− genotype,20 suggesting that the 2-fold increase in ser40 TH phosphorylation would increase TH activity. However, the relative amount of remaining DA tissue content was less than remaining TH protein, suggesting that TH activity was decreased, although this was not directly tested herein by assessment of L-DOPA. Yet, this observation would be consistent with the decline in ser31 in both the DAT +/− genotype (which had increased TH expression but DA levels equal to the WT) and the DAT −/− genotype. In our previous work investigating the relationship of TH phosphorylation with L-DOPA biosynthesis in the four regions examined here, there was evidence that ser31 phosphorylation has significant influence upon L-DOPA biosynthesis. With normalization of L-DOPA against inherent TH protein recovery ser31, but not ser40, phosphorylation paralleled differences in L-DOPA levels against TH in the four regions examined here.5 We also speculate that the reason for decreased TH protein expression only in the terminal field regions of the DAT −/− genotype may be associated with increased ser40 phosphorylation. Increased ser40 TH phosphorylation can be associated with TH ubiquitination and proteosomal degradation31,32 and even irreversible TH inactivation.33 Furthermore, increased ser40 phosphorylation occurs in conjunction with a decrease in TH and DAT expression, decreased DA tissue content, and increased DA turnover, in mice with genetic alteration of iron regulatory protein.34 Given these recent observations, the increase in ser40 phosphorylation may contribute to TH protein loss and partially contribute to accelerated DA turnover in the terminal fields of DAT −/− mice.
The particularly selective impact upon TH phosphorylation in the terminal field compartments in the DAT +/− and DAT −/− genotypes may be related to endogenous compartmental differences (as would be reflected in the DAT-WT genotype) in DA release capacity, DA D2 autoreceptor function, VMAT2 expression, DAT expression, or endogenous DA stores. The loss of DAT can affect D2 autoreceptor function.21 DA D1 receptor antagonist effects on TH phosphorylation are limited to ser31 TH phosphorylation in the VTA and nucleus accumberns.18 However, much evidence supports that DA D2 autoreceptors affect TH phosphorylation16–18 following either acute or chronic treatment with agonist or antagonists. Thus, major differences in DA uptake (as controlled by differences in DAT expression in our study) and DA release capacity (also affected by DAT expression) could affect DA D2 autoreceptor function and, therefore, TH phosphorylation among the genotypes. As expression of DAT is decreased relative to that in the WT, two relevant observations affecting autoreceptor function have been reported in the striatum. First, DA release capacity decreases to ~25% of that observed in the WT.20,23 Second, the efficacy of autoreceptor-mediated regulation of DA release is diminished.21 Notably, diminished autoreceptor function is also observed with continuous stimulant exposure.35 Therefore, given that there is less extracellular DA release (with the caveat of a greatly protracted DA clearance time in the DAT −/−20,23 and the efficacy of DA D2 receptor function is decreased), there is the possibility that the DA D2 receptor-mediated effects on TH phosphorylation would be decreased. The major increase in ser40 phosphorylation in the DAT −/− genotype suggests this possibility, given that DA agonists reduce16 and antagonists increase18 ser40 phosphorylation. However, the decrease in striatal ser31 TH phosphorylation in the DAT +/− and DAT −/− indicates that some DA-mediated D2 regulation is still present, as antagonists also increase ser31 in striatum.18 The DA D2 receptor is coupled to ERK function,10 and given the reported decrease in autoreceptor function in DAT +/− and DAT −/− genotypes, the decrease in ser31 TH phosphorylation, a target of ERK under depolarizing conditions,7 would be expected. This differential response in ser31 and ser40 phosphorylation in both nucleus accumbens and striatum indicates that, under the conditions of DA neurotransmission imposed by the loss of DAT, autoreceptor-mediated control of only ser40 may be abrogated, and the segregation of phosphorylation differences in response to physiological stimuli at these two sites has been observed in other paradigms studying TH regulation in vivo.11,36,37 Alternatively, differences in TH phosphorylation response to DAT deficiency between the somatodendritic and terminal field compartments may be related to the influence of cytosolic DA from DAT-mediated uptake. Inhibition of L-DOPA decarboxylase by NSD-1015 (which would decrease DA content) may increase ser31 and ser40 phosphorylation in the tissues studied here.5 The compartment specific increase in ser40 in the DAT −/− seen only in the terminal field regions may therefore be related to the decrease in cytosolic DA we report in both regions. This terminal field specific effect is further supported by the evidence that L-DOPA content per DA content is significantly less in the terminal field compartments,5 indicating that the much greater quantity of DAT normally expressed likely accounts for the normal pool of DA. Thus, removal of the DAT would carry greater impact on DA homeostasis in the terminal fields.
