Abstract
Background
Neuroimaging studies of patients with major depression have revealed abnormal intrinsic functional connectivity measured during the resting state in multiple, distributed networks. However, it is unclear whether these findings reflect the state of major depression or reflect trait neurobiological underpinnings of risk for major depression.
Methods
We compared resting-state functional connectivity, measured with functional magnetic resonance imaging (fMRI), between unaffected children of parents who had documented histories of major depression (at-risk, n = 27; 8–14 years of age) and age-matched children of parents with no lifetime history of depression (controls, n = 16).
Results
At-risk children exhibited hyperconnectivity between the default mode network (DMN) and subgenual anterior cingulate cortex (sgACC) / orbital frontal cortex (OFC), and the magnitude of connectivity positively correlated with individual symptom scores. At-risk children also exhibited (1) hypoconnectivity within the cognitive control network, which also lacked the typical anticorrelation with the DMN; (2) hypoconnectivity between left dorsolateral prefrontal cortex (DLPFC) and sgACC; and (3) hyperconnectivity between the right amygdala and right inferior frontal gyrus, a key region for top-down modulation of emotion. Classification between at-risk children and controls based on resting-state connectivity yielded high accuracy with high sensitivity and specificity that was superior to clinical rating scales.
Conclusions
Children at familial risk for depression exhibited atypical functional connectivity in the default-mode, cognitive-control, and affective networks. Such task-independent functional brain measures of risk for depression in children could be used to promote early intervention to reduce the likelihood of developing depression.
Keywords: resting-state fMRI, depression, default network, subgenual ACC, children, familial risk
Introduction
Neuroimaging in patients with major depression (MDD) has revealed abnormal activation patterns in multiple brain networks, including the default mode (DMN), cognitive control, and affective networks. The DMN, anchored in the medial prefrontal cortex (MPFC) and posterior cingulate cortex (PCC), is suppressed in healthy adults during tasks that demand external attention, but does not show the typical pattern of task-induced deactivation in adults and adolescents with MDD (1–3). The cognitive control network, including dorsal lateral prefrontal cortex (DLPFC), which is typically activated during cognitively demanding tasks, has shown decreased activations in adults with MDD (4, 5). The affective network includes the amygdala and other limbic-region structures (6, 7), and most saliently for MDD, the subgenual anterior cingulate cortex (sgACC), which is considered a core region in the functional and structural pathophysiology of MDD (8–10). The affective network exhibits abnormal activation patterns during emotion processing in adults with MDD (11–13). These abnormal activations in distributed networks may account for cortico-limbic dysregulation in MDD (8,14).
Mirroring these brain activation abnormalities, patients of different ages with MDD have shown abnormal intrinsic functional connectivity of the brain measured via resting-state fMRI (rs-fMRI) (15). First, increased resting-state connectivity within the DMN and between the DMN and sgACC has been reported in adults (16,17) and adolescents (18) with MDD. Hyperconnectivity of sgACC correlated with duration of current depressive episodes in adults (16) and with emotional dysregulation in pediatric depression (19). These results support the possibility that DMN-sgACC hyperconnectivity might underlie depressive rumination (20). Second, several studies reported decreased resting-state connectivity within the cognitive control network in adult patients with MDD (21–23). In line with this evidence, MDD has been conceptualized as an imbalance between the DMN and the cognitive control network (24–26). Third, atypical connectivity between the amygdala and cortical structures has been found in adults (27,28) and children (29) with MDD and is thought to reflect deficits in emotion regulation.
Despite evidence of abnormal functional connectivity across distributed brain networks in patients with MDD, it is unclear whether these differences reflect the state of current depression versus neurobiological traits that predispose individuals to be at risk for MDD. One approach to distinguishing between current state and predisposing traits is the study of unaffected individuals at heightened risk for MDD, such as unaffected children at familial risk for MDD by virtue of having a parent with MDD. Such familial history increases the risk of MDD in offspring by three- to five fold (30), and increases the risk of a broader spectrum of mood and anxiety disorders (31). Understanding whether rs-fMRI findings represent trait or state markers of MDD in the young can lead to the identification of informative neural biomarkers of risk for mood and anxiety disorders and help develop early intervention strategies to mitigate this risk. Rs-fMRI also possesses significant translational strengths in its short duration of scanning, and the lack of task performance demands that can complicate interpretation of activations.
In the present study, we examined rs-fMRI in unaffected children at familial risk for MDD and other mood and anxiety disorders by virtue of being offspring of parents with MDD (at-risk group) and compared them with age-matched children who were offspring of parents with no lifetime history of any mood disorder (control group). Two previous studies examining at-risk children and adolescents found decreased connectivity between amygdala and frontal-parietal network in unaffected children of depressed mothers and in children with early onset depression (29), and decreased connectivity within the frontal-parietal cognitive control network in unaffected adolescent girls with parental depression (32).
Based on previous functional connectivity results in patients with MDD, we focused on functional connectivity differences between at-risk and control children in the DMN, the cognitive control network, and the affective network, using a seed-based functional connectivity approach. We examined connectivity differences from the two midline anchor regions of the DMN (MPFC and PCC), which are associated with self-referential processing (33) and self-focused rumination in MDD (20,34), and from seed regions in left and right DLPFC and amygdala. We tested: 1) whether unaffected at-risk children exhibit patterns of abnormal functional connectivity similar to those reported in patients with MDD, and 2) whether connectivity of DMN-sgACC is related to symptom scores in at-risk children. To further test whether resting-state connectivity can be a useful neural biomarker for risk for MDD, we built classification models based on resting-state data to discriminate at-risk versus control children.
Methods and Materials
Participants
We initially recruited 38 offspring ages 8–14 years of parents with lifetime history of MDD (at-risk group) and 30 age-matched offspring of parents with no lifetime mood disorder (control group). The study was approved by the Institutional Review Boards at the Massachusetts General Hospital and at the Massachusetts Institute of Technology. Parents provided written informed consent for their and their child’s participation, and youths provided written assent. Exclusion criteria included the presence of acute psychosis or suicidality in a parent or a child; the presence at any point in the lifespan of bipolar disorder in the parent, autism in the child, or a lifetime history of a traumatic brain injury or neurological disorder in the child.
