Abstract
The Src-family kinases (SFKs), an intracellularly located group of non-receptor tyrosine kinases are involved in oncogenesis. The importance of SFKs has been implicated in the promotion of tumor cell motility, proliferation, inhibition of apoptosis, invasion and metastasis. Recent evidences indicate that specific effects of SFKs on epithelial-to-mesenchymal transition (EMT) as well as on endothelial and stromal cells in the tumor microenvironment can have profound effects on tumor microinvasion and metastasis. Although, having been studied extensively, these novel features of SFKs may contribute to greater understanding of benefits from Src inhibition in various types of cancers. Here we review the novel role of SFKs, particularly c-Src in mediating EMT, modulation of tumor endothelial-barrier, transendothelial migration (microinvasion) and metastasis of cancer cells, and discuss the utility of Src inhibitors in vascular normalization and cancer therapy.
Keywords: Src, EMT, vascular permeability, microinvasion, metastasis
Graphical abstract
1. Introduction
Src family kinases (SFKs) that include isoforms such as c-Src (Sarcoma), Blk (B-lymphoid tyrosine kinase), Fgr (Gardner-Rasheed feline sarcoma), Fyn, Frk (Fyn-related kinase), Hck (Hematopoietic cell kinase), Lck (Lymphocyte specific kinase), Lyn, Yes (Yamagichi sarcoma) and Yrk (Yes-related kinase), each with a unique domain [1] have been implicated in oncogenesis. SFKs are non-receptor tyrosine kinases (nRTKs) that act downstream of receptor tyrosine kinases (RTKs) and integrins in the regulation of various stages of tumor cell proliferation and survival [2]. Among these, c-Src is the most characterized isoform that plays a definitive role in tumor metastasis by regulating earlier stages of cell proliferation such as cell migration, adhesion, and invasion [2]. Src interacts extensively with transmembrane RTKs such as epidermal growth factor receptor (EGFR), platelet derived growth factor receptor (PDGFR), vascular endothelial growth factor receptor (VEGFR) and others [3, 4] in transducing its signal. Although well known for its tumor promoting effects via activation of cellular function, recent studies have identified novel roles for SFKs in epithelial-to-mesenchymal transition (EMT) of cancer cells and in the modulation of tumor microenvironment, particularly the vascular compartment and myofibroblast differentiation. These new reports render reasonable optimism on the development of drugs targeting SFKs for cancer therapy.
2. Activity modulation of SFKs
All SFK members are made of well-characterized protein domains. Lipid modifications such as N-terminal myristoylation and palmitoylation make Src suitable for the interaction with transmembrane receptor tyrosine kinases (RTKs) [5]. The general structure of SFKs includes lipophilic N-terminus, followed by the regulatory SH3 and SH2 domains, catalytic protein tyrosine kinase (PTK) core and C-terminus regulatory tail that contains the hallmark tyrosine residue (Tyr527 in c-Src) [6]. Phosphorylated Tyr527 interacts and binds with the SH2 domain, keeping the SFK in the inactive conformation. This inactive conformation of SFKs is attached to the inner leaflet of the cell membrane to be activated by signal transductions initiated at the transmembrane or extracellular receptor [5](Figure 1). Thus, SFKs are auto-inhibited by multiple intermolecular interactions. SFKs are activated by protein tyrosine phosphatases that dephosphorylate Tyr527. In addition to dephosphorylation of Tyr527, phosphorylation of a key Tyr416 residue in the activation loop is essential for Src activation. Upon growth factor stimulation, RTKs undergo autophosphorylation on a number of its tyrosine residues at the cytoplasmic domain that serve as docking sites for the SH2 domain of SFKs. As the SH2 domain of SFKs binds to phosphorylated docking sites on RTKs, it exposes the Tyr527 for dephosphorylation by protein tyrosine phosphatases such as PTPα, allowing them to become fully activated and ready to perform cellular functions [5, 7] (Figure 1).
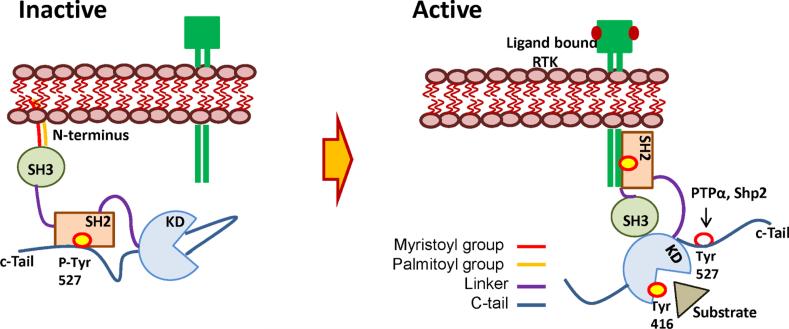
Figure 1. Activity modulation of SFKs.
In inactive conformation, SFKs bind to the cell membrane via lipids in the N-terminus. Phosphorylation of Tyr527 on c-terminal regulatory tail keeps them inactive by binding to the SH2 domain. Upon ligand binding to RTKs, SH2 domain of SFK is recruited to the phosphorylated docking sites on the cytosolic domain of RTKs exposing the Tyr527 for dephosphorylation by protein tyrosine phosphatases such as PTPα, Shp2 etc. This allows the activation of Tyr416 in the activation loop leading to the complete activation of SFKs to perform cellular functions. KD-kinase domain; RTK-receptor tyrosine kinase; PTyr527-phosphorylated tyrosine 527; PTPα- protein tyrosine phosphatase alpha; KD-kinase domain.
3. Role of Src in epithelial-to-mesenchymal transition (EMT) in solid tumors
In order to invade and metastasize to distant tissues, cancer cells transform themselves via EMT, modulate the microenvironment, induce tumor angiogenesis as well as undergo proliferation, detachment, migration, invasion, and adhesion thus establishing a new niche through secretion of various tumor derived factors [8, 9]. Activation of Src signaling is crucial to all of the above described aspects of tumor development and tumor microenvironment remodeling [2]. Src interaction with integrins, erythropoietin receptor (EpoR) and various RTKs such as EGFR, HER2, PDGFR, IGF-IR and HGFR leads to survival signal transduction downstream [10]. Src has a prominent role in regulating the cytoskeleton and cell migration. Activated Src interaction with p120 catenin causes dissociation of cell-cell junctions, facilitating cell mobility [11]. Actin based membrane protrusions are promoted through Src via induction of PDGFRα by Twist1 transcription factor [12]. This leads to formation of invadopodia and degeneration of extracellular matrix. While activation of Src enhances invadopodia formation, inhibition of Src activity significantly increases tubulin-based microtentacle (McTN) formation, which enhances capillary retention of circulating tumor cells in vivo [2, 13], indicating involvement of Src in additional cellular mechanisms leading to tumor cell proliferation [14]. In addition to the various mechanisms described, Src is very important in regulating tumor microenvironment and tumor cell survival. Src mediates VEGF secretion, matrix metalloproteases (MMPs) and interleukin 8 (IL-8) expression that promote angiogenesis and osteolytic bone metastasis through osteoclast activation [2]. In prostate and breast cancer cells, EGF signaling is regulated by androgen receptor (AR)/Src complex [15]. Blockade of AR/Src complex by either Casodex, an androgen antagonist, or S1 peptide, an androgen-receptor derived peptide, impaired AR/Src complex formation thus suppressing Src-mediated, EGF-induced mitogenesis [16]. Src activation promotes downstream kinase stimulation such as extracellular signal-regulated kinase (ERK) dependent formation of actin stress fibers allowing activation of survival signals. Other pro-survival mechanisms include resistance to the programmed cell death (anoikis) due to increased Src-mediated Akt activation and expression of angiopoietin-like 4 protein (ANGPTL4) which hijacks integrin signaling [2].
EMT is required for tissue structure and function of developing organs during embryonic development [17] and is a feature of pluripotent stem cells [18]. Epithelial cancer cells acquire mesenchymal features that permit their invasion from the primary tumor [19]. Because mesenchymal cells are highly mobile and invasive, this mechanism permits local invasion of tumor cells, a necessary first step in metastasis. Additionally, while epithelial cells are usually subject to anoikis upon detachment from the basal lamina, mesenchymal cells have no such limitation, thereby promoting cancer cell survival [20, 21]. EMT is one of the early steps in the process of cancer metastasis [18].
The process of EMT includes dissolution and disassembly of barrier adherens- and tight-junctions resulting in baso-lateral polarity [22]. During EMT, cell surface E-cadherin that maintain epithelial connections to neighboring cells will be replaced with N-cadherin that provide more transient adhesive properties as well as reorganization of cytoskeletal elements due to up-regulation of vimentin and downregulation of cytokeratins [23-27]. Combinations of these changes allow the cell to acquire the ability to leave the primary tumor and invade into the local tissue and blood vessels [19, 28, 29]. EMT may also induce a stem cell-like state in cancer cells; this may enable disseminated cancer cells to develop into macroscopic metastases [30-32]. A recent report indicates that changes in the expression of cadherins and EMT, which are critical in tumor cell invasion and migration, is controlled by SFKs [19, 33-35].
