Abstract
Background and Aims
Corticosteroids are effective rescue therapies for patients with inflammatory bowel disease (IBD), but have significant side effects, which may be amplified in the growing population of elderly IBD patients. We aimed to compare the use of steroids and steroid-sparing therapies (immunomodulators and biologics) and rates of complications among elderly (≥65) and younger patients in a national cohort of veterans with IBD.
Methods
We used national Veterans Health Administrative (VHA) data to conduct a retrospective study of Veterans with IBD between 2002 and 2010. Medications and the incidence of complications were obtained from the VHA Decision Support Systems. Multivariate logistic regression accounting for facility-level clustering was used to identify predictors of use of steroid-sparing medications.
Results
We identified 30,456 Veterans with IBD. Of these, 94% were men and 40% were over 65, and 32% were given steroids. Elderly Veterans were less likely to receive steroids (23.8% vs. 38.3%, p<0.001) and were less likely to be prescribed steroid-sparing medications (25.5% vs 46.9%, respectively, p<0.001). In multivariate analysis controlling for gender, age <65 (OR 2.19 95%CI: 1.54–3.11) and GI care (OR 8.42 95%CI 6.18–11.47) were associated with initiation of steroid-sparing medications. After starting steroids, fracture rates increased in the elderly IBD patients, while increases in VTE and infections after starting steroids affected both age groups.
Conclusions
Elderly Veterans are less likely to receive steroids and steroid-sparing medications than younger Veterans; elderly patients exposed to steroids were more likely to have fractures than the younger population.
Keywords: corticosteroids, elderly, escalation
Background
While inflammatory bowel disease (IBD), an idiopathic disease of the gastrointestinal tract, most commonly presents in patients in the second to third decade of life1,2, 10–15% of new diagnoses are made in patients above the age of 603. Due to the low mortality of this disease and the aging of the population, providers are caring for an increasing number of patients with IBD over the age of 60. Patients diagnosed at an advanced age often have a more severe initial presentation, but their overall course is milder than the younger population4,5.
Corticosteroids are a critical part of the medical armamentarium in the treatment of IBD. These medications reduce inflammation in the gastrointestinal tract, ameliorating symptoms rapidly. Corticosteroids are not effective in maintaining remission however, and have a number of undesirable side effects including increased risk of diabetes6, reduced bone density7, immunosuppression8, and increased risk of venous thromboembolism (VTE)9. For these reasons, many experts recommend escalation to steroid sparing therapy such as immunomodulators or anti-tumor necrosis factors (TNFs) for patients who receive more than two courses of steroids within 12 months10. These medications are effective in maintaining remission but have other associated risks including increased risk of infection and a very small absolute increased risk of lymphoma11. The use of anti-TNFs in the elderly has been associated with higher adverse outcomes including infection and death12 so providers may often elect to maintain patients on corticosteroids for lengthy periods of time13.
The Veteran’s Health Administration (VHA) is the largest national health care system in the United States, caring for more than 6 million Veterans14. We aimed to compare the use of corticosteroids among elderly veterans to younger veterans, the rate of escalation to steroid sparing medications between these two groups, and the associated side effects of prolonged steroid use.
Methods
We used national Veterans Health Administrative (VHA) electronic data to conduct a retrospective comparison of the use of steroids among elderly versus younger patients. Patients were identified based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for Crohn’s disease (CD 555.x), and ulcerative colitis (UC 556.x). Patients were included in the cohort if they had 2 or more ICD-9-CM codes in ≥2 separate encounters between 2002–2009 with at least 1 outpatient encounter. This approach has been validated with a positive predictive value of Crohn’s disease of 0.84 and UC of 0.9115. The date of the first visit with an IBD ICD-9 code was considered the index date. In order to follow outcomes and medication use patterns, patients identified between 2002 and 2009 were followed through 2010.
Medication data was electronically abstracted from the VHA Decision Support System (DSS). Corticosteroid use was classified as dispensation of either oral or intravenous steroids (see Appendix 1) for IBD of at least 2 weeks duration and at least 10mg of prednisone. To ensure that patients were receiving steroids for IBD and not for other indications, we examined the ICD-9-CM codes for the week prior to prescription fill date looking for other common inflammatory conditions which might have also led to steroid prescriptions (see supplementary methods). A manual chart review was conducted on patients from 3 VHA sites in Michigan and a 10% random sample of other VHA facilities to ensure that the prescriptions were for IBD.