Differences in DAT expression were without effect on TH phosphorylation in the somatodendritic compartments of either the SN or VTA, suggesting that the influence of TH regulation on DA content therein is largely independent of any contributions by DA uptake. Dopamine release in the substantia nigra has been established for 40 years,38,39 and this study further supports that TH function may be a primary component of how much DA is available for release, particularly since evidence also shows that DA D2 agonists are much less effective in inhibiting DA release therein40 and shows less efficiency of DA uptake in the midbrain1–3,41 and less DA release in the midbrain.2–4,42 The expression of DAT protein relative to TH protein is nearly 10–20-fold less in the somatodendritic compartments versus the terminal fields,19 and DA tissue content is more dependent upon TH function in the somatodendritic regions.5 Our results seem to further extend the critical differences in DA regulation between the two compartments, which may come into play to determine how DAT affects TH activity in DA-dependent behavioral outcomes,43–47 particularly if considering the influence of DA neurotransmission in the somatodendritic compartments upon behavior25–28,48–50 and in disease states affected by DA loss like Parkinson’s disease.51,52 Thus, given the major loss of DA in the striatum, it is reasonable to ask if hyperactivity in DAT−/− is driven by elevated extracellular DA levels in striatum or in the substantia nigra. From the perspective of reward, recent work indicates that amphetamine self-administration was more associated with expression of DA D2/D3 receptors in the VTA rather than the nucleus accumbens.53 Methamphetamine, which acts in part via DAT, also produces a differential impact on DA regulation in the VTA versus NAc19 and upon TH phosphorylation.54
SUMMARY
The influence of monoamine transporters upon the components of the biosynthesis and catabolism steps of mono-aminergic neurotransmitters is a critical question to resolve to understand the interdependent nature of the steps involved in neurotransmission. This question has been also addressed in serotoninergic terminal field regions in serotonin transporter +/− and −/− genotypes.55 This study expands insight into the relative impact of DAT removal in the DAT −/−, and partial DAT expression in the DAT +/−, to reveal a dichotomous plasticity in TH and DA regulation between the terminal field and somatodendritic compartments of the nigrostriatal and mesoaccumbens pathways. The comparative lack of effect of either the DAT +/− or DAT −/− genotypes on TH regulation in the somatodendritic compartments may be related to the relatively minor contribution of DAT function to DA homeostasis in the somatodendritic compartments, and may be due to the major differences in the relative abundance of DAT and TH between the terminal field and somatodendritic compartments.19 The divergent changes in TH phosphorylation in the terminal fields in conjunction with effects on DA tissue content likely substantiate the major role of DAT function on DA homeostasis in DA terminals, but also its comparatively mitigated influence on TH function and DA tissue content in the somatodendritic compartments.
METHODS
Breeding of Mice
Male DAT −/− (KO) (Giros et al., 1996), DAT +/− (HET), and wild-type (WT) littermate mice on a C57BL/ 6J background (bred in house for >10 generations) were maintained on a 12:12 h light/dark cycle (6:00 a.m. lights on; 6:00 p.m. lights off) with food and water ad libitum. Two- to three-month-old male mice were used in all experiments. All animals were maintained according to the National Institutes of Health guidelines in Association for Assessment and Accreditation of Laboratory Animal Care accredited facilities. The experimental protocol was approved by the Institutional Animal Care and Use Committee at Wake Forest School of Medicine.