Src has been implicated in EMT because it mediates many of the processes the tumor cell undergoes in order to acquire the ability to invade and disseminate. Drake et al 2011 demonstrated that castration-resistant prostate cancer (CRPC) in men exhibits increased tyrosine phosphorylation due to increased expression of the tyrosine kinase Src in addition to many other tyrosine kinases [36]. Activated SFK expression correlates with the presence of distant metastases in patients with androgen-independent prostate cancer [37]. When enhanced c-Src expression is coupled with enhanced expression of androgen receptor (AR), it results in invasive prostate carcinoma with associated EMT as the initiation of invasive carcinoma is coupled with dynamic alterations in prostate tubule structure. Data from Cai et al 2011 also indicate that the over-expression of constitutively active Src kinase alone is capable of transforming luminal epithelial cell features into mesenchymal type [38]. Ectopic expression of constitutively activated Src and other SFKs such as Fyn and Lyn exhibits distinct differential response to paracrine signals in the initiation of prostate cancer, setting stages for EMT, and presenting a possible specific target of SFK isoforms rather than the entire class of SFKs [39]. Characteristic features of cells that have undergone EMT are evident via phosphorylation of paxillin at Tyr118 by focal adhesion kinase (FAK), which increases cell motility and survival [40]. Increased Src activity allows EMT while Src inhibition suppresses this process; for this reason, Src is a potentially effective target in preventing tumor metastasis [41, 42]. Data from Cai et al 2011 also show that the Src kinase inhibitor, dasatinib, effectively inhibits active Src kinase-induced invasive carcinoma and EMT in vivo [38]. Other previously known effects of SFKs in EMT are reviewed by Nagathihalli and Merchant [43].
Studies have also shown activity of Src in relation to EMT in other solid malignancies. In a study performed by Liu et al, breast cancer cells with high metastatic potential that were treated with Src-inhibitor showed altered epithelial morphology and inhibited cancer cell migration [44]. Cells with high metastatic potential became more clustered with each other after treatment with Src-inhibitor. Such changes imply inhibition of EMT via c-Src suppression. In addition, inhibition of EMT by c-Src suppression resulted in changes in expression of transcription factors in breast carcinoma cells as shown by an increase of E-cadherin and decrease of vimentin [44].
Further evidence suggests contribution of Src in invasiveness of pancreatic ductal carcinoma by inducing E-cadherin repressors that lead to E-cadherin down regulation [43]. Loss of E-cadherin allows epithelial cells to differentiate into mesenchymal type. While normal pancreatic ductal cells have high expression of E-cadherin and β-catenin but reduced expression of mesenchymal markers N-cadherin and vimentin, pancreatic tumor cells have increased expression of these mesenchymal markers [43]. Nagathihalli et al. have discussed unpublished data that show high expression of E-cadherin in tumor tissues treated with dasatinib, a Src family kinase (SFK) inhibitor, when compared to treatment with control in mice tumor tissues [43]. Therefore, Src renders itself as a plausible target for treating pancreatic ductal adenocarcinoma by targeting its effects on E-cadherin. Together, these recent findings open up a new area of research on many other cancers to investigate the role of SFKs in EMT and characterize the downstream signaling (Figure 2).
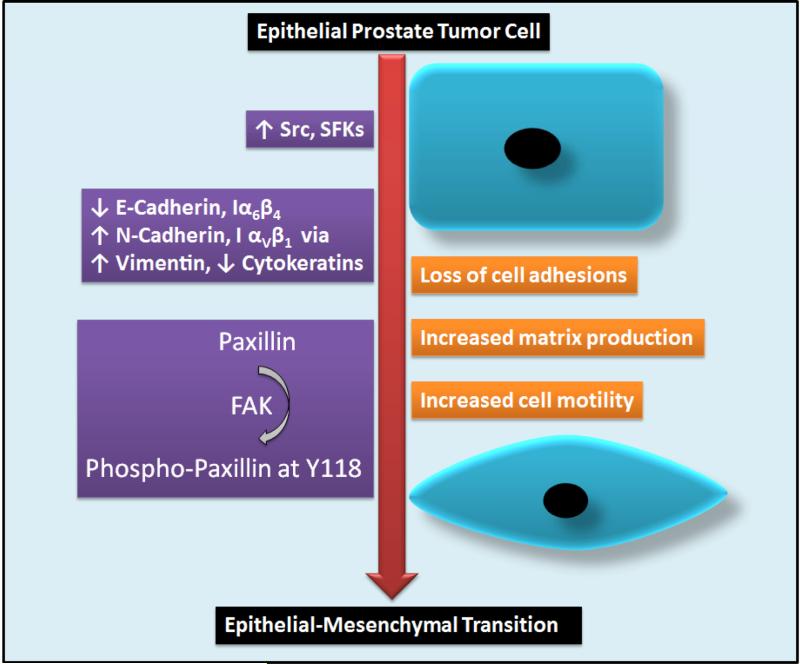
Figure 2. Role of SFKs in promoting epithelial-to-mesenchymal transition (EMT) in prostate cancer.
Enhanced expression of Src (and other SFKs) in prostate cancer is associated with disassembly of AJs and TJs due to decrease in E-cadherin and Integrin α6β4 (Iα6β4) as well as increase in N-cadherin and Integrin α5β1 (Iα5β1) associated with increase in the expression of mesenchymal markers such as vimentin and decrease in epithelial markers such as cytokeratins. Phosphorylation of paxillin at Y118 by FAK enhances cell motility and survival facilitating the cells to acquire mesenchymal properties and ability to invade local tissues. [SFK, Src family of kinases; FAK, focal adhesion kinase; AJ, adherens junction; TJ, tight junction]
4. Modulation of tumor vascular permeability and tumor cell microinvasion by Src
The series of events leading to transition between epithelial cells and mesenchymal cells parallel characteristics of alterations in endothelial cell-barrier function and tumor vascular permeability involved in cancer [45]. Integrity of endothelial monolayer, intercellular contacts between adjacent cells, and focal adhesions anchoring the endothelial lining to its surroundings in the vascular wall are important aspects in the maintenance of normal endothelial-barrier [46]. Studies suggest that activation of Src family protein tyrosine kinases is critical in disrupting endothelial barrier and inducing vascular permeability [46].
In endothelial cells, binding of vascular endothelial growth factor (VEGF) to VEGFR-2 regulates mitogenic, chemotactic and hyperpermeability signals which renders the vessels leaky [47]. VEGF induces endothelial permeability by two mechanisms. One of them is transcellular transport of substances through activation of Src kinase which phosphorylates Tyr14 on caveolin-1, a primary structural protein of caveolae [48] and phosphorylation of caveolin-2 so that caveolin-1 and caveolin-2 hetero-oligomer formation is inhibited favoring the formation of caveolin-1 structured caveolae [49]. Also, Src is reported to phosphorylate dynamin-2, a GTPase protein that migrates to the neck of caveolae facilitating their pinching from plasma membrane as vescicles [46, 50]. The other mechanism involves paracellular transport which results from alterations in structure and spatial arrangement of endothelial cells by affecting either cytoskeletal association or junctional complex association of SFKs. Junctional complexes comprised of adherens junctions (AJs) and tight junctions (TJs) are important in maintaining endothelial cell-barrier integrity. VE-cadherin (vascular endothelial cadherin) is the fundamental constituent of AJs that provide a molecular bond between cells and link the plasma membrane with the intracellular actin cytoskeleton through catenin family proteins [41, 51, 52]. The adjacent endothelial cells are connected through calcium dependent homophilic adhesion of fifth subunit of their extracellular domains of VE-cadherins. The intracellular domain is anchored to the plasma membrane and linked to cytoskeleton through β-, p120- and α-catenins. β- and p120-catenins bind within the VE-cadherin C-terminal and juxta-membrane domains, respectively. In this mechanism SFKs associate with VE-cadherin and VEGFR2 (Flk) to form junctional complexes in order to phosphorylate VE-cadherin [53].
Apart from the physiological levels produced by normal cells, tumor cells exhibit enhanced production of VEGF-A, which upon binding to its receptors activates the intrinsic tyrosine kinase activity of the receptors causing their transphosphorylation [54] resulting in binding of SH-2 domain of SFKs, c-Src in particular to the phosphotyrosines on the receptors. This leads to activation of Src and ultimate phosphorylation of VE-cadherin resulting in dissociation of all junctional complex proteins such as VEGFR, β-catenin subsequently disrupting the endothelial barrier [55]. β-catenin is also subjected to phosphorylation by Src and Fyn on Tyr654 and Tyr142, respectively [56], causing disruption of complex formed between intracellular domains of VE-cadherin, adaptor proteins and cytoskeleton [57]. VEGF-induced VE-cadherin Tyr685 phosphorylation mediated by Src is also critical in regulating VEGF-induced migration of endothelial cells and tumor angiogenesis [58].