Corticosteroid users were classified into continuous steroid users (CS), intermittent steroid users (IS), or any IBD steroid users (AS) based on duration and pattern of use. Patients were classified as CS if they were initiated on steroids and treated for at least 2 weeks on 2 occasions with no more than a 90 day interval. Intermittent use was defined as patients who received at least 2 weeks of steroids twice within a 365 day period with at least 90 days between courses. Patients who were started on steroid sparing therapy in the 365 day period prior to the 2nd course of therapy and those prescribed only 1 course of steroid for at least 2 weeks were classified as AS users. For comparison, we also created a category of patients who required at least one corticosteroid prescription for a non-IBD condition and labeled them other steroids (OS).
Escalation of therapy was defined as the addition of steroid-sparing therapy to the patient’s regimen within 365 days after the initial corticosteroid fill. Steroid-sparing therapy included immunomodulators such as azathioprine, 6-mercaptopurine or methotrexate or biologic therapy (infliximab, adalimumab, or certolizumab).
We evaluated 3 steroid related complications (VTE, infections and fractures) in this cohort to determine the influence of steroid use on the rates in the year prior to diagnosis, after diagnosis but before steroid initiation and the year after steroid initiation. We compared patients who received no steroids to patients who received any steroids for IBD (CS, IS and AS). The ICD-9-CM codes used to identify (VTE), infections and fragility fractures are located in the supplementary material.
Statistical Analysis
Descriptive statistics were used to compare characteristics between the young and elderly population. Pearson’s chi-square tests and non-parametric Mann-Whitney U tests were used to compare categorical and continuous measures, respectively. To assess escalation of therapy within one year of corticosteroid initiation, we used logistic regression adjusting for clustering by facility. All data analysis was performed using Stata 13.1 (StataCorp, College Station, TX).
Results
During the time period studied, 30,456 patients were identified who met the criteria for IBD. Of these, 93.6% were male, 69% were Caucasian and 40.3% were 65 years or older at the time of their first visit with an IBD diagnosis (Table 1). Examining the breakdown of steroid use by age category, the elderly population was less likely to be exposed to steroids. A breakdown of steroid use and escalation rates by age shows that this does not occur abruptly at age 65 (Table 2). Among the younger patients, 38.3% received at least one course of steroids during the study period compared to 23.8% of the elderly population (p<0.001).
Table 1.
Patient Characteristics of No Corticosteroid and All Corticosteroid Users among Young and Elderly Veterans with IBD.
| Total | No Corticosteroid Users (NS) | All Corticosteroid Users * |
p | |||
|---|---|---|---|---|---|---|
| < 65 y/o | ≥ 65 y/o | < 65 y/o | ≥ 65 y/o | |||
| No. of Patients | 30,456 (100) |
11,224 (54.6) |
9,351 (45.5) |
6,956 (70.4) |
2,925 (29.6) |
<0.001 |
| Male | 28,500 (93.6) |
10,222 (52.7) |
9,167 (47.3) |
6,247 (68.6) |
2,864 (31.4) |
<0.001 |
| Race | <0.001 | |||||
| Caucasian | 21,010 (69.0) |
7,697 (68.6) |
6,201 (66.3) |
5,010 (72.0) |
2,102 (71.9) |
|
| African American | 2,097 (6.9) |
1,011 (8.9) |
198 (2.1) |
797 (11.5) |
101 (3.5) |
|
| Other | 491 (1.6) |
216 (1.9) |
82 (0.9) |
147 (2.1) |
46 (1.6) |
|
| Unknown or Missing | 6,858 (22.5) |
2,310 (20.6) |
2,870 (30.7) |
1,002 (14.4) |
676 (23.1) |
|
Includes patients on steroids for any duration or indication y/o- years old
Table 2.