The final sample included in the analyses consisted of 27 at-risk and 16 control participants with no prior history of depression or current clinical-range symptom scores. Participants who did not complete the scan, had excessive head movement during the scan, or had a history of depression or clinical range symptom scores were excluded. See Supplementary Information for details.
Diagnostic Assessment
At enrollment for the present study, each child and both parents in each family were assessed for current and lifetime mood disorders (MDD, bipolar disorder, and dysthymia), using structured clinical interviews in which the mother was the informant. Interviews about parents used the depression, mania, dysthymia modules, and psychosis modules from the Structured Interview for DSM-IV (35) and those about the child used the depression, mania, dysthymia, and psychosis modules from the Schedule of Affective Disorders and Schizophrenia for School-Aged Children–Epidemiological Version (KSADS-E) for DSM-IV (36).
Other Assessments
Cognitive Function
To compare cognitive function between groups, we used the Kaufman Brief Intelligence Test-2 (KBIT-2), a 20-minute screen for verbal and nonverbal cognitive functioning (37).
Current Symptoms, Parent Report
To assess current behavioral and emotional symptoms in the children, we asked mothers to complete the Child Behavior Checklist (CBCL) (38) (see Supplementary Information for details) about all children. The CBCL includes a total problems score, as well as scores reflecting internalizing (affective and anxiety) and externalizing symptoms (attentional problems and disruptive behavior). T-scores of 70 and above have been shown to discriminate clinical-range from non-clinical range children (38).
Current Symptoms, Self-Report
To assess current depressive symptoms by self-report, we administered the Child Depression Inventory (CDI) (39) to all children. See Supplementary Information for details of the CDI.
Participant Demographics (Table 1)
Table 1.
Participant demographic and clinical information. Mean ± SD where appropriate. F, female; M, male; CBCL, Child Behavior Checklist; CDI, total score on the Child Depression Inventory; t(df), between-group t-statistic and degrees of freedom; p, between-group test p value.
| Control (N = 16) | At-risk (N = 27) | Statistical Evaluation | |
|---|---|---|---|
| Age | 11.3 ± 2.14 | 11.2 ± 1.67 | t(41) = .17, p =.86 |
| Gender | 8 F, 8 M | 13 F, 14 M | χ2 =. 14, p = .9 |
| 4.44 | |||
| < .001 | |||
| IQ (KBIT) | 117 ± 10.5 | 120.6 ± 12.0 | t(41)=.99, p = .33 |
| 40 | |||
| 4.47 | |||
| < .001 | |||
| Mother affected | 0 | 18 | |
| 40 | |||
| 4.50 | |||
| < .001 | |||
| Father affected | 0 | 14 | |
| Both parents affected | 0 | 5 | |
| CBCL total | 41.0 ± 11.8 | 48.8 ± 10.0 | t(35) = 2.07, p = .046 |
| CBCL internalizing | 44.3 ± 8.50 | 50.1 ± 9.83 | t(35) = 1.71, p = .096 |
| CBCL externalizing | 45.1 ± 10.5 | 47.8 ± 9.30 | t(35) = 0.96, p = .34 |
| CBCL anxiety | 51.5 ± 2.78 | 55.2 ± 6.56 | t(35) = 1.79, p = .08 |
| CDI | 4.33 ± 5.54 | 6.57 ± 4.64 | t(35) = 1.16, p=.26 |
Children in the at-risk and control groups did not differ significantly in age, gender distribution, or IQ (ps > .3). The at-risk group had marginally higher CBCL total (p = .05), internalizing (p = .096), and anxiety scores (p= .08), but did not differ significantly in CBCL external problem scores (p= .34). None of the children had clinical-range CBCL scores (> 70). CDI total scores did not differ significantly between the two groups (p = .26). Additionally, by parent report, the children were largely pre-pubertal (with the exceptions of 4 at-risk and 3 control children).
Imaging Procedure
Data were acquired on a 3T TrioTim Siemens scanner using a 32-channel head coil. T1-weighted whole brain anatomical images (MPRAGE sequence, 256x256 voxels, 1x1.3-mm in-plane resolution, 1.3-mm slice thickness) were acquired. After the anatomical scan, participants underwent a resting fMRI scan in which participants were instructed to keep their eyes open and the screen was blanked. Resting scan images were obtained in 67 2-mm thick transverse slices, covering the entire brain (interleaved EPI sequence, T2*-weighted images; repetition time = 6 s, echo time = 30 ms, flip angle = 90, 2x2x2 mm voxels). The resting scan lasted 6.2 min (62 volumes). Online prospective acquisition correction (PACE) was applied to the EPI sequence (40) (see Supplementary Information). Two dummy scans were included at the start of the sequence.
Functional connectivity analysis
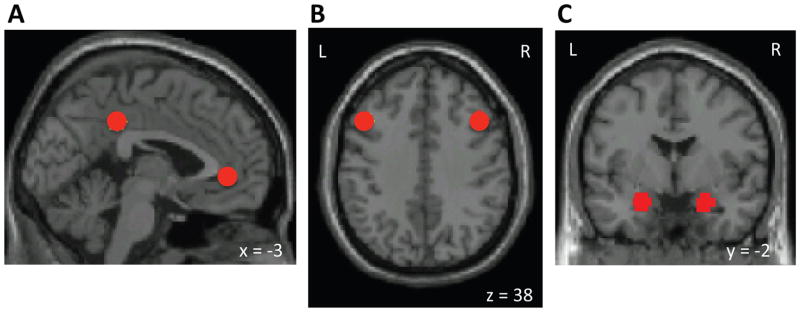
Rs-fMRI data were first preprocessed in SPM8, using standard spatial preprocessing steps. Images were slice-time corrected, realigned to the first image of the resting scan, resampled such that they matched the first image of the resting scan voxel-for-voxel, normalized in MNI space, and smoothed with a 6-mm kernel (full width at half maximum). Functional connectivity analysis was performed using a seed-driven approach with in-house, custom software “CONN” (41,42). We performed seed-voxel correlations by estimating maps showing temporal correlations between the BOLD signal from our a priori regions of interest and that at every brain voxel. We performed resting-state connectivity analysis from the DMN seeds (MPFC, PCC), cognitive control network seeds (bilateral DLPFC), and bilateral amygdala seeds (Figure 1). The DMN and DLPFC seeds were defined as 6-mm spheres around peak coordinates from (43). The amygdala seeds were defined from the WFU Pick Atlas (44).