VE-cadherin also drives SFK-mediated activation of the PI3K (phosphotidyl inositol 3-kinase) by phosphorylating Tyr688 on p85 regulatory subunit of PI3K resulting in the release of p110 catalytic subunit [59]. Through PI3K/Akt (protein kinase B) signaling pathway, Src regulates the endothelial-barrier through FoxO1 phosphorylation and cytosolic accumulation. This activation of PI3K governs claudin-5 expression, contributing to junctional organization and permeability control [60-62]. In such ways, VE-cadherin interaction with the molecules involved in adhesion and cytoskeleton dynamics reinforces cell-cell adhesion and cytoskeletal anchoring, along with modulating intracellular signaling and gene expression.
In addition to AJs formed by VE-cadherins, endothelial barrier function requires TJs formed by claudins and zona occludens (ZO) [52]. The AJs and TJs in endothelial cells are intermingled throughout cell-cell contact areas. The formation, maintenance and remodeling of the intercellular contacts require a functional interaction between these two adhesive structures [63]. This functional relationship involves expression of claudin-5 by VE-cadherin; VE-cadherin mediated adhesion enables claudin-5 expression in endothelial cells by preventing the nuclear accumulation of transcriptional regulators FoxO1 and β-catenin, as described in the previous paragraph. Since VE-cadherin is upstream of claudin-5, any changes in VE-cadherin adhesive properties will affect TJs and other levels of endothelial barrier function. Dissociation of β-catenin from the AJs, also due to SFK-mediated phosphorylations of VE-cadherin and β-catenin results in its nuclear localization and suppression of claudin gene transcription.
A recent study revealed that capsiate, a capsaicin-like compound, exerted a direct inhibitory effect on VEGF-induced activation of c-Src and phosphorylation of downstream signaling molecules, such as p125(FAK) and VE-cadherin, hence suppressing VEGF-induced angiogenesis and vascular permeability [64]. Endothelial and hematopoietic cells express DEP-1/CD148, a receptor-like protein tyrosine phosphatase that regulates SFKs. DEP-1 is phosphorylated on Src SH2 domain bound Tyr1311 and Tyr1320, in a c-Src- and Fyn-dependent manner catalyzing the dephosphorylation of inhibitory Tyr529 on c-Src resulting in VEGF induced phosphorylation of VE-Cadherin and cortactin to mediate responses such as capillary formation, invasion and permeability [65].
Phosphorylation of myosin light chain kinase (MLCK) and VE-cadherin by SFKs regulate intercellular junctions. C-Src binding to MLCK causes activation of MLCK allowing cytoskeletal rearrangements and cellular contraction [46]. This contraction may result in dissociation of AJ causing formation of gaps. Focal adhesion kinase (FAK) and paxillin located in focal adhesion complexes are also regulated through phosphorylation by SFKs [46]. Crosstalk between Src and focal adhesion kinase interferes with integrin adhesion and signaling, thus alters endothelial-barrier integrity.
Vascular endothelial growth factor secreted by tumor cells is involved in alteration of endothelial cell integrity via its interaction with c-Src and other SFKs. VEGF stimulation leads to SFKs involved dissociation of protein complex composed of VEGF receptor-2 (Flk-1), VE-cadherin, and β-catenin. Active c-Src causes tyrosine phosphorylation of VE-cadherin and β-catenin, which accounts for dissociation of the junctional protein complex. In addition, VEGF may regulate endothelial permeability and VE-cadherin endocytosis through activation of VEGFR2, Src and Vav2 guanine nucleotide exchange factor in that order. C-Src linked with stimulated VEGFR2 promotes VE-cadherin endocytosis by enhancing tyrosine phosphorylation of Vav2, ultimately disassembling endothelial cell junctions and increasing microvascular permeability [46, 66] (Figure 3).
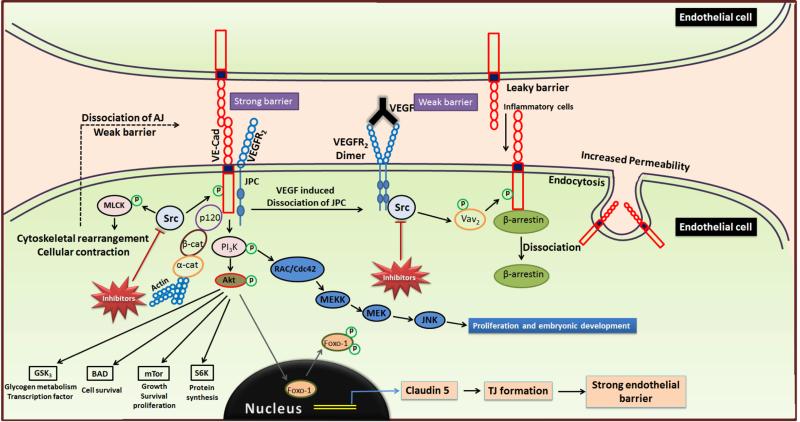
Figure 3. Modulation of tumor vascular permeability and microinvasion by SFKs.
VEGF-A binding to VEGFR2 activates intrinsic tyrosine kinase activity of the receptors resulting in their transphosphorylation, recruitment of Src and subsequent phosphorylation of VE-cadherin by Src in the junctional complex. This results in the dissociation of junctional complex proteins such as VE-cadherin and β-catenin, thus disrupting endothelial barrier, and releasing β-arrestin from VE-cadherin causing internalization of VE-cadherins into vesicles through endocytosis, thus rendering the barrier permeable to intravasation of inflammatory cells. Phosphorylation of MLCK by Src results in cytoskeletal rearrangements and cellular contractions leading to dissociation of AJs, thus contributing to leaky endothelial barrier. SFK-mediated activation of PI3K/Akt signaling pathway regulates phosphorylation of FoxO1 and its cytosolic accumulation thereby preventing claudin-5 expression and permeability control in addition to promoting growth, survival and transcriptional regulation of tumor cells through phosphorylation of BAD, mTOR, S6K and GSK3. (VEGF, Vascular endothelial growth factor; MLCK, Myosin light chain kinase; AJ, Adherens junction; BAD, Bcl-2 associated death promoter; mTOR, Mammalian target of rapamycin; S6K, Ribosomal S6 kinase; GSK-3, Glycogen synthase kinase-3)
Transcription factor Stat5 induces a decrease in the expression of E-cadherin in prostate cancer cells. Treatment of prostate cancer cells with PP2, a Src inhibitor, results in inhibition of Stat5 and its effect of decreased E-cadherin; inhibition also results in decreased binding of prostate cancer cells to endothelial cells [67].
Depletion of c-Src in triple negative breast cancer cells decreased Cyr61 levels and inhibited, and with its resultant diminished activation of Fak, caveolin-1, paxillin, and p130CAS, c-Src depletion inhibited cellular migration, invasion, and transendothelial migration [68]. MUC1/ICAM-1 binding, which appears throughout the migratory tract of metastatic breast cancer cells, results in signaling events that promotes in cytoskeletal reorganization and simulated TEndM, and those signal events are dependent on the activity of Src [34]. The MUC1/ICAM-1 interaction initiates lamellipodial and filopodial protrusion, but this requires the formation of a Src/CrkL signaling complex. Src is required for the recruitment of CrkL and inhibition of Src blocks this signaling cascade [69].
5. Src-mediated regulation of Glycogen Synthase Kinase-3 (GSK-3) activity in EMT
Protein kinase B (Akt), a Ser/Thr kinase greatly implicated in oncogenic transformation, tumor growth and metastasis of various cancers negatively regulates the activity of GSK-3 [70, 71]. Akt phosphorylates GSK-3 at Serine 21 and Serine 9 in two different isoforms GSK-3 and GSK-3 respectively and inhibits its activity [72, 73]. These observations raise a question how Akt and GSK-3β can concurrently be active in cancers. We recently provided the first evidence that both Akt and GSK-3 can simultaneously be active in prostate cancer cells and mouse embryonic fibroblasts via another activating phosphorylation of a tyrosine residue in GSK-3 (Tyrosine 216) by Src family of kinases [74].