Dissection of Tissue and Analysis
Mice were lightly anesthetized with isofluorane and decapitated. Brains were rapidly removed and held in ice-cold PBS solution while the dissection occurred. Striatum, NAc, SN, or VTA punches (1 mm, 14-gauge) from DAT −/−, DAT +/−, and WT animals were freshly dissected on ice and snap frozen in isopentane. The dissection of the discrete midbrain tissue regions follows published methodology.56 The VTA is segregated from the SN by triangular cuts freehand by using a #11 scalpel, cutting away from the midline of the midbrain to isolate the VTA from the SN. The SN is dissected away from the midbrain by cutting diagonally upward to end midway in the lateral edge of the midbrain. Tissue was kept frozen at −80 °C until analysis for DA and DOPAC tissue content, TH protein and phosphorylation, and DAT expression.
Analysis of Dopamine and Tyrosine Hydroxylase
An established procedure was followed to ascertain the combination of DA tissue content, tyrosine hydroxylase expression and phosphorylation, and DAT protein expression in each tissue sample.55 Briefly, tissue samples were sonicated in 0.1 M perchloric acid solution, and protein precipitates were isolated from the supernatants. The supernatants were subsequently analyzed for DA and dihydroxyphenylacetic acid (DOPAC) quantity by HPLC.55 The ratio of DOPAC to DA was determined from these analyses to determine any change in DA turnover among the three groups. The protein precipitate was sonicated in 1% SDS solution and total tissue protein was determined prior to sample preparation for blot-immunolabeling. The expression levels of DAT were determined using Santa Cruz antibody sc-1433 (goat host, 1 μg/mL use dilution, rabbit anti-goat secondary antibody (DAKO), TH protein (rabbit host, Millipore, AB152), and phosphorylation at ser31 (Salvatore et al., 2009) and ser40 (cat. no. p1580-40, Phosphosolutions, Aurora, CO)) using established methodology.
Statistics
A Grubb’s outlier test was performed on data sets to determine any outliers in results from each genotype. After confirmation of no detection of DAT protein in the DAT −/−, DAT expression between the WT and DAT-HET was evaluated with a one-tailed unpaired t test, on the hypothesis that DAT expression has been previously reported to decrease in the DAT-HET genotype.57 For all other statistical analyses, a one-way ANOVA was run on each dependent measure obtained from the 3 groups followed by a Holm–Sidak’s multiple comparisons post hoc test to compare DA, DA turnover, and DAT and TH expression and TH phosphorylation differences among the three groups. Significance was set at p < 0.05.
Acknowledgments
Funding
This work was fully funded by the National Institute on Aging (R01AG040261 awarded to M.F.S.) and the National Institute on Drug Abuse (R01DA030161 awarded to S.R.J.).
The authors thank Victoria L. Fields and Brian Latimer for outstanding technical support in Western blot and HPLC assays.
ABBREVIATIONS
- DAT
dopamine transporter
- TH
tyrosine hydroxylase
- HET
heterozygote
- KO
knockout
- WT
wildtype
- SN
substantia nigra
- VTA
ventral tegmental area
- L-DOPA
dihydroxyphenylalanine
- VMAT2
vesicular monoamine transporter 2
- ERK
extracellular signal-regulated kinase
Footnotes
Author Contributions
Conceived and designed the experiments: M.F.S, S.R.J. Performed data analysis: M.F.S. Contributed reagents/materials/analysis tools: M.F.S., S.R.J., E.S.C. Wrote manuscript: M.F.S., S.R.J., E.S.C.
Notes
The authors declare no competing financial interest.