Figure 1.
Seeds (regions of interest) used in the study. A) Default network (DMN) seeds (posterior cingulate cortex and medial prefrontal cortex), B) left and right dorsolateral prefrontal cortex (DLPFC) seeds, C) left and right amygdala seeds. L, left hemisphere. R, right hemisphere. Images are presented in neurological convention in all figures (left side of the brain is on the left side of the image).
Physiological and other spurious sources of noise were estimated and regressed out using the anatomical CompCor method (aCompCor) (45). Global signal regression (GSR), a widely used preprocessing method was not used because it artificially creates negative correlations which prevents the interpretation of anticorrelation (46) and can contribute to spurious group differences in positive correlations (47). Instead, aCompCor allows for interpretation of anticorrelations and yields higher specificity and sensitivity compared to GSR (41). See Supplementary Information for details on the aCompCor. A temporal band-pass filter of 0.008 Hz to 0.083 Hz was applied simultaneously to all regressors in the model. Residual head motion parameters (3 rotation and 3 translation parameters, plus another 6 parameters representing their first-order temporal derivatives) were regressed out. Artifact/outlier scans (average intensity deviated more than 3 SD from the mean intensity in the session or composite head movement exceeded 1mm from the previous image) were also regressed out. Head displacement across the resting scan did not differ significantly between the two groups for either frame-to-frame translations in x, y, z directions (at-risk: mean = .19 mm ± .11; control: mean = .16mm ± .11; p = .33) or frame-to-frame rotations (at-risk: mean = .0044 ± .002; control: mean = .004 ± .003; p = .66). The number of outliers also did not differ significantly between the groups (range: 0 to 9; at-risk: mean = 2.7 ± 2.2; control: mean = 2.1 ± 3.1; p = .47). Outlier images were modeled as nuisance covariates. Each outlier image was represented by a single regressor in the GLM, with a 1 for the outlier time point and 0s elsewhere.
Time series of all the voxels within each seed were averaged, and first-level correlation maps were produced by extracting the residual BOLD time course from each seed and computing Pearson’s correlation coefficients between that time course and the time course of all other voxels. Correlation coefficients were converted to normally distributed z-scores using the Fisher transformation to allow for second-level General Linear Model analyses. DMN connectivity was calculated from the averages of the time series from MPFC and PCC seeds (48,49), given their similar connectivity patterns. Functional connectivity of left and right DLPFC were analyzed separately, as were left and right amygdala due to evidence of differential roles in emotion processing (50). First-level connectivity maps for each participant were entered into a between-group t-test to determine connectivity differences for each seed between groups. Clusters-level threshold was set at p < .05 using false discovery rate (FDR) correction for multiple comparisons (51), with voxel-wise t-value threshold of 2.42 (df = 41; p < .01). Bonferroni correction was applied to the FDR-corrected cluster-level p-values to correct for multiple comparisons of the five a priori seeds tested (DMN, left and right DLPFC, and left and right amygdala). Regions that showed significant connectivity differences between groups were further examined for their connectivity values (significantly above or below zero) using one sample t-tests in each group. Based on prior evidence of DMN-sgACC hyperconnectivity in MDD and its implication in depressive rumination (20), we examined the within group correlations between DMN-sgACC connectivity values and CBCL scores. Given the higher CBCL total score in the at-risk group, we re-tested group differences by including CBCL total scores as a covariate.
Classification models of at-risk children and controls discrimination
We trained two linear classification models using logistic regression, implemented in machine learning software Weka (52), in order to categorize individual participants to the at-risk or control groups based on their rs-fMRI or behavioral data. To create robust prediction models that can be generalized to new cases, we performed leave-one-out cross-validation so that each individual was classified on the basis of data from the other individuals. Specifically, data from all participants except one were used as the training set to build a classification model, and the remaining participant was classified with the model and used as the validation case. This procedure was iterated for each participant and used to estimate specificity/sensitivity from the out-of-sample predictions. In the first model, we used anatomically defined regions-of-interest (ROIs) that were independent from the regions that showed between-group connectivity differences. Connectivity values between the five a priori seeds and 116 clusters defined by the AAL atlas (53) were estimated and used in the prediction model. We constructed a second model based on CBCL scores (total, internalizing, externalizing, anxiety), to compare with classification accuracies from the model based on rs-fMRI data in anatomically defined ROIs.