Role of GSK-3 in cancer progression has been highly debated following the observations such as local inhibition of GSK-3 enhanced invasiveness, metastasis while global inhibition impaired cell spreading, and migration [75]. In addition, the effects of GSK-3 inhibition on apoptosis and proliferation were controversial [76, 77]. As Akt negatively regulates GSK-3 and since Akt is essential for cancer development, one would expect that inhibition of GSK-3 would promote cancer progression. Surprisingly, inhibition of GSK-3 activity is reported to have tumor suppressive effect in various cancers [78-82]. Our laboratory recently provided the first evidence that GSK-3α is involved in the regulation of cell survival, proliferation, and rate of tumor growth in both early and advanced prostate cancer cells in vitro, which was evident from the increased expression of pro-apoptotic markers cleaved caspase-3 and cleaved caspase-9 upon silencing GSK-3α in these cells. In contrast, GSK-3β is predominantly involved in the promotion of EMT and acquisition of invasive and metastatic property in advanced prostate cancer cells as silencing GSKβ resulted in the inhibition of cell scattering, establishment of cell-cell contacts, increased expression and membrane localization of β-catenin, and reduced expression of EMT markers such as Snail and MMP-9 [83]. We have also shown that GSK activation as a result of Src-mediated phosphorylation of GSK tyrosine 216 is necessary for prostate cancer cell function in vitro and inhibition of Src activity by Dasatinib, a Src-Abl inhibitor resulted in the inhibition of GSK tyrosine 216 phosphorylation in prostate cancer cell lines. This resulted in the impairment of proliferation, survival and invasion of prostate cancer cells in vitro and impaired tumor growth in vivo [74].
6. Regulation of matrix metalloproteinase expression and activity by Src
Matrix metalloproteinases (MMPs) belong to a structurally related family of at least 20 zinc-dependent endopeptidases that cleave extracellular matrix (ECM) [84]. MMPs have been implicated not only in numerous physiological processes such as fibrillogenesis, wound healing, tissue remodeling and organogenesis, but also in pathological conditions including inflammatory, vascular, fibrotic and auto-immune disorders, as well as carcinogenesis [85-89]. A wealth of information has been accumulated on the involvement of MMPs in altering the tumor microenvironment, thus aiding cancer progression [87, 90]. Hence, MMPs are proposed to be potential diagnostic and prognostic biomarkers for many cancers [91]. In humans, MMP-2 and MMP-9 exhibit substrate specificity toward type IV collagen, a predominant component of the basement membrane [86, 92]. Intriguingly, their expressions are directly correlated with the metastatic potential [93, 94], and the incidence of secondary metastasis [95-97]. Hence, MMPs are being considered as potential therapeutic targets for treatment of cancer [96, 98].
A lot has been studied on Src-mediated regulation of MMP-1, −2, −9, membrane type of MMP-1 and tissue inhibitor of MMP-1 expression in different cell types [99-102]. Collagen-IV has been reported to regulate MMP-9 secretion via a Src and FAK dependent pathway in human breast cancer cells [103]. TNF-α has been shown to induce MMP-1 expression via c-Src/EGFR-dependent ERK/AP-1 signaling pathway in human fibroblasts [104]. In another study, gallic acid inhibited the activation of EGFR/Src-mediated Akt and ERK, leading to reduced levels of p65/c-Jun-mediated DNA looping and thus inhibiting MMP-9 expression in EGF-treated MCF-7 breast cancer cells [105]. It was reported that the knockdown of Abl interactor 1 (Abl1), a key regulator of actin polymerization/depolymerization that confers abnormal cytoskeletal functions to cancer cells inhibits the Src-Id1-MMP-9 pathway, impede tumor cell proliferation and migration in breast cancer cells, subsequently limiting tumor growth in xenograft model in mice [106].
The underlying mechanism through which Src regulates the activation of MMPs varies across the cell types. Src induces the expression of MMP-9 via the c-Jun N-terminal kinase (JNK) signaling pathway and the AP-1 and GT box homologous to retinoblastoma control elements [107, 108]. In 2006, Lee at al., showed that TNF-α induces the expression of MMP-9 by Src mediated EGFR, PDGFR/PI3K/Akt signaling pathway in human tracheal smooth muscle cells [109]. Src has also shown to enhance MMP-1 by regulating the extracellular signal-regulated kinases (ERKs), PEA3 and STAT transcription factors [102]. On the other hand, Src induces MMP-2 expression via transcription activation and ERK/Sp1 signaling [110]. In contrast, the small heat shock protein 27 (hsp27) upregulates MMP-9 in breast cancer cells and promotes invasiveness via inhibition of a Src tyrosine kinase, Yes [111]. Together, these studies demonstrate the indispensable role of Src kinases in the regulation of MMP expression and activation, thus modulating the course of cancer progression.
7. Importance of Src in myofibroblast differentiation, inflammation and homing of bone-marrow derived cells
Endothelial-barrier integrity is tightly controlled under normal physiological conditions to prevent leaking of inflammatory cells from the blood. Due to the barrier's disruption in cancer, inflammation remains as one of the seven hallmarks of cancer [112]. Pro-inflammatory mediators like IL-8, VEGF, histamine, thrombin and fibrinogen have all been shown to impact VE-cadherin function because they are potent pro-permeability factors [41]. Src mediated destabilization of VE-cadherin is a major opportunity for inflammatory cells such as leukocytes to extravasate into the tissue, as well access of nutrients and oxygen needed for angiogenesis and tumor proliferation.
Tumor progression, invasion, and eventual metastasis require the activity of many adhesion proteins, including the integrin superfamily [9]. As in inflammatory cell trafficking, key steps of bone marrow derived cell (BMDC) recruitment are potentially mediated by cell adhesion molecules, such as integrin αvβ3 [113]. Integrin αvβ3 is a receptor for extracellular matrix (ECM) proteins that are important bone matrix proteins, and αvβ3 has been identified as a critical integrin in breast cancer and prostate cancer skeletal metastasis. Bone metastatic cells have a higher expression of αvβ3 than the primary tumor, promoting adherence to the bone matrix by binding to osteopontin expressed by bone stromal cells [9]. Adhesion-dependent effects in tumor cells promoted by integrins are attributed to activation of focal adhesion kinase (FAK), which recruits other signaling molecules including c-Src. After adhesion, c-Src phosphorylates Crk-associated substrate (CAS), a large adaptor protein implicated in cell invasion and survival. A study conducted by Feng et al [113] revealed that the defective tyrosine phosphorylation of β3 integrin in a mouse model resulted in impaired endothelial cell adhesion, spreading, migration, tumor angiogenic defects potentially affected by recruitment of other BMDCs [113]. Effects of Src inhibition on individual phosphorylation of integrin and VEGFR2 and VEGF-induced VEGFR2-integrin αvβ3 complex formation etc. are reviewed elsewhere [4]. These findings clearly demonstrate the involvement of SFKs in the phosphorylation of β3 integrin in the recruitment of BMDCs, and that targeting SFKs could be viable option for halting actions of BMDCs in cancer (Figure 4).
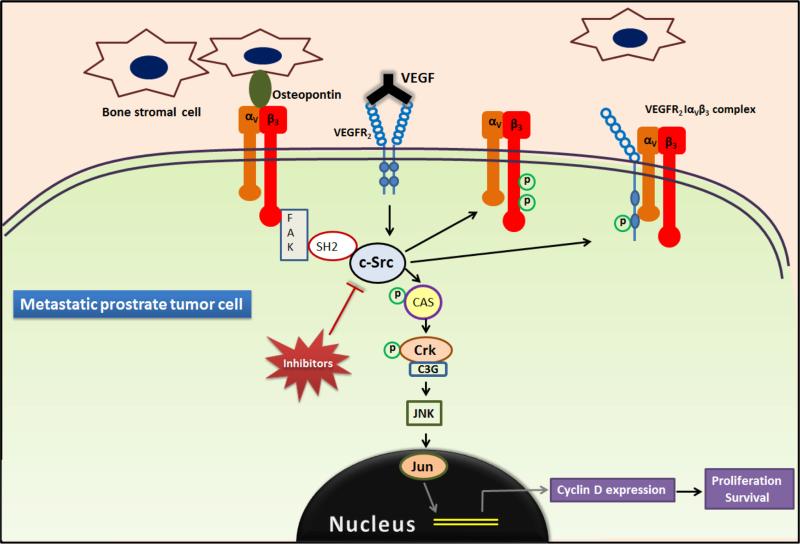
Figure 4. Role of SFKs in recruiting inflammatory and bone marrow derived cells.