References
- 1.Hoffman AF, Lupica CR, Gerhardt GA. Dopamine transporter activity in the substantia nigra and striatum assessed by high-speed chronoamperometric recordings in brain slices. J Pharmacol Exp Ther. 1998;287:487–496. [PubMed] [Google Scholar]
- 2.Chen BT, Rice ME. Novel Ca2+ dependence and time course of somatodendritic dopamine release: substantia nigra versus striatum. J Neurosci. 2001;21:7841–7847. doi: 10.1523/JNEUROSCI.21-19-07841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.John CE, Jones SR. Exocytotic release of dopamine in ventral tegmental area slices from C57BL/6 and dopamine transporter knockout mice. Neurochem Int. 2006;49:737–45. doi: 10.1016/j.neuint.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Ford CP, Gantz SC, Phillips PEM, Williams JT. Control of extracellular dopamine at dendrite and axon terminals. J Neurosci. 2010;30:6975–6983. doi: 10.1523/JNEUROSCI.1020-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salvatore MF, Pruett BS. Dichotomy of tyrosine hydroxylase and dopamine regulation between somatodendritc and terminal field areas of nigrostriatal and mesoaccumbens pathways. PLoS One. 2012;7:e29867. doi: 10.1371/journal.pone.0029867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haycock JW, Haycock DA. Tyrosine hydroxylase in rat brain dopaminergic nerve terminals: Multiple-site phosphorylation in vivo and in synaptosomes. J Biol Chem. 1991;266:5650–5657. [PubMed] [Google Scholar]
- 7.Salvatore MF, Waymire JC, Haycock JW. Depolarization-stimulated catecholamine biosynthesis: involvement of protein kinases and tyrosine hydroxylase phosphorylation sites in situ. J Neurochem. 2001;79:349–360. doi: 10.1046/j.1471-4159.2001.00593.x. [DOI] [PubMed] [Google Scholar]
- 8.Harada K, Wu J, Haycock JW, Goldstein M. Regulation of L-DOPA biosynthesis by site-specific phosphorylation of tyrosine hydroxylase in AtT-20 cells expressing wild-type and serine 40-substituted enzyme. J Neurochem. 1996;67:629–635. doi: 10.1046/j.1471-4159.1996.67020629.x. [DOI] [PubMed] [Google Scholar]
- 9.Nunez C, Laorden ML, Milanes MV. Regulation of serine (Ser)-31 and Ser40 tyrosine hydroxylase phosphorylation during morphine withdrawal in the hypothalamic paraventricular nucleus and nucleus tractus solitarius-A2 cell group: role of ERK1/2. Endocrinology. 2007;148:5780–5793. doi: 10.1210/en.2007-0510. [DOI] [PubMed] [Google Scholar]
- 10.Dadalko OI, Siuta M, Poe A, Erreger K, Matthies HJ, Niswender K, Galli A. mTORC2/rictor signaling disrupts dopamine-dependent behaviors via defects in striatal dopamine neurotransmission. J Neurosci. 2015;35:8843–8855. doi: 10.1523/JNEUROSCI.0887-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salvatore MF. Ser31 tyrosine hydroxylase phosphorylation parallels differences in dopamine recovery in nigrostriatal pathway following 6-OHDA lesion. J Neurochem. 2014;129:548–558. doi: 10.1111/jnc.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haycock JW, Lew JY, Garcia-Espana A, Lee KY, Harada K, Meller E, Goldstein M. Role of Serine-19 phosphorylation in regulating tyrosine hydroxylase studied with site-and phosphospecific antibodies and site-directed mutagenesis. J Neurochem. 1998;71:1670–1675. doi: 10.1046/j.1471-4159.1998.71041670.x. [DOI] [PubMed] [Google Scholar]
- 13.Chotibut T, Davis RW, Arnold JC, Frenchek Z, Gurwara S, Bondada V, Geddes JW, Salvatore MF. Ceftriaxone increases glutamate uptake and reduces striatal tyrosine hydroxylase loss in 6-OHDA Parkinson’s model. Mol Neurobiol. 2014;49:1282–1292. doi: 10.1007/s12035-013-8598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickinson SD, Sabeti J, Larson GA, Giardina K, Rubinstein M, Kelly MA, Grandy DK, Low MJ, Gerhardt GA, Zahniser NR. Dopamine D2 receptor-deficient mice exhibit decreased dopamine transporter function but no changes in dopamine release in dorsal striatum. J Neurochem. 1999;72:148–156. doi: 10.1046/j.1471-4159.1999.0720148.x. [DOI] [PubMed] [Google Scholar]
