Abstract
The basolateral nucleus of the amygdala (BLA) is critical to the pathophysiology of anxiety-driven alcohol drinking and relapse. The endogenous cannabinoid/type 1 cannabinoid receptor (eCB/CB1) system curbs BLA-driven anxiety and stress responses via a retrograde negative feedback system that inhibits neurotransmitter release, and BLA CB1 activation reduces GABA release and drives anxiogenesis. Additionally, decreased amygdala CB1 is observed in abstinent alcoholic patients and ethanol withdrawn rats. Here we investigated the potential disruption of eCB/CB1 signaling on GABAergic transmission in BLA pyramidal neurons of rats exposed to 2–3 weeks intermittent ethanol. In the naïve rat BLA, the CB1 agonist WIN 55,212-2 (WIN) decreased GABA release and this effect was prevented by the CB1 antagonist AM251. AM251 alone increased GABA release via a mechanism requiring postsynaptic calcium-dependent activity. This retrograde tonic eCB/CB1 signaling was diminished in chronic ethanol exposed rats, suggesting a functional impairment of the eCB/CB1 system. In contrast, acute ethanol increased GABAergic transmission similarly in naïve and chronic ethanol exposed rats, via both pre- and postsynaptic mechanisms. Notably, CB1 activation impaired ethanol’s facilitation of GABAergic transmission across both groups, but the AM251- and ethanol-induced facilitation of GABA release were additive, suggesting independent presynaptic sites of action. Collectively, the present findings highlight a critical CB1 influence on BLA GABAergic transmission that is dysregulated by chronic ethanol exposure and thus, may contribute to the alcohol dependent state.
Keywords: basolateral amygdala, endocannabinoid (eCB), ethanol, GABA, type 1 cannabinoid receptor (CB1), whole cell recordings
INTRODUCTION
The basolateral amygdala (BLA) is critical to the pathophysiology of stress, anxiety disorders and addiction (Azad et al., 2004; Quirk and Gehlert, 2003; Stamatakis et al., 2014), and dysregulation of its aversive emotional learning processes is implicated in several alcohol drinking-related behaviors, such as cue-induced alcohol seeking (Sciascia et al., 2015) and reinstatement (Sinclair et al., 2012), and withdrawal-associated anxiety-like behaviors (Lack et al., 2007). The BLA receives highly processed sensory input from the hippocampus, medial prefrontal cortex (mPFC), thalamus and ventral tegmental area (VTA), and assigns a negative emotional value to salient events (Stamatakis et al., 2014). The majority of BLA neurons are glutamatergic pyramidal cells (90–95% of BLA neurons), but local GABAergic interneurons (5–10%) play a critical role in regulating overall BLA activity due to their robust inhibition of pyramidal neurons. The BLA transmits this information to several addiction-related downstream regions, including the bed nucleus of the stria terminalis (BNST), central nucleus of the amygdala (CeA), hippocampus, mPFC and nucleus accumbens (NAc) (Azad et al., 2004; Stamatakis et al., 2014). Previous work has shown that acute alcohol increases GABA release onto BLA pyramidal neurons (Silberman et al., 2008; Zhu and Lovinger, 2005), thereby limiting excitatory output to downstream effector regions (Perra et al., 2008), and that chronic ethanol and withdrawal alter BLA GABAergic transmission (Diaz et al., 2011b; McCool et al., 2003).
Within the BLA, the endogenous cannabinoid/type 1 cannabinoid receptor (eCB/CB1) system plays a well-established role in gating stress and anxiety responses by modulating GABA and glutamate neurotransmission (Hill et al., 2010; Morena et al., 2015; Serrano and Parsons, 2011). Specifically, eCBs are released “on demand” from postsynaptic terminals and act retrogradely at presynaptic CB1 receptors to decrease neurotransmitter release (Hill et al., 2010; Morena et al., 2015). CB1 is expressed widely throughout the brain; in the BLA, it is highly expressed in cholecystokinin (CCK)-positive GABAergic interneurons (Katona et al., 2001; Yoshida et al., 2011) and at lower levels in glutamatergic pyramidal cells (Yoshida et al., 2011). Previous work has demonstrated that CB1 activity decreases BLA GABAergic transmission (Azad et al., 2004; Katona et al., 2001; Marsicano et al., 2002; Zhu and Lovinger, 2005), which can affect emotional processing (Hill et al., 2010; Tan et al., 2014).
Several studies have linked human allelic variants of the gene encoding CB1 (CNR1) with an altered patient susceptibility to alcohol dependence, though some of these data are conflicting (Marcos et al., 2012; Preuss et al., 2003; Schmidt et al., 2002; van den Wildenberg et al., 2007). Furthermore, alterations of the eCB/CB1 system have been observed in the amygdala of alcoholic patients; CB1 receptor availability decreased in the amygdala of heavy drinkers with 2–4 weeks of abstinence (Ceccarini et al., 2014; Hirvonen et al., 2013) but increased after 4 weeks of abstinence (Neumeister et al., 2012). Similarly, chronic ethanol exposed rats had reduced amygdala CB1 gene expression after 6 hours of withdrawal (Serrano et al., 2012). No studies to date have examined the functional effects of chronic ethanol on BLA eCB/CB1 signaling, but in the CeA we recently reported that 2–3 week intermittent ethanol significantly reduced eCB/CB1 influence on basal GABA release (Varodayan et al., 2015). Additionally, CB1 activation blocked ethanol’s facilitation of GABA release in the CeA of naive and chronic ethanol exposed rats, even though ethanol and a CB1 antagonist acted independently to increase GABA release (Roberto et al., 2010; Varodayan et al., 2015). Given the substantial evidence that alcohol alters BLA CB1 expression and function, here we investigated the interaction of the eCB/CB1 system with the effects of acute ethanol on GABAergic transmission, and its dysregulation by chronic ethanol exposure.
MATERIALS AND METHODS
All procedures were approved by The Scripps Research Institutional Animal Care and Use Committee and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Slice preparation
We prepared slices from 78 adult male Sprague Dawley rats (250–350 g) (Roberto et al., 2004; Varodayan et al., 2015). Rats were anesthetized with 3–5% isoflurane, decapitated and the brains placed in ice-cold high-sucrose solution (pH 7.3–7.4; in mM): sucrose 206; KCl 2.5; CaCl2 0.5; MgCl2 7; NaH2PO4 1.2; NaHCO3 26; glucose 5; HEPES 5. 300 μm coronal brain slices containing the BLA were cut on a vibrating microtome (Leica VT1000S, Leica Microsystems, Buffalo Grove, IL). Slices were incubated (30 min at 35–37 °C, 30 min at room temperature) in oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid (aCSF; in mM): NaCl 130; KCl 3.5; CaCl2 2; NaH2PO4 1.25; MgSO4 1.5; NaHCO3 24; glucose 10. For each experiment, an individual slice was placed in a chamber on an upright microscope stage (Olympus BX50WI, Tokyo, Japan) and superfused at 2–5 ml/min with oxygenated aCSF.
Electrophysiological recording
BLA pyramidal neurons were visualized using IR-DIC optics, a CCD camera (EXi Aqua and ROLERA-XR, QImaging, Surrey, BC, Canada) and a w60 or w40 water immersion objective (Olympus). Recordings were performed in gap-free acquisition mode (sampling rate/signal of 10 kHz) and low-pass filtered at 10 kHz, using the Multiclamp 700B amplifier, Digidata 1440A and pClamp 10 software (Molecular Devices, Sunnyvale, CA). Patch pipettes (3–6 M′Ω; Warner Instruments, Hamden, CT and King Precision, Claremont, CA) were filled with internal solution (in mM): KCl 145; EGTA 5; MgCl2 5; HEPES 10; Na-ATP 2; Na-GTP 0.2. Action potential-dependent spontaneous inhibitory GABAA postsynaptic currents (sIPSCs) were isolated using the glutamate receptor blockers 6,7-dinitroquinoxaline-2,3-dione (DNQX, 20 μM) and DL-2-amino-5-phosphonovalerate (AP-5, 30 μM) and the GABAB receptor antagonist CGP55845A (1 μM), and for action potential-independent miniature IPSCs (mIPSCs) recordings 0.5 μM tetrodotoxin (TTX) was added. All 139 cells were clamped at −60 mV and experiments with series resistance >15 M′Ω or a >20% change, as monitored with a 10 mV pulse, were excluded. The s/mIPSC frequencies, amplitudes and kinetics were analyzed with MiniAnalysis software (Synaptosoft Inc., Fort Lee, NJ). A cell’s s/mIPSC characteristics were averaged from a minimum 60 events across 3–5 min, and the final s/mIPSC values from at least 4 animals.