Results
Increased connectivity between DMN and sgACC/OFC in at-risk children
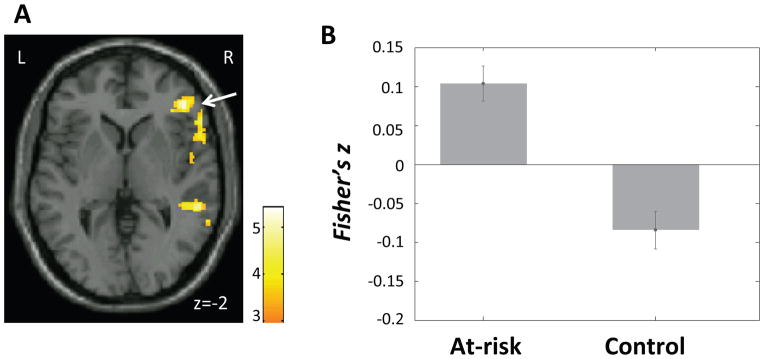
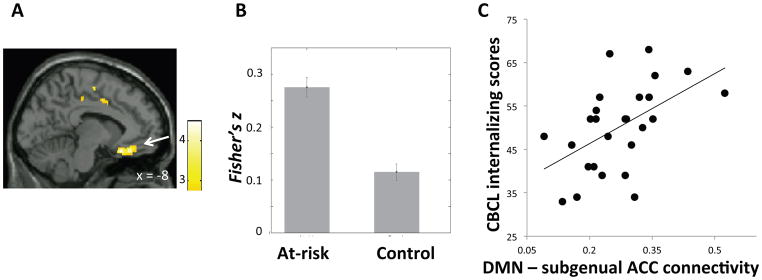
Compared to the control group, the at-risk group exhibited increased positive DMN connectivity with a cluster in the sgACC extending into medial orbital frontal cortex (OFC) bilaterally (Figure 2A–B; Table 2). Among the at-risk children, connectivity between the DMN and sgACC/OFC correlated significantly and positively with CBCL internalizing scores (at-risk: r = .53, p = .003, Figure 2C) and CBCL total scores (at-risk: r = .39, p = .04); there was no such correlation among the control children. Connectivity strengths within the DMN did not differ significantly between groups.
Figure 2.
A) Region in subgenual anterior cingulate cortex (ACC)/orbital frontal cortex (OFC) (white arrow) that exhibited higher connectivity with the default mode network (DMN) in the at-risk than the control group. Color bar represents t-values from between-group t-test (at-risk > control). B) Mean DMN-sgACC/OFC connectivity (Fisher’s z) in each group. Error bars represent standard errors of the means. C) DMN-sgACC/OFC connectivity plotted against CBCL internalizing scores within the at-risk group.
Table 2.
Between-group connectivity differences from default mode network (DMN), right dorsolateral prefrontal cortex (DLPFC), left DLPFC, and right amygdala. BA, Brodmann area; k, cluster size in mm3. Peak coordinates (x y z) based on MNI (Montreal Neurologic Institute) brain. t, peak t value from the cluster (degrees of freedom = 41); p-value, FDR-corrected cluster-level p value; sgACC, subgenual anterior cingulate cortex; OFC, orbital prefrontal cortex; SMG, supramarginal gyrus; STG, superior temporal gyrus; All reported clusters survived Bonferroni correction of p < .05 for the number of seeds tested (five).
| BA | k(mm3) | x, y, z | t | p-value | |
|---|---|---|---|---|---|
| DMN connectivity | |||||
| At-risk > Control | |||||
| L sgACC/OFC | 25/11 | 2544 | −8, 22, −20 | 4.44 | < .001 |
| R supramarginal gyrus | 40 | 2152 | 64, −40, 26 | 4.47 | < .001 |
| R mid cingulum | 24/31 | 2808 | 18, −34, 38 | 4.50 | < .001 |
| Control > At-risk | |||||
| None | |||||
| R DLPFC connectivity | |||||
| At-risk > Control | |||||
| None | |||||
| Control > At-risk | |||||
| R DLPFC | 46/9 | 2920 | 42, 28, 22 | 4.57 | < .001 |
| R inferior parietal lobule | 40 | 1424 | 46, −50, 58 | 3.89 | .01 |
| L DLPFC connectivity | |||||
| At-risk > Control | |||||
| Medial frontal gyrus | 6/24 | 2072 | 0, −2, 48 | 3.66 | .005 |
| Control > At-risk | |||||
| R sgACC | 25/11 | 2480 | 10, 18, −18 | 4.62 | < .001 |
| L inferior parietal lobule | 40 | 2248 | −50, −56, 54 | 4.93 | .003 |
| L lingual gyrus | 18 | 2760 | −14, −82, −14 | 5.77 | < .001 |
| R lingual gyrus | 18 | 1976 | 32, −70, −14 | 4.24 | < .001 |
| R superior frontal gyrus | 8/6 | 8616 | 14, 34, 60 | 5.25 | < .001 |
| R inferior temporal gyrus | 21 | 8392 | 60, −14, −20 | 6.88 | < .001 |
| L inferior temporal gyrus | 21 | 3120 | −60, −14, −20 | 5.00 | < .001 |
| R Amygdala connectivity | |||||
| At-risk > Control | |||||
| R Inferior frontal gyrus | 47 | 2608 | 44, 40, 4 | 4.41 | < .001 |
| R SMG/STG | 40/22 | 1456 | 42, −40, 16 | 3.94 | < .001 |
| Control > At-risk | |||||
| None | |||||
Decreased anticorrelation between DMN and inferior parietal lobule in at-risk children
Compared to the control group, the at-risk group exhibited higher positive connectivity between the DMN and the right inferior parietal lobule (IPL) (Figure 3; Table 2). Instead of the anticorrelation exhibited in the control group (t(15) = −5.99, p = .004), the at-risk group exhibited a positive correlation between the DMN and the right IPL (t(29) = 2.25, p = .03).
Figure 3.
A) Region in the right inferior parietal lobule that exhibited higher connectivity with the default mode network (DMN) in the at-risk than the control group. Color bar represents t-values from between-group t-test (at-risk > control). B) Mean connectivity between DMN and the inferior parietal lobule cluster shown in A) in each group. Error bars represent standard errors of the means.
