Abstract
Experimental approaches to genetic studies of complex traits evolve with technological advances. How do discoveries using different approaches advance our knowledge of the genetic architecture underlying complex diseases/traits? Do most of the findings of newer techniques, such as genome-wide association study (GWAS), provide more information than older ones, e.g., genome-wide linkage study? In this review, we address these issues by developing a nicotine dependence (ND) genetic susceptibility map based on the results obtained by the approaches commonly used in recent years, namely, genome-wide linkage, candidate gene association, GWAS, and targeted sequencing. Converging and diverging results from these empirical approaches have elucidated a preliminary genetic architecture of this intractable psychiatric disorder and yielded new hypotheses on ND aetiology. The insights we obtained by putting together results from diverse approaches can be applied to other complex diseases/traits. In sum, developing a genetic susceptibility map and keeping it updated are effective ways to keep track of what we know about a disease/trait and what the next steps might be with new approaches.
Keywords: Addiction, GWAS, Linkage, Smoking, Susceptibility genes
INTRODUCTION
Along with technological advances, experimental approaches for the genetic study of complex diseases/traits have evolved from genome-wide linkage study to candidate gene association study and from genome-wide association study (GWAS) to targeted sequencing. With improvements in accuracy, coverage, and cost, whole-exome and whole-genome sequencing studies seem to be the next mainstream approaches. Are the discoveries from all of these approaches consistent? Should we focus on results obtained with newer approaches; e.g., GWAS, and abandon findings from older ones, such as genome-wide linkage study, in the literature sea? How can we make the findings guide our understanding of the genetic architecture of the disease/trait in question? In this review, we use nicotine dependence (ND) as an example to investigate these issues.
Tobacco smoking poses significant threats to public health and kills more than 6 million people annually worldwide, making it one of the three leading components of the global disease burden in 2010.1 Despite 50 years of prevention efforts, smoking remains the greatest cause of preventable diseases and deaths; each year, nearly 500,000 Americans die prematurely from smoking, and more than 16 million Americans suffer from a disease caused by smoking. Even though today's users smoke fewer cigarettes than those 50 years ago, they are at higher risk of developing adenocarcinoma, possibly because of ventilated filters and greater amounts of tobacco-specific nitrosamines in cigarettes.2
Since the 1980s, a broad scientific consensus has been established that nicotine dependence (ND) is the primary factor maintaining smoking behaviour.3 We and others have shown strong evidence for the involvement of genetics in ND, with an average heritability of 0.56.4, 5 In the past dozen years, considerable efforts have been exerted to identify the genetic factors underlying ND. However, only three widely accepted “successes;” i.e., the neuronal nicotinic acetylcholine receptor gene clusters on chromosomes 15 (CHRNA5/A3/B4)6-20 and 8 (CHRNB3/A6)13, 15, 18, 21-25 and the genes encoding nicotine-metabolizing enzymes on chromosome 19 (CYP2A6/A7),16, 18, 26-28 meet community standards for significance and replication.29 These few triumphs stand in contrast to the limited heritability they explain; e.g., the most significant synonymous single-nucleotide polymorphism (SNP) rs1051730 (p = 2.75 × 10−73) in CHRNA3 accounted for only 0.5% of the variance in cigarettes smoked per day (CPD) in a meta-analysis of 73,853 subjects.16 Researchers have suggested that “missing heritability” is merely hidden and that additional loci can be discovered in GWAS with larger samples,30, 31 not to mention that the largest ND GWAS to date included 143,023 subjects,16 and many relevant genetic loci have been revealed with other experimental approaches, such as genome-wide linkage, hypothesis-driven candidate gene association, and targeted sequencing. Despite the fact that many non-GWAS findings have an uncertain yield or failed to be replicated, sorting out genetic loci with evidence from multiple approaches is not only essential but also more cost effective than pursuing a formidable sample size for GWAS.
In this communication, we first review the literature on genetics studies for all smoking-related phenotypes using different approaches by highlighting the converging results from different approaches and then offer new hypotheses that have emerged across the allelic spectrum, including common and rare variants. These findings provide insights into the preliminary genetic architecture of ND, data that are essential for guiding future research. Crucially, we show that developing a genetic susceptibility map with data from various approaches is an effective means of knowledge integration, research progress evaluation, and research direction forecast.
GENOME-WIDE LINKAGE STUDIES
For many years, linkage analysis was the primary approach for the genetic mapping of both Mendelian and complex traits with familial aggregation.32, 33 This method was largely supplanted by the wide adoption of GWAS in the middle 2000s. In 2008, we published a comprehensive review of more than 20 published genome-wide linkage studies of smoking behaviour and identified 13 regions, located on chromosomes 3–7, 9–11, 17, 20, and 22, suggestively or significantly linked with various ND measurements in at least two independent samples.34 Since then, only one genome-wide linkage study has been reported, by Hardin et al.,35 finding a linked spot in the same region as in their previous analysis (6q26) using the same sample but a different phenotype.36 In addition, Han et al.37 conducted a meta-analysis of 15 genome-wide linkage scans of smoking behaviour and identified two suggestive (5q33.1–5q35.2 and 17q24.3–q25.3) and one significant (20q13.12–q13.32) linkage regions. In fact, the regions on chromosomes 5 and 20 expand two of the regions reported in our 2008 review. The region on chromosome 17 reported by Han et al.37 verified one of the regions detected in only one sample before 2008, which makes it a newly nominated linkage peak (Table 1).34 Please refer to Li34 and Table 1 for detailed information on the 14 nominated linkage regions. Figure 1 also shows updated linkage results after incorporating the findings reported after 2008 by Han et al.37
Table 1.
Information on the Nominated Linkage Regions Updated According to Li 34
| Chromosome | Marker or marker region | Position | Chr. bands | Phenotype |
|---|---|---|---|---|
| 3 | D3S1763–D3S1262 | 167,239,681–186,223,727 | 3q26–q27 | DSM-IVND, SQ |
| 4 | D4S403–D4S2632, D4S244 | 13,750,828–65,491,728 | 4p15–q13.1 | FTND, CPD |
| 5 (region 1) | D5S1969, D5S647, D5S428 | 53,242,832–85,410,963 | 5q11.2–q14 | SQ, smoking status, FTND |
| 5 (region 2)* | D5S400, D5S1354 | 149,800,001–179,631,902 | 5q33.1–q35* | FTND, CPD |
| 6 | D6S1009, D6S1581–D6S281, D6S446 | 137,302,085–170,552,657 | 6q23.3–q27 | Smoking status, FTND, withdrawal severity |
| 7 | D7S486, D7S636 | 115,894,675–150,699,599 | 7q31.2–q36.1 | FTND, DSM-IV |
| 9 (region 1) | D9S2169–D9S925, D9S925–D9S319 | 5,200,390–29,560,115 | 9p21–p24.1 | FTND, HSI, SQ |
| 9 (region 2) | D9S257–D9S910, D9S283, D9S64, D9S1825 | 90,290,735–127,888,281 | 9q21.33–q33 | SQ, FTND, smoking status |
| 10 | D10S1432, D10S2469/CYP17, D10S597, D10S1652-D10S1693, D10S129-D10S217 | 64,407,495–129,540,525 | 10q21.2–q26.2 | SQ, FTND, smoking status |
| 11 | D11S4046, D11S4181, D11S2362-D11S1981, D11S1999-D11S1981, D11S2368–D11S2371, D11S1392–D11S1344, D11S1985–D11S2371 | 1,963,635–73,505,374 | 11p15–q13.4 | FTND, SQ |
| 17 (region 1) | GATA 193, D17S974–D17S2196, D17S799–D17S2196, D17S799–D17S1290 | 10,518,666–56,331,730 | 17p13.1–q22 | CPD, SQ, HSI |
| 17 (region 2)* | D17S968 | 67,100,001–81,195,210 | 17q24.3–q25.3* | Smoking status |
| 20* | D20S119–D20S178, D20S481–D20S480 | 43,648,850–58,400,000 | 20q13.12–q13.32* | CPD, SQ |
| 22 | D22S345–D22S315, D22S315–D22S1144 | 24,488,587–27,683,302 | 22q11.23–12.1 | CPD, age at first cigarette |
denotes linkage regions expanded or newly ascertained after evaluating results published after our 2008 review. Genomic positions for microsatellite markers and corresponding chromosome bands were obtained through the UCSC Genome Browser (http://genome.ucsc.edu/), which are in the GRCh37/hg19 assembly.
Abbreviations: Chr., chromosome; CPD, cigarettes smoked per day; DSM = Diagnostic and Statistical Manual (American Psychiatric Association), FTND, Fagerström Test for Nicotine Dependence; HSI, heaviness of smoking index; SQ, smoking quantity.
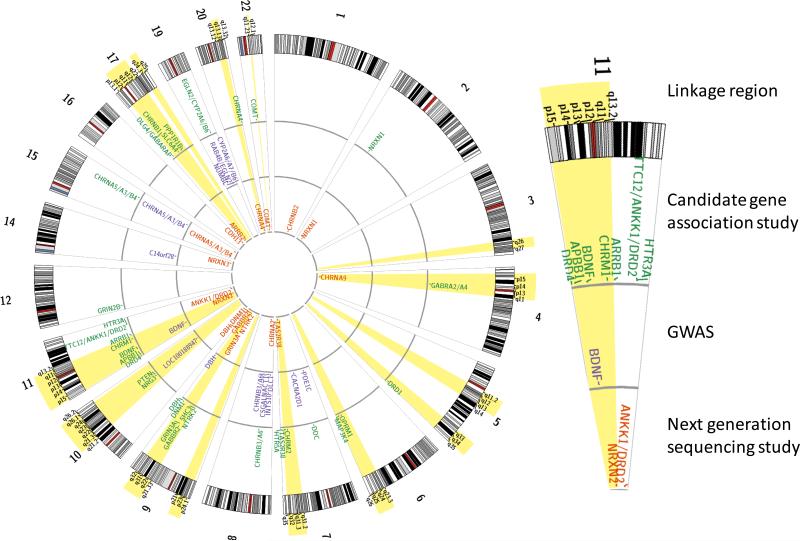
Figure 1.
The ND genetic susceptibility map with nominated linkage peaks and candidate genes, as suggested by genome-wide linkage, hypothesis-driven candidate gene association (CAS), genome-wide association (GWAS), and targeted sequencing (next-generation sequencing; NGS) studies. Linkage peaks are marked in light gray; CAS, GWAS, and NGS results are presented as gene names at the outer, middle, and inner rings, respectively.
HYPOTHESIS-DRIVEN CANDIDATE GENE ASSOCIATION STUDIES
Candidate gene association studies usually have moderate sample sizes and are much cheaper than GWAS, where the genes examined are selected according to the linkage/GWAS study results or biological hypotheses. However, because of population heterogeneity and liberal statistical thresholds (compared with GWAS) that often are applied, hypothesis-driven candidate gene association studies generally are considered to have an uncertain yield.38 On the other hand, the abundant results obtained using this approach provide greater depth of exploration of potential targets and offer valuable replication for other unbiased approaches; e.g., genome-wide linkage study and GWAS.
To eliminate concerns about potential false-positive results, especially for studies reported in earlier years, we focused primarily on the genes showing significance in at least two independent studies with a sample size of ≥ 1,000 or within (or close to) nominated linkage regions or overlapping with GWAS results but with a sample size of ≥ 500 based on the statistical thresholds set by each study. Because the reported sex-average recombination rate is 1.30 ± 0.80 cM/Mbp,39 in this report, we defined candidate genes within 2 megabase pairs (Mbp) of any linkage region as “within” and 2–5 Mbp as “close to.” The sample size requirement was determined with the following parameters: two-tailed α = 0.05, population risk = 0.30, minor allele frequencies = 0.20, and genotypic relative risk = 1.3 with an approximate odds ratio (OR) of 1.5 or 0.7, which is similar to the statistics usually found in candidate gene association studies. For a statistical power of 0.80 (β = 0.20) using the allelic test, the minimum sample size for a case-control study is 1,062, with equal numbers of cases and controls. Of the reported 201 candidate gene association studies, only 88 have had a sample size of 1,000 or more. Considering the detected power of 0.54 for a sample size of 500 under the dominant genetic model, we also included genes implicated in studies with 500–1,000 subjects if the genes were located in a nominated linkage peak34 or overlapped with GWAS signals. In total, 34 genetic loci with 43 genes met the criteria (Table 2 and Figure 1), which were assigned to the following four groups. For details on those studies that failed to pass the thresholds but show positive associations, please see Supplementary Table 1.
Table 2.