Chronic Intermittent Ethanol Exposure
Chronic intermittent ethanol (CIE) exposed rats (n=33) were generated using standard cages kept in chambers into which ethanol vapor (14 h) or air (10 h) was intermittently injected for 2–3 weeks. Blood alcohol levels (BALs) were determined by twice weekly tail-bleedings, with the terminal BAL measured upon sacrifice, and the mean BAL of all CIE animals was 172±22 mg/dL. Naive rats were treated with continuous air (24 h). For sacrifice the rats were removed from the ethanol vapor-filled chambers and slice preparation occurred in ethanol-free solutions. Thus, the brain slices were in acute withdrawal (1–8 hours) during recording.
Drugs
We purchased BAPTA (1,2-Bis(2-Aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid) from Sigma (St. Louis, MO), CGP 55845A, DL-AP5 and DNQX from Tocris (Ellisville, MO), ethanol from Remet (La Mirada, CA) and TTX from Biotium (Hayward, CA). WIN (WIN55,212-2; [(3R)-2,3-dihydro-5-methyl-3-(4-morpholinylmethyl) pyrrolo [1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenyl-methanone, monomethanesulfonate) and AM251 (N-(Piperidin-1-yl)-5-(4-iodophenyl)-1-2,4-dichloro phenyl)-4-methyl-1H-pyrazole-3-carboxa-mide) were purchased from Cayman Chemical (Ann Arbor, MI), and dissolved in dimethylsulfoxide (final concentrations of 0.05–0.1%), which had no effect on synaptic responses in control experiments.
Statistics
We used GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA) for statistical analyses. The results were evaluated with cumulative probability analysis, with the Kolmogorov-Smirnov non-parametric two-sample test used to determine significance (Van der Kloot, 1991). To control for cell-to-cell variation in baseline IPSC properties, drug effects were normalized to their own neuron’s baseline (presented as control) prior to group analyses. We used t-test analyses for individual means comparisons. Two-way ANOVAs were used to assess treatment (naïve and CIE) and drug interactions (ethanol, WIN and AM251) with the Bonferroni test to determine significance. Data are presented as mean±SEM and n, reported in the text and graphs, represents the cell number of each experimental group.
RESULTS
Chronic ethanol exposure alters GABAergic transmission in the BLA via a postsynaptic mechanism
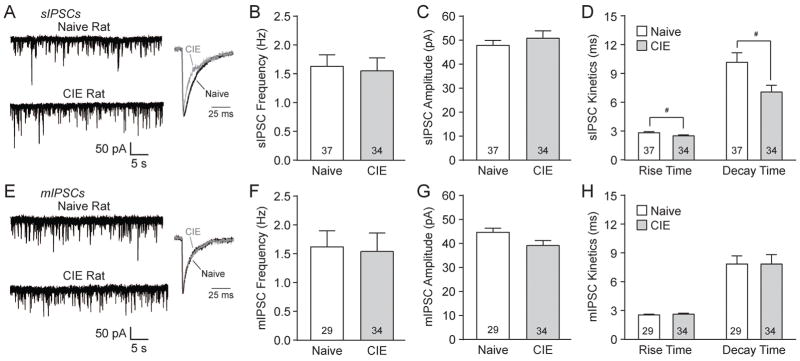
Action potential-dependent spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded from BLA pyramidal neurons of naïve or 2–3 week CIE rats (BAL: 172±22 mg/dL). The mean sIPSC frequencies and amplitudes were comparable. In naïve animals the sIPSC frequency was 1.63±0.20 Hz and amplitude was 47.82±2.12 pA (n=37), whereas in CIE rats the sIPSC frequency was 1.56±0.23 Hz and amplitude was 50.81±3.12 pA (n=34; Fig. 1A–C). With CIE there was, however, a significant decrease in sIPSC rise (#p<0.05 by unpaired t-test; t(69)=2.06) and decay times (#p<0.05; t(69)=2.50); in naïve rats the rise time was 2.85±0.11 ms and decay time was 10.19±1.00 ms, while in CIE rats the rise time was 2.54±0.09 ms and decay time was 7.10±0.70 ms (Fig. 1A and D).
Figure 1.
Baseline s/mIPSCs characteristics in BLA neurons of naive and CIE rats A: Representative sIPSC traces (left panel) and scaled average sIPSCs (right panel) from naive and CIE rat BLA neurons. B: Mean sIPSC frequencies are similar in naive and CIE rats. C: sIPSC amplitudes are similar in both groups. D: Chronic ethanol exposure significantly decreased sIPSC rise and decay times compared to naïve rats by unpaired t-test (#p<0.05). E: Representative mIPSC traces (left panel) and scaled average mIPSCs (right panel) from naive and CIE rat BLA neurons. F–G: Mean mIPSC frequencies, amplitudes, rise times and decay times are similar in both groups.
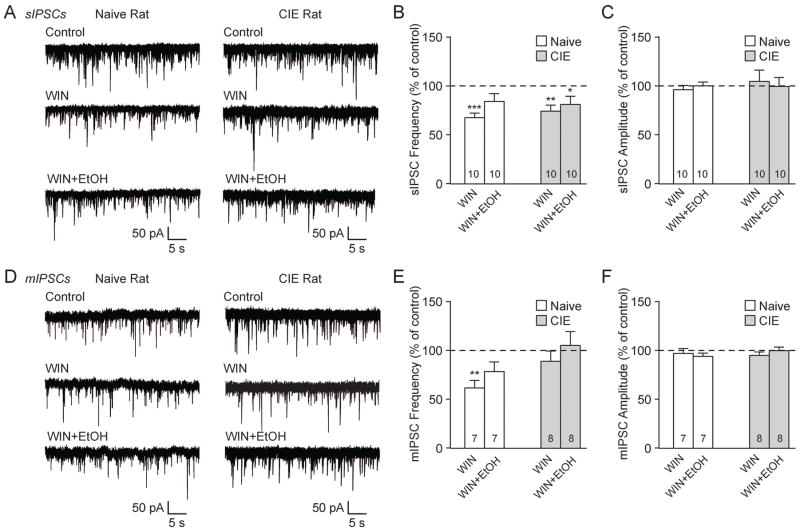
Action potential-independent miniature IPSCs (mIPSCs), recorded in TTX, were similar across groups. Naïve rats had a mIPSC frequency of 1.62±0.28 Hz, amplitude of 44.67±1.78 pA, rise time of 2.56±0.09 ms and decay time of 7.87±0.83 ms (n=29), while CIE rats had a mIPSC frequency of 1.51±0.28 Hz, amplitude of 39.20±2.10 pA, rise time of 2.64±0.09 ms and decay time of 7.87±0.97 ms (n=34; Fig. 1E–H). In these experiments, a change in s/mIPSC frequency reflects presynaptic changes that lead to an altered probability of GABA release, while changes in s/mIPSC amplitude and kinetics reflect altered postsynaptic receptor sensitivity. Therefore, our data suggests that chronic ethanol exposure alters GABAA receptor subunit composition and/or number in the rat BLA (De Koninck and Mody, 1994; Otis et al., 1994).
Acute ethanol increases BLA GABAergic transmission in naïve and chronic ethanol exposed rats
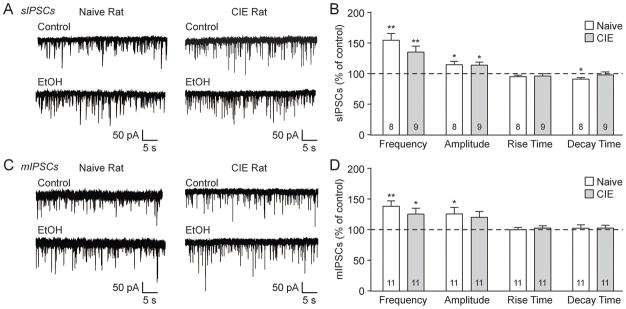
We then assessed the effects of acute ethanol (44 mM) on spontaneous GABAergic transmission. In the naïve rat BLA, ethanol increased the sIPSC frequency to 155.1±11.0% of baseline (**p<0.01 by one-sample t-test; n=8) and amplitude to 115.1±5.4% (*p<0.05; Fig. 2A and B). Similarly, in CIE rats the sIPSC frequency (135.8±9.7%; **p<0.01; n=9) and amplitude (114.4±4.8%; *p<0.05) were increased by ethanol (Fig. 2A and B). Apart from the decreased sIPSC decay time in naïve rats (91.4±2.5%; *p<0.05; Fig. 2B), there were no significant effects of ethanol on sIPSC kinetics. Acute ethanol also increased the naïve rat BLA mIPSC frequency to 138.6±9.0% of baseline (**p<0.01; n=11) and amplitude to 126.3±10.7% (*p<0.05; Fig. 2C and D). In CIE rats, there was a significant increase in mIPSC frequency (125.9±9.7%; *p<0.05; n=11) and a trend towards an increased amplitude (120.7±9.6%; p=0.058) with acute ethanol (Fig. 2C and D). There were no differences in mIPSC kinetics after ethanol. Thus acute ethanol increased BLA GABA transmission, via both pre- and postsynaptic mechanisms, in naïve and chronic ethanol exposed rats.