Decreased connectivity within cognitive control network in at-risk children
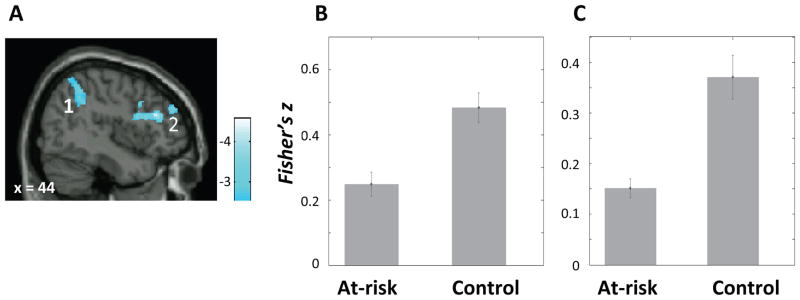
Compared to the control group, the at-risk group exhibited decreased positive connectivity between the right DLPFC seed and the right frontal-parietal control network regions including the right IPL and the right DLPFC (BA46) (Figure 4; Table 2), and decreased connectivity between left DLPFC seed and the left IPL (Table 2).
Figure 4.
A) Regions that exhibited lower connectivity with right dorsolateral prefrontal cortex (DLPFC) seed in the at-risk than the control group; (1) right inferior parietal lobule; (2) right DLPFC. Color bar represents t-values from between-group t-test (control > at-risk). B) Mean connectivity (Fisher’s z) between the right DLPFC seed and the right inferior parietal lobule cluster (1) in each group. C) Mean connectivity (Fisher’s z) between the right DLPFC seed and a cluster in the right DLPFC (2) in each group. Error bars represent standard errors of the means.
Decreased connectivity between L DLPFC and sgACC in at-risk children
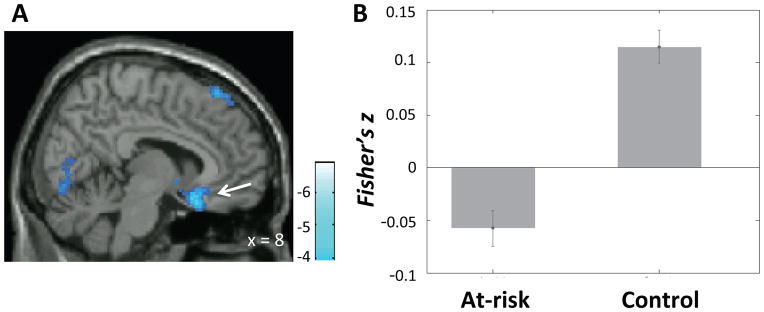
Compared to the control group, the at-risk group exhibited decreased connectivity between the left DLPFC seed and sgACC (bilateral), right lingual gyrus, right superior frontal gyrus, and bilateral inferior temple gyri, and increased connectivity between left DLPFC and supplementary motor cortex (Table 2). Left DLPFC and sgACC were anticorrelated in at-risk children only (t(29) = −3.36, p=.002; Figure 5).
Figure 5.
A) Region in the subgenual anterior cingulate cortex (sgACC) (white arrow) that exhibited lower connectivity with left dorsolateral prefrontal cortex (DLPFC) seed in the at-risk than the control group. Color bar represents t-values from between-group t-test (control > at-risk). B) Mean connectivity (Fisher’s z) between the left DLPFC seed and the sgACC cluster shown in A) in each group. Error bars represent standard errors of the means.
Increased connectivity between amygdala and inferior frontal gyrus (IFG) in at-risk children
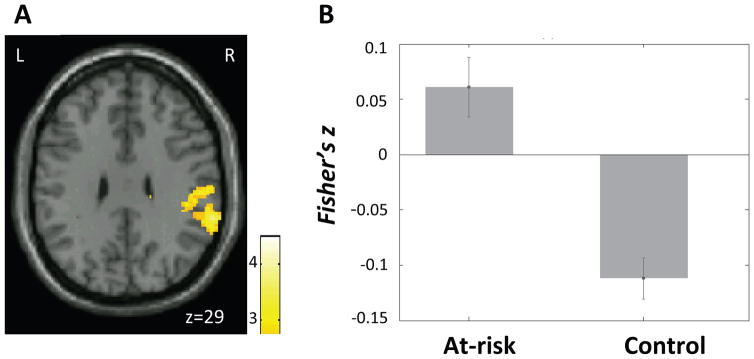
Compared to the control group, the at-risk group exhibited increased connectivity between the right amygdala and both the right IFG and the right supramarginal gyrus (SMG) (Figure 6; Table 2). Instead of the of the negative correlations exhibited in the control group, the at-risk group exhibited positive correlations between right amygdala and right IFG (controls: t(15) = −3.54, p = .003; at-risk: t(29) = 4.67, p < .001), and between right amygdala and right SMG (controls: t(15) = −2.53, p = .02 at-risk: t(29) = 4.53, p < .001). Connectivity from the left amygdala did not differ between the two groups.
Figure 6.
A) Region in the right inferior frontal gyrus (IFG) (white arrow) that exhibited higher connectivity with right amygdala seed in the at-risk than the control group. Color bar represents t-values from between-group t-test (at-risk > control). B) Mean connectivity (Fisher’s z) between the right amgydala seed and the right IFG cluster shown in A) in each group. Error bars represent standard errors of the means.
Group differences after controlling for symptom scores
After controlling for CBCL total scores, differences between the at-risk and control groups remained largely similar to the above reported results (Table SI).
Classification of at-risk children and controls
The classification model based on connectivity data in ROIs defined from the AAL atlas yielded 79% accuracy, 81% sensitivity, and 78% specificity. The model based on CBCL scores yielded only 64% accuracy with 80% sensitivity, and 27% specificity.
Discussion
We found differential intrinsic functional connectivity patterns in unaffected children with familial risk for MDD compared to children without such familial risk in the DMN, the cognitive control network, and the amygdala. At-risk children showed hyperconnectivity between the DMN and the sgACC/OF. Furthermore, although none of the at-risk children was clinically depressed, DMN-sgACC/OFC connectivity was positively correlated with individual CBCL scores among those children. At-risk children also showed hypoconnectivity within the cognitive control network, lacked the typical anticorrelation between the DMN and the right parietal region, and exhibited lower connectivity between left DLPFC and sgACC. In addition, at-risk children showed hyperconnectivity between amygdala and the right IFG. Finally, classification between at-risk children and controls based on resting-state connectivity yielded high sensitivity and specificity. These findings appear to identify trait neurobiological underpinnings of risk for major depression in the absence of the state of depression.