- 15.Lindgren N, Xu ZQD, Lindskog M, Herrera-Marschitz M, Goiny M, Haycock JW, Goldstein M, Hokfelt T, Fisone G. Regulation of tyrosine hydroxylase activity and phosphorylation at Ser19 and Ser40 via activation of glutamate NMDA receptors in rat striatum. J Neurochem. 2000;74:2470–2477. doi: 10.1046/j.1471-4159.2000.0742470.x. [DOI] [PubMed] [Google Scholar]
- 16.Lindgren N, Xu Z-QD, Herrera-Marschitz M, Haycock J, Hökfelt T, Fisone G. Dopamine D2 receptors regulate tyrosine hydroxylase activity and phosphorylation at ser40 in rat striatum. Eur J Neurosci. 2001;13:773–780. doi: 10.1046/j.0953-816x.2000.01443.x. [DOI] [PubMed] [Google Scholar]
- 17.Håkansson K, Pozzi L, Usiello A, Haycock JW, Borrelli E, Fisone G. Regulation of striatal tyrosine hydroxylase phosphorylation by acute and chronic haloperidol. Eur J Neurosci. 2004;20:1108–1112. doi: 10.1111/j.1460-9568.2004.03547.x. [DOI] [PubMed] [Google Scholar]
- 18.Salvatore MF, Garcia-Espana A, Goldstein M, Deutch AY, Haycock JW. Stoichiometry of tyrosine hydroxylase phosphorylation in the nigrostriatal and mesolimbic systems in vivo: Effects of acute haloperidol and related compounds. J Neurochem. 2000;75:225–232. doi: 10.1046/j.1471-4159.2000.0750225.x. [DOI] [PubMed] [Google Scholar]
- 19.Keller CM, Salvatore MF, Pruett BS, Guerin GF, Goeders NE. Biphasic dopamine regulation in mesoaccumbens pathway in response to non-contingent binge and escalating methamphetamine regimens in the Wistar rat. Psychopharmacol. 2011;215:513–526. doi: 10.1007/s00213-011-2301-9. [DOI] [PubMed] [Google Scholar]
- 20.Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci U S A. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones SR, Gainetdinov RR, Hu XT, Cooper DC, Wightman RM, White FJ, Caron MG. Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat Neurosci. 1999;2:649–655. doi: 10.1038/10204. [DOI] [PubMed] [Google Scholar]
- 22.Jaber M, Dumartin B, Sagne C, Haycock JW, Roubert C, Giros B, Bloch B, Caron MG. Differential regulation of tyrosine hydroxylase in the basal ganglia of mice lacking the dopamine transporter. Eur J Neurosci. 1999;11:3499–3511. doi: 10.1046/j.1460-9568.1999.00764.x. [DOI] [PubMed] [Google Scholar]
- 23.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 24.Spielewoy C, Roubert C, Hamon M, Nosten-Bertrand M, Betancur C, Giros B. Behavioural disturbances associated with hyperdopaminergia in dopamine-transporter knockout mice. Behav Pharmacol. 2000;11:279–290. doi: 10.1097/00008877-200006000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trevitt JT, Carlson BB, Nowend K, Salamone JD. Substantia nigra pars reticulata is a highly potent site of action for the behavioral effects of the D1 antagonist SCH23390 in the rat. Psychopharmacology. 2004;156:32–41. doi: 10.1007/s002130100708. [DOI] [PubMed] [Google Scholar]
- 26.Andersson DR, Nissbrandt H, Bergquist F. Partial depletion of dopamine in substantia nigra impairs motor performance without altering striatal dopamine neurotransmission. Eur J Neurosci. 2006;24:617–624. doi: 10.1111/j.1460-9568.2006.04953.x. [DOI] [PubMed] [Google Scholar]
- 27.Kliem MA, Maidment NT, Ackerson LC, Chen S, Smith Y, Wichmann T. Activation of nigral and pallidal dopamine D1-like receptors modulates basal ganglia outflow in monkeys. J Neurophysiol. 2007;98:1489–1500. doi: 10.1152/jn.00171.2007. [DOI] [PubMed] [Google Scholar]