Significant Candidate Gene Association Results for ND-Related Phenotypes
| Gene | Chr. | Linkage (distance)/GWAS | Sample size | Variant | Position | Variant Type | P value | Effect size | Phenotype | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| Neurotransmitter system genes | ||||||||||
| Dpaminergic system | ||||||||||
|
TTC12- ANKK1- DRD2 |
11q23.2 | Within the modest linkage peak on 11q23 (0 bp)41 |
638 (270 AAs+368 EAs) |
rs2303380-rs4938015-rs11604671 (TTC12)-(ANKK1)-(ANKK1) |
0.01 | OR = 1.6 |
Regular smoking initiation |
43 | ||
| 752 (European) | rs1800497 (Taq1A) |
113270828 | Missense (ANKK1) |
4.0 × 10−3 (interaction with sex and treatment) |
Smoking cessation |
145 | ||||
| 755 (European) | rs1800497 (Taq1A) |
113270828 | Missense (ANKK1) |
0.04 (interaction with treatment) |
Smoking cessation |
146 | ||||
| 782 (European) | rs1799732 (−141C Ins/Del) |
113346251: 113346252 |
Near Gene-5 (DRD2) |
0.01 (interaction with treatment) |
Smoking cessation |
147 | ||||
| 1,026 (European) | rs1800497 (Taq1A) |
113270828 | Missense (ANKK1) |
<1.0 × 10−8 | Smoking status |
148 | ||||
| 1,615 (854 AAs+761 EAs) |
rs4938012 | 113259654 | 5′-UTR (ANKK1) |
8.0 × 10−6 (pooled) |
DSM-IV ND | 44 | ||||
| 1,900 (European and other) |
rs1800497 (Taq1A) |
113270828 | Missense (ANKK1) |
0.01 (interaction with ADHD symptoms) |
Initial subjective response to nicotine |
82 | ||||
| 1,929 (European | rs4245150 | 113364647 | Intergenic | 0.01 | FTND ≥ 4 vs. FTND = 0 in smokers |
13 | ||||
| rs17602038 | 113364691 | Intergenic | 0.01 | |||||||
| 2,037 (1,366 AAs+671 EAs) |
rs2734849 | 113270160 | Missense (ANKK1) |
5.3 × 10−4 (AA) |
HSI | 45 | ||||
| 4,762 (European) | rs10502172 | 113199146 | Intronic (TTC12) |
9.1 × 10−6 | OR = 1.3 |
Smoking status |
46 | |||
| DRD1 | 5q35.2 | Within the nominated linkage peak on 5q34–q35 (0 bp) |
2,037 (1,366 AAs+671 EAs) |
rs686 | 174868700 | 3′-UTR | 4.8 × 10−3 (AA) |
FTND | 48 | |
| DRD4 | 11p15.5 | Within the nominated linkage peak on 11p15– q13.4 (1.3 Mbp) |
720 (European) | VNTR | Exon 3 | 0.03 | Smoking cessation |
49 | ||
| 839 (59% European) |
VNTR | Exon 3 | 2.0 × 10−3 (interaction with neuroticism) |
OR = 3.5 |
Progression to ND |
50 | ||||
| 2,274 (European) | VNTR | Exon 3 | 6.0 × 10−3 | B = 0.1 | CPD | 51 | ||||
| DBH | 9q34.2 | GWAS16 | 793 (European) | rs1541333 | 136511385 | Intronic | 4.0 × 10−4 (interaction with ND) |
Smoking cessation |
52 | |
| 1,608 (European) | rs3025382 | 136502321 | Intronic | 3.3 × 10−4 | FTND ≥4 vs. 0 in smokers |
90 | ||||
| 1,929 (European) | rs4531 | 136509370 | Missense | 5.1 × 10−3 | FTND ≥4 vs. 0 in smokers |
13 | ||||
| 2,521 149 | rs5320 | 136507473 | Missense | 7.0 × 10−3 (male) |
CPD | 53 | ||||
| DDC | 7p12.1 | 1,590 (854 AAs+736 EAs) |
rs12718541 | 50550144 | Intronic | 2.0 × 10−4 (pooled) |
FTND | 54 | ||
| 2,037 (1,366 AAs+671 EAs) |
rs921451 | 50623285 | Intronic | 0.01 (EA) |
CPD | 55 | ||||
| COMT | 22q11.21 | Close to the nominated linkage peak on 22q11.23– q12.1 (4.5 Mbp) |
511 (81 AAs+430 EAs) |
rs737865-rs165599 | 4.3 × 10−3 (EA) |
Smoking cessation |
56 | |||
| 614 (91% European) |
rs4680 | 19951271 | Missense | <0.05 | OR = 2.1 |
Increased smoking |
57 | |||
| 657 (European) | rs4680 | 19951271 | Missense | 0.02 (male) | Smoking status |
58 | ||||
| 2,037 (1,366 AAs+671 EAs) |
rs4680 | 19951271 | Missense | 9.0 × 10−3 (EA) |
CPD | 59 | ||||
| 6,310 (European) | rs4680 | 19951271 | Missense | 3.0 × 10−3 | OR = 0.7 |
Smoking cessation |
60 | |||
| 13,312 (European) |
rs4680 | 19951271 | Missense | 7.0 × 10−3 (meta) |
OR = 1.1 |
Smoking status before pregnancy |
61 | |||
| PPP1R1B | 17q12 | Within the nominated linkage peak on 17p13.1– q22 (0 bp) |
2,037 (1,366 AAs+671 EAs) |
rs2271309-rs907094-rs3764352-rs3817160 | 0.01 (EA) | CPD | 150 | |||
| OPRM1 | 6q25.2 | Within the nominated linkage peak on 6q23.3– q27 (0 bp) |
710 (European) | rs1799971 | 154360797 | Missense | 5.0 × 10−3 (interaction with sex) |
Smoking cessation |
151 | |
| 1,929 (European) | rs510769 | 154362019 | Intronic | 9.8 × 10−3 | FTND ≥ 4 vs. 0 in smokers |
13 | ||||
| GABAergic system | ||||||||||
| GABBR2 | 9q22.33 | Within the nominated linkage peak on 9q21.33– q33 (0 bp) |
1,276 (793 AAs+483 EAs) |
rs1435252 | 101103591 | Intronic | 3.0 × 10−3 (EA) |
CPD | 73 | |
| rs3750344 | 101340316 | Synonymous | 3.0 × 10−3 (EA) |
|||||||
|
DLG4- GABARAP |
17p13.1 | Within the nominated linkage peak on 17p13.1– q22 (0 bp) |
2,037 (1,366 AAs+671 EAs) |
rs222843 | 7145981 | nearGene-5 (GABARAP) |
9.0 × 10−3 (EA) |
FTND | 74 | |
|
GABRA2- GABRA4 |
4p12 | Within the nominated linkage peak on 4p15– q13.1 (0 bp) |
1,929 (European) | rs3762611 | 46997288 | nearGene-5 (GABRA4) |
9.0 × 10−4 | OR = 0.5 |
FTND ≥ 4 vs. 0 in smokers |
13, 75, 76 |
| Serotonergic system | ||||||||||
| HTR3A | 11q23.2 | Within the modest linkage peak on 11q23 (0 bp)41 |
2,037 (1,366 AAs+671 EAs) |
rs1150226-rs1062613-rs33940208- rs1985242-rs2276302-rs10160548 |
2.0 × 10−3 (AA) |
HSI | 79 | |||
| HTR5A | 7q36.2 | Close to the nominated linkage peak on 7q31.2– q36.1 (4.2 Mbp) |
1,929 (European) | rs6320 | 154862621 | Synonymous | 6.5 × 10−3 | FTND ≥ 4 vs. 0 in smokers |
13 | |
| SLC6A4 | 17q11.2 | Within the nominated linkage peak on 17p13.1– q22 (0 bp) |
782 (European) | 5- HTTLPR+intronic VNTR |
1.0 × 10−4 | OR = 1.4 |
Smoking status |
80 | ||
| 1,098 (41% European) |
5-HTTLPR | <1.0 × 10−3 (interaction with peer smoking) |
HR = 5.7 |
Regular smoking initiation |
81 | |||||
| 1,900 (European and other) |
5-HTTLPR | 0.02 (interaction with ADHD symptoms) |
Initial subjective response to nicotine |
82 | ||||||
| Glutamatergic system and other | ||||||||||
| GRIN3A | 9q31.1 | Within the nominated linkage peak on 9q21.33– q33 (0 bp) |
2,037 (1,366 AAs+671 EAs) |
rs17189632 | 104368002 | Intronic | 2.0 × 10−4 (pooled) |
FTND | 89 | |
| GRIN2B | 12p13.1 | GWAS,87 close to the modest linkage peak on 12p13.31– 13.32 (3.6 Mbp)88 |
1,608 (European) | rs17760877 | 13819473 | Intronic | 1.5 × 10−5 (interaction with age of onset) |
FTND ≥ 4 vs. 0 in smokers |
90 | |
| NRXN1 | 2p16.3 | GWAS92 | 2,037 (1,366 AAs+671 EAs) |
rs6721498 | 50713012 | Intronic | 8.6 × 10−6 (AA) |
FTND | 93 | |
| 2,516149 | rs2193225 | 51079482 | Intronic | 6.0 × 10−3 | Smoking status |
94 | ||||
| Nicotinic receptor (nAChR) subunit & other cholinergic system genes | ||||||||||
|
CHRNA5- CHRNA3- CHRNB4 |
15q25.1 | GWAS12, 16-19 | 516 (European) | rs1051730 | 78894339 | Synonymous (CHRNA3) |
3.0 × 10−6 | B = 0.3 | Cotinine concentration |
9 |
| 965 (European) | rs578776 | 78888400 | 3′-UTR (CHRNA3) |
8.0 × 10−3 | Neural response |
121 | ||||
| 1,073 (European) | rs16969968-rs680244 (CHRNA5)-(CHRNA5) |
2.7 × 10−3 (interaction with treatment) |
OR = 3.1 |
Smoking cessation |
122 | |||||
| 1,030 (European) | rs1051730 | 78894339 | Synonymous (CHRNA3) |
4.0 × 10−3 | B = 0.1 | CPD | 152 | |||
| 1,075 (775 EAs+169 Hispanics+131 others) |
rs1948 | 78917399 | 3′-UTR (CHRNB4) |
<1.0 × 10−3 | HR = 1.3 |
Age of initiation |
125 | |||
| 1,118 (European) | rs3743078 | 78894759 | Intronic (CHRNA3) |
1.0 × 10−4 (ADHD patients) |
OR = 1.8 |
Smoking status |
153 | |||
| 1,450 (European) | rs16969968 | 78882925 | Missense (CHRNA5) |
2.0 × 10−3 (adolescents who tried smoking before 18) |
OR = 2.4 |
Smoking status |
154 | |||
| 1,608 (European) | rs578776 | 78888400 | 3′-UTR (CHRNA3) |
3.8 × 10−4 | FTND ≥ 4 vs. 0 in smokers |
90 | ||||
| 1,689 (European) | rs1051730 | 78894339 | Synonymous (CHRNA3) |
0.01 (meta) | OR = 0.8 |
Smoking cessation |
123 | |||
| 1,929 (European) | rs578776 | 78888400 | 3′-UTR (CHRNA3) |
1.1 × 10−4 | OR = 1.3 |
FTND ≥ 4 vs. 0 in smokers |
13, 127 | |||
| 1,936 (815 discovery+1,121 replication) |
rs16969968 | 78882925 | Missense (CHRNA5) |
2.8 × 10−3 (replication) |
FTND | 155 | ||||
| 2,038 (European) | rs16969968 | 78882925 | Missense (CHRNA5) |
7.7 × 10−3 (interaction with peer smoking) |
FTND ≥ 4 vs. 0 in smokers |
156 | ||||
| 2,047 (European) | rs16969968 | 78882925 | Missense (CHRNA5) |
5.2 × 10−8 | B = 0.2 | FTND | 106 | |||
| 2,206 (European) | rs16969968 | 78882925 | Missense (CHRNA5) |
4.4 × 10−3 (interaction with childhood adversity in male) |
OR=1.8 | DSM-IV ND | 157 | |||
| 2,284 (European) | rs17487223 | 78923987 | Intronic (CHRNB4) |
1.0 × 10−3 | Habitual smoking |
7 | ||||
| 2,474 (European) | rs1051730 | 78894339 | Synonymous (CHRNA3) |
3 × 10−4 | OR=1.3 | CPD during pregnancy |
126 | |||
| 2,633 (European) | rs1051730 | 78894339 | Synonymous (CHRNA3) |
<0.01 (NRT at 6 months) |
OR=2.5 | Smoking cessation |
124 | |||
| 2,772 (710 AAs+2,602 EAs) |
rs16969968 | 78882925 | Missense (CHRNA5) |
4.5 × 10−8 | OR=1.4 | FTND ≥4 vs. 0 in smokers |
158 | |||
| 2,827 (European) | rs680244-rs569207-rs16969968-rs578776- rs1051730 (CHRNA5)-(CHRNA5)-(CHRNA5)- (CHRNA3)-(CHRNA3) |
2.0 × 10−5 (age of daily smoking ≤16) |
OR=1.8 | FTND≤4 vs. FTND≥6 |
14 | |||||
| 2,847 (European) | rs3743078 | 78894759 | Intronic (CHRNA3) |
5.0 × 10−9 | OR=0.7 | Heavy vs. light smokers |
159 | |||
| rs11637630 | 78899719 | Intronic (CHRNA3) | 5.0 × 10−9 | OR=0.7 | ||||||
| 4,150 (European) | rs1051730 | 78894339 | Synonymous (CHRNA3) |
5.7 × 10−3 | OR=1.3 | CPD≤10 vs. CPD>10 |
160 | |||
| 4,153 (European) | rs16969968 | 78882925 | Missense (CHRNA5) |
5.0 × 10−3 | FTND | 161 | ||||
| 4,762 (European) | rs1051730 | 78894339 | Synonymous (CHRNA3) |
1.1 × 10−5 | OR=1.3 | Smoking status |
46 | |||
| 8,842 149 | rs951266 | 78878541 | Intronic (CHRNA5) |
1.0 × 10−3 (male) |
OR=1.7 | Indexed CPD | 11 | |||
| rs11072768 | 78929478 | Intronic (CHRNB4) |
1.0 × 10−3 (male) |
OR=1.2 | Smoking status |
|||||
| 32,587 (10,912 AAs + 6,889 Asians + 14,786 European) |
rs16969968 | 78882925 | Missense (CHRNA5) |
1.1 × 10−17 (meta) |
OR=1.3 | CPD≤10 vs. CPD≥20 |
8 | |||
| 32,823 (European) |
rs1051730 | 78894339 | Synonymous (CHRNA3) |
<1.0 × 10−3 | Pack-years | 162 | ||||
| 33,348 (European) |
rs16969968 | 78882925 | Missense (CHRNA5) |
0.01 | OR=1.5 (early- onset) OR=1.3 (late- onset) |
CPD ≤10 vs. >20 |
163 | |||
| 38,617 (European) |
rs16969968 | 78882925 | Missense (CHRNA5) |
6.0 × 10−31 | OR=1.3 | CPD ≤10 vs. >20 |
164 | |||
|
CHRNB3- CHRNA6 |
8p11.21 | GWAS18, 25, 92 | 965 (European) | rs4950 | 42552633 | 5′-UTR (CHRNB3) |
<1.0 × 10−4 (patients with Parkinson's disease) |
OR=1.5 | Smoking status |
129 |
| 1,051 (132 AAs + 860 EAs + 28 Hispanics + 31 others) |
rs7004381 | 42551161 | nearGene-5 (CHRNB3) |
2.4 ×10−3 (pooled) |
Quit attempt | 23 | ||||
| 1,076 (189 AAs + 631 EAs + 154 Hispanics + 102 others) |
rs892413 | 42614378 | Intronic (CHRNA6) |
<1.0 × 10−3 (interaction with ADHD symptoms) |
β=−0.3 | CPD | 128 | |||
| 1,929 (European) | rs13277254 | 42549982 | nearGene-5 (CHRNB3) |
4.0 × 10−5 | OR=1.4 | FTND ≥4 vs. 0 in smokers |
127 | |||
| 2,047 (European) | rs6474412 | 42550498 | nearGene-5 (CHRNB3) |
1.3 × 10−4 | β=−0.2 | WISDM tolerance |
106 | |||
| 2,580 (74% European) |
rs4950 | 42552633 | 5′-UTR (CHRNB3) |
<1.0 × 10−3 | Initial subjective response to nicotine |
24 | ||||
| rs13280604 | 42559586 | Intronic (CHRNB3) | <1.0 × 10−3 | |||||||
| 5,092 (1,661 AAs + 3,431 EAs) |
rs13273442 | 42544017 | NearGene-5 (CHRNB3) |
8.6 × 10−5 (meta) |
OR=0.8 | FTND ≥4 vs. 0 or 1 in smokers |
22 | |||
| 22,654 (4,297 AAs + 9,515 EAs + 8,842 Asians) |
rs4736835 | 42547033 | nearGene-5 (CHRNB3) |
5.1 × 10−8 (meta) |
β=0.16 | FTND, indexed CPD |
21 | |||
| CHRNA4 | 20q13.33 | Close to the nominated linkage peak on 20q13.12– 13.32 (3.6 Mbp) |
621 (Asian male) | rs1044397 | 61981104 | Synonymous | <1.0 × 10−3 | FTND | 97 | |
| 1,608 (European) | rs2236196 | 61977556 | 3′-UTR | 9.3 × 10−4 | FTND ≥4 vs. 0 in smokers |
90 | ||||
| 2,037 (1,366 AAs + 671 EAs) |
rs2236196 | 61977556 | 3′-UTR | 9.0 × 10−4 (AA female) |
FTND | 98 | ||||
| 3,695 (2,394 EAs + 1,301 Hispanics) |
rs1044396 | 61981134 | Missense | 0.02 (pooled) |
DSM-IV ND symptom count |
100 | ||||
| 5,561 (European) | rs2236196 | 61977556 | 3′-UTR | 2.3 × 10−3 | β=0.1 | FTND | 99 | |||
| CHRNB1 | 17p13.1 | Close to the nominated linkage peak on 17p13.1– q22 (3.2 Mbp) |
1,608 (European) | rs17732878 | 7362359 | nearGene-3 | 1.7 × 10−3 | FTND ≥4 vs. 0 in smokers |
90 | |
| 2,037 (1,366 AAs + 671 EAs) |
rs2302763 | 7359277 | Intronic | 0.01 (EA) |
CPD | 101 | ||||
| CHRM1 | 11q12.3 | Within the nominated linkage peak on 11p15– q13.4 (0 bp) |
2,037 (1,366 AAs + 671 EAs) |
rs2507821-rs4963323-rs544978-rs542269- rs2075748-rs1938677 |
8.0 × 10−3 (AA) |
CPD | 101 | |||
| CHRM2 | 7q33 | Within the nominated linkage peak on 7q31.2– q36.1 (0 bp) |
1,608 (European) | rs1378650 | 136705151 | nearGene-3 | 2.1 × 10−3 | FTND ≥4 vs. 0 in smokers |
90 | |