Increased connectivity between DMN and sgACC in at-risk children, and the positive correlation between DMN-sgACC connectivity and current symptom scores, are consistent with findings reported in adult (16,17) and pediatric (19) patients with MDD. The fact that these findings were observed in unaffected children at familial risk for MDD suggests that hyperconnectivity with sgACC is not a consequence or manifestation of MDD, but instead may be a biomarker of predisposed risk for MDD. The at-risk children also exhibited an atypical anticorrelation between sgACC and left DLPFC. In line with our finding, stimulation of the sgACC resulted in attenuation of hyperactivation in sgACC and increased activation in previously underactive DLPFC in adults with MDD (54). The left DLPFC region that showed maximum anticorrelation with the sgACC has been identified as a target for TMS treatment of MDD (55). A prospective study would be needed to determine if atypical sgACC connectivity at this age predicts later development of MDD.
The lack of typical anticorrelation between the DMN and supramarginal gyrus / inferior parietal lobule, an important attention control region (56,57), in at-risk children is consistent with cognitive control deficits in depressed adult patients (58,59) and reduced DMN deactivation during an emotional identification task in depressed adolescents (3). Greater anticorrelation between DMN and cognitive control networks in healthy adults has been linked to better performance in cognitive control and working memory tasks (60,61) and may reflect an individual’s capacity to switch between internally and externally focused attention (62). This dynamic interplay between DMN and cognitive control networks in MDD was examined in a task-based connectivity study. During an external attention condition, adults with MDD exhibited increased DMN connectivity and decreased cognitive control network connectivity (25). The present study suggests that an imbalance between DMN and cognitive-control networks is a developmental risk factor for MDD.
With regards to decreased connectivity within the cognitive control regions in at-risk children, a previous study of adolescents with familial risk for depression also reported reduced connectivity between cognitive control regions (32). In that study, lower connectivity in the control network was associated with more severe parental depression symptoms. These results in at-risk children and adolescents are consistent with findings from depressed adults of reduced connectivity in attention control regions including the DLPFC (23). Studies consistently show that the DLPFC is under-activated in depressed adults (63), which might contribute to their difficulty in cognitive control and emotion regulation (64). It is possible that children at-risk for depression have an under-connected control network that is also a developmental risk factor for MDD.
There was increased connectivity between the right amygdala and the right IFG and supramarginal gyrus in at-risk children. The right IFG is a key region in emotion regulation (65). The top-down IFG-amygdala circuitry is disrupted during emotion regulation in adults with mood disorders (66,67). A study of children with MDD and children of mothers with MDD also reported reduced negative correlation between the amygdala and lateral parietal regions including the supramarginal gyrus (29). The atypically high level of connectivity between amygdala and emotion regulation and cognitive-control regions might reflect emotion dysregulation in MDD.
To test whether intrinsic functional organization of the brain, as measured by rs-fMRI, can be a potential biomarker for risk for depression in children, we performed a classification analysis to discriminate children in the at-risk group and control group based their resting-state functional connectivity data. This classification based on functional connectivity yielded high accuracy, sensitivity, and specificity in discriminating between children at risk for MDD and controls compared to classification based on CBCL scores. Importantly, the rs-fMRI classification was based on analyses that, at the level of each individual child, were independent of the group differences in functional connectivity. Such generalizable and individually robust classification is important if brain measures are to be used for early identification (68). Future prospective and longitudinal studies can determine whether such biomarkers predict which high-risk children progress to MDD and whether early intervention reduces the likelihood of developing MDD. Also, perhaps such biomarkers may be helpful in identifying children at risk for developing depression independent of parental histories of depression.
Our findings need to be viewed in light of some methodological limitations. First, we did not exclude children born prematurely, and premature births can lead to neurological complications. However, we did exclude children with known developmental delays such as autism and intellectual disability. Second, because parental MDD confers a spectrum of risk to offspring (31,69), the at-risk children were also at risk for anxiety and other disorders. Parents with MDD also have higher rates of comorbid anxiety than the general population. Thus we cannot rule out that the brain differences we found were due to the children being at risk for anxiety and other disorders. Third, although our sample size of at-risk children (N=27) was moderate, the control group was small (N=16). Lastly, our resting-state scans were acquired with a repetition time (TR) of 6 seconds, which is longer than most resting state fMRI studies so that we could acquire high-resolution whole-brain data (2mm isotropic voxels) without the use of parallel imaging. A previous study found there was no significant difference in correlation strengths within and between resting-state functional networks when comparing TR = 2.5 and 5 seconds resting scans, and that correlation strengths stabilized with acquisition time of 5 min (TR = 5) (70). In the current and previous studies using the same acquisition parameters (TR = 6 s) (71), we observed the typical resting-state network patterns observed in other studies. Nonetheless, an additional issue of the long TR is that cognitive and emotional processes internally initiated at the beginning and the end of each scan can be different. We cannot rule out the possibility that the group difference observed here might be in part due to systematic differences in chronometry between the two groups.
The present study consisted of a sample of pre-adolescent children who were at familial risk for depression but not currently affected with depression and therefore functional connectivity differences cannot reflect an expression of depression as could be the case in patients with ongoing MDD. Rather, the differences in intrinsic functional brain architecture likely reflect neural traits that predispose children towards MDD or related disorders. Importantly, we demonstrated that discrimination between at-risk and control children occurred with high sensitivity and specificity based on resting-state functional connectivity. Future studies that track the development of children at familial risk for MDD and determines which children develop MDD or other mood and anxiety disorders are needed to build predictive models based on findings from the present study so as to identify high-risk individuals for early intervention.
