Abstract
For some patient subgroups, HIV-infection has been associated with worse outcomes after kidney transplantation (KT); potentially modifiable factors may be responsible. The study goal was to identify factors that predict a higher risk of graft loss among HIV+ KT recipients compared with a similar transplant among HIV− recipients. 82,762 deceased donor KT (HIV+: 526; HIV−: 82,236) reported to SRTR (2001–2013) were studied by interaction term analysis. Compared to HIV− recipients, HCV amplified risk 2.72-fold among HIV+ KT recipients (aHR: 2.72, 95%CI: 1.75–4.22, p<0.001); and 43% of the excess risk was attributable to the interaction between HIV and HCV (AP: 0.43, 95%CI: 0.23–0.63, p=0.02). Among HIV+ recipients with >3 HLA mismatches (MM), risk was amplified 1.80-fold compared to HIV− (aHR: 1.80, 95% CI: 1.31–2.47, p < 0.001); and 42% of the excess risk was attributable to the interaction between HIV and >3 HLA MM (AP: 0.42, 95%CI: 0.24–0.60, p=0.01). High-HIV-risk (HIV+/HCV+ & >3 HLA MM) recipients had a 3.86-fold increased risk compared to low-HIV-risk (HIV+/HCV− & ≤3 HLA MM) recipients (aHR: 3.86, 95%CI: 2.37–6.30, p< 0.001). Avoidance of >3 HLA mismatches in HIV+ KT recipients, particularly among co-infected patients, may mitigate the increased risk of graft loss associated with HIV-infection.
INTRODUCTION
Studies have demonstrated that some individuals infected with human immunodeficiency virus (HIV) have increased risk for graft loss following kidney transplantation (KT) compared to their HIV-negative counterparts. This increased risk has been attributed to higher rates of acute rejection and co-infection with HCV compared to the general HIV-negative KT population.1–4 While significant, these population-based studies have drawn conclusions from multivariate regression models designed to explain or predict the outcomes of all patients in the cohort, not a specific high-risk subgroup. Conclusions from these studies are said to be generalizable, and as such, these findings have begun to effect clinical practice.
However, population-based studies may not apply to specific high-risk subgroups or individual patients. Despite predictions from population-based studies, a number of centers have reported good outcomes among HIV-infected and the even higher risk subgroup of co-infected KT recipients. In separate studies, both Stock et al. and Gasser et al. reported 100% graft survival at 1-year in case series that included both mono-infected and co-infected recipients.5,6 Excellent outcomes in these single center series may reflect experienced recipient and donor selection, and it would seem that some recipient-donor combinations may be more favorable than others. Although there is a general sense that outcomes among HIV-infected KT recipients could be optimized by carefully matching donor and recipient factors, the interaction or effect modification between HIV-infection and modifiable risk factors have not been fully elucidated.
The goal of this study was to comprehensively investigate effect modification among HIV patients and within the higher risk subgroup of co-infected recipients in order to elucidate potentially modifiable risk factors for graft loss, and as such, identify optimal donor-recipient combinations.
METHODS
Data Source
This study uses data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data, submitted by members of the Organ Procurement and Transplantation Network (OPTN), on all donors, waitlisted candidates, and transplant recipients in the United States. The Health Resources and Services Administration of the US Department of Health and Human Services provides the oversight to the activities of the OPTN and SRTR contractors.
Study Design
A cohort of 82,762 kidney-only deceased donor transplant recipients (≥18years old) between January 1, 2001 and December 31, 2013 was identified (HIV+: 526, HIV−: 82,236). The primary outcome was graft survival which was defined as time from transplantation to graft loss, return to dialysis, or death and was censored for administrative end-of-study. Comparisons of recipient and donor characteristics by HIV-status were analyzed using Wilcoxon rank-sum tests for continuous variables and chi-squared tests for categorical variables.
Regression Model Development
Risk factors for graft loss within the HIV+ cohort were identified using univariate Cox proportional hazards with statistical significance set at 0.1. The proportional hazards assumption was assessed using complementary log-log plots. As a sensitivity analysis, a stratified model was fit to minimize Schoenfeld residuals, and the hazard ratio (HR) for covariates in the stratified model were compared with estimates from the non-stratified model; HRs varied by less than 1% and thus, for ease of interpretation, results from non-stratified models are presented. Furthermore, as another sensitivity analysis, a model including time-dependent variables was fit, with similar inferences.