| Nicotine metabolism genes | ||||||||||
|
EGLN2- CYP2A6- CYP2B6 |
19q13.2 | GWAS16, 18, 26, 130 | 545 (European) | rs1801272 | 41354533 | Missense (CYP2A6) |
<1.0 × 10−4 | Nicotine metabolite ratio |
105 | |
| rs28399433 | 41356379 | nearGene-5 (CYP2A6) |
<1.0 × 10−4 | |||||||
| CYP2A6*12 | crossover with CYP2A7 |
<1.0 × 10−4 | ||||||||
| CYP2A6*1B | conversion | <1.0 × 10−4 | ||||||||
| 709 (European) | genotype-based metabolism (CYP2A6) | 2.0 × 10−8 (interaction with treatment) |
HR=0.4 | Time to relapse |
27 | |||||
| 1,355 (European) | rs3733829 | 41310571 | Intronic (EGLN2) |
3.8 × 10−5 | β=2.0 | Carbon monoxide (CO) |
28 | |||
| 1,900 (European and other) |
rs1801272 | 41354533 | Missense (CYP2A6) |
0.02 (interaction with ADHD symptoms) |
Initial subjective response to nicotine |
82 | ||||
| 1,929 (European) | rs4802100 | 41496025 | nearGene-5 (CYP2B6) |
6.8 × 10−3 | FTND ≥4 vs. 0 in smokers |
13 | ||||
| 2,047 (European) | rs3733829 | 41310571 | Intronic (EGLN2) |
1.5 × 10−3 | β=0.1 | CPD | 106 | |||
| MAPK signaling pathway & other genes | ||||||||||
| BDNF | 11p14.1 | GWAS,16 within the nominated linkage peak on 11p15–q13.4 (0 bp) |
628 149 | rs6265 | 27679916 | Missense | <0.05 (male) |
Age of initiation |
108 | |
| 2,037 (1,366 AAs + 671 EAs) |
rs6484320-rs988748-rs2030324-rs7934165 | 9.0 × 10−4 (EA) |
CPD | 109 | ||||||
| NTRK2 | 9q21.33 | GWAS,87 close to the nominated linkage peak on 9q21.33–33 (2.7 Mbp) |
2,037 (1,366 AAs + 671 EAs) |
rs1187272 | 87404086 | Intronic | 1.0 × 10−3 (EA) |
HSI | 110 | |
| ARRB1 | 11q13.4 | Within the nominated linkage peak on 11p15– q13.4 (1.5 Mbp) |
2,037 (1,366 AAs + 671 EAs) |
rs528833-rs1320709-rs480174-rs5786130- rs611908-rs472112 |
8.0 × 10−4 (EA) |
FTND | 111 | |||
| MAP3K4 | 6q26 | Within the nominated linkage peak on 6q23.3– q27 (0 bp) |
1,608 (European) | rs1488 | 161538250 | 3′-UTR | 2.7 × 10−4 | OR=1.4 | FTND ≥4 vs. 0 in smokers |
90 |
| SHC3 | 9q22.1 | Within the nominated linkage peak on 9q21.33– 33 (0 bp) |
2,037 (1,366 AAs + 671 EAs) |
rs1547696 | 91694120 | Intronic | 9.0 × 10−3 (pooled) |
CPD | 112 | |
| DNM1 | 9q34.11 | Close to the nominated linkage peak on 9q21.33– 33 (3.1 Mbp) |
2,037 (1,366 AAs + 671 EAs) |
rs3003609 | 130984755 | Synonymous | 3.1 × 10−3 (EA) |
CPD | 113 | |
| TAS2R38 | 7q34 | Within the nominated linkage peak on 7q31.2– q36.1 (0 bp) |
567 (European) | Haplotype conferring intermediate taste sensitivity (AAV) |
1.0 × 10−3 | Smoking status |
165 | |||
| 2,037 (1,366 AAs + 671 EAs) |
Taster (PAV) and non-taster (AVI) haplotypes |
3.0 × 10−3 (AA female) |
CPD | 114 | ||||||
| APBB1 | 11p15.4 | Within the nominated linkage peak on 11p15- q13.4 (0 bp) |
2,037 (1,366 AAs + 671 EAs) |
rs4758416 | 6434149 | Intronic | 3.0 × 10−3 (pooled) |
CPD | 115 | |
| PTEN | 10q23.1 | Within the nominated linkage peak on 10q21.2– q26.2 (0 bp) |
688 (European) | rs1234213 | 89689321 | Intronic | 2.0 × 10−4 | Smoking status |
116 | |
| NRG3 | 10q23.1 | Within the nominated linkage peak on 10q21.2– q26.2 (0 bp) |
614 (European) | rs1896506 | 83874383 | Intronic | 4.0 × 10−4 | Smoking cessation |
117 | |
Notes: Genes significantly associated with ND-related phenotypes in at least two hypothesis-driven candidate gene association studies with a sample size of more than 1,000 or in studies with a sample size of 500 or more but overlapped with linkage or GWAS findings. The “Linkage (distance)/GWAS” column indicates whether a gene is within (< 2 Mbp) or close to (2–5 Mbp) any reported linkage region (Table 1) or found significant in GWAS. All the linkage peaks are based on the review by Li in 200834 unless otherwise noted. Distances between candidate genes and nearby linkage regions are in parentheses. For genes with more than one significant variant in a particular study, only the variant(s) with the smallest p value(s) is presented, and only the most significant p value is shown for each variant if multiple phenotypes were tested in different ethnic/gender groups. Corresponding ethnic/gender group or special analysis methods, such as meta-analysis and interaction, for each p value are noted in parentheses right after. Variants composing the most significant haplotype are given if none of the single variants tested was statistically significant. Corresponding effect sizes are provided whenever available. The general term “smoking cessation” was used in the “Phenotype” column for ease of summarization, which represents abstinence at different time points for different studies. Variant positions are based on NCBI Build 37/hg19. For loci with multiple genes, symbols of the gene variants are indicated in parentheses following the variant type.
Abbreviations: Smoking status, smokers vs. non-smokers; HSI, heaviness of smoking index (0-6 scale); FTND, Fagerström Test for Nicotine Dependence (0-10 scale); CPD, cigarettes smoked per day; indexed CPD,11, 21 CPD categorized as non-smoking, <10, 11-20, 21-30, and >31 CPD; habitual smoking,7 ever smoking 20 CPD for 6 months or more; heavy vs. light smokers,159 heavy smokers, defined as smoking at least 30 CPD for at least 5 years, and light smokers, defined as smoking <5 CPD for at least 1 year; WISDM, Wisconsin Inventory of Smoking Dependence motives; NRT, nicotine replacement therapy; AA, African American; EA, European American; VNTR, variable number tandem repeat; 5-HTTLPR, serotonin-transporter-linked polymorphic region; OR, odds ratio; HR, hazard ratio; ADHD, attention-deficit/hyperactivity disorder; bp, base pair; Mbp, megabase pair.
Neurotransmitter system genes
Dopaminergic system
The dopaminergic system has long been acknowledged to play a critical role in nicotine addiction.40 The most studied gene in this system is DRD2, located on chromosome 11q23.2 within a modest linkage peak.41 The intriguing polymorphism Taq1A is located in ANKK1 near DRD2, leading to an amino acid change in ANKK1.42 Several other variants and haplotypes in regions adjacent to DRD2, within TTC12 and ANKK1, or downstream of DRD2 have been associated with smoking-related phenotypes.13, 43-47 Besides DRD2, a modest number of studies have shown significant associations between ND traits and other dopamine receptor genes, such as DRD148 and DRD4,49-51 and genes involved in dopamine metabolism, including dopamine β-hydroxylase (DBH),13, 52, 53 DOPA decarboxylase (DDC),54, 55 and catechol-O-methyl transferase (COMT).56-61 All of these genes are within or close to the nominated linkage peaks34 except for DBH and DDC, which have received support from GWAS results16 and as ND-associated genes from two independent studies with sample sizes ≥ 1,000.13,50-53
Huang et al.62 implicated DRD3 as a susceptibility gene for ND, but this result has not yet been replicated. Meanwhile, Stapleton et al.63 showed a significant association of a dopamine transporter gene (SLC6A3) with smoking cessation in a meta-analysis of 2,155 subjects (80% of European ancestry), although this finding received only weak support from another study on age at smoking initiation in 668 Asians.64 This gene group includes two others, protein phosphatase 1 regulatory subunit 1B (PPP1R1B) and μ-opioid receptor (OPRM1), on the basis of their functional connections with dopamine in studies of other addictive substances. PPP1R1B, also known as dopamine- and cAMP-regulated neuronal phosphatase (DARPP-32), encodes a key phosphoprotein involved in the regulation of several signaling cascades for dopaminoceptive neurons in several areas of the brain, which also is required for the biochemical effects of cocaine.65 Activation of OPRM1 in the ventral tegmental area suppresses the activity of inhibitory GABAergic interneurons, resulting in disinhibition of dopamine neurons and dopamine release from terminals in the ventral striatum.66 OPRM1 A118G variation is a genetic determinant of the striatal dopamine response to alcohol in men,66 with a preliminary study of tobacco smoking confirming this result.67 Although we believe in the importance of the above-mentioned genes in ND based on rigorous scientific evidence, the inconsistent results are worth further examination.68-72
GABAergic and serotonergic systems
For the GABAergic system, variants in the GABAB receptor subunit 2 (GABBR2),73 GABAA receptor-associated protein (GABARAP),74 and GABAA receptor subunits alpha-2 (GABRA2) and −4 (GABRA4)13, 75, 76 were significantly associated with different ND phenotypes. Cui et al.77 reviewed the significance of the GABAergic system in ND and alcohol dependence. In addition, the serotonergic system is implicated in susceptibility to ND because nicotine increases serotonin release in the brain, and symptoms of nicotine withdrawal are associated with diminished serotonergic neurotransmission.78 Genes encoding serotonin receptor 3A, ionotropic (HTR3A),79 5A, G protein-coupled (HTR5A),13 and serotonin transporter (SLC6A4)80-82 showed significant association with smoking-related behaviors. All of these seven genes of the GABAergic and serotonergic systems are within or close to the nominated linkage peaks,34 which strengthens the validity of the identified associations, although two studies reported negative results for association between serotonin transporter gene (SLC6A4) and smoking behaviour.83, 84 Another gene worth mentioning for this group is serotonin receptor 2A, G protein-coupled (HTR2A), which is within a modest linkage peak (13q14) suggested by Li et al.85 and was significantly associated with smoking status in a Brazilian sample of 625 subjects.86 Replication in larger samples is needed to confirm association of this gene with ND.
Glutamatergic system and related genes
Two glutamate receptors, ionotropic, NMDA 3A (GRIN3A), within the nominated linkage peak on 9q21.33-q33,34 and NMDA 2B (GRIN2B), suggested by one GWAS87 and close to a modest linkage peak on 12p13.31-13.32,88 were significantly associated with scores on the Fagerström Test for Nicotine Dependence (FTND).89, 90 More genes in the glutamatergic system, such as GRIN2A, GRIK2, GRM8, and SLC1A2, showed suggestive association with smoking behaviour in the GWAS reported by Vink et al.87 but without significant replication in candidate gene association studies. Accumulating evidence suggests that blockade of glutamatergic transmission attenuates the positive reinforcing and incentive motivational aspects of nicotine, inhibits the reward-enhancing and conditioned rewarding effects of nicotine, and blocks nicotine-seeking behaviour.91 More attention may be paid to this neurotransmitter system in the future.
In the catch-all part, after showing suggestive association in the first ND GWAS,92 neurexin 1 (NRXN1) association has been replicated in two independent studies with more than 2,000 subjects of three ancestries: African, Asian, and European.93, 94 Although neurexin 3 (NRXN3) also showed a significant association with the risk of being a smoker,95 this finding has not been verified in any other ND samples, and NRXN3 is not within any detected linkage peak.34 Neurexins are cell-adhesion molecules that play a key role in synapse formation and maintenance and have been implicated in polysubstance addiction.96
Nicotinic receptor (nAChR) subunit and other cholinergic system genes
As nAChR subunit gene clusters on chromosomes 15 (CHRNA5/A3/B4) and 8 (CHRNB3/A6) are major discoveries from ND GWAS, their candidate association results will be discussed together with the GWAS results. Significant association of variants in two other subunit genes (CHRNA4 and CHRNB1) did not approach genome-wide significance (p < 5 × 10−8), but they are both close to nominated linkage peaks.34 Association of CHRNA4 with ND, close to the nominated linkage peak on 20q13.12–13.32,34 has been demonstrated in five independent studies (Table 2).90, 97-100 Variants within CHRNB1, located close to the nominated linkage peak on 17p13.1-q22,34 are significantly associated with FTND and CPD scores.90, 101 Two other genes encoding nAChR subunits, CHRNB2 and CHRNA2, although associated with ND-related phenotypes in two studies,102, 103 are not within any detected linkage peaks and have no replication studies reported that are of the required sample size. Thus, these two genes are considered to have only weak evidence of involvement in ND and therefore are not included in Figure 1 and Table 2. Besides nAChR subunit genes, two cholinergic receptors, muscarinic 1 (CHRM1) and 2 (CHRM2), were found to be significantly associated with CPD and FTND, respectively.90, 101 They are within nominated linkage peaks as well.34 However, because of the inadequacy of knowledge of their biological functions, they have been less investigated.