Figure 2.
Acute ethanol increases BLA GABA neurotransmission in naive and CIE rats. A: Representative sIPSCs from BLA neurons in naive (left) and CIE (right) rats in control conditions and during ethanol (EtOH; 44 mM) application. B: Acute EtOH increased sIPSC frequencies and amplitudes in naive and CIE rats (*p<0.05; **p<0.01 by one-sample t-test). Additionally, EtOH decreased the sIPSC decay time in naive rats (*p<0.05). C: Representative BLA mIPSCs from naive (left) and CIE (right) rats in control conditions and during EtOH application. D: Acute EtOH increased mIPSC frequencies in naive and CIE rats, and increased the mIPSC amplitude in naive rats (*p<0.05; **p<0.01). EtOH did not alter the mIPSC kinetics in either group.
CB1 activation decreases BLA GABA release and chronic ethanol exposure dampens this effect
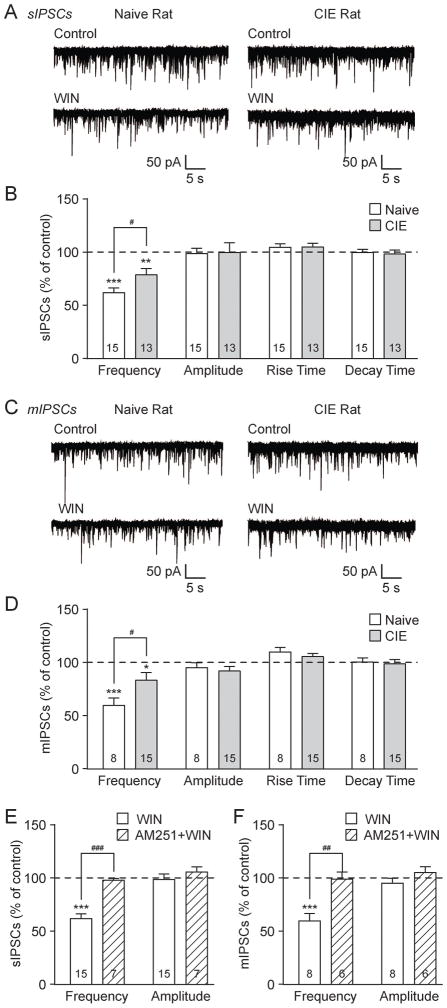
To investigate the influence of the eCB/CB1 system on BLA GABA transmission, we used the cannabinoid receptor agonist WIN55,212-2 (WIN; 2 μM) (Roberto et al., 2010; Varodayan et al., 2015). WIN significantly decreased sIPSC frequencies to 62.1±4.6% of baseline (***p<0.001 by one-sample t-test; n=15) in the BLA of naïve rats and 79.0±6.0% in CIE rats (**p<0.01; n=13; Fig. 3A and B). Notably, chronic ethanol exposure significantly reduced the magnitude of the WIN effect compared to naïve rats (#p<0.05 by unpaired t-test; t(216)=2.29). WIN had no effects on sIPSC amplitudes and kinetics (Fig. 3B). Similar to the sIPSCs, WIN decreased BLA mIPSC frequencies to 59.5±7.0% of baseline in naïve rats (***p<0.001 by one-sample t-test; n=8) and 83.1±7.1% in CIE rats (*p<0.05; n=15), though the WIN effect was significantly diminished in CIE vs. naïve rats (#p<0.05 by unpaired t-test; t(21)=2.13; Fig. 3C and D). There were no WIN-induced effects on mIPSC amplitudes and kinetics (Fig. 3D). Collectively, these data indicate that WIN decreased GABA release and the magnitude of this effect is dampened by chronic ethanol exposure.
Figure 3.
CB1 activation decreases GABA release in the BLA and chronic ethanol exposure dampens this effect. A: Representative sIPSCs in control conditions and during WIN 55,212-2 (WIN; 2 μM) application from naive (left) and CIE (right) rat BLA neurons. B: WIN significantly decreased sIPSC frequencies in naive and CIE rats (**p<0.01; ***p<0.001 by one-sample t-test). The effect of WIN on sIPSC frequency is significantly reduced in CIE rats vs. naive rats (#p<0.05 by unpaired t-test). WIN did not alter the sIPSC amplitudes and kinetics. C: Representative mIPSCs in control conditions and during WIN application from naive (left) and CIE (right) rat BLA. D: The mIPSC frequency was significantly decreased by WIN in naive and CIE rats (*p<0.01; ***p<0.001 by one-sample t-test). CIE reduced the magnitude of WIN’s effect on mIPSC frequency vs. naive rats (#p<0.05 by unpaired t-test). WIN did not alter the mIPSC amplitudes and kinetics. E: Bar graphs plotting the WIN-induced changes in sIPSCs in normal conditions (same neurons in panel B) or in the presence of the CB1 antagonist, AM251 (2 μM). In naive neurons, AM251 abolished the WIN-induced decrease in sIPSC frequency (###p<0.001 by unpaired t-test) and had no effect on sIPSC amplitude. F: In naive rats, the WIN-induced decrease in mIPSC frequency is lost with AM251 pretreatment vs. WIN alone (same neurons in panel D; ##p<0.01 by unpaired t-test). There were no drug-induced changes in the mIPSC amplitude.
To verify that these WIN effects were mediated by CB1, we repeated these experiments after 15 min pretreatment with the CB1 antagonist AM251 (2 μM; see Fig 5. for the per se effects of AM251). In naive rat BLA neurons WIN alone decreased the sIPSC frequency to 62.1±4.6% of baseline, and AM251 pretreatment prevented this WIN-induced effect (97.7±1.6%; ###p<0.001 by unpaired t-test; t(20)=5.22; Fig. 3E). Similarly, the WIN-induced effect on naïve rat BLA mIPSC frequency (59.5±7.0% of baseline) was lost with AM251 pretreatment (98.7±6.7%; ##p<0.01; t(12)=3.93; Fig. 3F). The s/mIPSC amplitudes were unaffected by drug treatment across all groups (Fig. 3E and F). Thus, the actions of WIN to decrease GABA release in the BLA occur via CB1 localized on the presynaptic terminal.
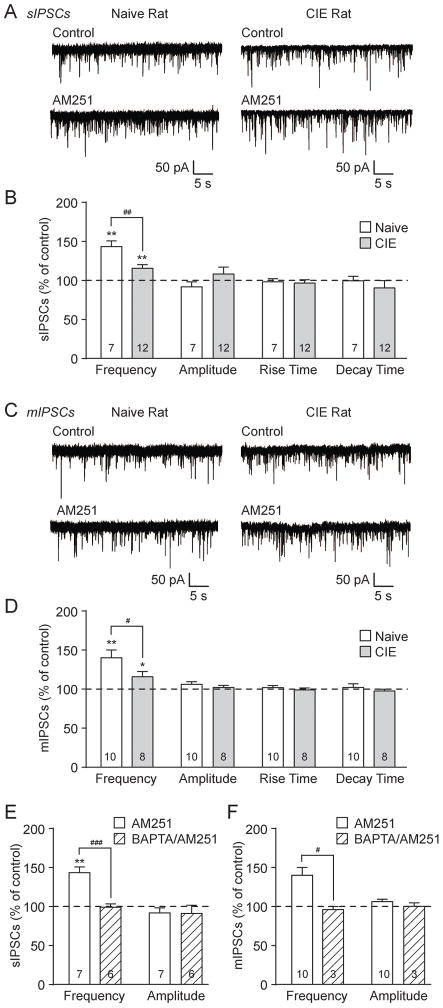
Figure 5.