Exploration of Effect Modification
We further explored the potential for individual donor and transplant factors to modify the effect of HIV infection on risk for graft loss. Specifically, effect modification occurs when the magnitude of the effect or association of a particular risk factor (e.g. HCV, CIT, HLA mismatch, etc) on the risk for graft loss differs depending on recipient HIV-infection status. In this situation, computing an overall estimate of association is misleading, and interaction term analyses are necessary to better understand the influence of HIV-infection in mitigating or amplifying risk of graft loss associated with a particular risk factor.
To this end, univariate Cox proportional hazards models were used to identify relevant donor and transplant factors. First-degree interaction terms with those factors were analyzed in univariate and multivariate Cox regression models adjusted for age, race, HLA mismatches, PRA, history of diabetes, donor age, and donor race. To allow for interpretation of each interaction term and to avoid overfitting the model, eight different models for each factor were fit in which each model contained the covariates for adjustment and one interaction term of HIV-infection and a covariate of interest.
The first-degree interaction terms allowed for exploration of interaction on the multiplicative scale. Interaction on the additive scale was investigated by calculating the relative excess risk due to the interaction (RERI) and the attributable proportion of risk due to the interaction (AP) using the methods outlined by Li et al.7 The lowest risk group for each interaction is presented as the referent group, as recommended by Knol and VanderWeele, with the exception of the interaction between HIV and HLA mismatches to assist with interpretation.8
Sensitivity Analyses
To further assess the robustness of our inferences additional sensitivity analyses were performed. Specifically, multivariate model adjusting for all clinically and biologically relevant factors was constructed. Effect modification was then assessed using the interaction term approach. In addition to the covariates adjusted for in the parsimonious model reported in the results, the full model also controlled for transplant era (pre and post introduction of integrase inhibitors), maintenance immunosuppression regimen, induction immunosuppression, cold ischemia time, and kidney donor profile index. Inferences were confirmed.
All analyses were performed using SAS 9.3 (Cary, NC).
RESULTS
Patient and Donor Characteristics
During the study period, 526 HIV-infected and 82,236 HIV-negative patients underwent deceased donor KT. Compared to their HIV-negative counterparts, HIV-infected recipients were more commonly male (79.1% vs. 60.3%), African American (79.5% vs. 33.0%), under 50 years of age (55.3% vs. 38.2%), and infected with HCV (26.4% vs. 5.9%). Median time on dialysis was longer among HIV-infected recipients (5.6 years (IQR: 3.4–8.2) vs. 3.2 years (IQR: 1.6–5.2)). Fewer HIV-infected patients had diabetes (16.7% vs. 34.9%), were highly sensitized (PRA>80%) (7.7% vs. 13.1%), or underwent re-transplantation (3.2% vs. 13.0%). There were no differences in utilization of kidneys with KDPI>85% or with cold ischemia times (CIT) >12hours based on HIV status (Table 1).
Table 1.