Nicotine metabolism genes
Of the nicotine metabolism genes, those encoding nicotine-metabolizing enzymes (CYP2A6 and CYP2B6) have been most investigated.104 Six studies have provided consistent evidence that variants leading to reduced or absent CYP2A6 activity are associated with various smoking-related phenotypes, including the nicotine metabolite ratio,105 time to smoking relapse,27 exhaled carbon monoxide (CO),28 initial subjective response to nicotine,82 FTND,13 and CPD.106 All six samples consisted of subjects of European descent (Table 1). The negative result of CYP2A6 in the 2004 meta-analytic review contrasts with the findings from more recent studies, which we believe offer stronger statistical evidence.107 Such significant association of variants in the EGLN2-CYP2A6-CYP2B6 region with ND is corroborated by GWAS results, as discussed in the next section.18, 26
MAPK signalling pathway and other genes
Although space limitations do not permit an exhaustive review, we want to acknowledge studies implicating other genes in ND, including brain-derived neurotrophic factor (BDNF),108, 109 neurotrophic tyrosine kinase, receptor type 2 (NTRK2),110 arrestin, beta 1 (ARRB1),111 MAP3K4,90 SHC3,112 dynamin 1 (DNM1),113 taste receptor type 2, member 38 (TAS2R38),114 amyloid beta precursor protein-binding, family B, member 1 (APBB1),115 PTEN,116 and neuregulin 3 (NRG3).117 It is worth noting that the first five of these genes belong to the MAPK signalling pathway, which was identified as significantly enriched in involvement with four drugs subject to abuse, namely, cocaine, alcohol, opioids, and nicotine.118
GENOME-WIDE ASSOCIATION STUDIES
Although the concept of GWAS was initially proposed in 1996, 119 no GWAS was conducted until 2005. 120 Since then, this technique became the preferred mapping tool for complex diseases/traits.32 As of October 2015, nine published GWASs and meta-GWASs have yielded 11 genetic loci carrying variants of genome-wide significance (GWS; p < 5×10−8) associated with relevant ND phenotypes in subjects of European, African, and East Asian ancestries (Table 3 and Figure 1). However, only three loci were replicated in more than two independent GWASs or meta-GWASs, among which the CHRNA5/A3/B4 gene cluster has the most evidence of significance.
Table 3.
Significant Genome-Wide Association Study (GWAS) Findings for ND-Related Phenotypes
| Population | Phenotype | Nearest gene | Chr. | SNP [Effect Allele] | Physical Position | Variant Type | Sample size | P value | Effect size | Refs |
|---|---|---|---|---|---|---|---|---|---|---|
| European | CPD | CHRNA5/A3/B4 | 15q25.1 | rs1051730[A] | 78894339 | Synonymous | 73,853 | 2.8 × 10−73 | β = 1.02 | 12, 16-18 |
| rs16969968[G] | 78882925 | Missense | 73,853 | 5.6 × 10−72 | β = 1.00 | 12, 16 | ||||
| rs6495308[T] | 78907656 | Intronic | 136,090 | 5.8 × 10−44 | β = 0.73 | 12 | ||||
| rs55853698 | 78857939 | 5′-UTR | 136,090 | 1.3 × 10−16 | 12 | |||||
| CYP2A6, EGLN2, RAB4B | 19q13.2 | rs4105144[C] | 41358624 | Intergenic | 83,317 | 2.2 × 10−12 | β = 0.39 | 18 | ||
| rs7937[T] | 41302706 | 3′-UTR | 86,319 | 2.4 × 10−9 | β = 0.24 | 18 | ||||
| rs3733829[G] | 41310571 | Intronic | 73,853 | 1.0 × 10−8 | β = 0.33 | 16 | ||||
| LOC100188947 | 10q23.32 | rs1329650[G] | 93348120 | Intronic | 73,853 | 5.7 × 10−10 | β = 0.37 | 16 | ||
| rs1028936[A] | 93349797 | Intronic | 73,853 | 1.3 × 10−9 | β = 0.45 | 16 | ||||
| PDE1C | 7p14.3 | rs215605[G] | 32336965 | Intronic | 77,012 | 5.4 × 10−9 | β = 0.26 | 18 | ||
| CHRNB3/A6 | 8p11.21 | rs13280604[A] | 42559586 | Intronic | 76,670 | 1.3 × 10−8 | β = 0.31 | 18 | ||
| rs6474412[T] | 42550498 | Intergenic | 84,956 | 1.4 × 10−8 | β = 0.29 | 18 | ||||
| FTND | CACNA2D1 | 7q21.11 | rs13225753 | 82158523 | Intergenic | 4,117 | 3.5 × 10−8 | NA | 166 | |
| Smoking initiation | BDNF | 11p14.1 | rs6265[C] | 27679916 | Missense | 143,023 | 1.8 × 10−8 | OR = 1.06 | 16 | |
| Smoking cessation | DBH | 9q34.2 | rs3025343[G] | 136478355 | Intergenic | 64,924 | 3.6 × 10−8 | OR = 1.12 | 16 | |
| NMR | CYP2A6, CYP2B6, CYP2A7, EGLN2, NUMBL | 19q13.2 | rs56113850[C] | 41353107 | Intronic | 1,518 | 5.8 × 10−86 | β = −0.65 | 130 | |
| African American | CPD | CHRNA5/A3/B4 | 15q25.1 | rs2036527[A] | 78851615 | Intergenic | 15,554 | 1.8 × 10−8 | β < 1.00 | 19 |
| FTND | C14orf28 | 14q21.2 | rs117018253 | 45337321 | Intergenic | 3,529 | 4.7 × 10−10 | NA | 166 | |
| CSGALNACT1, INTS10 | 8p21.3 | rs6996964 | 19623911 | Intergenic | 3,529 | 1.1 × 10−9 | NA | 166 | ||
| DLC1 | 8p22 | rs289519 | 13237048 | Intronic | 3,529 | 4.5 × 10−8 | NA | 166 | ||
| European & African American | Dichotomized FTND | CHRNB3 | 8p11.21 | rs1451240[A] | 42546711 | Intergenic | 4,200 | 6.7 × 10−16 | OR = 0.65 | 25 |
| Japanese | CPD | CYP2A6, CYP2A7 | 19q13.2 | rs8102683[0 copy] | 41363765 | CNV | 17,158 | 3.8 × 10−42 | β = −4.00 | 26 |
| rs11878604[C] | 41333284 | Intergenic | 17,158 | 9.7 × 10−30 | β = −2.69 | 26 |
This table focuses on results achieving genome-wide significance (GWS). We used the significance threshold of 5 × 10−8. The most significant GWAS finding from different studies for any specific variant is given. If numerous tightly mapped markers showed GWS in one study, only the most significant one is provided. Variant positions are based on NCBI Build 37/hg19. For many studies, it was not possible to extract the exact sample size used for each locus, so the sizes above are approximate. “Effect sizes” refers to beta coefficients for CPD and NMR and odds ratios for smoking initiation and cessation.
Abbreviations: CNV, copy number variation; CPD, cigarettes smoked per day; dichotomized Fagerström Test for Nicotine Dependence (FTND): scores ≥ 4 vs. < 4; NA, not available; NMR, nicotine metabolite ratio; OR, odds ratio; Smoking cessation, whether regular smokers had quit at the time of interview; Smoking initiation, ever versus never began smoking.
Before the GWAS reports, Saccone et al.13 reported significant association of a 3′-UTR variant (rs578776) in CHRNA3 with dichotomized FTND in smokers in a candidate gene association study examining 348 genes. Then, in the GWAS era, five variants in this region reached genome-wide significance in five GWAS and meta-GWAS,12, 16-19 among which four (rs1051730, rs16969968, rs64952308, and rs55853698) were found to be significant in Europeans, and one (rs2036527) was significantly associated with CPD in AAs. The SNPs rs1051730, rs16969968, and rs55853698 are close-tagging proxies (all pairwise r2 > 0.96), 12 and rs2036527 is correlated with rs1051730.19 All the r2s reported in the main text were extracted from the original studies. Thus, these variants were predicted to either tag or potentially cause the principal risk for high smoking quantity attributable to the 15q25 locus, with approximately one CPD step increase for each risk allele.12, 16, 19 Although the synonymous SNP rs1051730 (Y188Y) in CHRNA3 showed the strongest association, the nonsynonymous SNP rs16969968 (D398N) in CHRNA5 and rs55853698 in the 5′-UTR of CHRNA5 hold more promise of functional importance. In the European samples, conditional on rs16969968 or rs55853698, residual association was detected at rs588765, tagging high expression of CHRNA5 and rs6495308 within CHRNA3 as showing significant association with CPD unconditionally. Liu et al.12 discovered better model fitting when conditioning on rs55853698 and rs6495308 compared with rs16969968 and rs588765 using the Bayesian information criteria (BIC). Both rs588765 and rs6495308 were reported to be in low linkage disequilibrium (LD) with each other (r2 = 0.21) and both to be in only modest LD with the principal SNPs (maximum r2 = 0.47) in subjects of European ancestry.12 However, in the AA samples, no second association signal was detected in this region after conditioning on rs2036527, suggesting that rs20356527 and correlated SNPs in populations of African ancestry define a single common haplotype.19 At the same time, the finding of importance of this gene cluster has been replicated by candidate gene association studies in persons of Asian ancestry8, 11 and different ND phenotype-cotinine concentrations,9 neural responses,121 smoking cessation successes,122-124 ages of initiation,125 and CPD during pregnancy.126 The two most replicated variants in candidate gene association studies, rs16969968 and rs1051730, are consistent with the GWAS results. Please refer to Table 2 for details.
The three GWS SNPs on chromosome 8p11 in samples of African and European ancestries—rs13280604, rs6474412, and rs1451240—are in perfect LD with each other18, 25 and also with a variant (rs13277254) suggestively associated with the ND status of smokers in the first ND GWAS.92 As noted by Rice et al.,25 although the dichotomized FTND appeared to have an equivalent relation with rs1451240 across ethnicities, the relation between this SNP and CPD was much weaker in AAs than in EAs. The other two SNPs were both significantly associated with CPD in Europeans.18 These associated SNPs are either intergenic or intronic, which may tag causal variation(s) within the LD block that contains CHRNB3 and CHRNA6 or regulate the expression of the two genes directly. Significant association of variants in CHRNB3 and CHRNA6 with ND was confirmed in eight candidate gene association studies with diverse population ancestries and smoking traits (Table 2).21-24, 106, 127-129 Cui et al.21 obtained a close to GWS meta-p value for an upstream variant of CHRNB3 (rs4736835) in a candidate gene association study of 22,654 subjects with African, European, and East Asian ancestries.
The last region detected by more than one GWAS or meta-GWAS is on chromosome 19q13.2 and includes genes such as CYP2A6/A7/B6, EGLN2, RAB4B, and NUMBL. Thorgeirsson et al.18 identified rs4105144 and rs7937 as significantly associated with CPD in European samples. These two SNPs were reported to be in LD with each other (r2 = 0.32 and D′ = 0.82 in the HapMap CEU samples). Rs4105144 was also in LD with CYP2A6*2 (rs1801272; r2 = 0.13 and D′ = 1.0 in the HapMap CEU samples), which reduces CYP2A6's enzymatic activity.18 The SNP identified by the Tobacco and Genetics Consortium16 (rs3733829) lies between these sites and was reported to show moderate LD with rs4105144 and rs7937. Besides association signals in samples with European ancestry, Kumasaka et al.26 found a copy-number variant (CNV; rs8102683) with a strong effect on CPD (β = −4.00) in a Japanese population and another significantly associated SNP (rs11878604; β = −2.69) located 30 kb downstream of the CYP2A6 gene after adjustment of the CNV. Rs8102683 shared a deletion region with other CNVs ranging from the 3′ end of the CYP2A6 gene to the 3′ end of the CYP2A7 gene; however, this common deletion was not significant in a European population.26 Very recently, Loukola et al.130 conducted the first GWAS on nicotine metabolite ratio (NMR) and identified 719 GWS SNPs within this region. Strikingly, the significant CYP2A6 variants explain a large fraction of variance (up to 31%) in NMR in their sample.
All the other signals reported by only one GWAS or meta-GWAS can be found in Table 3 and Figure 1, among which a missense variant rs6265 in BDNF was significantly associated with smoking initiation and an intergenic variant rs3025343 close to DBH was implicated in smoking cessation.16 It is worth noting that GWASs without GWS variant identification still render valuable information in determining susceptibility loci for ND. The first ND GWAS, performed by Bierut et al.,92 nominated NRXN1 in the development of ND, which was validated by a subsequent candidate gene association study.93 By using a network-based genome-wide association approach, Vink et al.87 discovered susceptibility genes encoding groups of proteins, such as glutamate receptors, proteins involved in tyrosine kinase receptor signaling, transporters, and cell-adhesion molecules, many of which were confirmed in later candidate gene association studies.89, 110 Please refer to Supplementary Table 1 for a list of GWASs without GWS results.
TARGETED SEQUENCING STUDIES
As the “missing heritability” issue emerged in each field, researchers suspected that much of the missing heritability is attributable to genetic variants that are too rare to be detected by GWAS but may have relatively large effects on risk and thus are important to study using next-generation sequencing technologies.131 Both population genetic theories and empirical studies of several complex traits suggest that rare alleles are enriched for functional and deleterious effects and thus are disproportionately represented among disease alleles.132
For the field of ND genetics, rare variant investigation started with the nAChR subunit genes, which not only are biologically important but also have yielded the most replicable results in both GWASs and candidate gene association studies, as presented above. Wessel et al.133 first examined the contribution of common and rare variants in 11 nAChR genes to FTND in 448 EA smokers, which revealed significant effects of common and rare variants combined in CHRNA5 and CHRNB2, as well as of rare variants only in CHRNA4. Xie et al.134 followed up on the CHRNA4 finding by sequencing exon 5, where most of the nonsynonymous rare variants were detected, in 1,000 ND cases and 1,000 non-ND controls with equal numbers of EAs and AAs. They discovered that functional rare variants within CHRNA4 may reduce ND risk. Also, Haller et al.135 detected protective effects of missense rare variants at conserved residues in CHRNB4. They examined in vitro the functional effects of the three major association signal contributors (i.e., T375I and T91I in CHRNB4 and R37H in CHRNA3), finding that the minor alleles of the studied SNPs increased the cellular response to nicotine. The two rare variants in CHRNB4 were confirmed to augment nicotine-mediated α3β4 nAChR currents in hippocampal neurons, as did a third variant, D447X, in the report of Slimak et al.136 The fourth SNP they analyzed, R348C, reduced nicotine currents. They also observed that habenular expression of the β4 gain-of-function allele T374I resulted in strong aversion to nicotine in mice, whereas transduction of the β4 loss-of-function allele R348C failed to induce nicotine aversion. Later, Doyle et al.137 reported an interesting rare variant in CHRNA5 that could result in nonsense-mediated decay of aberrant transcripts in 250 AA heavy smokers. And recently, Yang et al.138 performed a targeted sequencing study with the goal of determining both the individual and the cumulative effects of rare and common variants in 30 candidate genes implicated in ND. Rare variants in NRXN1, CHRNA9, CHRNA2, NTRK2, GABBR2, GRIN3A, DNM1, NRXN2, NRXN3, and ARRB2 were found to be significantly associated with smoking status in 3,088 AA samples, and a significant excess of rare variants exclusive to EA smokers was observed in NRXN1, CHRNA9, TAS2R38, GRIN3A, DBH, ANKK1/DRD2, NRXN3, and CDH13. The 18 genetic loci implicated in targeted sequencing studies are marked in Figure 1.