Corticosteroid Use and Escalation Rates to Corticosteroid-Sparing Mediations by Age
| No Corticosteroid Use (NS) |
Corticosteroid use for IBD (CS,IS,AS) |
All Corticosteroid Users (CS,IS,AS,OS) |
Escalated (Among Corticosteroid Users for IBD) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | |
| Total Patients | 30,456 | 100 | 20,575 | 100 | 6,054 | 100 | 9,881 | 100 | 2528 | 41.8 |
| Age <25 | 707 | 2.3 | 323 | 45.7 | 341 | 48.2 | 384 | 54.3 | 212 | 62.2 |
| Age 25–30 | 1009 | 3.3 | 483 | 47.9 | 450 | 44.6 | 526 | 52.1 | 261 | 58.0 |
| Age 30–35 | 867 | 2.9 | 470 | 54.2 | 323 | 37.3 | 397 | 45.8 | 168 | 52.0 |
| Age 35–40 | 1002 | 3.3 | 560 | 55.9 | 320 | 31.9 | 442 | 44.1 | 169 | 52.8 |
| Age 40–45 | 1360 | 4.5 | 796 | 58.5 | 404 | 29.7 | 564 | 41.5 | 175 | 43.3 |
| Age 45–50 | 1734 | 5.7 | 986 | 56.9 | 468 | 27.0 | 748 | 43.1 | 216 | 46.2 |
| Age 50–55 | 2591 | 8.5 | 1626 | 62.8 | 597 | 23.0 | 965 | 37.2 | 238 | 39.9 |
| Age 55–60 | 4498 | 14.8 | 2925 | 65.0 | 932 | 20.7 | 1573 | 35.0 | 401 | 43.0 |
| Age 60–65 | 4412 | 14.5 | 3055 | 69.2 | 775 | 17.6 | 1357 | 30.8 | 320 | 41.3 |
| Age 65–70 | 3266 | 10.7 | 2412 | 73.9 | 450 | 13.8 | 854 | 26.1 | 147 | 32.7 |
| Age 70–75 | 3408 | 11.2 | 2605 | 76.4 | 398 | 6.6 | 803 | 23.6 | 114 | 28.6 |
| Age 75–80 | 3174 | 10.4 | 2455 | 77.3 | 360 | 11.3 | 719 | 22.7 | 80 | 22.2 |
| Age 80+ | 2428 | 8.0 | 1879 | 77.4 | 236 | 3.9 | 549 | 5.6 | 27 | 11.4 |
While the elderly group was less likely to be exposed to steroids, the median exposure among the elderly population was longer by 12 days among patients taking steroids for their IBD (Table 3). Accounting for the number of days that patients were included in the study, the median exposure to steroids for the elderly population was 6% of the study days compared to a median of 5.7% among the younger population (p<0.001). Overall, 16.3% of the patients exposed to steroids for their IBD were prescribed anti-TNFs in the year after their steroid initiation. The rate of anti-TNF use was significantly lower among the elderly (6.4% versus 19.4%). Examining the use of steroid-sparing medications more broadly to include immunomodulators, the rate of escalation remained lower among the elderly at 25.5% compared to 46.9% of the younger patients (p<0.001).
Table 3.
Corticosteroid User Characteristics among Veterans using Corticosteroids for IBD Only.
| Total | IBD Corticosteroid Users * |
p | ||
|---|---|---|---|---|
| < 65 y/o | ≥ 65 y/o | |||
| No. of Patients | 6,054 (100) |
4,610 (76.2) |
1,444 (23.9) |
|
| Study Days | 2097 (540 – 3,182) |
1,985 (528 – 3,158) |
2,454 (630 – 3,217) |
<0.001 |
| Corticosteroid Days |
90 (14 – 900) |
90 (13 – 750) |
102 (14 – 1260) |
<0.001 |
| Percent of Study Days on Steroids |
5.7 (0.0 – 54.7) |
5.7 (0.0 – 49.9) |
6.0 (0.1 – 68.7) |
<0.001 |
| Anti-TNF Use | 985 (16.3) |
893 (19.4) |
92 (6.4) |
<0.001 |
| Initiation of steroid-sparing therapy |
2,528 (41.8) |
2,160 (46.9) |
368 (25.5) |
<0.001 |
Includes patients on steroids of any duration
For study days, corticosteroid days, proportion days on steroids, median (5 – 95 percentile) is shown and for other variables, n (%) is shown.
Patients on prolonged steroids (IS or CS) were more likely to be escalated if they saw a GI provider (Table 4). Among elderly patients who did not see a GI provider, the escalation rate was 5% whereas those who did see a gastroenterologist were escalated in 43.7% of cases. Among younger patients, a visit with a GI provider also had a significant influence with 63% of younger patients escalated to steroid-sparing therapy.
Table 4.