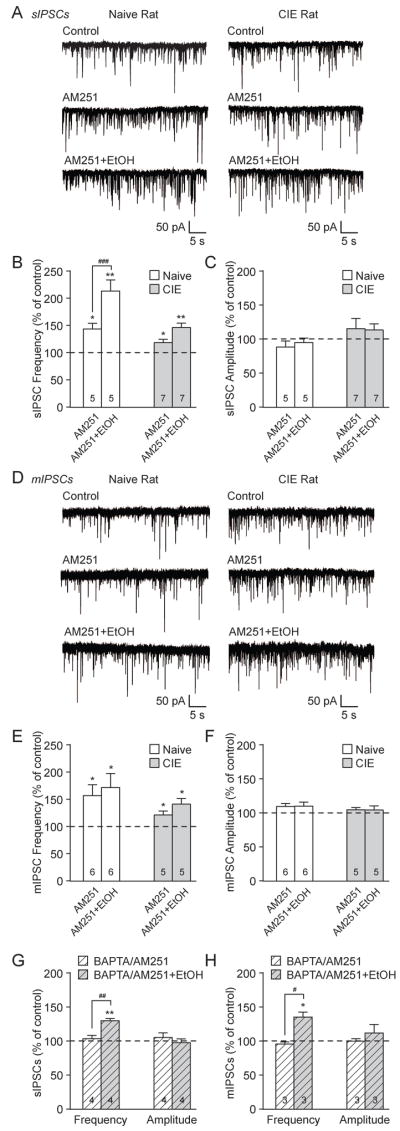
Tonic eCB/CB1 signaling regulates BLA GABA release, an effect dampened by chronic ethanol exposure. A: Representative sIPSCs in control conditions and during AM251 (2 μM) application in BLA neurons from naive (left) and CIE (right) rats. B: AM251 significantly increased the sIPSC frequency in naive and CIE rats (**p<0.01 by one-sample t-test). CIE significantly reduced the magnitude of the AM251-induced effect vs. naive rats (##p<0.01 by unpaired t-test). There were no AM251-induced alterations in sIPSC amplitudes and kinetics. C: Representative BLA mIPSCs from naive (left) and CIE (right) rats in control conditions and during AM251 application. D: AM251 significantly increased mIPSC frequencies in naive and CIE rats (*p<0.05; **p<0.01 by one-sample t-test). The AM251 effect on mIPSC frequency is dampened in CIE rats vs. naive rats (#p<0.05 by unpaired t-test). AM251 did not alter the mIPSC amplitudes and kinetics. E: Bar graphs plotting the AM251-induced changes in sIPSCs in normal conditions (same neurons in panel B) or with high BAPTA in the recording pipette. In naive neurons, BAPTA abolished the AM251-induced enhancement of sIPSC frequency (###p<0.001 by unpaired t-test) and had no effect on the sIPSC amplitude. F: The AM251-induced changes in mIPSC frequency were lost with BAPTA in the recording pipette vs. normal conditions (same neurons in panel D; #p<0.05 by unpaired t-test), with no change in amplitudes.
CB1 activation impairs ethanol facilitation of GABAergic transmission in the BLA
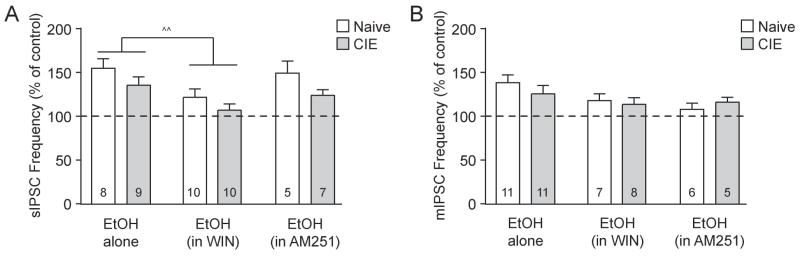
To investigate the interaction of CB1 activation and ethanol in the BLA of naïve and CIE rats we first applied WIN and then co-applied ethanol in the continued presence of WIN. We found a significant main effect of acute ethanol (in the presence of WIN) on sIPSC frequency (p<0.01 by two-way repeated measures (RM) ANOVA; F(1,18)=12.38), indicating that ethanol increases BLA GABA release after CB1 activation. Specifically, in naïve rats the sIPSC frequency was reduced by WIN to 68.1±4.6% of baseline and increased by subsequent ethanol co-application to 84.7±8.0% of baseline (n=10; Fig. 4A and B). Similarly, in CIE rats the sIPSC frequency was changed by WIN to 74.2±6.1% of baseline and by ethanol co-application to 81.1±8.2% of baseline (n=10; Fig. 4A and B). Although the sIPSC amplitudes were unchanged by CB1 activation, WIN blocked their increase by ethanol (Fig. 4C). We next assessed whether CB1 activation altered ethanol’s effect on sIPSC frequency vs. ethanol alone (from Fig 2B) in naïve and CIE rats. There was a significant main effect of WIN (^^p<0.01 by two-way ANOVA; F(1,32)=10.94; see summary in Fig 7A), indicating that the magnitude of the ethanol effect on sIPSC frequency was impaired by CB1 activation across both animal groups.
Figure 4.
Ethanol still increases BLA GABA release after CB1 activation. A: Representative sIPSCs at baseline, during WIN 55,212-2 (WIN; 2 μM) application and co-application of WIN + ethanol (EtOH; 44 mM) from naive (left) and CIE (right) rat BLA neurons. B: Ethanol increased spontaneous GABA release after CB1 activation in the BLA of both naïve and CIE rats. Bar graphs plotting the changes in sIPSC frequency induced by WIN and WIN + EtOH (*p<0.05; **p<0.01; ***p<0.001 by one-sample t-test). A two-way RM ANOVA revealed a significant main effect of acute ethanol (in the presence of WIN) on sIPSC frequency (p<0.01), with no significant main effect of CIE and no significant interaction (p>0.05). C: WIN and WIN + EtOH did not alter sIPSC amplitudes. D: Representative mIPSCs in control, WIN and WIN + EtOH conditions in naive (left) and CIE (right) rat BLA neurons. E: Ethanol (in the presence of WIN) increased action potential-independent GABA release across animal groups. Bar graphs plotting the changes in mIPSC frequency induced by WIN and WIN + EtOH (**p<0.01 by one-sample t-test). Similar to sIPSCS, there was a significant main effect of acute ethanol (in WIN) on mIPSC frequency (p<0.01 by two-way RM ANOVA), with no significant main effect of CIE and no significant interaction (p>0.05). F: WIN and WIN + EtOH did not alter mIPSC amplitudes.
Figure 7.
Summary of eCB/CB1 and ethanol interactions on GABA release in the BLA. CB1 activation impairs the ethanol response, but the effects of ethanol and a CB1 antagonist are additive. A: Bar graphs plotting sIPSC frequencies of EtOH alone (44 mM; normalized to the pre-ethanol baseline; from Fig. 2B), EtOH in the presence of WIN (2 μM; normalized to WIN alone), and EtOH in the presence of AM251 (2 μM; normalized to AM251 alone) in naive and CIE rats. WIN-induced activation of CB1 impairs the effects of EtOH on BLA GABA release (main effect: ^^p<0.01 by two-way ANOVA). In contrast, CB1 antagonism and ethanol act independently to increase spontaneous GABA release (p>0.05 two-way ANOVA). B: Bar graphs plotting mIPSC frequencies of EtOH alone (from Fig. 2D), EtOH in the presence of WIN, and EtOH in the presence of AM251 in naive and CIE rats. CB1 agonism and antagonism do not affect EtOH facilitation of action potential-independent GABA transmission (p>0.05 two-way ANOVA).
Similar to the sIPSCs, we found a significant main effect of acute ethanol (in the presence of WIN) on mIPSC frequency across both naïve and CIE rats (p<0.05 by two-way RM ANOVA; F(1,13)=7.55). Specifically, in naïve rats ethanol co-application increased the WIN effect on mIPSC frequency from 61.6±7.8% to 78.1±9.9% of baseline (n=7; Fig. 4D and E). Additionally, in CIE rats the mIPSC frequency was changed by WIN to 88.8±10.6% of baseline and by subsequent ethanol co-application to 104.9±14.2% of baseline (n=8; Fig. 4D and E). WIN also blocked the ethanol-induced increase of BLA mIPSC amplitude in naïve rats (Fig. 4F). Further analysis of the effects of ethanol alone (from Fig 2D) vs. ethanol plus WIN on mIPSC frequency identified no significant main effects of WIN and CIE (p>0.05 by two-way ANOVA; see summary in Fig 7B). Thus, CB1 activation impaired ethanol’s facilitation of GABA transmission in the BLA of both naïve and chronic ethanol exposed rats.