Patient characteristics by HIV status
| Demographics | HIV+(n=526) | HIV-(n=82,236) | P value |
|---|---|---|---|
| Recipient characteristics | |||
| Age ≥ 50 yr | 44.7% (235) | 61.8% (50,853) | <0.001 |
| Male | 79.1% (416) | 60.3% (49,569) | <0.001 |
| African-American race | 79.5% (418) | 33.0% (27,122) | <0.001 |
| BMI (kg/m2) | 25.5 (22.9–29.1) | 27.6 (24.0–31.8) | <0.001 |
| HLA mismatch > 3 | 79.5% (418) | 70.3% (57,771) | <0.001 |
| Diabetes | 16.7% (86) | 34.9% (28,401) | <0.001 |
| Time on dialysis (yrs): median, IQR | 5.6 (3.4–8.2) | 3.2 (1.6–5.2) | <0.001 |
| PRA >80% | 7.7% (39) | 13.1% (10,600) | 0.003 |
| HCV infection | 26.4% (134) | 5.9% (4,692) | <0.001 |
| Retransplant | 3.2% (17) | 13.0% (10,698) | <0.001 |
| Donor characteristics | |||
| Age ≥ 50 yr | 25.1% (132) | 30.1% (24,724) | 0.01 |
| African-American | 15.4% (81) | 14.2% (11,691) | 0.44 |
| HCV+* | 55.2% (74) | 35.5% (1,662) | <0.001 |
| KDPI: median, IQR | 46 (24–70) | 46 (23–70) | 0.69 |
| KDPI ≥ 85% | 11.0% (58) | 11.8% (9,714) | 0.56 |
| CIT (hrs): median, IQR | 18.0 (12.0–25.0) | 17.0 (11.75–23.0) | 0.01 |
| CIT ≥ 12 h | 76.4% (386) | 74.6% (58,606) | 0.33 |
Among HCV+ recipients only
BMI, body mass index; CIT, cold ischemia time; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HLA, human leukocyte antigen; IQR, interquartile range; KDPI, Kidney Donor Profile Index; PRA, panel-reactive antibody.
Risk Factors Stratified by HIV-status
On unadjusted analyses, HCV in the setting of HIV-infection was associated with a higher risk of graft loss (hazard ratio (HR): 2.22, 95%CI: 1.58–3.14, p<0.001) than HCV among HIV-negative recipients (HR: 1.48, 95%CI: 1.40–1.57, p<0.001). Similarly, >3 HLA mismatches was associated with higher risk of graft loss among HIV-infected recipients (HR: 1.94, 95%CI: 1.31–2.87, p=0.001) than HIV-negative recipients (HR: 1.21, 95%CI: 1.17–1.25, p<0.001); and high KDPI (>85%) kidneys were associated with slightly higher risk of graft loss among HIV-infected recipients (HR: 1.93, 95%CI: 1.16–3.21, p=0.01) than HIV-negative recipients (HR: 1.87, 95%CI: 1.80–1.95, p<0.001) (Table 2).
Table 2.
Univariate hazard ratios for all-cause graft loss after transplant
| HIV+ | HIV− | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Recipient characteristics | ||||
| Age ≥ 50 yr | 1.22 (0.97–1.53) | 0.08 | 1.44 (1.40–1.49) | <0.001 |
| Male | 1.30 (0.88–1.92) | 0.20 | 1.15 (1.12–1.18) | <0.001 |
| African-American race | 1.46 (1.04–2.04) | 0.03 | 1.26 (1.21–1.32) | <0.001 |
| BMI (kg/m2) | 1.00 (0.97–1.03) | 0.97 | 1.01 (1.01–1.02) | <0.001 |
| HLA mismatch > 3 | 1.94 (1.31–2.87) | 0.001 | 1.21 (1.17–1.25) | <0.001 |
| PRA >80% | 1.33 (0.79–2.22) | 0.28 | 1.06 (1.01–1.10) | 0.01 |
| HCV infection | 2.22 (1.58–3.14) | <0.001 | 1.48 (1.40–1.57) | <0.001 |
| Diabetes | 1.15 (0.80–1.65) | 0.44 | 1.54 (1.49–1.58) | <0.001 |
| Time on dialysis (yrs) | 1.02 (0.98–1.07) | 0.34 | 1.03 (1.03–1.04) | <0.001 |
| Previous transplant | 1.24 (0.62–2.46) | 0.38 | 0.99 (0.95–1.03) | 0.68 |
| Donor characteristics | ||||
| Age ≥ 50 yr | 1.20 (0.92–1.56) | 0.18 | 1.61 (1.55–1.66) | <0.001 |
| African-American | 1.01 (0.67–1.53) | 0.95 | 1.22 (1.17–1.26) | <0.001 |
| HCV+* | 1.01 (0.61–1.66) | 0.97 | 1.32 (1.20–1.46) | <0.001 |
| KDPI ≥ 85 | 1.93 (1.16–3.14) | 0.01 | 1.87(1.80–1.95) | <0.001 |
| KDPI | 1.01 (1.00–1.02) | 0.04 | 1.01 (1.01–1.02) | <0.001 |
| CIT (hr) | 0.99 (0.99–1.02) | 0.82 | 1.01 (1.00–1.01) | 0.001 |
| CIT ≥ 12 | 1.26 (0.79–2.00) | 0.34 | 1.13 (1.08–1.19) | <0.001 |
| DGF | 1.84 (1.35–2.51) | <0.001 | 1.91 (1.84–1.98) | <0.001 |
Among HCV+ recipients only
BMI, body mass index; CIT, cold ischemia time; DGF, delayed graft function; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HLA, human leukocyte antigen; HR, hazard ratio; IQR, interquartile range; KDPI, Kidney Donor Profile Index; PRA, panel-reactive antibody.