IMPLICATIONS
According to our list, 242 candidate gene association, 22 genome-wide linkages, 18 GWAS, and 5 targeted sequencing, making a total of 287 studies, have been conducted in the ND genetics field. The numbers for genome-wide linkage and candidate gene association studies before 2004 are based on Li34 and Munafò et al.,139 respectively. As a summary and refining of the 286 ND genetic studies, we developed an ND genetic susceptibility map with 14 linkage regions and 47 unique loci of 60 susceptibility genes (Figure 1).
Both genome-wide linkage and GWAS are considered “unbiased” exploratory approaches. By comparing their results, we found that only two GWS signals are within the nominated linkage peaks, which are LOC100188947 and BDNF.34, 140 The other nine loci, including the three most replicable ones, are all outside of the linkage peaks, and the rest of the 12 linkage regions do not contain any GWS signal (Tables 1 and 2). This discrepancy might reflect not only the different natures of the two genome-wide approaches but also different ND measures used among those studies. Genome-wide linkage studies usually investigate sparse microsatellites segregated with the trait of interest in different families, whereas GWAS takes advantage of dense common variants and thousands of unrelated individuals. Because of the distinct characteristics of family and case control samples and known locus heterogeneity for ND, we might not expect same sets of susceptibility alleles to be detected by both approaches. The relatively large nominated linkage regions tagged by microsatellites may implicate common or rare variants or both within the region of interest, on the other hand, it is generally believed that only common variants can be detected by GWAS. Moreover, even if a linkage region is driven by common variants, we may still not be able to locate them in GWAS because of the stringent p values applied for defining significance in GWAS. The presence of GWAS signals outside linkage peaks might also result from the lack of power for linkage studies to detect weak genetic effects exhibited by the loci involved in complex diseases compared with association studies.119 As one can see, these unbiased approaches are powerful in marking areas in the genome; nevertheless, the areas they indicate are often large and may not be complete. In this case, hypothesis-driven studies are useful and necessary tools not only to scrutinize marked areas, but also to explore promising false-negative results and biologically plausible targets.
Both candidate gene association and targeted sequencing studies serve this purpose. Candidate gene association studies replicated and extended 5 of the 11 GWAS results; i.e., CHRNB3/A6, DBH, BDNF, CHRNA5/A3/B4, and EGLN2/CYP2A6/B6. For the other 29 non-GWS candidate genetic loci, 20 and 7 were selected from within and close to linkage peaks, respectively, the exceptions being NRXN1 and DDC (Table 2), which reminds us of the importance of examining suggestive results in GWAS,92 the other two examples being GRIN2B and NTRK2,87 and biologically plausible genes separately. Although we have localized candidate genes within most of the nominated linkage regions, four linkage peaks, on chromosomes 3q26-q27, 5q11.2-q14, 9p21-p24.1, and 17q24.3-q25.3, are still empty, suggesting there are novel susceptibility genes to be discovered in the future. Overlaps and distinctions from the two unbiased approaches and the significant number of loci reproduced or proposed in candidate gene studies suggest that we have many more study targets with good statistical evidence besides the three most replicable GWAS loci. The fourth “immature” approach is also hypothesis driven and has verified the importance of rare variants in ND genetics.133-135, 138 Besides the demonstrated aggregate effects of rare variants in 12 genetic loci implicated in previous studies, biological candidates showing equivocal or no association beforehand were found to be significantly associated with ND-related phenotypes, such as CHRNB2, CHRNA9, CHRNA2, NRXN2, NRXN3, and CDH13, among which CHRNA9 and NRXN2 are within linkage regions.34, 141 Thus, we believe whole-exome and whole-genome sequencing studies focusing on rare variants, as the third unbiased experimental approach, will reveal new susceptibility genes/variants and further dissect the existing targets.
It is worth noting that to establish a replication of a genotype–phenotype association, every effort should be made to analyze phenotypes comparable to those reported in the original study.29 However, the ND genetics studies mentioned above involved a plethora of smoking-related phenotypes. Generally speaking, they can be classified into the following groups: 1) categorical variables along smoking trajectories; e.g., smoking initiation, status, and cessation; 2) ND assessed using DSM-IV or FTND; 3) smoking quantity such as CPD; and 4) endophenotypes such as NMR, cotinine and CO concentrations, or functional imaging results. At least two of the four phenotype groups have been used in genome-wide linkage studies (Table 1),34 candidate gene association studies (Table 2), and GWASs (Table 3). Because of the sample source and size requirement differences, DSM- or FTND-ascertained ND definitions were commonly used in linkage studies, whereas CPD was more often applied in GWAS. For candidate gene association studies, more comprehensive smoking profiles were usually tested for association with positive results from unbiased studies as replication, or more importantly, extension by using different phenotypes (Table 2), because there is considerable evidence that the various smoking measures are not highly related to one another.142 Even for measures with relatively high correlation, such as FTND and CPD, the slight change of phenotype from FTND-based ND to CPD would change the results.25 Therefore, although several loci, such as TTC12-ANKK1-DRD2, CHRNA5/A3/B4, and CYP2A6/B6 showed associations with different phenotypes (Tables 2 and 3), we should not expect positive associations with one phenotype to be replicated in samples with other phenotypes. It is important to keep in mind that a small change in phenotype may expose previously undiscovered variants, which underlie different biological processes and may have specific roles in distinguishing phenotypes.25
Additionally, gene–gene and gene–environment interactions are two pieces of information missing from the current map because of the small number of reported studies. We expect more results in these two areas will be published with the development of efficient algorithms and become important parts of the susceptibility map. It also is worth noting that half of the 48 ND loci were significantly associated with alcohol-related phenotypes, and ~30% were involved in illicit drug dependence (Supplementary Table 2), suggesting that the 60 genes on the ND map are good candidates for addiction studies of other drugs as well.
FUTURE DIRECTIONS
Technological advances enable the development of different experimental approaches. A genetic susceptibility map, as put together in this review, contains scientific evidence from diverse approaches and can serve as a draft of the “parts list” to be updated periodically until complete.38 We hope such an enumeration will catalyze an array of specific targeted and nuanced scientific studies, as suggested by Sullivan et al.;38 e.g., calculating the heritability explained by the 47 genetic loci, replicating association signals currently inadequately supported, identifying causal variant(s) within each locus through expression data integration and functional characterization, selecting appropriate phenotypic measures of ND, elucidating biological mechanisms between the genotype and ND, exploring gene–gene and gene–environment interactions, understanding the part played by epigenetic modifications, developing and evaluating treatment prediction models, and so forth.
Although the sample size of candidate gene association studies has increased over the years (Supplementary Figure 1A), genetic power calculation and corresponding sample size ascertainment should always be a top priority before conducting genetic studies. Additionally, only 18% and 10% of the 287 studies investigated subjects with African and Asian ancestries, respectively, compared with 69% for European ancestry (Supplementary Figure 1B). Studying different populations is necessary to understand the genetic causes of ND in various ethnic groups. Concurrently, given the importance of rare variants suggested by targeted sequencing study results, thorough and well-powered genomic evaluations at the lower end of the allelic spectrum are needed. Whole-exome and whole-genome sequencing studies with enough statistical rigor would enable a substantial update of the ND genetic susceptibility map in the near future.
However, it is important to acknowledge that the genetic liability accounted for by each of the 47 loci is probably less than 1% of the phenotypic variance, considering their respective effect sizes, which may also explain why they can be identified through one type of unbiased study, but not the other. Anticipating future studies on the predictive power of these loci cumulatively, we are inclined to project that the amount of heritability explained will still be limited, which renders the susceptibility map as only a beginning. Furthermore, functional studies have been conducted for limited genetic variants with certain or uncertain smoking associations (Table 4). Nevertheless, the TTC12/ANKK1-DRD2 cluster shows consistent association with smoking-related behaviors (Table 2), and the function of the most prominent variation in this region, Taq1A, still is largely unknown. 47 On the other hand, we have understood the molecular and neurobehavioral functional consequences of BDNF Met66Val polymorphism (rs6265) for more than a decade,143 although its association with ND phenotypes is still relatively weak (Table 2). Combining the susceptibility map results with relevant functional annotations will facilitate determination of variations bearing higher translational values.144 All in all, this map empowers us to sift through existing accomplishments and ponder future research strategies, an approach that may serve as a useful tool for other complex diseases/traits also.
Table 4.
Functional studies of variations associated with smoking in the 47 ND susceptibility loci
| Chr. | Gene | Experiment | Variation [Effect Allele] | Effect | Ref. |
|---|---|---|---|---|---|
| 1 | CHRNB2 | In vitro gene expression assay | rs2072658 [A] | Reduced expression | 167 |
| 6 | OPRM1 | PET brain imaging | rs1799971 [G] | Binding potential & receptor availability change | 67, 168, 169 |
| 8 | CHRNA2 | Electrophysiology assay | rs141072985 rs56344740 rs2472553 |
nAChR function change | 170, 171 |
| CHRNB3 | In vitro gene expression assay | rs6474413 [C] | Reduced expression | 172 | |
| ChIP and in vitro gene expression assay | rs4950 [G] | Eliminated TF binding and reduced promoter activity | 129 | ||
| 9 | DNM1 | In vitro gene expression assay | rs3003609 [T] | Reduced expression | 113 |
| 11 | BDNF | fMRI, 1H-MRSI, and immunoenzyme assays | rs6265 | Different brain activation, BDNF secretion, and subcellular distribution | 143 |
| DRD4 | fMRI | exon 3 VNTR | Different brain activation | 173 | |
| 15 | CHRNA5/A3/B4 | Imaging Series of in vitro assays Electrophysiology and FLEX station |
rs16969968 [A] | Brain circuit strength prediction Altered response to nicotine agonist Lower Ca permeability and increased short-term desensitization |
7, 174, 175 |
| 17 | SLC6A4 |
In vitro gene expression assay In situ hybridization SPECT imaging |
5-HTTLPR | Transcriptional efficiency and expression change | 176-178 |
| 19 | CYP2A6/B6 | Please refer to Tricker179 for a comprehensive summary | |||
| 20 | CHRNA4 | Electrophysiology assay | exon 5 haplotype | Different receptor sensitivity | 180 |
| 22 | COMT | Enzyme activity assay | rs4680 [A] | Less enzyme activity | 181 |
Abbreviations: ChIP, chromatin immunoprecipitation; fMRI, functional magnetic resonance imaging; 1H-MRSI, 1H magnetic resonance spectroscopic imaging; nAChR, nicotinic acetylcholine receptor; PET, positron emission tomography; SPECT, single-photon emission computed tomography.
Supplementary Material
ACKNOWLEDGMENT
The preparation of this communication was supported by U.S. National Institutes of Health grant DA012844 to MDL. We thank Dr. David L Bronson for his excellent editing of this report.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest relating to this report.