Integrin αvβ3 a receptor for extracellular matrix proteins on bone metastatic tumor cells bind to osteopontin expressed by bone stromal cells causing activation of FAK, which recruits other signaling molecules including c-Src. C-Src enhances proliferation and survival through JNK pathway by phosphorylating CAS in addition to the recruitment of BMDCs by phosphorylating integrin β3. [FAK, focal adhesion kinase; CAS, Crk-associated substrate; BMDC, bone marrow derived cells]
Myofibroblasts are the activated fibroblasts characterized by the expression of alpha smooth muscle actin (α-SMA) and contribute to excess extra cellular matrix deposition [114, 115]. They are greatly implicated in health (wound healing and tissue repair) [116-118] and disease (tissue fibrosis, inflammatory diseases, and cancer) [119-124]. Although the mechanisms of the pathologic activation of fibroblasts are still under investigation, transforming growth factor β (TGFβ), pro-fibrotic cytokine, is proven to be a potent trigger and promoter of myofibroblast differentiation [115, 125]. Some studies have reported the involvement of Src kinases in the non-canonical signaling of TGFβ contributing to fibroblast adhesion, migration, and myofibroblast-mediated ECM assembly suggesting that Src Kinases are pro-fibrotic [126-130]. Our laboratory has shown that selective Src kinase inhibition using PP2 mimicked the effect of dasatinib (Src-abl inhibitor) in blunting the αSMA expression and modest, but significant inhibition of fibronectin assembly via modulation of the expression of serum response factor (SRF) and hence in attenuating myofibroblast differentiation in both mouse and human fibroblasts [131].
8. Potential role of Src in vascular normalization and anti-angiogenic therapy
Angiogenesis, a tightly regulated process is triggered by stimuli such as hypoxia, acidosis, growth factors, oncogenic signaling, hormones, cytokines [132-135] and is necessary to meet the demands of normal cell or solid tumors. Tumor-induced angiogenesis fuels the growth by providing the tumor with adequate oxygen and nutrients, enabling them to proliferate and metastasize. It also supports cancer-associated inflammation by allowing passage of inflammatory mediators to the tumor site as well as physical process of tumor cell extravasation and metastasis [66]. C-Src is thought to activate signal transducer and activator of transcription (STAT)-3, an important mediator of angiogenesis through VEGF activation [136]. Nestler et al reported the involvement of SFKs in integrin βvβ5-mediated re-endothelialization and neovascularization. Overexpression of human integrin β5 in circulating angiogenic cells resulted in an enhanced phosphorylation of integrin αvβ5 and activation of c-Src leading to increased activation of STAT-3 via phosphorylation of Tyr705 followed by a signaling cascade involving CXCL8, CCL2 gene expression, and angiogenesis [137, 138]. STAT3 is found to be constitutively upregulated in many types of cancers, including hepatocellular carcinoma (HCC), breast cancer, prostate cancer, multiple myeloma, head and neck squamous cell carcinoma etc. Src and other tyrosine kinases mediated activation of STAT-3 regulates the expression of many genes involved in initiation, progression and chemoresistance [139] as well as survival (Bcl-xl, Bcl-2 and survivin), proliferation (cyclin D1) and angiogenesis (VEGF) [140], thus implicating the role of Src in promoting different cancers via enhanced survival supported by angiogenesis.
Studies performed by Soldi et al [141] showed that phosphorylation of VEGFR2 is enhanced when endothelial cells are plated onto ECM proteins which are ligands for integrin αvβ3. Further studies showed that interaction between integrin αvβ3 and VEGFR2 stimulated by interplay between ECM ligands and VEGF is essential for the activation of endothelial cells and stimulation of VEGF-induced angiogenesis both in vitro and in vivo [4]. VEGF levels are increased in tumor cells. VEGF induces initial VEGFR2 phosphorylation followed by c-Src recruitment and both events lead to the complex formation between VEGFR2 and β3 integrin. These events promote activation of αvβ3 leading to integrin activation and phosphorylation of integrin β3 by c-Src. Altogether, these observations point out the direct and specific involvement of c-Src in the regulation of αvβ3-VEGFR2 cross- talk and the resulting integrin and VEGF-dependent cellular responses underlying angiogenesis [4].
Pre-clinical studies with anti-VEGF agent show that neutralization of tumor cell-derived VEGF can reverse some of the abnormalities responsible for lack of normalization in microvasculature [142]. However, discontinuation of this therapy yields loss of vascular normalization and pronounced vascular regression, demonstrating transient nature of drug induced normalization phenotype and possibly a “normalization window,” which begins with the appearance of normalized vasculature shortly after starting the therapy and ends when the features of normalization are lost [142]. Normalization phenotype may have been lost due to increased anti-angiogenic stimuli leading to extensive vascular regression. However, extensive vascular regression does not correlate with improved response to chemotherapy seen when administered with anti-VEGF therapy, because that would require proper tumor perfusion. Multiple animal model studies indicate that soon after beginning the anti-VEGF treatment, microvessel density is decreased; however, several days after commencing the therapy, tumor perfusion is improved, leading to synergistic effect when used with traditional chemotherapeutics [142]. This paradox may suggest involvement of additional mechanisms and potential for alternative molecular target, such as Src, for vascular normalization. Vascular maturation/normalization demonstrated by experiments on murine model of pancreatic β-cell derived tumors [142, 143] shows evidence of vascular maturation with decrease in endothelial fenestration with tightened associations between endothelial cells and pericyte coverage. Rice et al. in 2012 showed that use of the Src-inhibitor dasatinib on human prostate cancer cells showed reduced induction of angiogenesis in vivo [144].
9. Conclusions and Perspectives
As described earlier, Src inhibition is important in maintaining endothelial cell integrity. In order to promote vascular normalization without potential compensatory mechanisms that may be involved after extended VEGF therapy, use of Src inhibitor Dasatinib, in conjunction with anti-VEGF and/or chemotherapy might help to improve the efficacy of anti-angiogenic cancer therapy. Dasatinib may prove to be helpful by avoiding compensatory mechanisms, as it works more downstream in the signaling pathway than VEGF. By inhibiting Src, activity of VEGF receptor and other RTKs that are involved in reducing vascular normalization also can be modulated. A number of Src inhibitors are being tested clinically as monotherapy and in combination with other chemotherapeutic agents for treatment of CML and other hematologic malignancies, castration resistant prostate cancer, bone metastatic prostate cancer, and metastatic solid tumors refractory to conventional therapies (Table 1). Although pre-clinical and additional clinical studies have yet to determine viability of this notion (Table 2), using Src inhibitors may show promise in cancer therapeutics by inducing vascular normalization, in addition to many potential benefits discussed in this article.
Table 1.
Src inhibitors in clinical trials
| Compound | Cancer clinical trials | Kinase target | Reference(s) |
|---|---|---|---|
| Bosutinib (SKI-606) | Phase I and II in solid tumors; phase III in Hematologic malignancies | Src, Bcr-Abl | [117-120] |
| Saracatinib (AZD0530) | Phase I and II as monotherapy and in combination; multiple solid malignancies | Src, Bcr-Abl | [121] |
| Dasatinib (BMS354825) | Phase I and II as monotherapy and in Combination for prostate cancer; biomarker selected trials; phase III trials in hematologic malignancies | Src, Bcr-Abl, c-Kit, PDGFR, c-FMS, EphA2, SFKs | [122-125] |
| Ponatinib (AP24534) | Phase I in CML/other hematological malignancies | Src, Bcr-Abl | [126, 127] |
| KX2–391 | metastatic solid tumors refractory to conventional therapies; bone metastatic prostate cancer, castration resistant prostate cancer | Selective Src, substrate binding pocket inhibitor | [128, 129] |
| XL-228 | Phase I in advanced solid tumor malignancies/lymphoma and CML | SFKs, IGF-1R, Aurora A, and Bcr-Abl | [130] |
Table 2.
Src inhibitors in pre-clinical trials
| Compound | Kinase target | Type of compound | Reference(s) |
|---|---|---|---|
| DCC-2036 (Rebastinib) | Abl inhibitor that also inhibits two of the SFKs including Lyn and Hck | Proprietary | [131] |
| TG100435/TG100855 | Src inhibitor | 3-aminobenzotriazine compound | [132, 133] |
| AP23464 | ATP-based inhibitor of Src and Abl kinases | purine | [134-136] |
| AP23846 | ATP-based SFK inhibitor | purine | [137] |
| AZM-475271 | Src tyrosine kinase inhibitor | quinazoline | [138] |
| PD180970 | ATP-competitive inhibitor of p210bcr/abl tyrosine kinase | pyridopyrimidine | [139-141] |
Acknowledgements
Research in the laboratory is funded by the National Institutes of Health grant (R01HL103952) to PRS, and in part by the University of Georgia Research Foundation, UGA-College of Pharmacy Dean's Foundation and Departmental Translational Research Initiative grants to PRS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
10. Conflict of Interest
The authors declare that they have no conflict of interest.