Supplementary Material
Acknowledgments
We thank Gretchen Reynolds, Daniel O’Young and Jiahe Zhang for their help with collecting imaging data. This research was carried out at the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research at the Massachusetts Institute of Technology and Massachusetts General Hospital. The study was supported by the Tommy Fuss Fund, the Poitras Center for Affective Disorders Research, and the MGH Pediatric Psychopharmacology Council Fund. The funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Financial Disclosures
Dr. Joseph Biederman is currently receiving research support from the following sources: The Department of Defense, AACAP, Alcobra, Forest Research Institute, Ironshore, Lundbeck, Magceutics Inc., Merck, PamLab, Pfizer, Shire Pharmaceuticals Inc., SPRITES, Sunovion, Vaya Pharma/Enzymotec, and NIH. In 2014, Dr. Joseph Biederman received honoraria from the MGH Psychiatry Academy for tuition-funded CME courses. He has a US Patent Application pending (Provisional Number #61/233,686) through MGH corporate licensing, on a method to prevent stimulant abuse. Dr. Biederman received departmental royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Ingenix, Prophase, Shire, Bracket Global, Sunovion, and Theravance; these royalties were paid to the Department of Psychiatry at MGH. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, et al. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology. 2009;34:932–843. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- 2.Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho TC, Connolly CG, Henje Blom E, LeWinn KZ, Strigo Ia, Paulus MP, et al. Emotion-Dependent Functional Connectivity of the Default Mode Network in Adolescent Depression. Biol Psychiatry. 2014:1–13. doi: 10.1016/j.biopsych.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008;63:377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitterschiffthaler MT, Williams SCR, Walsh ND, Cleare AJ, Donaldson C, Scott J, Fu CHY. Neural basis of the emotional Stroop interference effect in major depression. Psychol Med. 2008;38:247–256. doi: 10.1017/S0033291707001523. [DOI] [PubMed] [Google Scholar]
- 6.Sheline YI, Price JL, Yan Z, Mintun Ma. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci USA. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeng LL, Shen H, Liu L, Wang L, Li B, Fang P, et al. Identifying major depression using whole-brain functional connectivity: A multivariate pattern analysis. Brain. 2012;135:1498–1507. doi: 10.1093/brain/aws059. [DOI] [PubMed] [Google Scholar]
- 8.Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 9.Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 10.Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gotlib IH, Sivers H, Gabrieli JDE, Whitfield-Gabrieli S, Goldin P, Minor KL, Canli T. Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport. 2005;16:1731–1734. doi: 10.1097/01.wnr.0000183901.70030.82. [DOI] [PubMed] [Google Scholar]
- 12.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 13.Suslow T, Konrad C, Kugel H, Rumstadt D, Zwitserlood P, Schöning S, et al. Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biol Psychiatry. 2010;67:155–160. doi: 10.1016/j.biopsych.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 14.Disner SG, Beevers CG, Haigh EAP, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- 15.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder. JAMA Psychiatry. 2015;02478:1–10. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y, Yu C, Zheng H, Liu Y, Song M, Qin W, et al. Increased neural resources recruitment in the intrinsic organization in major depression. J Affect Disord. 2010;121:220–230. doi: 10.1016/j.jad.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 18.Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S, et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol Psychiatry. 2013;74:898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaffrey MS, Luby JL, Repovš G, Belden AC, Botteron KN, Luking KR, Barch DM. Subgenual cingulate connectivity in children with a history of preschool-depression. Neuroreport. 2010;21:1182–8. doi: 10.1097/WNR.0b013e32834127eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive Rumination, the Default-Mode Network, and the Dark Matter of Clinical Neuroscience. Biol Psychiatry. 2015;78:224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veer IM, Beckmann CF, van Tol M-J, Ferrarini L, Milles J, Veltman DJ, et al. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci. 2010;4:1–10. doi: 10.3389/fnsys.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. 2012;139:56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye T, Peng J, Nie B, Gao J, Liu J, Li Y, et al. Altered functional connectivity of the dorsolateral prefrontal cortex in first-episode patients with major depressive disorder. Eur J Radiol. 2012;81:4035–4040. doi: 10.1016/j.ejrad.2011.04.058. [DOI] [PubMed] [Google Scholar]
- 24.Marchetti I, Koster EHW, Sonuga-Barke EJ, De Raedt R. The default mode network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychol Rev. 2012;22:229–51. doi: 10.1007/s11065-012-9199-9. [DOI] [PubMed] [Google Scholar]
- 25.Belleau EL, Taubitz LE, Larson CL. Imbalance of default mode and regulatory networks during externally focused processing in depression. Soc Cogn Affect Neurosci. 2014:1–8. doi: 10.1093/scan/nsu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton JP, Chen MC, Gotlib IH. Neural systems approaches to understanding major depressive disorder: An intrinsic functional organization perspective. Neurobiol Dis. 2013;52:4–11. doi: 10.1016/j.nbd.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, et al. Activity and connectivity of brain mood regulating circuit in depression: A functional magnetic resonance study. Biol Psychiatry. 2005;57:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 28.Chen C-H, Suckling J, Ooi C, Fu CHY, Williams SCR, Walsh ND, et al. Functional coupling of the amygdala in depressed patients treated with antidepressant medication. Neuropsychopharmacology. 2008;33:1909–18. doi: 10.1038/sj.npp.1301593. [DOI] [PubMed] [Google Scholar]
- 29.Luking KR, Repovs G, Belden AC, Gaffrey MS, Botteron KN, Luby JL, Barch DM. Functional connectivity of the amygdala in early-childhood-onset depression. J Am Acad Child Adolesc Psychiatry. 2011;50:1027–41.e3. doi: 10.1016/j.jaac.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williamson DE, Birmaher B, Axelson DA, Ryan ND, Dahl RE. First episode of depression in children at low and high familial risk for depression. J Am Acad Child Adolesc Psychiatry. 2004;43:291–297. doi: 10.1097/00004583-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Lieb R, Isensee B, Höfler M, Pfister H, Wittchen H-U. Parental major depression and the risk of depression and other mental disorders in offspring: a prospective-longitudinal community study. Arch Gen Psychiatry. 2002;59:365–374. doi: 10.1001/archpsyc.59.4.365. [DOI] [PubMed] [Google Scholar]