REFERENCES
- 1.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.USDHHS . A Report of the Surgeon General. US Department of Health & Human Services, Center for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promortion; Atlanta, Georgia: 2014. The Health Consequences of Smoking—50 Years of Progress. [Google Scholar]
- 3.Gunby P. Surgeon General emphasizes nicotine addiction in annual report on tobacco use, consequences. Jama. 1988;259(19):2811. [PubMed] [Google Scholar]
- 4.Carmelli D, Swan GE, Robinette D, Fabsitz R. Genetic influence on smoking--a study of male twins. N Engl J Med. 1992;327(12):829–833. doi: 10.1056/NEJM199209173271201. [DOI] [PubMed] [Google Scholar]
- 5.Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98(1):23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- 6.Berrettine W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, et al. alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatr. 2008;13(4):368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, et al. Variants in Nicotinic Receptors and Risk for Nicotine Dependence. Am J Psychiatry. 2008;165(9):1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen LS, Saccone NL, Culverhouse RC, Bracci PM, Chen CH, Dueker N, et al. Smoking and genetic risk variation across populations of European, Asian, and African American ancestry--a meta-analysis of chromosome 15q25. Genet Epidemiol. 2012;36(4):340–351. doi: 10.1002/gepi.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keskitalo K, Broms U, Heliovaara M, Ripatti S, Surakka I, Perola M, et al. Association of serum cotinine level with a cluster of three nicotinic acetylcholine receptor genes (CHRNA3/CHRNA5/CHRNB4) on chromosome 15. Hum Mol Genet. 2009;18(20):4007–4012. doi: 10.1093/hmg/ddp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li MD, Xu Q, Lou XY, Payne TJ, Niu T, Ma JZ. Association and interaction analysis of variants in CHRNA5/CHRNA3/CHRNB4 gene cluster with nicotine dependence in African and European Americans. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(3):745–756. doi: 10.1002/ajmg.b.31043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li MD, Yoon D, Lee JY, Han BG, Niu T, Payne TJ, et al. Associations of variants in CHRNA5/A3/B4 gene cluster with smoking behaviors in a Korean population. PLoS One. 2010;5(8):e12183. doi: 10.1371/journal.pone.0012183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu JZ, Tozzi F, Waterworth DM, Pillai SG, Muglia P, Middleton L, et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet. 2010;42(5):436–440. doi: 10.1038/ng.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16(1):36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss RB, Baker TB, Cannon DS, von Niederhausern A, Dunn DM, Matsunami N, et al. A Candidate Gene Approach Identifies the CHRNA5-A3-B4 Region as a Risk Factor for Age-Dependent Nicotine Addiction. Plos Genetics. 2008;4(7) doi: 10.1371/journal.pgen.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saccone NL, Schwantes-An TH, Wang JC, Grucza RA, Breslau N, Hatsukami D, et al. Multiple cholinergic nicotinic receptor genes affect nicotine dependence risk in African and European Americans. Genes Brain Behav. 2010;9(7):741–750. doi: 10.1111/j.1601-183X.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.TAG. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42(5):441–447. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452(7187):638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, Geller F, et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet. 2010;42(5):448–453. doi: 10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.David SP, Hamidovic A, Chen GK, Bergen AW, Wessel J, Kasberger JL, et al. Genome-wide meta-analyses of smoking behaviors in African Americans. Translational psychiatry. 2012;2:e119. doi: 10.1038/tp.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen L, Jiang K, Yuan W, Cui W, Li MD. Contribution of Variants in CHRNA5/A3/B4 Gene Cluster on Chromosome 15 to Tobacco Smoking: From Genetic Association to Mechanism. Molecular neurobiology. 2016;53(1):472–484. doi: 10.1007/s12035-014-8997-x. [DOI] [PubMed] [Google Scholar]
- 21.Cui WY, Wang S, Yang J, Yi SG, Yoon D, Kim YJ, et al. Significant association of CHRNB3 variants with nicotine dependence in multiple ethnic populations. Mol Psychiatry. 2013;18(11):1149–1151. doi: 10.1038/mp.2012.190. [DOI] [PubMed] [Google Scholar]
- 22.Culverhouse RC, Johnson EO, Breslau N, Hatsukami DK, Sadler B, Brooks AI, et al. Multiple distinct CHRNB3-CHRNA6 variants are genetic risk factors for nicotine dependence in African Americans and European Americans. Addiction. 2014;109(5):814–822. doi: 10.1111/add.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoft NR, Corley RP, McQueen MB, Schlaepfer IR, Huizinga D, Ehringer MA. Genetic association of the CHRNA6 and CHRNB3 genes with tobacco dependence in a nationally representative sample. Neuropsychopharmacology. 2009;34(3):698–706. doi: 10.1038/npp.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeiger JS, Haberstick BC, Schlaepfer I, Collins AC, Corley RP, Crowley TJ, et al. The neuronal nicotinic receptor subunit genes (CHRNA6 and CHRNB3) are associated with subjective responses to tobacco. Hum Mol Genet. 2008;17(5):724–734. doi: 10.1093/hmg/ddm344. [DOI] [PubMed] [Google Scholar]
- 25.Rice JP, Hartz SM, Agrawal A, Almasy L, Bennett S, Breslau N, et al. CHRNB3 is more strongly associated with Fagerstrom test for cigarette dependence-based nicotine dependence than cigarettes per day: phenotype definition changes genome-wide association studies results. Addiction. 2012;107(11):2019–2028. doi: 10.1111/j.1360-0443.2012.03922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumasaka N, Aoki M, Okada Y, Takahashi A, Ozaki K, Mushiroda T, et al. Haplotypes with copy number and single nucleotide polymorphisms in CYP2A6 locus are associated with smoking quantity in a Japanese population. PLoS One. 2012;7(9):e44507. doi: 10.1371/journal.pone.0044507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen LS, Bloom AJ, Baker TB, Smith SS, Piper ME, Martinez M, et al. Pharmacotherapy effects on smoking cessation vary with nicotine metabolism gene (CYP2A6). Addiction. 2014;109(1):128–137. doi: 10.1111/add.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bloom AJ, Baker TB, Chen LS, Breslau N, Hatsukami D, Bierut LJ, et al. Variants in two adjacent genes, EGLN2 and CYP2A6, influence smoking behavior related to disease risk via different mechanisms. Hum Mol Genet. 2014;23(2):555–561. doi: 10.1093/hmg/ddt432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chanock SJ, Manolio T, Boehnke M, Boerwinkle E, Hunter DJ, Thomas G, et al. Replicating genotype-phenotype associations. Nature. 2007;447(7145):655–660. doi: 10.1038/447655a. [DOI] [PubMed] [Google Scholar]
- 30.Yang J, Benyamin B, McEvoy BP, Gordon S, Henders AK, Nyholt DR, et al. Common SNPs explain a large proportion of the heritability for human height. Nat Genet. 2010;42(7):565–569. doi: 10.1038/ng.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SH, DeCandia TR, Ripke S, Yang J, Sullivan PF, Goddard ME, et al. Estimating the proportion of variation in susceptibility to schizophrenia captured by common SNPs. Nat Genet. 2012;44(3):247–250. doi: 10.1038/ng.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ott J, Wang J, Leal SM. Genetic linkage analysis in the age of whole-genome sequencing. Nat Rev Genet. 2015;16(5):275–284. doi: 10.1038/nrg3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gelernter J. Genetics of complex traits in psychiatry. Biol Psychiatry. 2015;77(1):36–42. doi: 10.1016/j.biopsych.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKay JD, Hung RJ, Gaborieau V, Boffetta P, Chabrier A, Byrnes G, et al. Lung cancer susceptibility locus at 5p15.33. Nat Genet. 2008;40(12):1404–1406. doi: 10.1038/ng.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardin J, He Y, Javitz HS, Wessel J, Krasnow RE, Tildesley E, et al. Nicotine withdrawal sensitivity, linkage to chr6q26, and association of OPRM1 SNPs in the SMOking in FAMilies (SMOFAM) sample. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18(12):3399–3406. doi: 10.1158/1055-9965.EPI-09-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swan GE, Hops H, Wilhelmsen KC, Lessov-Schlaggar CN, Cheng LS, Hudmon KS, et al. A genome-wide screen for nicotine dependence susceptibility loci. Am J Med Genet B Neuropsychiatr Genet. 2006;141(4):354–360. doi: 10.1002/ajmg.b.30315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han S, Gelernter J, Luo X, Yang BZ. Meta-analysis of 15 genome-wide linkage scans of smoking behavior. Biological psychiatry. 2010;67(1):12–19. doi: 10.1016/j.biopsych.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sullivan PF, Daly MJ, O'Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet. 2012;13(8):537–551. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu A, Zhao C, Fan Y, Jang W, Mungall AJ, Deloukas P, et al. Comparison of human genetic and sequence-based physical maps. Nature. 2001;409(6822):951–953. doi: 10.1038/35057185. [DOI] [PubMed] [Google Scholar]
- 40.Dani JA. Roles of dopamine signaling in nicotine addiction. Mol Psychiatry. 2003;8(3):255–256. doi: 10.1038/sj.mp.4001284. [DOI] [PubMed] [Google Scholar]
- 41.Gelernter J, Panhuysen C, Weiss R, Brady K, Poling J, Krauthammer M, et al. Genomewide linkage scan for nicotine dependence: identification of a chromosome 5 risk locus. Biological psychiatry. 2007;61(1):119–126. doi: 10.1016/j.biopsych.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 42.Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Hum Mutat. 2004;23(6):540–545. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- 43.David SP, Mezuk B, Zandi PP, Strong D, Anthony JC, Niaura R, et al. Sex differences in TTC12/ANKK1 haplotype associations with daily tobacco smoking in Black and White Americans. Nicotine Tob Res. 2010;12(3):251–262. doi: 10.1093/ntr/ntp201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gelernter J, Yu Y, Weiss R, Brady K, Panhuysen C, Yang BZ, et al. Haplotype spanning TTC12 and ANKK1, flanked by the DRD2 and NCAM1 loci, is strongly associated to nicotine dependence in two distinct American populations. Hum Mol Genet. 2006;15(24):3498–3507. doi: 10.1093/hmg/ddl426. [DOI] [PubMed] [Google Scholar]
- 45.Huang W, Payne TJ, Ma JZ, Beuten J, Dupont RT, Inohara N, et al. Significant association of ANKK1 and detection of a functional polymorphism with nicotine dependence in an African-American sample. Neuropsychopharmacology. 2009;34(2):319–330. doi: 10.1038/npp.2008.37. [DOI] [PubMed] [Google Scholar]
- 46.Ducci F, Kaakinen M, Pouta A, Hartikainen AL, Veijola J, Isohanni M, et al. TTC12-ANKK1-DRD2 and CHRNA5-CHRNA3-CHRNB4 influence different pathways leading to smoking behavior from adolescence to mid-adulthood. Biol Psychiatry. 2011;69(7):650–660. doi: 10.1016/j.biopsych.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma Y, Yuan W, Jiang X, Cui WY, Li MD. Updated findings of the association and functional studies of DRD2/ANKK1 variants with addictions. Molecular neurobiology. 2015;51(1):281–299. doi: 10.1007/s12035-014-8826-2. [DOI] [PubMed] [Google Scholar]
- 48.Huang W, Ma JZ, Payne TJ, Beuten J, Dupont RT, Li MD. Significant association of DRD1 with nicotine dependence. Hum Genet. 2008;123(2):133–140. doi: 10.1007/s00439-007-0453-9. [DOI] [PubMed] [Google Scholar]
- 49.David SP, Munafo MR, Murphy MF, Proctor M, Walton RT, Johnstone EC. Genetic variation in the dopamine D4 receptor (DRD4) gene and smoking cessation: follow-up of a randomised clinical trial of transdermal nicotine patch. Pharmacogenomics J. 2008;8(2):122–128. doi: 10.1038/sj.tpj.6500447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ellis JA, Olsson CA, Moore E, Greenwood P, Van De Ven MO, Patton GC. A role for the DRD4 exon III VNTR in modifying the association between nicotine dependence and neuroticism. Nicotine Tob Res. 2011;13(2):64–69. doi: 10.1093/ntr/ntq210. [DOI] [PubMed] [Google Scholar]
- 51.Das D, Tan X, Easteal S. Effect of model choice in genetic association studies: DRD4 exon III VNTR and cigarette use in young adults. Am J Med Genet B Neuropsychiatr Genet. 2011;156B(3):346–351. doi: 10.1002/ajmg.b.31169. [DOI] [PubMed] [Google Scholar]
- 52.Leventhal AM, Lee W, Bergen AW, Swan GE, Tyndale RF, Lerman C, et al. Nicotine dependence as a moderator of genetic influences on smoking cessation treatment outcome. Drug Alcohol Depend. 2014;138:109–117. doi: 10.1016/j.drugalcdep.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ella E, Sato N, Nishizawa D, Kageyama S, Yamada H, Kurabe N, et al. Association between dopamine beta hydroxylase rs5320 polymorphism and smoking behaviour in elderly Japanese. J Hum Genet. 2012;57(6):385–390. doi: 10.1038/jhg.2012.40. [DOI] [PubMed] [Google Scholar]
- 54.Yu Y, Panhuysen C, Kranzler HR, Hesselbrock V, Rounsaville B, Weiss R, et al. Intronic variants in the dopa decarboxylase (DDC) gene are associated with smoking behavior in European-Americans and African-Americans. Hum Mol Genet. 2006;15(14):2192–2199. doi: 10.1093/hmg/ddl144. [DOI] [PubMed] [Google Scholar]
- 55.Ma JZ, Beuten J, Payne TJ, Dupont RT, Elston RC, Li MD. Haplotype analysis indicates an association between the DOPA decarboxylase (DDC) gene and nicotine dependence. Hum Mol Genet. 2005;14(12):1691–1698. doi: 10.1093/hmg/ddi177. [DOI] [PubMed] [Google Scholar]
- 56.Berrettini WH, Wileyto EP, Epstein L, Restine S, Hawk L, Shields P, et al. Catechol-O-methyltransferase (COMT) gene variants predict response to bupropion therapy for tobacco dependence. Biological psychiatry. 2007;61(1):111–118. doi: 10.1016/j.biopsych.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 57.Amstadter AB, Nugent NR, Koenen KC, Ruggiero KJ, Acierno R, Galea S, et al. Association between COMT, PTSD, and increased smoking following hurricane exposure in an epidemiologic sample. Psychiatry. 2009;72(4):360–369. doi: 10.1521/psyc.2009.72.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nedic G, Nikolac M, Borovecki F, Hajnsek S, Muck-Seler D, Pivac N. Association study of a functional catechol-O-methyltransferase polymorphism and smoking in healthy Caucasian subjects. Neuroscience letters. 2010;473(3):216–219. doi: 10.1016/j.neulet.2010.02.050. [DOI] [PubMed] [Google Scholar]
- 59.Beuten J, Payne TJ, Ma JZ, Li MD. Significant association of catechol-O-methyltransferase (COMT) haplotypes with nicotine dependence in male and female smokers of two ethnic populations. Neuropsychopharmacology. 2006;31(3):675–684. doi: 10.1038/sj.npp.1300997. [DOI] [PubMed] [Google Scholar]
- 60.Omidvar M, Stolk L, Uitterlinden AG, Hofman A, Van Duijn CM, Tiemeier H. The effect of catechol-O-methyltransferase Met/Val functional polymorphism on smoking cessation: retrospective and prospective analyses in a cohort study. Pharmacogenet Genomics. 2009;19(1):45–51. doi: 10.1097/fpc.0b013e328317f3f8. [DOI] [PubMed] [Google Scholar]
- 61.Munafo MR, Freathy RM, Ring SM, St Pourcain B, Smith GD. Association of COMT Val(108/158)Met genotype and cigarette smoking in pregnant women. Nicotine Tob Res. 2011;13(2):55–63. doi: 10.1093/ntr/ntq209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang W, Payne TJ, Ma JZ, Li MD. A functional polymorphism, rs6280, in DRD3 is significantly associated with nicotine dependence in European-American smokers. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(7):1109–1115. doi: 10.1002/ajmg.b.30731. [DOI] [PubMed] [Google Scholar]
- 63.Stapleton JA, Sutherland G, O'Gara C. Association between dopamine transporter genotypes and smoking cessation: a meta-analysis. Addict Biol. 2007;12(2):221–226. doi: 10.1111/j.1369-1600.2007.00058.x. [DOI] [PubMed] [Google Scholar]