Identification of Effect Modifiers
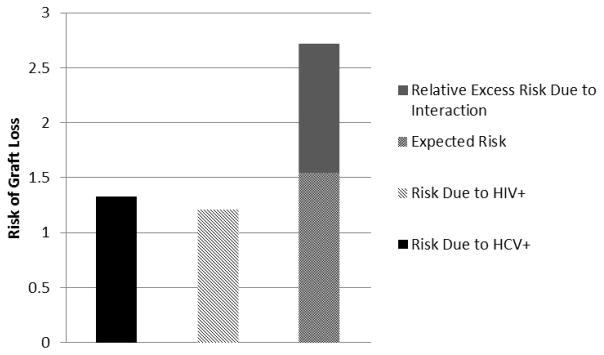
Infection with both HIV and HCV increased the risk of graft loss 2.72-fold compared to uninfected recipients (HIV−/HCV−) (adjusted HR (aHR): 2.72, 95%CI: 1.75–4.22, p < 0.001). The joint effects of co-infection resulted in a positive multiplicative interaction of 1.68 or 68% greater risk than expected (HR: 1.68, 95% CI: 1.19–2.36, p=0.003). Moreover, the joint effects of co-infection also resulted in a positive interaction on the additive scale, such that of the observed increased risk, 43% (AP: 0.43, 95%CI: 0.23–0.63, p=0.02) was attributable to the interaction between HIV and HCV infections and exceeded the expected effect of the sum of the individual exposures (RERI: 1.17; 95%CI: 0.15–2.19, p=0.02) (Table 3 & Figure 1).
Table 3.
Modification of the effect of recipient HIV status on likelihood of graft loss by recipient and donor characteristics (adjusted)
| RECIPIENT FACTORS | HIV+ | HIV− | HR within strata | ||
|---|---|---|---|---|---|
|
| |||||
| N with/without graft loss | N with/without graft loss | ||||
| HR (95% CI) | HR (95% CI) | ||||
| HCV− A | 102/272 | 1.21 (0.92, 1.60) | 22,222/53,267 | Ref | 1.21 (0.92, 1.60) |
| HCV+ | 69/65 | 2.72 (1.75, 4.22) | 1,950/2,742 | 1.33 (1.26, 1.41) | 2.04 (1.32, 3.16) |
| HCV+*HIV+ | 1.68 (1.19, 2.36) | P=0.003 | |||
|
| |||||
| AP | 0.43 (0.23, 0.63) | RERI | 1.17 (0.15, 2.19) | p=0.02 | |
|
| |||||
| HLA mismatch ≤3 A | 27/81 | 0.91 (0.58, 1.43) | 7,021/17,444 | Ref | 0.91 (0.58, 1.43) |
| HLA mismatch >3 | 151/267 | 1.80 (1.31, 2.47) | 17,900/39,871 | 1.13 (1.10, 1.17) | 1.59 (1.15, 2.19) |
| HIV+*HLA>3 | 1.74 (1.15, 2.65) | ||||
|
| |||||
| AP | 0.42 (0.24, 0.60) | RERI | 0.75 (0.22, 1.28) | p=0.01 | |
|
| |||||
| DONOR FACTORS | |||||
| Age < 50B | 128/266 | 1.64 (1.20, 2.24) | 14,471/42,041 | Ref | 1.64 (1.20, 2.24) |
| Age ≥ 50 | 50/82 | 1.66 (1.07, 2.57) | 9,450/15,274 | 1.50 (1.45, 1.55) | 1.11 (0.72, 1.70) |
| HIV+*Age≥ 50 | 0.68 (0.49, 0.94) | ||||
|
| |||||
| AP | −0.29 (−0.71, 0.14) | RERI | −0.48 (−1.02, 0.07) | p=0.09 | |
|
| |||||