Bibliography
- 1.Li S. Src-family kinases in the development and therapy of Philadelphia chromosome- positive chronic myeloid leukemia and acute lymphoblastic leukemia. Leuk Lymphoma. 2008;49(1):19–26. doi: 10.1080/10428190701713689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang S, Yu D. Targeting Src family kinases in anti-cancer therapies: turning promise into triumph. Trends Pharmacol Sci. 2012;33(3):122–8. doi: 10.1016/j.tips.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene. 2000;19(49):5636–42. doi: 10.1038/sj.onc.1203912. [DOI] [PubMed] [Google Scholar]
- 4.Somanath PR, Malinin NL, Byzova TV. Cooperation between integrin alphavbeta3 and VEGFR2 in angiogenesis. Angiogenesis. 2009;12(2):177–85. doi: 10.1007/s10456-009-9141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlessinger J. New roles for Src kinases in control of cell survival and angiogenesis. Cell. 2000;100(3):293–6. doi: 10.1016/s0092-8674(00)80664-9. [DOI] [PubMed] [Google Scholar]
- 6.Williams JC, Wierenga RK, Saraste M. Insights into Src kinase functions: structural comparisons. Trends Biochem Sci. 1998;23(5):179–84. doi: 10.1016/s0968-0004(98)01202-x. [DOI] [PubMed] [Google Scholar]
- 7.Courtneidge SA, Fumagalli S. A mitotic function for Src? Trends Cell Biol. 1994;4(10):345–7. doi: 10.1016/0962-8924(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 8.Soki FN, Park SI, McCauley LK. The multifaceted actions of PTHrP in skeletal metastasis. Future Oncol. 2012;8(7):803–17. doi: 10.2217/fon.12.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider JG, Amend SR, Weilbaecher KN. Integrins and bone metastasis: integrating tumor cell and stromal cell interactions. Bone. 2011;48(1):54–65. doi: 10.1016/j.bone.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zwick E, et al. The EGF receptor as central transducer of heterologous signalling systems. Trends in pharmacological sciences. 1999;20(10):408–412. doi: 10.1016/s0165-6147(99)01373-5. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds AB, Roczniak-Ferguson A. Emerging roles for p120-catenin in cell adhesion and cancer. Oncogene. 2004;23(48):7947–7956. doi: 10.1038/sj.onc.1208161. [DOI] [PubMed] [Google Scholar]
- 12.Eckert MA, et al. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer cell. 2011;19(3):372–386. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balzer EM, et al. c-Src differentially regulates the functions of microtentacles and invadopodia. Oncogene. 2010;29(48):6402–8. doi: 10.1038/onc.2010.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balzer E, et al. c-Src differentially regulates the functions of microtentacles and invadopodia. Oncogene. 2010;29(48):6402–6408. doi: 10.1038/onc.2010.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Migliaccio A, et al. Steroid receptor regulation of epidermal growth factor signaling through Src in breast and prostate cancer cells: steroid antagonist action. Cancer Res. 2005;65(22):10585–93. doi: 10.1158/0008-5472.CAN-05-0912. [DOI] [PubMed] [Google Scholar]
- 16.Castoria G, et al. Targeting androgen receptor/src complex impairs the aggressive phenotype of human fibrosarcoma cells. PLoS One. 2013;8(10):e76899. doi: 10.1371/journal.pone.0076899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. The Journal of clinical investigation. 2009;119(6):1417. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong D, et al. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS One. 2010;5(8):e12445. doi: 10.1371/journal.pone.0012445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15(2):117–34. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95(9):884–91. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- 21.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124(4):619–26. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whiteman EL, et al. The transcription factor snail represses Crumbs3 expression and disrupts apico-basal polarity complexes. Oncogene. 2008;27(27):3875–9. doi: 10.1038/onc.2008.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomita K, et al. Cadherin switching in human prostate cancer progression. Cancer Res. 2000;60(13):3650–4. [PubMed] [Google Scholar]
- 24.Bussemakers MJ, et al. Complex cadherin expression in human prostate cancer cells. Int J Cancer. 2000;85(3):446–50. [PubMed] [Google Scholar]
- 25.Strumane K, Berx G, Van Roy F. Cadherins in cancer. Handb Exp Pharmacol. 2004;(165):69–103. doi: 10.1007/978-3-540-68170-0_4. [DOI] [PubMed] [Google Scholar]
- 26.Thiery JP, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198(1):11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 28.Radisky DC. Epithelial-mesenchymal transition. J Cell Sci. 2005;118(Pt 19):4325–6. doi: 10.1242/jcs.02552. [DOI] [PubMed] [Google Scholar]
- 29.Avizienyte E, Frame MC. Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr Opin Cell Biol. 2005;17(5):542–7. doi: 10.1016/j.ceb.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morel AP, et al. Generation of breast cancer stem cells through epithelial mesenchymal transition. PLoS One. 2008;3(8):e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giannoni E, et al. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 2010;70(17):6945–56. doi: 10.1158/0008-5472.CAN-10-0785. [DOI] [PubMed] [Google Scholar]
- 33.Putzke AP, et al. Metastatic progression of prostate cancer and e-cadherin regulation by zeb1 and SRC family kinases. Am J Pathol. 2011;179(1):400–10. doi: 10.1016/j.ajpath.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernier AJ, et al. Non-cysteine linked MUC1 cytoplasmic dimers are required for Src recruitment and ICAM-1 binding induced cell invasion. Mol Cancer. 2011:93. doi: 10.1186/1476-4598-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan H, et al. Transmembrane-Bound IL-15-Promoted Epithelial-Mesenchymal Transition in Renal Cancer Cells Requires the Src-Dependent Akt/GSK-3beta/beta- Catenin Pathway. Neoplasia. 2015;17(5):410–20. doi: 10.1016/j.neo.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drake JM, et al. Oncogene-specific activation of tyrosine kinase networks during prostate cancer progression. Proc Natl Acad Sci U S A. 2012;109(5):1643–8. doi: 10.1073/pnas.1120985109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tatarov O, et al. SRC family kinase activity is up-regulated in hormone-refractory prostate cancer. Clin Cancer Res. 2009;15(10):3540–9. doi: 10.1158/1078-0432.CCR-08-1857. [DOI] [PubMed] [Google Scholar]
- 38.Cai H, et al. Invasive prostate carcinoma driven by c-Src and androgen receptor synergy. Cancer Res. 2011;71(3):862–72. doi: 10.1158/0008-5472.CAN-10-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai H, et al. Differential transformation capacity of Src family kinases during the initiation of prostate cancer. Proc Natl Acad Sci U S A. 2011;108(16):6579–84. doi: 10.1073/pnas.1103904108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chu PY, et al. Tyrosine phosphorylation of growth factor receptor-bound protein-7 by focal adhesion kinase in the regulation of cell migration, proliferation, and tumorigenesis. J Biol Chem. 2009;284(30):20215–26. doi: 10.1074/jbc.M109.018259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Guelte A, Dwyer J, Gavard J. Jumping the barrier: VE-cadherin, VEGF and other angiogenic modifiers in cancer. Biol Cell. 2011;103(12):593–605. doi: 10.1042/BC20110069. [DOI] [PubMed] [Google Scholar]
- 42.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29(34):4741–51. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagathihalli NS, Merchant NB. Src-mediated regulation of E-cadherin and EMT in pancreatic cancer. Front Biosci (Landmark Ed) 2012;17:2059–69. doi: 10.2741/4037. [DOI] [PubMed] [Google Scholar]
- 44.Liu X, Feng R. Inhibition of epithelial to mesenchymal transition in metastatic breast carcinoma cells by c-Src suppression. Acta Biochim Biophys Sin (Shanghai) 2010;42(7):496–501. doi: 10.1093/abbs/gmq043. [DOI] [PubMed] [Google Scholar]
- 45.Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437(7058):497–504. doi: 10.1038/nature03987. [DOI] [PubMed] [Google Scholar]
- 46.Hu G, Place AT, Minshall RD. Regulation of endothelial permeability by Src kinase signaling: vascular leakage versus transcellular transport of drugs and macromolecules. Chem Biol Interact. 2008;171(2):177–89. doi: 10.1016/j.cbi.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagy JA, et al. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11(2):109–19. doi: 10.1007/s10456-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li S, Seitz R, Lisanti MP. Phosphorylation of caveolin by src tyrosine kinases. The alpha-isoform of caveolin is selectively phosphorylated by v-Src in vivo. J Biol Chem. 1996;271(7):3863–8. [PubMed] [Google Scholar]
- 49.Li S, et al. Mutational analysis of caveolin-induced vesicle formation. Expression of caveolin-1 recruits caveolin-2 to caveolae membranes. FEBS Lett. 1998;434(1-2):127–34. doi: 10.1016/s0014-5793(98)00945-4. [DOI] [PubMed] [Google Scholar]