- 64.Ling D, Niu T, Feng Y, Xing H, Xu X. Association between polymorphism of the dopamine transporter gene and early smoking onset: an interaction risk on nicotine dependence. J Hum Genet. 2004;49(1):35–39. doi: 10.1007/s10038-003-0104-5. [DOI] [PubMed] [Google Scholar]
- 65.Farris SP, Harris RA, Ponomarev I. Epigenetic modulation of brain gene networks for cocaine and alcohol abuse. Frontiers in neuroscience. 2015;9:176. doi: 10.3389/fnins.2015.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, et al. A genetic determinant of the striatal dopamine response to alcohol in men. Mol Psychiatry. 2011;16(8):809–817. doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Domino EF, Evans CL, Ni L, Guthrie SK, Koeppe RA, Zubieta JK. Tobacco smoking produces greater striatal dopamine release in G-allele carriers with mu opioid receptor A118G polymorphism. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38(2):236–240. doi: 10.1016/j.pnpbp.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 68.Huang S, Cook DG, Hinks LJ, Chen XH, Ye S, Gilg JA, et al. CYP2A6, MAOA, DBH, DRD4, and 5HT2A genotypes, smoking behaviour and cotinine levels in 1518 UK adolescents. Pharmacogenet Genomics. 2005;15(12):839–850. doi: 10.1097/01213011-200512000-00002. [DOI] [PubMed] [Google Scholar]
- 69.Ton TG, Rossing MA, Bowen DJ, Srinouanprachan S, Wicklund K, Farin FM. Genetic polymorphisms in dopamine-related genes and smoking cessation in women: a prospective cohort study. Behav Brain Funct. 2007;3:22. doi: 10.1186/1744-9081-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Breitling LP, Dahmen N, Illig T, Rujescu D, Nitz B, Raum E, et al. Variants in COMT and spontaneous smoking cessation: retrospective cohort analysis of 925 cessation events. Pharmacogenet Genomics. 2009;19(8):657–659. doi: 10.1097/FPC.0b013e32832fabf3. [DOI] [PubMed] [Google Scholar]
- 71.Marteau TM, Aveyard P, Munafo MR, Prevost AT, Hollands GJ, Armstrong D, et al. Effect on adherence to nicotine replacement therapy of informing smokers their dose is determined by their genotype: a randomised controlled trial. PLoS One. 2012;7(4):e35249. doi: 10.1371/journal.pone.0035249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Munafo MR, Johnstone EC, Aveyard P, Marteau T. Lack of association of OPRM1 genotype and smoking cessation. Nicotine Tob Res. 2013;15(3):739–744. doi: 10.1093/ntr/nts174. [DOI] [PubMed] [Google Scholar]
- 73.Beuten J, Ma JZ, Payne TJ, Dupont RT, Crews KM, Somes G, et al. Single- and multilocus allelic variants within the GABA(B) receptor subunit 2 (GABAB2) gene are significantly associated with nicotine dependence. Am J Hum Genet. 2005;76(5):859–864. doi: 10.1086/429839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lou XY, Ma JZ, Sun D, Payne TJ, Li MD. Fine mapping of a linkage region on chromosome 17p13 reveals that GABARAP and DLG4 are associated with vulnerability to nicotine dependence in European-Americans. Hum Mol Genet. 2007;16(2):142–153. doi: 10.1093/hmg/ddl450. [DOI] [PubMed] [Google Scholar]
- 75.Agrawal A, Pergadia ML, Saccone SF, Hinrichs AL, Lessov-Schlaggar CN, Saccone NL, et al. Gamma-aminobutyric acid receptor genes and nicotine dependence: evidence for association from a case-control study. Addiction. 2008;103(6):1027–1038. doi: 10.1111/j.1360-0443.2008.02236.x. [DOI] [PubMed] [Google Scholar]
- 76.Agrawal A, Pergadia ML, Balasubramanian S, Saccone SF, Hinrichs AL, Saccone NL, et al. Further evidence for an association between the gamma-aminobutyric acid receptor A, subunit 4 genes on chromosome 4 and Fagerstrom Test for Nicotine Dependence. Addiction. 2009;104(3):471–477. doi: 10.1111/j.1360-0443.2008.02445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cui WY, Seneviratne C, Gu J, Li MD. Genetics of GABAergic signaling in nicotine and alcohol dependence. Hum Genet. 2012;131(6):843–855. doi: 10.1007/s00439-011-1108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iordanidou M, Tavridou A, Petridis I, Kyroglou S, Kaklamanis L, Christakidis D, et al. Association of polymorphisms of the serotonergic system with smoking initiation in Caucasians. Drug Alcohol Depend. 2010;108(1-2):70–76. doi: 10.1016/j.drugalcdep.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 79.Yang Z, Seneviratne C, Wang S, Ma JZ, Payne TJ, Wang J, et al. Serotonin transporter and receptor genes significantly impact nicotine dependence through genetic interactions in both European American and African American smokers. Drug Alcohol Depend. 2013 doi: 10.1016/j.drugalcdep.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kremer I, Bachner-Melman R, Reshef A, Broude L, Nemanov L, Gritsenko I, et al. Association of the serotonin transporter gene with smoking behavior. The American journal of psychiatry. 2005;162(5):924–930. doi: 10.1176/appi.ajp.162.5.924. [DOI] [PubMed] [Google Scholar]
- 81.Daw J, Boardman JD, Peterson R, Smolen A, Haberstick BC, Ehringer MA, et al. The interactive effect of neighborhood peer cigarette use and 5HTTLPR genotype on individual cigarette use. Addictive behaviors. 2014;39(12):1804–1810. doi: 10.1016/j.addbeh.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bidwell LC, Garrett ME, McClernon FJ, Fuemmeler BF, Williams RB, Ashley-Koch AE, et al. A preliminary analysis of interactions between genotype, retrospective ADHD symptoms, and initial reactions to smoking in a sample of young adults. Nicotine Tob Res. 2012;14(2):229–233. doi: 10.1093/ntr/ntr125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Trummer O, Koppel H, Wascher TC, Grunbacher G, Gutjahr M, Stanger O, et al. The serotonin transporter gene polymorphism is not associated with smoking behavior. Pharmacogenomics J. 2006;6(6):397–400. doi: 10.1038/sj.tpj.6500389. [DOI] [PubMed] [Google Scholar]
- 84.David SP, Johnstone EC, Murphy MF, Aveyard P, Guo B, Lerman C, et al. Genetic variation in the serotonin pathway and smoking cessation with nicotine replacement therapy: new data from the Patch in Practice trial and pooled analyses. Drug Alcohol Depend. 2008;98(1-2):77–85. doi: 10.1016/j.drugalcdep.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li MD, Payne TJ, Ma JZ, Lou XY, Zhang D, Dupont RT, et al. A genomewide search finds major susceptibility Loci for nicotine dependence on chromosome 10 in african americans. Am J Hum Genet. 2006;79(4):745–751. doi: 10.1086/508208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.do Prado-Lima PA, Chatkin JM, Taufer M, Oliveira G, Silveira E, Neto CA, et al. Polymorphism of 5HT2A serotonin receptor gene is implicated in smoking addiction. Am J Med Genet B Neuropsychiatr Genet. 2004;128B(1):90–93. doi: 10.1002/ajmg.b.30004. [DOI] [PubMed] [Google Scholar]
- 87.Vink JM, Smit AB, de Geus EJ, Sullivan P, Willemsen G, Hottenga JJ, et al. Genome-wide association study of smoking initiation and current smoking. Am J Hum Genet. 2009;84(3):367–379. doi: 10.1016/j.ajhg.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li MD, Ma JZ, Payne TJ, Lou XY, Zhang D, Dupont RT, et al. Genome-wide linkage scan for nicotine dependence in European Americans and its converging results with African Americans in the Mid-South Tobacco Family sample. Mol Psychiatry. 2008;13(4):407–416. doi: 10.1038/sj.mp.4002038. [DOI] [PubMed] [Google Scholar]
- 89.Ma JZ, Payne TJ, Li MD. Significant association of glutamate receptor, ionotropic N-methyl-D-aspartate 3A (GRIN3A), with nicotine dependence in European- and African-American smokers. Hum Genet. 2010;127(5):503–512. doi: 10.1007/s00439-010-0787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grucza RA, Johnson EO, Krueger RF, Breslau N, Saccone NL, Chen LS, et al. Incorporating age at onset of smoking into genetic models for nicotine dependence: evidence for interaction with multiple genes. Addict Biol. 2010;15(3):346–357. doi: 10.1111/j.1369-1600.2010.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li X, Semenova S, D'Souza MS, Stoker AK, Markou A. Involvement of glutamatergic and GABAergic systems in nicotine dependence: Implications for novel pharmacotherapies for smoking cessation. Neuropharmacology. 2014;76(Pt B):554–565. doi: 10.1016/j.neuropharm.2013.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bierut LJ, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, et al. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet. 2007;16(1):24–35. doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nussbaum J, Xu Q, Payne TJ, Ma JZ, Huang W, Gelernter J, et al. Significant association of the neurexin-1 gene (NRXN1) with nicotine dependence in European- and African-American smokers. Hum Mol Genet. 2008;17(11):1569–1577. doi: 10.1093/hmg/ddn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sato N, Kageyama S, Chen R, Suzuki M, Tanioka F, Kamo T, et al. Association between neurexin 1 (NRXN1) polymorphisms and the smoking behavior of elderly Japanese. Psychiatr Genet. 2010;20(3):135–136. doi: 10.1097/YPG.0b013e32833a21f9. [DOI] [PubMed] [Google Scholar]
- 95.Docampo E, Ribases M, Gratacos M, Bruguera E, Cabezas C, Sanchez-Mora C, et al. Association of neurexin 3 polymorphisms with smoking behavior. Genes Brain Behav. 2012;11(6):704–711. doi: 10.1111/j.1601-183X.2012.00815.x. [DOI] [PubMed] [Google Scholar]
- 96.Liu QR, Drgon T, Walther D, Johnson C, Poleskaya O, Hess J, et al. Pooled association genome scanning: validation and use to identify addiction vulnerability loci in two samples. Proc Natl Acad Sci U S A. 2005;102(33):11864–11869. doi: 10.1073/pnas.0500329102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feng Y, Niu TH, Xing HX, Xu X, Chen CZ, Peng SJ, et al. A common haplotype of the nicotine acetylcholine receptor alpha 4 subunit gene is associated with vulnerability to nicotine addiction in men. American Journal of Human Genetics. 2004;75(1):112–121. doi: 10.1086/422194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li MD, Beuten J, Ma JZ, Payne TJ, Lou XY, Garcia V, et al. Ethnic- and gender-specific association of the nicotinic acetylcholine receptor alpha4 subunit gene (CHRNA4) with nicotine dependence. Hum Mol Genet. 2005;14(9):1211–1219. doi: 10.1093/hmg/ddi132. [DOI] [PubMed] [Google Scholar]
- 99.Breitling LP, Dahmen N, Mittelstrass K, Rujescu D, Gallinat J, Fehr C, et al. Association of nicotinic acetylcholine receptor subunit alpha 4 polymorphisms with nicotine dependence in 5500 Germans. Pharmacogenomics J. 2009;9(4):219–224. doi: 10.1038/tpj.2009.6. [DOI] [PubMed] [Google Scholar]
- 100.Kamens HM, Corley RP, McQueen MB, Stallings MC, Hopfer CJ, Crowley TJ, et al. Nominal association with CHRNA4 variants and nicotine dependence. Genes Brain Behav. 2013;12(3):297–304. doi: 10.1111/gbb.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lou XY, Ma JZ, Payne TJ, Beuten J, Crew KM, Li MD. Gene-based analysis suggests association of the nicotinic acetylcholine receptor beta1 subunit (CHRNB1) and M1 muscarinic acetylcholine receptor (CHRM1) with vulnerability for nicotine dependence. Hum Genet. 2006;120(3):381–389. doi: 10.1007/s00439-006-0229-7. [DOI] [PubMed] [Google Scholar]
- 102.Ehringer MA, Clegg HV, Collins AC, Corley RP, Crowley T, Hewitt JK, et al. Association of the neuronal nicotinic receptor beta 2 subunit gene (CHRNB2) with subjective responses to alcohol and nicotine. Am J Med Genet B. 2007;144B(5):596–604. doi: 10.1002/ajmg.b.30464. [DOI] [PubMed] [Google Scholar]
- 103.Wang S, A DvdV, Xu Q, Seneviratne C, Pomerleau OF, Pomerleau CS, et al. Significant associations of CHRNA2 and CHRNA6 with nicotine dependence in European American and African American populations. Hum Genet. 2014;133(5):575–586. doi: 10.1007/s00439-013-1398-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ray R, Tyndale RF, Lerman C. Nicotine dependence pharmacogenetics: role of genetic variation in nicotine-metabolizing enzymes. Journal of neurogenetics. 2009;23(3):252–261. doi: 10.1080/01677060802572887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Johnstone E, Benowitz N, Cargill A, Jacob R, Hinks L, Day I, et al. Determinants of the rate of nicotine metabolism and effects on smoking behavior. Clin Pharmacol Ther. 2006;80(4):319–330. doi: 10.1016/j.clpt.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 106.Chen LS, Baker TB, Grucza R, Wang JC, Johnson EO, Breslau N, et al. Dissection of the phenotypic and genotypic associations with nicotinic dependence. Nicotine Tob Res. 2012;14(4):425–433. doi: 10.1093/ntr/ntr231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carter B, Long T, Cinciripini P. A meta-analytic review of the CYP2A6 genotype and smoking behavior. Nicotine Tob Res. 2004;6(2):221–227. doi: 10.1080/14622200410001676387. [DOI] [PubMed] [Google Scholar]
- 108.Zhang XY, Chen da C, Xiu MH, Luo X, Zuo L, Haile CN, et al. BDNF Val66Met variant and smoking in a Chinese population. PLoS One. 2012;7(12):e53295. doi: 10.1371/journal.pone.0053295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beuten J, Ma JZ, Payne TJ, Dupont RT, Quezada P, Huang W, et al. Significant association of BDNF haplotypes in European-American male smokers but not in European-American female or African-American smokers. Am J Med Genet B Neuropsychiatr Genet. 2005;139(1):73–80. doi: 10.1002/ajmg.b.30231. [DOI] [PubMed] [Google Scholar]
- 110.Beuten J, Ma JZ, Payne TJ, Dupont RT, Lou XY, Crews KM, et al. Association of Specific Haplotypes of Neurotrophic Tyrosine Kinase Receptor 2 Gene (NTRK2) with Vulnerability to Nicotine Dependence in African-Americans and European-Americans. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 111.Sun D, Ma JZ, Payne TJ, Li MD. Beta-arrestins 1 and 2 are associated with nicotine dependence in European American smokers. Mol Psychiatry. 2008;13(4):398–406. doi: 10.1038/sj.mp.4002036. [DOI] [PubMed] [Google Scholar]
- 112.Li MD, Sun D, Lou XY, Beuten J, Payne TJ, Ma JZ. Linkage and association studies in African- and Caucasian-American populations demonstrate that SHC3 is a novel susceptibility locus for nicotine dependence. Mol Psychiatry. 2007;12(5):462–473. doi: 10.1038/sj.mp.4001933. [DOI] [PubMed] [Google Scholar]
- 113.Xu Q, Huang W, Payne TJ, Ma JZ, Li MD. Detection of genetic association and a functional polymorphism of dynamin 1 gene with nicotine dependence in European and African Americans. Neuropsychopharmacology. 2009;34(5):1351–1359. doi: 10.1038/npp.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mangold JE, Payne TJ, Ma JZ, Chen G, Li MD. Bitter taste receptor gene polymorphisms are an important factor in the development of nicotine dependence in African Americans. J Med Genet. 2008;45(9):578–582. doi: 10.1136/jmg.2008.057844. [DOI] [PubMed] [Google Scholar]
- 115.Chen GB, Payne TJ, Lou XY, Ma JZ, Zhu J, Li MD. Association of amyloid precursor protein-binding protein, family B, member 1 with nicotine dependence in African and European American smokers. Hum Genet. 2008;124(4):393–398. doi: 10.1007/s00439-008-0558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang L, Kendler KS, Chen X. Association of the phosphatase and tensin homolog gene (PTEN) with smoking initiation and nicotine dependence. Am J Med Genet B Neuropsychiatr Genet. 2006;141B(1):10–14. doi: 10.1002/ajmg.b.30240. [DOI] [PubMed] [Google Scholar]
- 117.Turner JR, Ray R, Lee B, Everett L, Xiang J, Jepson C, et al. Evidence from mouse and man for a role of neuregulin 3 in nicotine dependence. Mol Psychiatry. 2014;19(7):801–810. doi: 10.1038/mp.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li CY, Mao X, Wei L. Genes and (common) pathways underlying drug addiction. PLoS computational biology. 2008;4(1):e2. doi: 10.1371/journal.pcbi.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Risch N, Merikangas K. The future of genetic studies of complex human diseases. Science. 1996;273(5281):1516–1517. doi: 10.1126/science.273.5281.1516. [DOI] [PubMed] [Google Scholar]