Tonic eCB/CB1 signaling regulates BLA GABA release, an effect dampened by chronic ethanol exposure
To further investigate the role of endogenously formed eCBs in basal GABAergic transmission, we assessed the effects of the CB1 antagonist AM251. Superfusion of AM251 (2 μM) significantly increased the BLA sIPSC frequency to 143.2±7.4% of baseline (**p<0.01 by one-sample t-test; n=7) in naïve rats and to 115.6±4.6% in CIE rats (**p<0.01; n=12; Fig. 5A and B). However, chronic ethanol significantly reduced the magnitude of the AM251 effect compared to naïve rats (##p<0.01 by unpaired t-test; t(17)=3.37). AM251 did not alter the sIPSC amplitudes or kinetics in either group (Fig. 5B). Similarly, AM251 increased the BLA mIPSC frequency to 139.9±10.0% of baseline (**p<0.01 by one-sample t-test; n=10) in naïve rats, and to 115.7±6.7% in CIE rats (*p<0.05; n=8; Fig. 5C and D), and the magnitude of the AM251 effect was significantly decreased in CIE vs. naïve rats (#p<0.05 by unpaired t-test; t(16)=2.14). There were no AM251-induced effects on mIPSC amplitudes and kinetics (Fig. 5D). We conclude that eCB/CB1 signalling tonically regulates inhibitory transmission in the BLA by decreasing basal GABA release, and chronic ethanol exposure dampens this influence.
To determine whether this tonic regulation resulted from constitutive CB1 activity (Turu and Hunyady, 2010), as opposed to eCB release, we buffered our recording cells by including 10 mM BAPTA in the internal solution to prevent postsynaptic calcium-dependent formation and/or mobilization of eCBs (Hentges et al., 2005; Neu et al., 2007; Roberto et al., 2010; Varodayan et al., 2015). There were significantly higher s/mIPSC baseline frequencies in the naive rat neurons loaded with BAPTA vs. normal internal solution; BAPTA increased the sIPSC frequency from 1.63±0.20 Hz (n=37) to 4.38±0.53 Hz (n=6; p<0.001 by unpaired t-test; t(41)=5.13) and the mIPSC frequency from 1.62±0.28 Hz (n=29) to 4.50±0.27 Hz (n=3; p<0.01; t(30)=3.24). Additionally, the AM251 effects on sIPSCs were lost in the BAPTA-loaded neurons as the sIPSC frequency decreased from 143.2±7.4% of baseline under normal conditions to 99.1±4.3% in BAPTA-loaded cells (n=6; ###p<0.001 by unpaired t-test; t(11)=4.96; Fig. 5E). Similarly, the AM251 effect on mIPSCs under normal conditions (139.9±10.0% of baseline) was lost in BAPTA-loaded neurons (95.8±3.2%; n=3; #p<0.05 by unpaired t-test; t(11)=2.24; Fig. 5F). There were no significant differences in s/mIPSC amplitudes (Fig. 5E and F). Collectively, these data indicate that local release of eCBs in the BLA act in a retrograde manner on presynaptic CB1 to regulate GABA release.
CB1 antagonism does not block the ethanol facilitation of GABA release
As AM251 and ethanol both increase GABA release in the BLA of naive and CIE rats, we investigated their interactions by first applying AM251 followed by co-application of ethanol in the continued presence of AM251. We found significant main effects of ethanol (in the presence of AM251; p<0.001 by two-way RM ANOVA; F(1,10)=30.19) and CIE on sIPSC frequency (p<0.01; F(1,10)=11.86), and a significant interaction (p<0.05; F(1,10)=5.58). A post-hoc Bonferroni test revealed that the effects of AM251 alone vs. ethanol and AM251 co-application are significantly different in naive rats (###p<0.001). Specifically in naive rats AM251 increased the sIPSC frequency to 143.8±10.6% of baseline and co-application of ethanol further increased it to 213.7±20.6% of baseline (n=5; Fig. 6A and B). In CIE rats, the BLA sIPSC frequency was increased to 118.9±6.2% of baseline by AM251 and to 146.8±7.9% of baseline by ethanol and AM251 (n=7; Fig. 6A and B). AM251 had no significant effects on sIPSC amplitudes in either animal group, but blocked their ethanol-induced enhancement (Fig. 6C). We next examined the effect of ethanol alone vs. ethanol plus AM251 and there were no significant main effects of AM251 or CIE (p>0.05 by two-way ANOVA; see summary in Fig 7A), indicating that CB1 antagonism and ethanol act independently to increase BLA GABA release.
Figure 6.
Ethanol still increases BLA GABA release after CB1 antagonism. A: Representative BLA sIPSCs in control conditions and during application of AM251 (2 μM) and AM251 + ethanol (EtOH; 44 mM) from naive (left) and CIE (right) rats. B: Ethanol increased spontaneous GABA release after CB1 antagonism in the BLA of both naïve and CIE rats. Bar graphs plotting the changes in sIPSC frequency induced by AM251 and AM251 + EtOH (*p<0.05; **p<0.01 by one-sample t-test). A two-way RMA ANOVA revealed significant main effects of ethanol (in AM251; p<0.001 by two-way RM ANOVA) and CIE (p<0.01) on sIPSC frequency, and a significant interaction (p<0.05). Specifically, the effects of AM251 + EtOH on sIPSC frequency were significantly different vs. AM251 alone in the naive rat BLA (###p<0.001 by Bonferroni post hoc test). C: AM251 and AM251 + EtOH did not alter sIPSC amplitudes. D: Representative mIPSCs in control conditions and during application of AM251 and AM251 + EtOH in naive (left) and CIE (right) rat BLA neurons. E: Ethanol (in the presence of AM251) increased action potential-independent GABA release across animal groups. Bar graphs plotting the changes in mIPSC frequency induced by AM251 and AM251 + EtOH (*p<0.05 by one-sample t-test). Similar to sIPSCS, there was a significant main effect of acute ethanol (in AM251) on mIPSC frequency (p<0.05 by two-way RM ANOVA), with no significant main effect of CIE and no significant interaction (p>0.05). F: AM251 and AM251 + EtOH did not alter mIPSC amplitudes. G: Bar graphs plotting sIPSC frequencies in the presence of AM251 and with BAPTA in the recording pipette (subset of neurons in Fig. 5E) and with the subsequent addition of EtOH (**p<0.01 by one-sample t-test). In naive neurons EtOH increased the sIPSC frequency vs. BAPTA/AM251 alone cells (##p<0.01 by unpaired t-test) and had no effect on the sIPSC amplitude. H: The effect of EtOH on mIPSC frequency in the presence of AM251 and with BAPTA in the recording pipette (same neurons in Fig. 5F) was significantly different vs. baseline (*p<0.05 by one-sample t-test) and also significantly different vs. BAPTA/AM251 alone (#p<0.05 by unpaired t-test). There were no significant differences in mIPSC amplitudes.
Similar additive effects of AM251 and ethanol were observed on action potential-independent GABA release, and across both naive and CIE rats there was a significant main effect of ethanol (in the presence of AM251) on mIPSC frequency (p<0.05 by two-way RM ANOVA; F(1,9)=5.81). Specifically, AM251 enhanced the mIPSC frequency to 157.4±20.0% of baseline (n=6) in naive rats and co-application of ethanol and AM251 further increased it to 172.3±25.8% of baseline (Fig. 6D and E). In CIE rats, the mIPSC frequency was increased by AM251 to 121.8±7.3% of baseline (n=5) and further increased to 141.7±10.4% of baseline with ethanol co-application (Fig. 6D and E). AM251 also blocked the ethanol-induced increase of mIPSC amplitude in naive rats (Fig. 6F). Further analysis of the effects of ethanol alone vs. ethanol plus AM251 on mIPSC frequency revealed no significant main effects of AM251 or CIE (p>0.05 by two-way ANOVA; see summary in Fig 7A). Thus, both CB1 blockade and ethanol increased GABA release in an additive manner and this AM251-EtOH interaction was not impaired by CIE, suggesting that the two drugs have different sites of action at the presynaptic terminal.