- 28.Pruett BS, Salvatore MF. Nigral GFRα1 infusion in aged rats increases locomotor activity, nigral tyrosine hydroxylase, and dopamine content in synchronicity. Mol Neurobiol. 2013;47:988–999. doi: 10.1007/s12035-013-8397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aumann TD, Egan K, Lim J, Boon WC, Bye CR, Chua HK, Baban N, Parish CL, Bobrovskaya L, Dickson PW, Horne MK. Neuronal activity regulates expression of tyrosine hydroxylase in adult mouse substantia nigra pars compacta neurons. J Neurochem. 2011;116:646–658. doi: 10.1111/j.1471-4159.2010.07151.x. [DOI] [PubMed] [Google Scholar]
- 30.Jedynak JP, Ali SF, Haycock JW, Hope BT. Acute administration of cocaine regulates the phosphorylation of ser19, −31, and −40 in tyrosine hydroxylase. J Neurochem. 2002;82:382–388. doi: 10.1046/j.1471-4159.2002.00982.x. [DOI] [PubMed] [Google Scholar]
- 31.Kawahata I, Ohtaku S, Tomioka Y, Ichinose H, Yamakuni T. Dopamine or biopterin deficiency potentiates phosphorylation at 40Ser and ubiquitination of tyrosine hydroxylase to be degraded by the ubiquitin proteasome system. Biochem Biophys Res Commun. 2015;465:53–58. doi: 10.1016/j.bbrc.2015.07.125. [DOI] [PubMed] [Google Scholar]
- 32.Shi X, Woodward WR, Habecker BA. Ciliary neurotrophic facor stimulates tyrosine hydroxylase activity. J Neurochem. 2012;121:700–704. doi: 10.1111/j.1471-4159.2012.07712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vrana KE, Roskoski R. Tyrosine hydroxylase inactivation following cAMP-dependent phosphorylation activation. J Neurochem. 1983;40:1692–1700. doi: 10.1111/j.1471-4159.1983.tb08144.x. [DOI] [PubMed] [Google Scholar]
- 34.Salvatore MF, Fisher B, Surgener SP, Gerhardt GA, Rouault TA. Neurochemical investigations of dopamine neuronal systems in iron-regulatory protein 2 (IRP-2) knockout mice. Mol Brain Res. 2005;139:341–347. doi: 10.1016/j.molbrainres.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Calipari ES, Sun H, Eldeeb K, Luessen DJ, Feng X, Howlett AC, Jones SR, Chen R. Amphetamine self-administration attenuates dopamine D2 autoreceptor function. Neuropsychopharmacology. 2014;39:1833–1842. doi: 10.1038/npp.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bobrovskaya L, Gilligan C, Bolster EK, Flaherty JJ, Dickson PW, Dunkley PR. Sustained phosphorylation of tyrosine hydroxylase at serine 40: a novel mechanism for maintenance of catecholamine synthesis. J Neurochem. 2007;100:479–489. doi: 10.1111/j.1471-4159.2006.04213.x. [DOI] [PubMed] [Google Scholar]
- 37.Ong LK, Guan L, Stutz B, Dickson PW, Dunkley PR, Bobrovskaya L. The effects of footshock and immobilization stress on tyrosine hydroxylase phosphorylation in the rat locus coeruleus and adrenal gland. Neuroscience. 2011;192:20–27. doi: 10.1016/j.neuroscience.2011.06.087. [DOI] [PubMed] [Google Scholar]
- 38.Geffen LB, Jessell TM, Cuello AC, Iversen LL. Release of dopamine from dendrites in rat substantia nigra. Nature. 1976;260:258–260. doi: 10.1038/260258a0. [DOI] [PubMed] [Google Scholar]
- 39.Korf J, Zieleman M, Westerink BH. Dopamine release in substantia nigra? Nature. 1976;260:257–258. doi: 10.1038/260257a0. [DOI] [PubMed] [Google Scholar]
- 40.Cragg SJ, Greenfield SA. Differential autoreceptor control of somatodendritic and axon terminal dopamine release in substantia nigra, ventral tegmental area, and striatum. J Neurosci. 1997;17:5738–5748. doi: 10.1523/JNEUROSCI.17-15-05738.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cragg SJ, Rice ME, Greenfield SA. Heterogeneity of electrically evoked dopamine release and reuptake in substantia nigra, ventral tegmental area, and striatum. J Neurophysiol. 1997;77:863–873. doi: 10.1152/jn.1997.77.2.863. [DOI] [PubMed] [Google Scholar]
- 42.Leviel V. Dopamine release mediated by the dopamine transporter, facts and consequences. J Neurochem. 2011;118:475–489. doi: 10.1111/j.1471-4159.2011.07335.x. [DOI] [PubMed] [Google Scholar]