- 50.Thomas CM, Smart EJ. Caveolae structure and function. J Cell Mol Med. 2008;12(3):796–809. doi: 10.1111/j.1582-4934.2008.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13:119–46. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- 52.Gavard J, Gutkind JS. VE-cadherin and claudin-5: it takes two to tango. Nat Cell Biol. 2008;10(8):883–5. doi: 10.1038/ncb0808-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weis S, et al. Src blockade stabilizes a Flk/cadherin complex, reducing edema and tissue injury following myocardial infarction. J Clin Invest. 2004;113(6):885–94. doi: 10.1172/JCI20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ito N, et al. Identification of vascular endothelial growth factor receptor-1 tyrosine phosphorylation sites and binding of SH2 domain-containing molecules. J Biol Chem. 1998;273(36):23410–8. doi: 10.1074/jbc.273.36.23410. [DOI] [PubMed] [Google Scholar]
- 55.Gumbiner BM. Signal transduction of beta-catenin. Curr Opin Cell Biol. 1995;7(5):634–40. doi: 10.1016/0955-0674(95)80104-9. [DOI] [PubMed] [Google Scholar]
- 56.Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin. Curr Opin Cell Biol. 2005;17(5):459–65. doi: 10.1016/j.ceb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 57.Roura S, et al. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem. 1999;274(51):36734–40. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- 58.Wallez Y, et al. Src kinase phosphorylates vascular endothelial-cadherin in response to vascular endothelial growth factor: identification of tyrosine 685 as the unique target site. Oncogene. 2007;26(7):1067–77. doi: 10.1038/sj.onc.1209855. [DOI] [PubMed] [Google Scholar]
- 59.Cuevas BD, et al. Tyrosine phosphorylation of p85 relieves its inhibitory activity on phosphatidylinositol 3-kinase. J Biol Chem. 2001;276(29):27455–61. doi: 10.1074/jbc.M100556200. [DOI] [PubMed] [Google Scholar]
- 60.Birdsey GM, et al. The endothelial transcription factor ERG promotes vascular stability and growth through Wnt/beta-catenin signaling. Dev Cell. 2015;32(1):82–96. doi: 10.1016/j.devcel.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Orsenigo F, et al. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun. 2012;3:1208. doi: 10.1038/ncomms2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu Y, Wang Y, Tao C. [Comparison of phospholipid in crude and fried semen Dolichos Lablab]. Zhongguo Zhong Yao Za Zhi. 1991;16(9):540–1. 574–5. [PubMed] [Google Scholar]
- 63.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5(4):261–70. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 64.Pyun BJ, et al. Capsiate, a nonpungent capsaicin-like compound, inhibits angiogenesis and vascular permeability via a direct inhibition of Src kinase activity. Cancer Res. 2008;68(1):227–35. doi: 10.1158/0008-5472.CAN-07-2799. [DOI] [PubMed] [Google Scholar]
- 65.Spring K, et al. Tyrosine phosphorylation of DEP-1/CD148 as a mechanism controlling Src kinase activation, endothelial cell permeability, invasion, and capillary formation. Blood. 2012;120(13):2745–56. doi: 10.1182/blood-2011-12-398040. [DOI] [PubMed] [Google Scholar]
- 66.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8(11):1223–34. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 67.Gu L, et al. Stat5 promotes metastatic behavior of human prostate cancer cells in vitro and in vivo. Endocr Relat Cancer. 2010;17(2):481–93. doi: 10.1677/ERC-09-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanchez-Bailon MP, et al. Cyr61 as mediator of Src signaling in triple negative breast cancer cells. Oncotarget. 2015;6(15):13520–38. doi: 10.18632/oncotarget.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shen Q, et al. MUC1 initiates Src-CrkL-Rac1/Cdc42-mediated actin cytoskeletal protrusive motility after ligating intercellular adhesion molecule-1. Mol Cancer Res. 2008;6(4):555–67. doi: 10.1158/1541-7786.MCR-07-2033. [DOI] [PubMed] [Google Scholar]
- 70.Somanath PR, et al. The role of PAK-1 in activation of MAP kinase cascade and oncogenic transformation by Akt. Oncogene. 2009;28(25):2365–9. doi: 10.1038/onc.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martini M, et al. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 2014;46(6):372–83. doi: 10.3109/07853890.2014.912836. [DOI] [PubMed] [Google Scholar]
- 72.Shaw M, Cohen P, Alessi DR. Further evidence that the inhibition of glycogen synthase kinase-3beta by IGF-1 is mediated by PDK1/PKB-induced phosphorylation of Ser-9 and not by dephosphorylation of Tyr-216. FEBS Lett. 1997;416(3):307–11. doi: 10.1016/s0014-5793(97)01235-0. [DOI] [PubMed] [Google Scholar]
- 73.ter Haar E, et al. Structure of GSK3beta reveals a primed phosphorylation mechanism. Nat Struct Biol. 2001;8(7):593–6. doi: 10.1038/89624. [DOI] [PubMed] [Google Scholar]
- 74.Goc A, et al. Targeting Src-mediated Tyr216 phosphorylation and activation of GSK-3 in prostate cancer cells inhibit prostate cancer progression in vitro and in vivo. Oncotarget. 2014;5(3):775–87. doi: 10.18632/oncotarget.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res. 2007;32(4-5):577–95. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kotliarova S, et al. Glycogen synthase kinase-3 inhibition induces glioma cell death through c-MYC, nuclear factor-kappaB, and glucose regulation. Cancer Res. 2008;68(16):6643–51. doi: 10.1158/0008-5472.CAN-08-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mamaghani S, et al. Glycogen synthase kinase-3 inhibition sensitizes pancreatic cancer cells to TRAIL-induced apoptosis. PLoS One. 2012;7(7):e41102. doi: 10.1371/journal.pone.0041102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bang D, et al. GSK-3alpha promotes oncogenic KRAS function in pancreatic cancer via TAK1-TAB stabilization and regulation of noncanonical NF-kappaB. Cancer Discov. 2013;3(6):690–703. doi: 10.1158/2159-8290.CD-12-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mishra R. Glycogen synthase kinase 3 beta: can it be a target for oral cancer. Mol Cancer. 2010:144. doi: 10.1186/1476-4598-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wilson W, 3rd, Baldwin AS. Maintenance of constitutive IkappaB kinase activity by glycogen synthase kinase-3alpha/beta in pancreatic cancer. Cancer Res. 2008;68(19):8156–63. doi: 10.1158/0008-5472.CAN-08-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang J, et al. Overexpression of Rab25 contributes to metastasis of bladder cancer through induction of epithelial-mesenchymal transition and activation of Akt/GSK- 3beta/Snail signaling. Carcinogenesis. 2013;34(10):2401–8. doi: 10.1093/carcin/bgt187. [DOI] [PubMed] [Google Scholar]
- 82.Remsing Rix LL, et al. GSK3 alpha and beta are new functionally relevant targets of tivantinib in lung cancer cells. ACS Chem Biol. 2014;9(2):353–8. doi: 10.1021/cb400660a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao F, Al-Azayzih A, Somanath PR. Discrete functions of GSK3alpha and GSK3beta isoforms in prostate tumor growth and micrometastasis. Oncotarget. 2015;6(8):5947–62. doi: 10.18632/oncotarget.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3(3):207–14. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 85.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 86.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 87.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8(3):221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4(8):617–29. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 89.Wu X, et al. Src promotes cutaneous wound healing by regulating MMP-2 through the ERK pathway. Int J Mol Med. 2016;37(3):639–48. doi: 10.3892/ijmm.2016.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27(31):5287–97. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011;41(2):271–90. doi: 10.1007/s00726-010-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brinckerhoff CE, Rutter JL, Benbow U. Interstitial collagenases as markers of tumor progression. Clin Cancer Res. 2000;6(12):4823–30. [PubMed] [Google Scholar]
- 94.Duffy MJ. Proteases as prognostic markers in cancer. Clin Cancer Res. 1996;2(4):613–8. [PubMed] [Google Scholar]
- 95.Liotta LA, et al. Degradation of basement membrane by murine tumor cells. J Natl Cancer Inst. 1977;58(5):1427–31. doi: 10.1093/jnci/58.5.1427. [DOI] [PubMed] [Google Scholar]
- 96.Liotta LA, et al. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980;284(5751):67–8. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- 97.Salo T, et al. Secretion of basement membrane collagen degrading enzyme and plasminogen activator by transformed cells--role in metastasis. Int J Cancer. 1982;30(5):669–73. doi: 10.1002/ijc.2910300520. [DOI] [PubMed] [Google Scholar]
- 98.Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278(1):16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 99.Chen JM, et al. Isolation and characterization of a 70-kDa metalloprotease (gelatinase) that is elevated in Rous sarcoma virus-transformed chicken embryo fibroblasts. J Biol Chem. 1991;266(8):5113–21. [PubMed] [Google Scholar]