In addition, in 4 naïve BAPTA-loaded neurons exposed to AM251 (see Fig. 5E), we subsequently tested the effects of acute ethanol. We found that in the continued presence of AM251 and postsynaptic calcium buffering, co-application of ethanol significantly increased the mean sIPSC frequency from 103.7±9.7% to 130.1±3.4% (**p<0.01 by one-sample t-test; n=4), and this ethanol-induced effect was significantly higher than AM251 alone (##p<0.01 by unpaired t-test; t(3)=6.28; Fig. 6G). We observed similar results in BLA mIPSCs recorded from 3 naïve BAPTA-loaded neurons continuously exposed to AM251 (see Fig. 5F). Specifically, ethanol increased the mIPSC frequency from 95.8±3.2% to 135.5±7.4% (*p<0.05 by one-sample t-test; n=3), and the effects of AM251 alone vs. ethanol plus AM251 were significantly different (#p<0.05 by unpaired t-test; t(2)=6.69; Fig. 6H). There were no changes in s/mIPSC amplitudes (Fig. 6G and H). These results further support our findings that the eCB/CB1 system and ethanol have additive effects on BLA GABA release.
DISCUSSION
The eCB/CB1 regulation of BLA neurotransmission appears critical in the neural basis of addiction due to the region’s prominent role in aversive emotional processing, learning and memory (Stamatakis et al., 2014; Tan et al., 2014). Here we report that eCB/CB1 retrograde signaling tonically inhibits GABA release onto BLA pyramidal neurons, and chronic intermittent ethanol exposure significantly diminishes this eCB/CB1 influence. Additionally, BLA GABAergic transmission is increased by acute ethanol via both pre- and postsynaptic mechanisms. While CB1 activation impairs ethanol’s facilitation of GABAergic transmission, the ethanol presynaptic site of action is likely independent of CB1 as acute ethanol further increases GABA release in the presence of a CB1 antagonist (Fig. 7).
The eCBs, synthesized postsynaptically in response to cellular activity, limit GABA release by binding to presynaptic CB1 and thereby activating intracellular Gi/o protein pathways to inhibit neurotransmitter release (Hill et al., 2010; Morena et al., 2015; Serrano and Parsons, 2011; Turu and Hunyady, 2010). Here we found that the CB1 agonist WIN decreased both action potential-dependent and -independent GABA release (sIPSCs and mIPSCs) in the BLA of naïve rats, and these effects were prevented by the CB1 antagonist AM251. AM251 alone increased both types of GABA release via a mechanism requiring postsynaptic calcium-dependent activity (as revealed by BAPTA blockade of the AM251 effect), strengthening our finding of basal eCB tone rather than constitutive CB1 activity (Turu and Hunyady, 2010). Collectively, these results indicate that endogenous eCB signaling acts exclusively on presynaptic CB1 to inhibit basal GABA release onto BLA pyramidal neurons. Other groups have consistently observed reduced BLA GABAergic transmission with CB1 activation (Azad et al., 2004; Katona et al., 2001; Marsicano et al., 2002; Talani and Lovinger, 2015; Zhu and Lovinger, 2005). However, studies on basal eCB tone produce more variable results; in young rats CB1 antagonism had no effect on GABA transmission in BLA slices, but increased GABA release in the majority of mechanically-isolated BLA neurons (Talani and Lovinger, 2015; Zhu and Lovinger, 2005). Additionally, Perra et al. did not observe basal eCB signaling in the adult rat BLA when performing in vivo extracellular single-unit electrophysiology (Perra et al., 2008). Similar to the present study, we have reported inhibitory effects of eCB tone on GABA release in the medial subdivision of the CeA (Roberto et al., 2010; Varodayan et al., 2015), but this effect is region-specific as CB1 activation did not significantly affect evoked GABAergic transmission in the lateral CeA (Ramikie et al., 2014).
In contrast to the effects of CB1 activation, acute ethanol increased GABA release and altered postsynaptic GABAA receptor function in the BLA of naïve and CIE rats. Previous studies have reported a similar ethanol facilitation of BLA GABAergic transmission in naïve neurons (Silberman et al., 2008; Talani and Lovinger, 2015; Zhu and Lovinger, 2005), and ethanol’s pre- and postsynaptic actions have been attributed to distinct BLA interneuron populations (local vs. paracapsular interneuron synapses with BLA pyramidal neurons) (Silberman et al., 2008). Additionally, we found that the CB1 agonist WIN impaired ethanol’s effects on GABAergic transmission in naïve and CIE rats, while AM251 only altered its actions at the postsynaptic terminal (Fig. 7). Interestingly, Talani et al. (2015) reported that WIN blocked ethanol-induced BLA GABA release in young and adult rats, but only assessed the interaction between CB1 antagonism and ethanol in young animals, reporting that a higher ethanol concentration (150 mM vs. 80 mM) was required to increase GABA release in the presence of the CB1 antagonist. In contrast, we observed that even though the CB1 agonist dampens ethanol’s actions, ethanol’s facilitation of adult rat BLA GABA release does not involve CB1. Instead, ethanol and WIN likely have independent presynaptic sites of action yet share common downstream signaling elements. It is tempting to speculate that the bidirectional effects of CB1 activation and ethanol on GABA release may derive from their opposing regulation of adenlylyl cyclase (AC). Specifically, eCB/CB1 binding activates Gi/o proteins that inhibit AC to suppress GABA release (Hill et al., 2010; Morena et al., 2015; Serrano and Parsons, 2011; Turu and Hunyady, 2010), while AC is activated by ethanol (Tabakoff et al., 2001; Yoshimura et al., 2006) and its activation with forskolin promotes GABA release (Talani and Lovinger, 2015). Additionally, Talani et al. (2015) recently reported that ethanol facilitation of BLA GABA release is blocked by AC inhibition; an effect previously observed in the CeA (Cruz et al., 2011) and cerebellum (Kelm et al., 2008). Therefore, we hypothesize that the initial actions of ethanol and eCBs may converge on the AC/PKA signaling pathway to antagonistically regulate BLA GABA release.
Chronic ethanol had no effect on baseline GABA release in the BLA, but significantly decreased sIPSC kinetics, indicating altered GABAA postsynaptic receptor function compared to naïve animals. Notably, significant changes in the membrane abundance of GABAA receptor subunits (increased α4 and γ, and decreased α1, α2 and δ levels) have been reported in the BLA of rats exposed to ethanol for 4 months, and this subunit composition profile was associated with shortened mIPSC kinetics (Lindemeyer et al., 2014). However, BLA mIPSC kinetics were modestly increased after 10 days of ethanol exposure of periadolescent rats (Diaz et al., 2011b). Interestingly, we found that chronic ethanol exposure did not alter acute ethanol’s effects on pre- and postsynaptic GABA terminals. A similar lack of acute ethanol tolerance with regards to BLA GABA transmission was previously observed in 10–12 day ethanol exposed rats (Diaz et al., 2011b; McCool et al., 2003) and this effect persisted 24 hours into withdrawal (Diaz et al., 2011b). Therefore, our 2–3 weeks of ethanol exposure likely alters the GABAA receptor subunit composition of BLA pyramidal neurons in adult male rats, but the acute ethanol sensitivity of these BLA pyramidal synapses is retained.
In contrast, the effects of WIN and AM251 on GABA release are greatly diminished in CIE rats, though WIN still dampens ethanol’s facilitation of GABAergic transmission. Importantly, these experiments were conducted during the initial hours of withdrawal from 2–3 weeks of ethanol exposure (brain slices were maintained in an ethanol-free aCSF), and it is currently unknown how rapidly and in which direction the BLA eCB/CB1 system responds to early withdrawal. Nevertheless, our observed decrease in BLA CB1 function in CIE rats likely stems from changes in receptor density; decreased amygdala CB1 mRNA were observed in rats undergoing 6 hours of withdrawal from 3 weeks of chronic ethanol exposure, though this effect was lost after 24 hours of withdrawal (Serrano et al., 2012). In these animals, the gene expression of eCB biosynthesis and clearance enzymes was also transiently altered, suggesting that the changes in CB1 mRNA levels may be compensatory (Serrano et al., 2012). Fluctuations in amygdala eCB/CB1 signalling also have been observed in alcoholic patients; CB1 receptor availability decreased with 2–4 weeks of abstinence (Ceccarini et al., 2014; Hirvonen et al., 2013) but increased after 4 weeks of abstinence (Neumeister et al., 2012). Alternatively, disrupted CB1 Gi/o-protein coupling has been observed after drug exposure (Serrano and Parsons, 2011; Turu and Hunyady, 2010), and this mechanism may also contribute to our finding of reduced CB1 influence. As BLA GABAergic transmission is regulated by an eCB tone in naive rats (discussed above) and chronic ethanol dampens CB1 influence in the BLA, it is surprising that basal GABA release was unaltered in the BLA of CIE rats. We suspect that the chronic ethanol-induced changes in CB1 function may be offset by enhanced BLA eCB levels (see (Serrano et al., 2012)), but dysregulation of other neuromodulatory systems, such as CCK (Chung and Moore, 2007), dopamine (Chu et al., 2012; Diaz et al., 2011a), norepinephrine (Miyajima et al., 2010; Silberman et al., 2012) and serotonin (Hofelmann et al., 2013), may also be involved in reestablishing normal levels of basal GABAergic transmission after 2–3 weeks of intermittent ethanol exposure.