Effect of GI Specialty Care on Initiation of Steroid-Sparing Therapy
| GI Visit | |||
|---|---|---|---|
| No | Yes | p | |
| Prolonged corticosteroid use, < 65 (N=1146) |
<0.001 | ||
| Not Escalated | 597 (83.4) | 159 (37.0) | |
| Escalated | 119 (16.6) | 271 (63.0) | |
| Total | 716 (62.5) | 430 (37.5) | |
| Prolonged corticosteroid use, ≥ 65 (N=549) |
<0.001 | ||
| Not Escalated | 429 (94.7) | 54 (56.3) | |
| Escalated | 24 (5.3) | 42 (43.7) | |
| Total | 453 (82.5) | 96 (17.5) | |
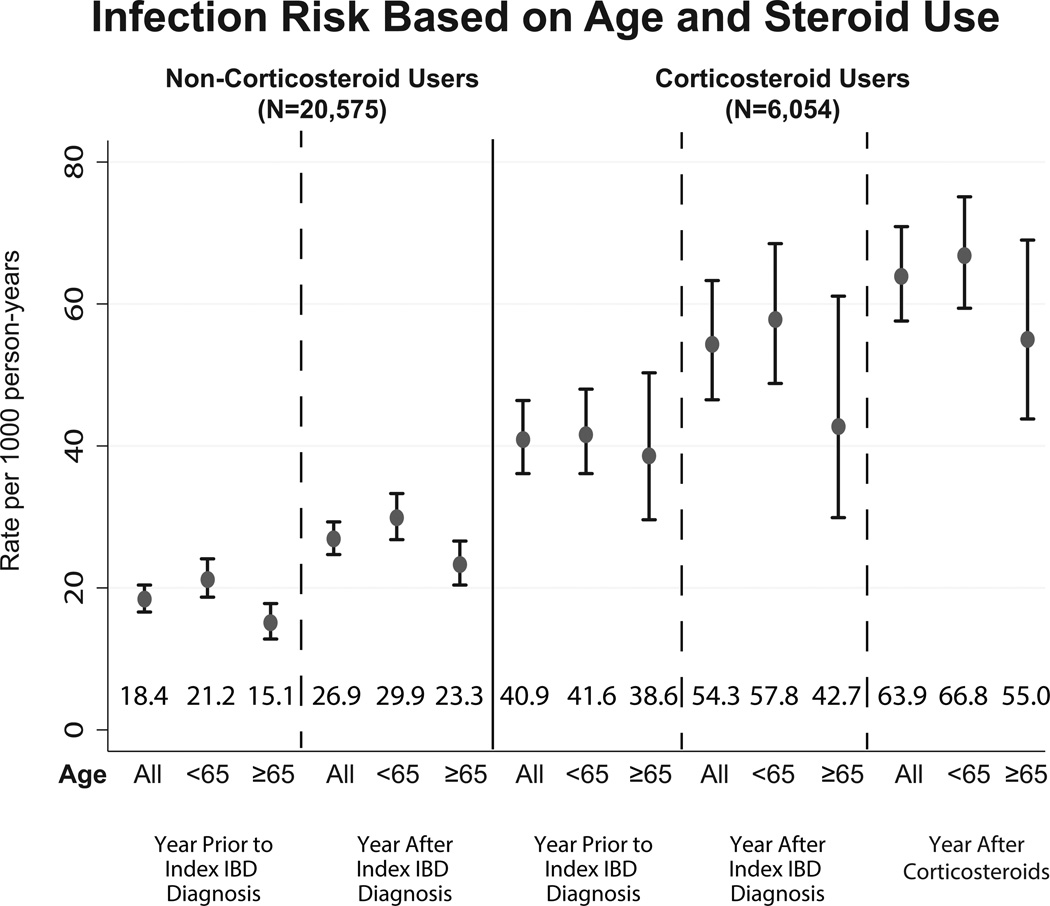
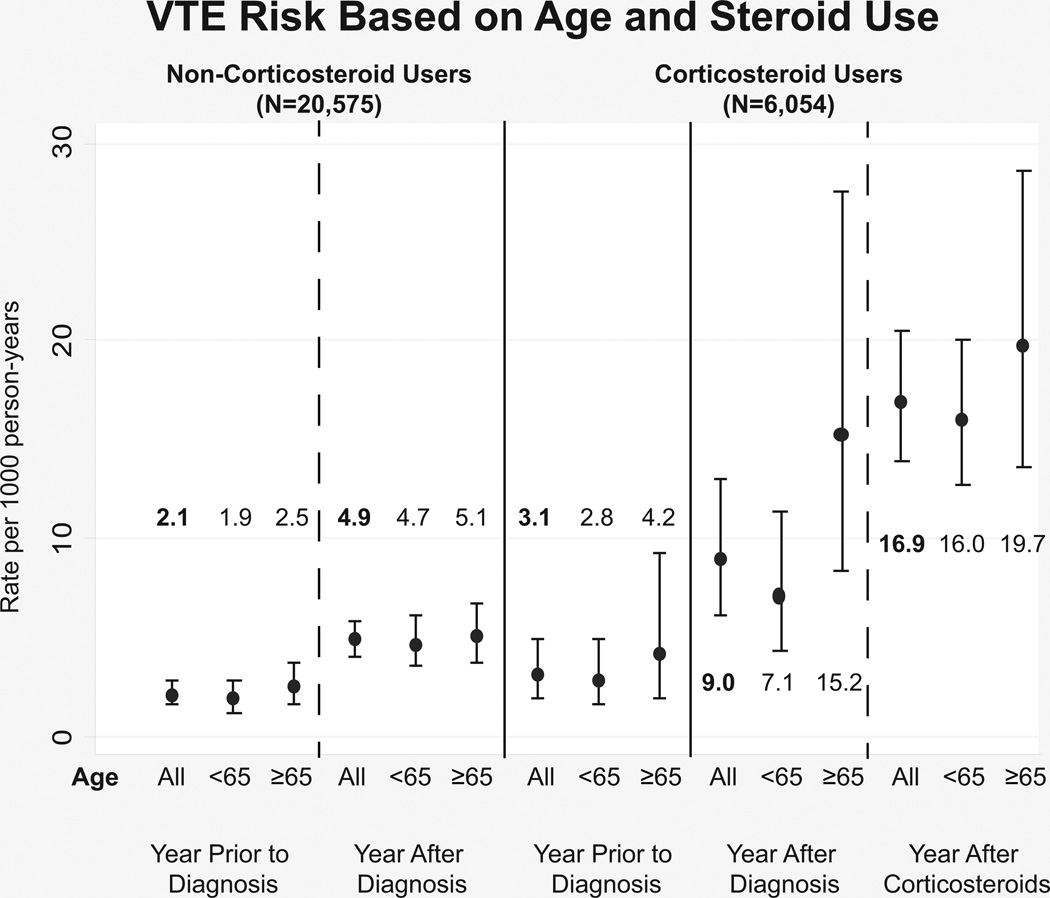
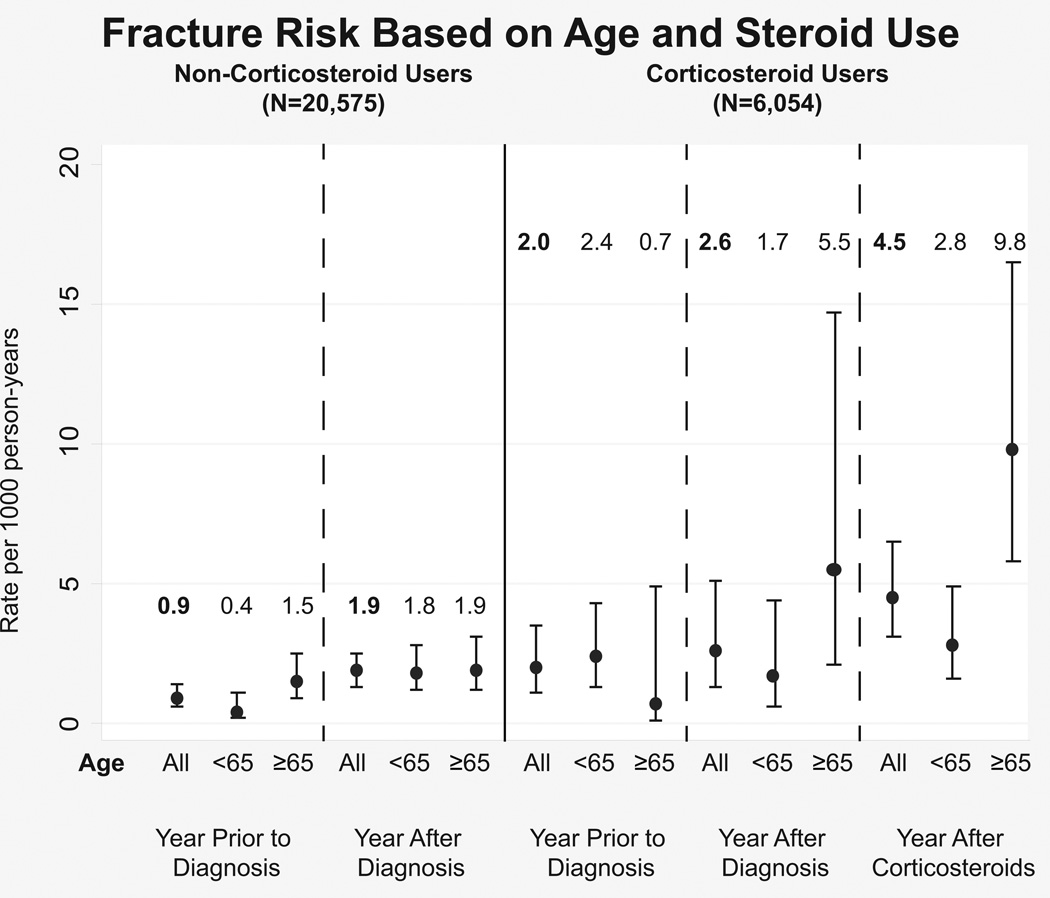
The rate of fracture, infection, and VTE per 1000 person years before IBD diagnosis, after diagnosis and after steroid initiation is displayed in Figure 1. After diagnosis of IBD, there was a statistically insignificant increase in fractures when comparing age groups. With initiation of steroids however, the rate of fractures among the elderly population increased significantly to 9.8 (95%CI: 5.8–16.5) per 1000 person years compared to 0.7 (95%CI: 0.1–4.9) during the year prior to diagnosis. Similarly, VTE rates among steroid users increased among patients exposed to steroids compared to these patients in the year prior to diagnosis, but this increase was seen in both age groups. Infection rates were higher among the patients