- 100.Noritake H, et al. Overexpression of tissue inhibitor of matrix metalloproteinases-1 (TIMP-1) in metastatic MDCK cells transformed by v-src. Clin Exp Metastasis. 1999;17(2):105–10. doi: 10.1023/a:1006596620406. [DOI] [PubMed] [Google Scholar]
- 101.Sato H, Kita M, Seiki M. v-Src activates the expression of 92-kDa type IV collagenase gene through the AP-1 site and the GT box homologous to retinoblastoma control elements. A mechanism regulating gene expression independent of that by inflammatory cytokines. J Biol Chem. 1993;268(31):23460–8. [PubMed] [Google Scholar]
- 102.Vincenti MP, et al. v-src activation of the collagenase-1 (matrix metalloproteinase-1) promoter through PEA3 and STAT: requirement of extracellular signal-regulated kinases and inhibition by retinoic acid receptors. Mol Carcinog. 1998;21(3):194–204. [PubMed] [Google Scholar]
- 103.Cortes-Reynosa P, et al. Src kinase regulates metalloproteinase-9 secretion induced by type IV collagen in MCF-7 human breast cancer cells. Matrix Biol. 2008;27(3):220–31. doi: 10.1016/j.matbio.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 104.Lee CS, et al. Compound K inhibits MMP-1 expression through suppression of c-Src- dependent ERK activation in TNF-alpha-stimulated dermal fibroblast. Exp Dermatol. 2014;23(11):819–24. doi: 10.1111/exd.12536. [DOI] [PubMed] [Google Scholar]
- 105.Chen YJ, et al. Gallic acid abolishes the EGFR/Src/Akt/Erk-mediated expression of matrix metalloproteinase-9 in MCF-7 breast cancer cells. Chem Biol Interact. 2016;252:131–40. doi: 10.1016/j.cbi.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 106.Sun X, et al. Abl interactor 1 regulates Src-Id1-matrix metalloproteinase 9 axis and is required for invadopodia formation, extracellular matrix degradation and tumor growth of human breast cancer cells. Carcinogenesis. 2009;30(12):2109–16. doi: 10.1093/carcin/bgp251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hsia DA, et al. Differential regulation of cell motility and invasion by FAK. J Cell Biol. 2003;160(5):753–67. doi: 10.1083/jcb.200212114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vincenti MP, et al. src-related tyrosine kinases regulate transcriptional activation of the interstitial collagenase gene, MMP-1, in interleukin-1-stimulated synovial fibroblasts. Arthritis Rheum. 1996;39(4):574–82. doi: 10.1002/art.1780390406. [DOI] [PubMed] [Google Scholar]
- 109.Lee CW, et al. TNF-alpha induces MMP-9 expression via activation of Src/EGFR, PDGFR/PI3K/Akt cascade and promotion of NF-kappaB/p300 binding in human tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292(3):L799–812. doi: 10.1152/ajplung.00311.2006. [DOI] [PubMed] [Google Scholar]
- 110.Kuo L, et al. Src oncogene activates MMP-2 expression via the ERK/Sp1 pathway. J Cell Physiol. 2006;207(3):729–34. doi: 10.1002/jcp.20616. [DOI] [PubMed] [Google Scholar]
- 111.Hansen RK, et al. Hsp27-induced MMP-9 expression is influenced by the Src tyrosine protein kinase yes. Biochem Biophys Res Commun. 2001;282(1):186–93. doi: 10.1006/bbrc.2001.4548. [DOI] [PubMed] [Google Scholar]
- 112.Mantovani A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 113.Feng W, et al. The angiogenic response is dictated by beta3 integrin on bone marrow- derived cells. J Cell Biol. 2008;183(6):1145–57. doi: 10.1083/jcb.200802179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hinz B, et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180(4):1340–55. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tomasek JJ, et al. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 116.Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990;63(1):21–9. [PubMed] [Google Scholar]
- 117.Mori N, et al. Role of multipotent fibroblasts in the healing colonic mucosa of rabbits. Ultrastructural and immunocytochemical study. Histol Histopathol. 1992;7(4):583–90. [PubMed] [Google Scholar]
- 118.Powell DW. Myofibroblasts: paracrine cells important in health and disease. Trans Am Clin Climatol Assoc. 2000;111:271–92. discussion 292-3. [PMC free article] [PubMed] [Google Scholar]
- 119.Birchmeier C, Birchmeier W. Molecular aspects of mesenchymal-epithelial interactions. Annu Rev Cell Biol. 1993;9:511–40. doi: 10.1146/annurev.cb.09.110193.002455. [DOI] [PubMed] [Google Scholar]
- 120.Gauldie J, et al. Transforming growth factor-beta gene transfer to the lung induces myofibroblast presence and pulmonary fibrosis. Curr Top Pathol. 1999;93:35–45. doi: 10.1007/978-3-642-58456-5_5. [DOI] [PubMed] [Google Scholar]
- 121.Pujuguet P, et al. Abnormal basement membrane in tumors induced by rat colon cancer cells. Gastroenterology. 1994;107(3):701–11. doi: 10.1016/0016-5085(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 122.Razzaque MS, Taguchi T. Pulmonary fibrosis: cellular and molecular events. Pathol Int. 2003;53(3):133–45. doi: 10.1046/j.1440-1827.2003.01446.x. [DOI] [PubMed] [Google Scholar]
- 123.Travers JG, et al. Cardiac Fibrosis: The Fibroblast Awakens. Circ Res. 2016;118(6):1021–40. doi: 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Martin M, Pujuguet P, Martin F. Role of stromal myofibroblasts infiltrating colon cancer in tumor invasion. Pathol Res Pract. 1996;192(7):712–7. doi: 10.1016/S0344-0338(96)80093-8. [DOI] [PubMed] [Google Scholar]
- 125.Hinz B, Gabbiani G. Fibrosis: recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol Rep. 2010;2:78. doi: 10.3410/B2-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hu M, et al. Therapeutic targeting of SRC kinase in myofibroblast differentiation and pulmonary fibrosis. J Pharmacol Exp Ther. 2014;351(1):87–95. doi: 10.1124/jpet.114.216044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Okutani D, et al. Src protein tyrosine kinase family and acute inflammatory responses. Am J Physiol Lung Cell Mol Physiol. 2006;291(2):L129–41. doi: 10.1152/ajplung.00261.2005. [DOI] [PubMed] [Google Scholar]
- 128.Schlaepfer DD, Hunter T. Focal adhesion kinase overexpression enhances ras- dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J Biol Chem. 1997;272(20):13189–95. doi: 10.1074/jbc.272.20.13189. [DOI] [PubMed] [Google Scholar]
- 129.Vittal R, et al. Modulation of prosurvival signaling in fibroblasts by a protein kinase inhibitor protects against fibrotic tissue injury. Am J Pathol. 2005;166(2):367–75. doi: 10.1016/S0002-9440(10)62260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Skhirtladze C, et al. Src kinases in systemic sclerosis: central roles in fibroblast activation and in skin fibrosis. Arthritis Rheum. 2008;58(5):1475–84. doi: 10.1002/art.23436. [DOI] [PubMed] [Google Scholar]
- 131.Abdalla M, et al. Dasatinib inhibits TGFbeta-induced myofibroblast differentiation through Src-SRF Pathway. Eur J Pharmacol. 2015;769:134–42. doi: 10.1016/j.ejphar.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Goel S, Wong AH, Jain RK. Vascular normalization as a therapeutic strategy for malignant and nonmalignant disease. Cold Spring Harb Perspect Med. 2012;2(3):a006486. doi: 10.1101/cshperspect.a006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10(8):789–99. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 134.Ferrara N. VEGF as a therapeutic target in cancer. Oncology. 2005;69(Suppl 3):11–6. doi: 10.1159/000088479. [DOI] [PubMed] [Google Scholar]
- 135.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Niu G, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21(13):2000–8. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 137.Leifheit-Nestler M, et al. Overexpression of integrin beta 5 enhances the paracrine properties of circulating angiogenic cells via Src kinase-mediated activation of STAT3. Arterioscler Thromb Vasc Biol. 2010;30(7):1398–406. doi: 10.1161/ATVBAHA.110.206086. [DOI] [PubMed] [Google Scholar]
- 138.Schindler C, Darnell JE., Jr. Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem. 1995;64:621–51. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- 139.Wang SC, et al. Targeting HER2: recent developments and future directions for breast cancer patients. Semin Oncol. 2001;28(6 Suppl 18):21–9. doi: 10.1053/sonc.2001.29724. [DOI] [PubMed] [Google Scholar]
- 140.Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21(1):11–9. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Soldi R, et al. Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 1999;18(4):882–92. doi: 10.1093/emboj/18.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Goel S, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91(3):1071–121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Inai T, et al. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol. 2004;165(1):35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rice L, et al. Impact of the SRC inhibitor dasatinib on the metastatic phenotype of human prostate cancer cells. Clin Exp Metastasis. 2012;29(2):133–42. doi: 10.1007/s10585-011-9436-2. [DOI] [PubMed] [Google Scholar]