- 32.Clasen PC, Beevers CG, Mumford Ja, Schnyer DM. Cognitive control network connectivity in adolescent women with and without a parental history of depression. Dev Cogn Neurosci. 2014;7:13–22. doi: 10.1016/j.dcn.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitfield-Gabrieli S, Moran JM, Nieto-Castañón A, Triantafyllou C, Saxe R, Gabrieli JDE. Associations and dissociations between default and self-reference networks in the human brain. Neuroimage. 2011;55:225–232. doi: 10.1016/j.neuroimage.2010.11.048. [DOI] [PubMed] [Google Scholar]
- 34.Nejad AB, Fossati P, Lemogne C. Self-referential processing, rumination, and cortical midline structures in major depression. Front Hum Neurosci. 2013;7:666. doi: 10.3389/fnhum.2013.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (Clinician Version) New York: New York State Psychiatric Institute Biometrics Department; 1995. [Google Scholar]
- 36.Orvaschel H. Schedule for Affective Disorder and Schizophrenia for School-Age children–Epidemiologic Version. 5. Fort Lauderdale, FL: Nova Southeastern University, Center for Psychological Studies; 1994. [Google Scholar]
- 37.Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test, Second Edition (KBIT-2) Bloomington, MN: Pearson, Inc; 2004. [Google Scholar]
- 38.Achenbach TM, Rescorla LA. Manual for ASEBA School-Age Forms & Profile. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- 39.Kovacs M. The children’s depression, Inventory (CDI) Psychopharmacol Bull. 1985;21:995–998. [PubMed] [Google Scholar]
- 40.Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med. 2000;44:457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 41.Chai XJ, Castañán AN, Öngür D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitfield-Gabrieli S, Nieto-Castanon A. Conn : A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2 doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 43.Fair Da, Cohen AL, Power JD, Dosenbach NUF, Church Ja, Miezin FM, et al. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5:14–23. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 45.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murphy K, Birn RM, Handwerker Da, Jones TB, Bandettini Pa. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2:25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dyck M, Loughead J, Kellermann T, Boers F, Gur RC, Mathiak K. Cognitive versus automatic mechanisms of mood induction differentially activate left and right amygdala. Neuroimage. 2011;54:2503–2513. doi: 10.1016/j.neuroimage.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 51.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 52.Hall M, National H, Frank E, Holmes G, Pfahringer B, Reutemann P, Witten IH. The WEKA data mining software: An update. SIGKDD Explor. 2009;11:10–18. [Google Scholar]
- 53.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 54.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 55.Fox MD, Liu H, Pascual-Leone A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage. 2013;66:151–160. doi: 10.1016/j.neuroimage.2012.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci. 2005;8:1603–10. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- 57.Ptak R. The Frontoparietal Attention Network of the Human Brain: Action, Saliency, and a Priority Map of the Environment. Neurosci. 2012;18:502–515. doi: 10.1177/1073858411409051. [DOI] [PubMed] [Google Scholar]
- 58.Hartlage S, Alloy LB, Vázquez C, Dykman B. Automatic and effortful processing in depression. Psychol Bull. 1993;113:247–278. doi: 10.1037/0033-2909.113.2.247. [DOI] [PubMed] [Google Scholar]
- 59.Harvey PO, Le Bastard G, Pochon JB, Levy R, Allilaire JF, Dubois B, Fossati P. Executive functions and updating of the contents of working memory in unipolar depression. J Psychiatr Res. 2004;38:567–576. doi: 10.1016/j.jpsychires.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 60.Keller JB, Hedden T, Thompson TW, Anteraper Sa, Gabrieli JDE, Whitfield-Gabrieli S. Resting-state anticorrelations between medial and lateral prefrontal cortex: Association with working memory, aging, and individual differences. Cortex. 2015;64:271–280. doi: 10.1016/j.cortex.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hampson M, Driesen N, Roth JK, Gore JC, Constable RT. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magn Reson Imaging. 2010;28:1051–1057. doi: 10.1016/j.mri.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8 doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- 63.Hooley JM, Gruber SA, Scott LA, Hiller JB, Yurgelun-Todd DA. Activation in dorsolateral prefrontal cortex in response to maternal criticism and praise in recovered depressed and healthy control participants. Biol Psychiatry. 2005;57:809–812. doi: 10.1016/j.biopsych.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 64.Gotlib IH, Hamilton JP. Neuroimaging and depression: Current status and unresolved issues. Curr Dir Psychol Sci. 2008;17:159–163. [Google Scholar]
- 65.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Townsend JD, Torrisi SJ, Lieberman MD, Sugar CA, Bookheimer SY, Altshuler LL. Frontal-amygdala connectivity alterations during emotion downregulation in bipolar I disorder. Biol Psychiatry. 2013;73:127–35. doi: 10.1016/j.biopsych.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gabrieli JDE, Ghosh SS, Whitfield-Gabrieli S. Prediction as a Humanitarian and Pragmatic Contribution from Human Cognitive Neuroscience. Neuron. 2015;85:11–26. doi: 10.1016/j.neuron.2014.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hirshfeld-Becker DR, Micco JA, Henin A, Petty C, Faraone SV, Mazursky H, et al. Psychopathology in adolescent offspring of parents with panic disorder, major depression, or both: A 10-year follow-up. Am J Psychiatry. 2012;169:1175–1184. doi: 10.1176/appi.ajp.2012.11101514. [DOI] [PubMed] [Google Scholar]
- 70.Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Redcay E, Moran JM, Mavros PL, Tager-Flusberg H, Gabrieli JDE, Whitfield-Gabrieli S. Intrinsic functional network organization in high-functioning adolescents with autism spectrum disorder. Front Hum Neurosci. 2013;7:573. doi: 10.3389/fnhum.2013.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.