exposed to steroids regardless of time period studied compared to the steroid naïve. The elderly patients who were steroid naïve had a significantly lower rate of infections while those who were steroid exposed had a trend towards lower infection rates in the 3 time periods studied.
Figure 1.
Complication rates (1a: infections, 1b: VTE, 1c: fractures) for Veterans with IBD comparing the year prior to diagnosis, the year after diagnosis and before steroid use, and the year after steroid initiation. The graph shows the difference in these rates for the elderly versus the younger population and those who received steroids versus those who were steroid naïve through the study period.
To determine the effect of age on the chance of initiation of a steroid-sparing medication, a multivariable model was constructed (Table 5). Among patients with a prolonged steroid exposure, the odds of steroid-sparing initiation was 2.19 (95%CI: 2.02–3.96) among younger patients compared to the elderly population controlling for gender and patients who saw a GI provider. Patients who saw a GI provider during the year after steroid initiation were eight times as likely (OR 8.42, 95%CI: 6.18–11.47) to be escalated to steroid sparing therapy.
Table 5.
Logistic Regression Analysis of Predictors of Escalation to Corticosteroid Sparing Medication
| OR (95% CI) | p | |
|---|---|---|
| Age < 65 (vs Age ≥ 65) | 2.19 (1.54, 3.11) | <0.001 |
| Male | 1.39 (0.80, 2.45) | 0.246 |
| GI visit | 8.42 (6.18, 11.47) | <0.001 |
Note. Analysis includes CS & IS groups only. Model adjusts for clustering by facility.
Discussion
In this study of a large cohort with IBD, we found that there were significant differences in the patterns of medication use among elderly patients compared to younger patients. Patients over the age of 65 were less likely to receive steroids compared to the younger population (23.8% vs. 38.3%, p<0.001), which corroborates prior findings suggesting that the elderly generally have a milder disease course16. When the elderly patients were exposed to steroids however, they were given the medications for longer. Additionally, the patients were significantly less likely to be given steroid sparing medications. Seeing a GI provider in the VA system did improve the rate of escalation to steroid sparing medications, but the overall prescription of anti-TNFs and immunomodulators remained lower in the elderly compared to the younger population. Fractures were much more common in the elderly population exposed to steroids compared to the younger population. Infection and VTE rates were both higher for steroid users, but the elderly population had similar rates to the younger population.