The BLA plays a central role in the pathophysiology of alcohol dependence by promoting anxiety-driven alcohol drinking (Quirk and Gehlert, 2003; Stamatakis et al., 2014; Tan et al., 2014), and in abstinent alcoholic patients, stress/anxiety leads to alcohol cravings and relapse susceptability (Cooney et al., 1997; Fox et al., 2007; Sinha et al., 2009). In the rodent BLA, the eCB/CB1 system regulates local GABAergic release (Azad et al., 2004; Katona et al., 2001; Marsicano et al., 2002; Talani and Lovinger, 2015; Zhu and Lovinger, 2005) to modulate glutamatergic output to downstream effector regions (Perra et al., 2008). Therefore, eCB/CB1 inhibitory control of BLA pyramidal neuron excitability (in addition to the cell’s excitatory synaptic inputs and intrinsic excitability) can play a critical role in maintaining appropriate emotional responses (Rau et al., 2015a; Rau et al., 2015b). While 2–3 weeks of ethanol exposure did not significantly alter baseline inhibitory synaptic inputs onto BLA pyramidal cells, 10 days of ethanol exposure increased BLA glutamatergic transmission (Lack et al., 2009; Lack et al., 2007). Critically, we found that eCB/CB1 signalling in the BLA is functionally dampened after chronic ethanol, presumably reducing its inhibitory control over pyramidal neuron excitability and potentially contributing to the increased overall BLA excitability observed in models of alcohol drinking and dependence (Lack et al., 2007; Sciascia et al., 2015; Sinclair et al., 2012). Thus, the diminished influence of the eCB/CB1 system after chronic ethanol exposure has direct effects on local inhibitory control that may significantly impact overall BLA excitability, to dysregulate aversive emotional learning processes and promote anxiety-driven drinking associated with alcohol dependence.
Acknowledgments
This is manuscript number 29202 from The Scripps Research Institute. We thank Maury Cole and Terese Kimber at The Scripps Research Institute for their technical support with the alcohol vapor chambers. This study was supported by grants from the NIH: AA006420, AA017447, AA015566, AA013498, AA021491.
Footnotes
AUTHOR CONTRIBUTIONS:
MR, FPV, MB and PS were responsible for the study concept and design. FPV, MB, NS, GL and SGM contributed to the acquisition, analysis and interpretation of the data. FPV and MR wrote and edited the manuscript. All authors reviewed the manuscript and approved the final version for publication.
DISCLOSURE:
The authors declare no conflict of interest.
References
- Azad SC, Monory K, Marsicano G, Cravatt BF, Lutz B, Zieglgansberger W, Rammes G. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:9953–9961. doi: 10.1523/JNEUROSCI.2134-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarini J, Hompes T, Verhaeghen A, Casteels C, Peuskens H, Bormans G, Claes S, Van Laere K. Changes in cerebral CB1 receptor availability after acute and chronic alcohol abuse and monitored abstinence. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:2822–2831. doi: 10.1523/JNEUROSCI.0849-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu HY, Ito W, Li J, Morozov A. Target-specific suppression of GABA release from parvalbumin interneurons in the basolateral amygdala by dopamine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:14815–14820. doi: 10.1523/JNEUROSCI.2997-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung L, Moore SD. Cholecystokinin enhances GABAergic inhibitory transmission in basolateral amygdala. Neuropeptides. 2007;41:453–463. doi: 10.1016/j.npep.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol. 1997;106:243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Cruz MT, Bajo M, Maragnoli ME, Tabakoff B, Siggins GR, Roberto M. Type 7 Adenylyl Cyclase is Involved in the Ethanol and CRF Sensitivity of GABAergic Synapses in Mouse Central Amygdala. Frontiers in neuroscience. 2011;4:207. doi: 10.3389/fnins.2010.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koninck Y, Mody I. Noise analysis of miniature IPSCs in adult rat brain slices: properties and modulation of synaptic GABAA receptor channels. Journal of neurophysiology. 1994;71:1318–1335. doi: 10.1152/jn.1994.71.4.1318. [DOI] [PubMed] [Google Scholar]
- Diaz MR, Chappell AM, Christian DT, Anderson NJ, McCool BA. Dopamine D3-like receptors modulate anxiety-like behavior and regulate GABAergic transmission in the rat lateral/basolateral amygdala. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011a;36:1090–1103. doi: 10.1038/npp.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MR, Christian DT, Anderson NJ, McCool BA. Chronic ethanol and withdrawal differentially modulate lateral/basolateral amygdala paracapsular and local GABAergic synapses. The Journal of pharmacology and experimental therapeutics. 2011b;337:162–170. doi: 10.1124/jpet.110.177121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcoholism, clinical and experimental research. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Hentges ST, Low MJ, Williams JT. Differential regulation of synaptic inputs by constitutively released endocannabinoids and exogenous cannabinoids. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:9746–9751. doi: 10.1523/JNEUROSCI.2769-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Patel S, Campolongo P, Tasker JG, Wotjak CT, Bains JS. Functional interactions between stress and the endocannabinoid system: from synaptic signaling to behavioral output. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:14980–14986. doi: 10.1523/JNEUROSCI.4283-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J, Zanotti-Fregonara P, Umhau JC, George DT, Rallis-Frutos D, Lyoo CH, Li CT, Hines CS, Sun H, Terry GE, Morse C, Zoghbi SS, Pike VW, Innis RB, Heilig M. Reduced cannabinoid CB1 receptor binding in alcohol dependence measured with positron emission tomography. Molecular psychiatry. 2013;18:916–921. doi: 10.1038/mp.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofelmann D, di Benedetto B, Azad SC, Micale V, Wotjak CT, Rammes G. Lack of interaction of endocannabinoids and 5-HT(3) neurotransmission in associative fear circuits of the amygdala: evidence from electrophysiological and behavioural experiments. Brain research. 2013;1527:47–56. doi: 10.1016/j.brainres.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. The role of protein kinase A in the ethanol-induced increase in spontaneous GABA release onto cerebellar Purkinje neurons. Journal of neurophysiology. 2008;100:3417–3428. doi: 10.1152/jn.90970.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack AK, Christian DT, Diaz MR, McCool BA. Chronic ethanol and withdrawal effects on kainate receptor-mediated excitatory neurotransmission in the rat basolateral amygdala. Alcohol (Fayetteville, NY) 2009;43:25–33. doi: 10.1016/j.alcohol.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. Journal of neurophysiology. 2007;98:3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemeyer AK, Liang J, Marty VN, Meyer EM, Suryanarayanan A, Olsen RW, Spigelman I. Ethanol-induced plasticity of GABAA receptors in the basolateral amygdala. Neurochem Res. 2014;39:1162–1170. doi: 10.1007/s11064-014-1297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos M, Pastor I, de la Calle C, Barrio-Real L, Laso FJ, Gonzalez-Sarmiento R. Cannabinoid receptor 1 gene is associated with alcohol dependence. Alcoholism, clinical and experimental research. 2012;36:267–271. doi: 10.1111/j.1530-0277.2011.01623.x. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- McCool BA, Frye GD, Pulido MD, Botting SK. Effects of chronic ethanol consumption on rat GABA(A) and strychnine-sensitive glycine receptors expressed by lateral/basolateral amygdala neurons. Brain research. 2003;963:165–177. doi: 10.1016/s0006-8993(02)03966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima M, Ozaki M, Wada K, Sekiguchi M. Noradrenaline-induced spontaneous inhibitory postsynaptic currents in mouse basolateral nucleus of amygdala pyramidal neurons: comparison with dopamine-induced currents. Neuroscience letters. 2010;480:167–172. doi: 10.1016/j.neulet.