- 120.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nees F, Witt SH, Lourdusamy A, Vollstadt-Klein S, Steiner S, Poustka L, et al. Genetic risk for nicotine dependence in the cholinergic system and activation of the brain reward system in healthy adolescents. Neuropsychopharmacology. 2013;38(11):2081–2089. doi: 10.1038/npp.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen LS, Baker TB, Piper ME, Breslau N, Cannon DS, Doheny KF, et al. Interplay of genetic risk factors (CHRNA5-CHRNA3-CHRNB4) and cessation treatments in smoking cessation success. The American journal of psychiatry. 2012;169(7):735–742. doi: 10.1176/appi.ajp.2012.11101545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Munafo MR, Johnstone EC, Walther D, Uhl GR, Murphy MF, Aveyard P. CHRNA3 rs1051730 genotype and short-term smoking cessation. Nicotine Tob Res. 2011;13(10):982–988. doi: 10.1093/ntr/ntr106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bergen AW, Javitz HS, Krasnow R, Nishita D, Michel M, Conti DV, et al. Nicotinic acetylcholine receptor variation and response to smoking cessation therapies. Pharmacogenet Genomics. 2013;23(2):94–103. doi: 10.1097/FPC.0b013e32835cdabd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schlaepfer IR, Hoft NR, Collins AC, Corley RP, Hewitt JK, Hopfer CJ, et al. The CHRNA5/A3/B4 gene cluster variability as an important determinant of early alcohol and tobacco initiation in young adults. Biological psychiatry. 2008;63(11):1039–1046. doi: 10.1016/j.biopsych.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Freathy RM, Ring SM, Shields B, Galobardes B, Knight B, Weedon MN, et al. A common genetic variant in the 15q24 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) is associated with a reduced ability of women to quit smoking in pregnancy. Hum Mol Genet. 2009;18(15):2922–2927. doi: 10.1093/hmg/ddp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Saccone NL, Saccone SF, Hinrichs AL, Stitzel JA, Duan W, Pergadia ML, et al. Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(4):453–466. doi: 10.1002/ajmg.b.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee CT, Fuemmeler BF, McClernon FJ, Ashley-Koch A, Kollins SH. Nicotinic receptor gene variants interact with attention deficient hyperactive disorder symptoms to predict smoking trajectories from early adolescence to adulthood. Addictive behaviors. 2013;38(11):2683–2689. doi: 10.1016/j.addbeh.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bar-Shira A, Gana-Weisz M, Gan-Or Z, Giladi E, Giladi N, Orr-Urtreger A. CHRNB3 c.-57A>G functional promoter change affects Parkinson's disease and smoking. Neurobiology of aging. 2014;35(9):2179, e2171–2176. doi: 10.1016/j.neurobiolaging.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 130.Loukola A, Buchwald J, Gupta R, Palviainen T, Hallfors J, Tikkanen E, et al. A Genome-Wide Association Study of a Biomarker of Nicotine Metabolism. PLoS Genet. 2015;11(9):e1005498. doi: 10.1371/journal.pgen.1005498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cirulli ET, Goldstein DB. Uncovering the roles of rare variants in common disease through whole-genome sequencing. Nat Rev Genet. 2010;11(6):415–425. doi: 10.1038/nrg2779. [DOI] [PubMed] [Google Scholar]
- 132.Sham PC, Purcell SM. Statistical power and significance testing in large-scale genetic studies. Nat Rev Genet. 2014;15(5):335–346. doi: 10.1038/nrg3706. [DOI] [PubMed] [Google Scholar]
- 133.Wessel J, McDonald SM, Hinds DA, Stokowski RP, Javitz HS, Kennemer M, et al. Resequencing of nicotinic acetylcholine receptor genes and association of common and rare variants with the Fagerstrom test for nicotine dependence. Neuropsychopharmacology. 2010;35(12):2392–2402. doi: 10.1038/npp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xie P, Kranzler HR, Krauthammer M, Cosgrove KP, Oslin D, Anton RF, et al. Rare nonsynonymous variants in alpha-4 nicotinic acetylcholine receptor gene protect against nicotine dependence. Biological psychiatry. 2011;70(6):528–536. doi: 10.1016/j.biopsych.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Haller G, Druley T, Vallania FL, Mitra RD, Li P, Akk G, et al. Rare missense variants in CHRNB4 are associated with reduced risk of nicotine dependence. Hum Mol Genet. 2012;21(3):647–655. doi: 10.1093/hmg/ddr498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Slimak MA, Ables JL, Frahm S, Antolin-Fontes B, Santos-Torres J, Moretti M, et al. Habenular expression of rare missense variants of the beta4 nicotinic receptor subunit alters nicotine consumption. Frontiers in human neuroscience. 2014;8:12. doi: 10.3389/fnhum.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Doyle GA, Chou AD, Saung WT, Lai AT, Lohoff FW, Berrettini WH. Identification of CHRNA5 rare variants in African-American heavy smokers. Psychiatric genetics. 2014;24(3):102–109. doi: 10.1097/YPG.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yang J, Wang S, Yang Z, Hodgkinson CA, Iarikova P, Ma JZ, et al. The contribution of rare and common variants in 30 genes to risk nicotine dependence. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Munafo MR, Clark TG, Johnstone EC, Murphy FG, Walton RT. The genetics basis for smoking behavior: A systematic review and meta-analysis. Nicotine Tob Res. 2004;6(4):583–597. doi: 10.1080/14622200410001734030. [DOI] [PubMed] [Google Scholar]
- 140.TGC. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42(5):441–447. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang J, Wang S, Yang Z, Hodgkinson CA, Iarikova P, Ma JZ, et al. The contribution of rare and common variants in 30 genes to risk nicotine dependence. Molecular psychiatry. 2015;20(11):1467–1478. doi: 10.1038/mp.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Piper ME, McCarthy DE, Baker TB. Assessing tobacco dependence: a guide to measure evaluation and selection. Nicotine Tob Res. 2006;8(3):339–351. doi: 10.1080/14622200600672765. [DOI] [PubMed] [Google Scholar]
- 143.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 144.Ducci F, Goldman D. The genetic basis of addictive disorders. The Psychiatric clinics of North America. 2012;35(2):495–519. doi: 10.1016/j.psc.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yudkin P, Munafo M, Hey K, Roberts S, Welch S, Johnstone E, et al. Effectiveness of nicotine patches in relation to genotype in women versus men: randomised controlled trial. BMJ. 2004;328(7446):989–990. doi: 10.1136/bmj.38050.674826.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Johnstone EC, Yudkin PL, Hey K, Roberts SJ, Welch SJ, Murphy MF, et al. Genetic variation in dopaminergic pathways and short-term effectiveness of the nicotine patch. Pharmacogenetics. 2004;14(2):83–90. doi: 10.1097/00008571-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 147.Lerman C, Jepson C, Wileyto EP, Epstein LH, Rukstalis M, Patterson F, et al. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: results of two randomized clinical trials. Neuropsychopharmacology. 2006;31(1):231–242. doi: 10.1038/sj.npp.1300861. [DOI] [PubMed] [Google Scholar]
- 148.Comings DE, Ferry L, Bradshaw-Robinson S, Burchette R, Chiu C, Muhleman D. The dopamine D2 receptor (DRD2) gene: a genetic risk factor in smoking. Pharmacogenetics. 1996;6(1):73–79. doi: 10.1097/00008571-199602000-00006. [DOI] [PubMed] [Google Scholar]
- 149.Liu YZ, Pei YF, Guo YF, Wang L, Liu XG, Yan H, et al. Genome-wide association analyses suggested a novel mechanism for smoking behavior regulated by IL15. Mol Psychiatry. 2009;14(7):668–680. doi: 10.1038/mp.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Beuten J, Ma JZ, Lou XY, Payne TJ, Li MD. Association analysis of the protein phosphatase 1 regulatory subunit 1B (PPP1R1B) gene with nicotine dependence in European- and African-American smokers. Am J Med Genet B Neuropsychiatr Genet. 2007;144(3):285–290. doi: 10.1002/ajmg.b.30399. [DOI] [PubMed] [Google Scholar]
- 151.Munafo MR, Elliot KM, Murphy MF, Walton RT, Johnstone EC. Association of the mu-opioid receptor gene with smoking cessation. Pharmacogenomics J. 2007;7(5):353–361. doi: 10.1038/sj.tpj.6500432. [DOI] [PubMed] [Google Scholar]
- 152.Falcone M, Jepson C, Benowitz N, Bergen AW, Pinto A, Wileyto EP, et al. Association of the nicotine metabolite ratio and CHRNA5/CHRNA3 polymorphisms with smoking rate among treatment-seeking smokers. Nicotine Tob Res. 2011;13(6):498–503. doi: 10.1093/ntr/ntr012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Polina ER, Rovaris DL, de Azeredo LA, Mota NR, Vitola ES, Silva KL, et al. ADHD diagnosis may influence the association between polymorphisms in nicotinic acetylcholine receptor genes and tobacco smoking. Neuromolecular medicine. 2014;16(2):389–397. doi: 10.1007/s12017-013-8286-2. [DOI] [PubMed] [Google Scholar]
- 154.Rodriguez S, Cook DG, Gaunt TR, Nightingale CM, Whincup PH, Day IN. Combined analysis of CHRNA5, CHRNA3 and CYP2A6 in relation to adolescent smoking behaviour. J Psychopharmacol. 2011;25(7):915–923. doi: 10.1177/0269881111405352. [DOI] [PubMed] [Google Scholar]
- 155.Chen X, Chen J, Williamson VS, An SS, Hettema JM, Aggen SH, et al. Variants in nicotinic acetylcholine receptors alpha5 and alpha3 increase risks to nicotine dependence. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(7):926–933. doi: 10.1002/ajmg.b.30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Johnson EO, Chen LS, Breslau N, Hatsukami D, Robbins T, Saccone NL, et al. Peer smoking and the nicotinic receptor genes: an examination of genetic and environmental risks for nicotine dependence. Addiction. 2010;105(11):2014–2022. doi: 10.1111/j.1360-0443.2010.03074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xie P, Kranzler HR, Zhang H, Oslin D, Anton RF, Farrer LA, et al. Childhood Adversity Increases Risk for Nicotine Dependence and Interacts with alpha5 Nicotinic Acetylcholine Receptor Genotype Specifically in Males. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Saccone NL, Wang JC, Breslau N, Johnson EO, Hatsukami D, Saccone SF, et al. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res. 2009;69(17):6848–6856. doi: 10.1158/0008-5472.CAN-09-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, Hinrichs AL, et al. Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17(12):3517–3525. doi: 10.1158/1055-9965.EPI-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sorice R, Bione S, Sansanelli S, Ulivi S, Athanasakis E, Lanzara C, et al. Association of a variant in the CHRNA5-A3-B4 gene cluster region to heavy smoking in the Italian population. European journal of human genetics : EJHG. 2011;19(5):593–596. doi: 10.1038/ejhg.2010.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Maes HH, Neale MC, Chen X, Chen J, Prescott CA, Kendler KS. A twin association study of nicotine dependence with markers in the CHRNA3 and CHRNA5 genes. Behavior genetics. 2011;41(5):680–690. doi: 10.1007/s10519-011-9476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rode L, Bojesen SE, Weischer M, Nordestgaard BG. High tobacco consumption is causally associated with increased all-cause mortality in a general population sample of 55,568 individuals, but not with short telomeres: a Mendelian randomization study. Int J Epidemiol. 2014;43(5):1473–1483. doi: 10.1093/ije/dyu119. [DOI] [PubMed] [Google Scholar]
- 163.Hartz SM, Short SE, Saccone NL, Culverhouse R, Chen L, Schwantes-An TH, et al. Increased genetic vulnerability to smoking at CHRNA5 in early-onset smokers. Arch Gen Psychiatry. 2012;69(8):854–860. doi: 10.1001/archgenpsychiatry.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Saccone NL, Culverhouse RC, Schwantes-An TH, Cannon DS, Chen X, Cichon S, et al. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet. 2010;6(8) doi: 10.1371/journal.pgen.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cannon DS, Baker TB, Piper ME, Scholand MB, Lawrence DL, Drayna DT, et al. Associations between phenylthiocarbamide gene polymorphisms and cigarette smoking. Nicotine Tob Res. 2005;7(6):853–858. doi: 10.1080/14622200500330209. [DOI] [PubMed] [Google Scholar]
- 166.Gelernter J, Kranzler HR, Sherva R, Almasy L, Herman AI, Koesterer R, et al. Genome-wide association study of nicotine dependence in American populations: identification of novel risk loci in both African-Americans and European-Americans. Biol Psychiatry. 2015;77(5):493–503. doi: 10.1016/j.biopsych.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hoft NR, Stitzel JA, Hutchison KE, Ehringer MA. CHRNB2 promoter region: association with subjective effects to nicotine and gene expression differences. Genes Brain Behav. 2011;10(2):176–185. doi: 10.1111/j.1601-183X.2010.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Domino EF, Hirasawa-Fujita M, Ni L, Guthrie SK, Zubieta JK. Regional brain [(11)C]carfentanil binding following tobacco smoking. Prog Neuropsychopharmacol Biol Psychiatry. 2015;59:100–104. doi: 10.1016/j.pnpbp.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ray R, Ruparel K, Newberg A, Wileyto EP, Loughead JW, Divgi C, et al. Human Mu Opioid Receptor (OPRM1 A118G) polymorphism is associated with brain mu-opioid receptor binding potential in smokers. Proc Natl Acad Sci U S A. 2011;108(22):9268–9273. doi: 10.1073/pnas.1018699108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dash B, Li MD. Two rare variations, D478N and D478E, that occur at the same amino acid residue in nicotinic acetylcholine receptor (nAChR) alpha2 subunit influence nAChR function. Neuropharmacology. 2014;85:471–481. doi: 10.1016/j.neuropharm.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dash B, Lukas RJ, Li MD. A signal peptide missense mutation associated with nicotine dependence alters alpha2*-nicotinic acetylcholine receptor function. Neuropharmacology. 2014;79:715–725. doi: 10.1016/j.neuropharm.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kamens HM, Miyamoto J, Powers MS, Ro K, Soto M, Cox R, et al. The beta3 subunit of the nicotinic acetylcholine receptor: Modulation of gene expression and nicotine consumption. Neuropharmacology. 2015;99:639–649. doi: 10.1016/j.neuropharm.2015.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xu X, Clark US, David SP, Mulligan RC, Knopik VS, McGeary J, et al. Effects of nicotine deprivation and replacement on BOLD-fMRI response to smoking cues as a function of DRD4 VNTR genotype. Nicotine Tob Res. 2014;16(7):939–947. doi: 10.1093/ntr/ntu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hong LE, Hodgkinson CA, Yang Y, Sampath H, Ross TJ, Buchholz B, et al. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc Natl Acad Sci U S A. 2010;107(30):13509–13514. doi: 10.1073/pnas.1004745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kuryatov A, Berrettini W, Lindstrom J. Acetylcholine receptor (AChR) alpha5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (alpha4beta2)(2)alpha5 AChR function. Mol Pharmacol. 2011;79(1):119–125. doi: 10.1124/mol.110.066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78(5):815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Little KY, McLauglin DP, Ranc J, Gilmore J, Lopez JF, Watson SJ, et al. Serotonin transporter binding sites and mRNA levels in depressed persons committing suicide. Biol Psychiatry. 1997;41(12):1156–1164. doi: 10.1016/s0006-3223(96)00301-0. [DOI] [PubMed] [Google Scholar]
- 178.Heinz A, Jones DW, Mazzanti C, Goldman D, Ragan P, Hommer D, et al. A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol Psychiatry. 2000;47(7):643–649. doi: 10.1016/s0006-3223(99)00171-7. [DOI] [PubMed] [Google Scholar]
- 179.Tricker AR. Nicotine metabolism, human drug metabolism polymorphisms, and smoking behaviour. Toxicology. 2003;183(1-3):151–173. doi: 10.1016/s0300-483x(02)00513-9. [DOI] [PubMed] [Google Scholar]
- 180.Eggert M, Winterer G, Wanischeck M, Hoda JC, Bertrand D, Steinlein O. The nicotinic acetylcholine receptor alpha 4 subunit contains a functionally relevant SNP Haplotype. BMC genetics. 2015;16:46. doi: 10.1186/s12863-015-0204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75(5):807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.