A smaller study from one center in the United States found that ~1/3 of elderly patients with IBD were treated with steroids for more than 6 months13. A very low percentage of patients were exposed to steroid sparing therapy (6.8% received thiopurines or methotrexate and 2.6% received anti-TNFs). During a large portion of the time period studied in that report, however, anti-TNFs were not approved for IBD. Our study focuses on the time period when anti-TNFs were approved for IBD, and also shows that national usage of steroid sparing therapy remains low. There are several factors at play which may influence the reluctance of providers to escalate patients to steroid-sparing therapy among the elderly. Patients at an advanced age are more likely to have heart failure, which is a contraindication to anti-TNF use when class III or IV heart failure is present17. Elderly patients are more likely to have a concurrent or previous cancer diagnosis. Experts suggest cautious use of these medications in the setting of a prior cancer18. The risk of lymphoma is higher in the elderly population exposed to these drugs compared to younger patients19. Finally, there is concern that the risk of infection may be higher in older patients treated with these medications compared to younger patients12. While steroid-sparing therapy comes with risks, we have shown here that continuous use of steroids is not without risk. It carries a significant risk of fracture, infection and VTE for all patient age groups. A visit with a gastroenterologist significantly increases the odds of escalation to steroid sparing therapy. These patients likely had more severe disease, leading to higher escalation rates in this group. Among those patients who saw a gastroenterologist and were not escalated, some patients may have been on lower doses of steroids, likely leading providers not to prescribe steroid-sparing therapy. As others have noted, there is a difference between the frail elderly patient and the fit elderly patient20; escalation is likely appropriate in patients at an advanced age with good health status.
The use of an administrative database does not allow us to control for severity of disease in the analysis of steroid use and escalation. Elderly patients with mild disease may have been on lower doses of steroids for lengthy periods of time, and therefore may not have been felt to be appropriate for escalation. Other limitations to our study include the fact that patients may obtain medications and care outside of the VHA. Patients over the age of 65 are eligible for Medicare coverage which increases patient’s access to care outside of the VA. Despite this limitation, we believe our findings to be a representative sampling of the cohort due to the high costs associated with these medications outside of the VA. In the single center study from a non-VA population, the use of biologics among the elderly was significantly lower than we found, likely due to the significant cost of these medications. We did not differentiate between elderly-onset IBD and elderly patients with long standing disease in our analysis. We did not adjust for comorbidities such as lymphoma, heart failure or cancers which may have factored into the decision not to escalate patients to steroid sparing therapy. Finally, this study is of the VA system which is mostly male and so may not be generalizable to the general population of elderly patients.
In conclusion, prolonged steroid use among the elderly remains common in a nationwide cohort of Veterans and patients are infrequently escalated to steroid sparing therapy. The elderly have significantly more fractures with steroid exposure compared to the younger population. Gastroenterology specialty care significantly impacts the decision to escalate to steroid sparing therapy. Prolonged steroids have significant risks in the elderly including an increased risk of fracture compared to younger patients. Further study of the risks and benefits of continued steroids versus escalation to steroid-sparing therapy is necessary to determine the safest approach in these patients.
Supplementary Material
Acknowledgments
Source of Funding.
Dr Waljee’s research is funded by a VA HSR&D CDA-2 Career Development Award 1IK2HX000775. Dr. Hou’s research is funded by the VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (#CIN 13-413), at the Michael E. DeBakey VA Medical Center, Houston, TX. Dr. Sussman is supported by VA CDA 13-021. The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Conflicts of Interest.
The authors have no personal or financial conflicts of interest to report.