- 43.Bergstrom BP, Sanberg SG, Andersson M, Mithyantha J, Carroll FI, Garris PA. Functional reorganization of the presynaptic dopaminergic terminal in parkinsonism. Neuroscience. 2011;193:310–322. doi: 10.1016/j.neuroscience.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Budygin EA, Brodie MS, Sotnikova TD, Mateo Y, John CE, Cyr M, Gainetdinov RR, Jones SR. Dissociation of rewarding and dopamine transporter-mediated properties of amphetamine. Proc Natl Acad Sci U S A. 2004;101:7781–7786. doi: 10.1073/pnas.0401418101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carpenter AC, Saborido TP, Stanwood GD. Development of hyperactivity and anxiety responses in dopamine transporter-deficient mice. Dev Neurosci. 2012;34:250–257. doi: 10.1159/000336824. [DOI] [PubMed] [Google Scholar]
- 46.Calipari ES, Ferris MJ, Melchior JR, Bermejo K, Salahpour A, Roberts DC, Jones SR. Methylphenidate and cocaine self-administration produce distinct dopamine terminal alterations. Addict Biol. 2014;19:145–155. doi: 10.1111/j.1369-1600.2012.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salvatore MF, Hudspeth O, Arnold LE, Wilson PE, Stanford JA, Mactutus CF, Booze RM, Gerhardt GA. Prenatal cocaine exposure alters potassium-evoked dopamine relase dynamics. Neuroscience. 2004;123:481–490. doi: 10.1016/j.neuroscience.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Rice ME, Patel JC. Somatodendritic dopamine release: recent mechanistic insights. Philos Trans R Soc, B. 2015;370:20140185. doi: 10.1098/rstb.2014.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Paradoxical striatal cellular signaling responses to psychostimulants in hyperactive mice. J Biol Chem. 2006;281:32072–32080. doi: 10.1074/jbc.M606062200. [DOI] [PubMed] [Google Scholar]
- 50.Rao A, Sorkin A, Zahniser NR. Mice expressing markedly reduced striatal dopamine transporters exhibit increased locomotor activity, dopamine uptake turnover rate, and cocaine responsiveness. Synapse. 2013;67:668–677. doi: 10.1002/syn.21671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, Halliday GM, Bartus RT. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain. 2013;136:2419–2431. doi: 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hansard MJ, Smith LA, Jackson MJ, Cheetham SC, Jenner P. Dopamine reuptake inhibition and failure to evoke dyskinesia in MPTP-treated primates. Eur J Pharmacol. 2002;451:157–160. doi: 10.1016/s0014-2999(02)02268-9. [DOI] [PubMed] [Google Scholar]
- 53.Sun H, Calipari ES, Beveridge TJ, Jones SR, Chen R. The brain gene expression profile of dopamine D2/D3 receptors and associated signaling proteins following amphetamine self-administration. Neuroscience. 2015;307:253–261. doi: 10.1016/j.neuroscience.2015.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shin EJ, Duong CX, Nguyen TX, Bing G, Bach JH, Park DH, Nakayama K, Ali SF, Kanthasamy AG, Cadet JL, Nabeshima T, Kim HJ. PKC inhibition enhances tyrosine hydroxylase phosphorylation in mice after methamphetamine treatment. Neurochem Int. 2011;59:39–50. doi: 10.1016/j.neuint.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim DK, Tolliver TJ, Huang SJ, Martin BJ, Andrews AM, Wichems C, Holmes A, Lesch KP, Murphy DL. Altered serotonin synthesis, turnover and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. Neuropharmacology. 2005;49:798–810. doi: 10.1016/j.neuropharm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 56.Salvatore MF, Pruett BS, Dempsey C, Fields V. Comprehensive profiling of dopamine regulation in susbtantia nigra and ventral tegmental area. J Visualized Exp. 2012 doi: 10.3791/4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ji J, Bourque M, Di Paolo T, Dluzen DE. Genetic alteration in the dopamine transporter differentially affects male and female nigrostriatal transporter systems. Biochem Pharmacol. 2009;78:1401–1411. doi: 10.1016/j.bcp.2009.07.004. [DOI] [PubMed] [Google Scholar]