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Morena M, Patel S, Bains JS, Hill MN. Neurobiological Interactions Between Stress and the Endocannabinoid System. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu A, Foldy C, Soltesz I. Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. The Journal of physiology. 2007;578:233–247. doi: 10.1113/jphysiol.2006.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeister A, Normandin MD, Murrough JW, Henry S, Bailey CR, Luckenbaugh DA, Tuit K, Zheng MQ, Galatzer-Levy IR, Sinha R, Carson RE, Potenza MN, Huang Y. Positron emission tomography shows elevated cannabinoid CB1 receptor binding in men with alcohol dependence. Alcoholism, clinical and experimental research. 2012;36:2104–2109. doi: 10.1111/j.1530-0277.2012.01815.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Otis TS, De Koninck Y, Mody I. Lasting potentiation of inhibition is associated with an increased number of gamma-aminobutyric acid type A receptors activated during miniature inhibitory postsynaptic currents. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:7698–7702. doi: 10.1073/pnas.91.16.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perra S, Pillolla G, Luchicchi A, Pistis M. Alcohol inhibits spontaneous activity of basolateral amygdala projection neurons in the rat: involvement of the endocannabinoid system. Alcoholism, clinical and experimental research. 2008;32:443–449. doi: 10.1111/j.1530-0277.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- Preuss UW, Koller G, Zill P, Bondy B, Soyka M. Alcoholism-related phenotypes and genetic variants of the CB1 receptor. Eur Arch Psychiatry Clin Neurosci. 2003;253:275–280. doi: 10.1007/s00406-003-0440-7. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Gehlert DR. Inhibition of the amygdala: key to pathological states? Annals of the New York Academy of Sciences. 2003;985:263–272. doi: 10.1111/j.1749-6632.2003.tb07087.x. [DOI] [PubMed] [Google Scholar]
- Ramikie TS, Nyilas R, Bluett RJ, Gamble-George JC, Hartley ND, Mackie K, Watanabe M, Katona I, Patel S. Multiple mechanistically distinct modes of endocannabinoid mobilization at central amygdala glutamatergic synapses. Neuron. 2014;81:1111–1125. doi: 10.1016/j.neuron.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau AR, Ariwodola OJ, Weiner JL. Postsynaptic adenosine A2A receptors modulate intrinsic excitability of pyramidal cells in the rat basolateral amygdala. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2015a;18 doi: 10.1093/ijnp/pyv017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau AR, Chappell AM, Butler TR, Ariwodola OJ, Weiner JL. Increased Basolateral Amygdala Pyramidal Cell Excitability May Contribute to the Anxiogenic Phenotype Induced by Chronic Early-Life Stress. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015b;35:9730–9740. doi: 10.1523/JNEUROSCI.0384-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Cruz M, Bajo M, Siggins GR, Parsons LH, Schweitzer P. The endocannabinoid system tonically regulates inhibitory transmission and depresses the effect of ethanol in central amygdala. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:1962–1972. doi: 10.1038/npp.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt LG, Samochowiec J, Finckh U, Fiszer-Piosik E, Horodnicki J, Wendel B, Rommelspacher H, Hoehe MR. Association of a CB1 cannabinoid receptor gene (CNR1) polymorphism with severe alcohol dependence. Drug and alcohol dependence. 2002;65:221–224. doi: 10.1016/s0376-8716(01)00164-8. [DOI] [PubMed] [Google Scholar]
- Sciascia JM, Reese RM, Janak PH, Chaudhri N. Alcohol-Seeking Triggered by Discrete Pavlovian Cues is Invigorated by Alcohol Contexts and Mediated by Glutamate Signaling in the Basolateral Amygdala. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano A, Parsons LH. Endocannabinoid influence in drug reinforcement, dependence and addiction-related behaviors. Pharmacology & therapeutics. 2011;132:215–241. doi: 10.1016/j.pharmthera.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano A, Rivera P, Pavon FJ, Decara J, Suarez J, Rodriguez de Fonseca F, Parsons LH. Differential effects of single versus repeated alcohol withdrawal on the expression of endocannabinoid system-related genes in the rat amygdala. Alcoholism, clinical and experimental research. 2012;36:984–994. doi: 10.1111/j.1530-0277.2011.01686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Ariwodola OJ, Weiner JL. beta1-adrenoceptor activation is required for ethanol enhancement of lateral paracapsular GABAergic synapses in the rat basolateral amygdala. The Journal of pharmacology and experimental therapeutics. 2012;343:451–459. doi: 10.1124/jpet.112.196022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Shi L, Brunso-Bechtold JK, Weiner JL. Distinct mechanisms of ethanol potentiation of local and paracapsular GABAergic synapses in the rat basolateral amygdala. The Journal of pharmacology and experimental therapeutics. 2008;324:251–260. doi: 10.1124/jpet.107.128728. [DOI] [PubMed] [Google Scholar]
- Sinclair CM, Cleva RM, Hood LE, Olive MF, Gass JT. mGluR5 receptors in the basolateral amygdala and nucleus accumbens regulate cue-induced reinstatement of ethanol-seeking behavior. Pharmacol Biochem Behav. 2012;101:329–335. doi: 10.1016/j.pbb.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Sparta DR, Jennings JH, McElligott ZA, Decot H, Stuber GD. Amygdala and bed nucleus of the stria terminalis circuitry: Implications for addiction-related behaviors. Neuropharmacology. 2014;76(Pt B):320–328. doi: 10.1016/j.neuropharm.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabakoff B, Nelson E, Yoshimura M, Hellevuo K, Hoffman PL. Phosphorylation cascades control the actions of ethanol on cell cAMP signalling. Journal of biomedical science. 2001;8:44–51. doi: 10.1007/BF02255970. [DOI] [PubMed] [Google Scholar]
- Talani G, Lovinger DM. Interactions between ethanol and the endocannabinoid system at GABAergic synapses on basolateral amygdala principal neurons. Alcohol. doi: 10.1016/j.alcohol.2015.08.006. Epub October 27 2015. http://dx.doi.org/10.1016/j.alcohol.2015.08.006. [DOI] [PMC free article] [PubMed]
- Tan H, Ahmad T, Loureiro M, Zunder J, Laviolette SR. The role of cannabinoid transmission in emotional memory formation: implications for addiction and schizophrenia. Front Psychiatry. 2014;5:73. doi: 10.3389/fpsyt.2014.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turu G, Hunyady L. Signal transduction of the CB1 cannabinoid receptor. Journal of molecular endocrinology. 2010;44:75–85. doi: 10.1677/JME-08-0190. [DOI] [PubMed] [Google Scholar]
- van den Wildenberg E, Janssen RG, Hutchison KE, van Breukelen GJ, Wiers RW. Polymorphisms of the dopamine D4 receptor gene (DRD4 VNTR) and cannabinoid CB1 receptor gene (CNR1) are not strongly related to cue-reactivity after alcohol exposure. Addiction biology. 2007;12:210–220. doi: 10.1111/j.1369-1600.2007.00064.x. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. The regulation of quantal size. Progress in neurobiology. 1991;36:93–130. doi: 10.1016/0301-0082(91)90019-w. [DOI] [PubMed] [Google Scholar]
- Varodayan FP, Soni N, Bajo M, Luu G, Madamba SG, Schweitzer P, Parsons LH, Roberto M. Chronic ethanol exposure decreases CB receptor function at GABAergic synapses in the rat central amygdala. Addiction biology. 2015 doi: 10.1111/adb.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Uchigashima M, Yamasaki M, Katona I, Yamazaki M, Sakimura K, Kano M, Yoshioka M, Watanabe M. Unique inhibitory synapse with particularly rich endocannabinoid signaling machinery on pyramidal neurons in basal amygdaloid nucleus. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3059–3064. doi: 10.1073/pnas.1012875108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Pearson S, Kadota Y, Gonzalez CE. Identification of ethanol responsive domains of adenylyl cyclase. Alcoholism, clinical and experimental research. 2006;30:1824–1832. doi: 10.1111/j.1530-0277.2006.00219.x. [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Retrograde endocannabinoid signaling in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:6199–6207. doi: 10.1523/JNEUROSCI.1148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]