Author Contributions:
SMG: Conception and interpretation of the data; drafting of the manuscript; final approval of the version to be published; agreement to be accountable for all aspects of the work
WLW: Conception, design, analysis and interpretation of the data; critical review of the manuscript; final approval of the version to be published; agreement to be accountable for all aspects of the work
RWS: Interpretation of the data; critical review of the manuscript; final approval of the version to be published; agreement to be accountable for all aspects of the work
SDS: Interpretation of the data; critical review of the manuscript; final approval of the version to be published; agreement to be accountable for all aspects of the work
JKH: Interpretation of the data; critical review of the manuscript; final approval of the version to be published; agreement to be accountable for all aspects of the work
LAF: Interpretation of the data; critical review of the manuscript; final approval of the version to be published; agreement to be accountable for all aspects of the work
JBS: Interpretation of the data; critical review of the manuscript; final approval of the version to be published; agreement to be accountable for all aspects of the work
PDRH: Interpretation of the data; critical review of the manuscript; final approval of the version to be published; agreement to be accountable for all aspects of the work
AKW: Conception of the study, interpretation of the data; drafting and critical review of the manuscript; final approval of the version to be published; agreement to be accountable for all aspects of the work
References
- 1.Thia KT, Loftus EV, Sandborn WJ, Yang S-K. An Update on the Epidemiology of Inflammatory Bowel Disease in Asia. The American Journal of Gastroenterology. 2008;103(12):3167–3182. doi: 10.1111/j.1572-0241.2008.02158.x. [DOI] [PubMed] [Google Scholar]
- 2.Loftus EV. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 3.Hussain SW, Pardi DS. Inflammatory bowel disease in the elderly. Drugs Aging. 2010;27(8):617–624. doi: 10.2165/11537340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Gisbert JP, Chaparro M. Systematic review with meta-analysis: inflammatory bowel disease in the elderly. Aliment Pharmacol Ther. 2014;39(5):459–477. doi: 10.1111/apt.12616. [DOI] [PubMed] [Google Scholar]
- 5.Lakatos PL, David G, Pandur T, et al. IBD in the elderly population: results from a population-based study in Western Hungary, 1977–2008. Journal of Crohn's and Colitis. 2011;5(1):5–13. doi: 10.1016/j.crohns.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Blackburn D, Hux J, Mamdani M. Quantification of the Risk of Corticosteroid-induced Diabetes Mellitus Among the Elderly. J Gen Intern Med. 2002;17(9):717–720. doi: 10.1046/j.1525-1497.2002.10649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lukert BP, Raisz LG. Glucocorticoid-Induced Osteoporosis: Pathogenesis and Management. Ann Intern Med. 1990;112(5):352–364. doi: 10.7326/0003-4819-112-5-352. [DOI] [PubMed] [Google Scholar]
- 8.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270(5234):286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 9.Higgins PDR, Skup M, Mulani PM, Lin J, Chao J. Increased Risk of Venous Thromboembolic Events With Corticosteroid vs Biologic Therapy for Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2015;13(2):316–321. doi: 10.1016/j.cgh.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Mowat C, Cole A, Windsor A, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60(5):571–607. doi: 10.1136/gut.2010.224154. [DOI] [PubMed] [Google Scholar]
- 11.Siegel CA. Risk of Lymphoma in Inflammatory Bowel Disease. Gastroenterology & Hepatology. 2009 [PMC free article] [PubMed] [Google Scholar]
- 12.Cottone M, Kohn A, Daperno M, et al. Advanced Age Is an Independent Risk Factor for Severe Infections and Mortality in Patients Given Anti–Tumor Necrosis Factor Therapy for Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2011;9(1):30–35. doi: 10.1016/j.cgh.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 13.Juneja M, Baidoo L, Schwartz MB, et al. Geriatric inflammatory bowel disease: phenotypic presentation, treatment patterns, nutritional status, outcomes, and comorbidity. Dig Dis Sci. 2012;57(9):2408–2415. doi: 10.1007/s10620-012-2083-x. [DOI] [PubMed] [Google Scholar]
- 14.Bagalman E. The Number of Veterans That Use VA Health Care Services: A Fact Sheet. [Accessed August 23, 2015];FAS. https://www.fas.org/sgp/crs/misc/R43579.pdf. [Google Scholar]
- 15.Hou JK, Tan M, Stidham RW, et al. Accuracy of diagnostic codes for identifying patients with ulcerative colitis and Crohn's disease in the Veterans Affairs Health Care System. Dig Dis Sci. 2014;59(10):2406–2410. doi: 10.1007/s10620-014-3174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gisbert JP, Chaparro M. Safety of Anti-TNF Agents During Pregnancy and Breastfeeding in Women With Inflammatory Bowel Disease. The American Journal of Gastroenterology. 2013;108(9):1426–1438. doi: 10.1038/ajg.2013.171. [DOI] [PubMed] [Google Scholar]
- 17.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107(25):3133–3140. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 18.Beaugerie L. Use of Immunosuppressants and Biologicals in Patients with Previous Cancer. Dig Dis. 2013;31(2):254–259. doi: 10.1159/000353382. [DOI] [PubMed] [Google Scholar]
- 19.Khan N, Abbas AM, Lichtenstein GR, Loftus EV, Bazzano LA. Risk of Lymphoma in Patients With Ulcerative Colitis Treated With Thiopurines: A Nationwide Retrospective Cohort Study. Gastroenterology. 2013;145(5):1007.e3–1015.e3. doi: 10.1053/j.gastro.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 20.Katz S, Feldstein R. Inflammatory bowel disease of the elderly: a wake-up call. Gastroenterology & Hepatology. 2008;4(5):337–347. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.