| Non-AAB | 153/292 | 1.52 (1.13, 2.05) | 21,032/49,513 | Ref | 1.52 (1.13, 2.05) |
| African-American | 25/56 | 1.36 (0.74, 2.53) | 3,889/7,802 | 1.20 (1.13, 2.14) | 1.14 (0.62, 2.11) |
| HIV+*AA Race | 0.75 (0.47, 1.19) | ||||
|
| |||||
| AP | −0.26 (−0.88, 0.36) | RERI | −0.35 (−0.98, 0.27) | p=0.27 | |
|
| |||||
| KDPI < 85 A | 186/459 | 1.46 (1.11, 1.91) | 30,545/92,514 | Ref | 1.46 (1.11, 1.91) |
| KDPI ≥85 | 32/26 | 2.31 (1.12, 4.76) | 4,369/5,345 | 1.65 (1.58, 1.73) | 1.40 (0.68, 2.86) |
| HIV+*KDPI≥85 | 0.96 (0.53, 1.72) | ||||
|
| |||||
| AP | 0.08 (−0.48, 0.65) | RERI | 0.20 (−1.25, 1.64) | p=0.79 | |
|
| |||||
| CIT <12 hrs A | 67/200 | 1.31 (0.93, 1.84) | 46,163/12,330 | Ref | 1.31 (0.93, 1.84) |
| CIT≥12 hrs | 134/256 | 1.64 (1.12, 2.41) | 18,300/41,162 | 1.10 (1.06, 1.16) | 1.49 (1.01, 2.18) |
| HIV+*CIT≥12 | 1.14 (0.70, 1.84) | ||||
|
| |||||
| AP | 0.14 (−0.23, 0.51) | RERI | 0.23 (−0.50, 0.96) | p=0.54 | |
adjusted for recipient age, race, HCV status, HLA mismatches, PRA, diabetes, CIT, and KDPI
adjusted for recipient age, race, HCV status, HLA mismatches, PRA, diabetes, donor age, and donor race
AA, African-American; AP, attributable proportion; CIT, cold ischemia time; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HLA, human leukocyte antigen; HR, hazard ratio; KDPI, Kidney Donor Profile Index; PRA, panel-reactive antibody; RERI, relative excess risk due to interaction.
Figure 1.
Risk of graft loss by exposure category (HIV-infection and HCV-infection). Models were adjusted for the following: recipient age, race, HCV status, HLA mismatches, PRA, history of diabetes, donor age, and donor race. HCV, hepatitis C virus; HIV, human immunodeficiency virus; HLA, human leukocyte antigen; PRA, panel-reactive antibody.
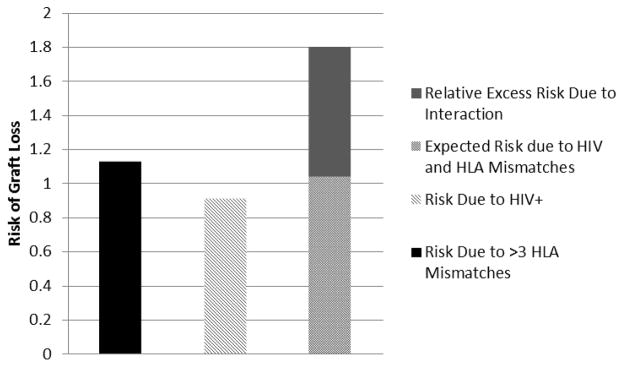
Among HIV recipients with >3 HLA mismatches, the risk of graft loss was 1.80-fold greater than patients with neither exposure (HIV−/HLA ≤3) (aHR: 1.80, 95%CI: 1.31–2.47, p < 0.001). The joint effects of both exposures resulted in a positive multiplicative interaction of 1.74 or 74% greater risk than expected (HR: 1.74, 95% CI: 1.15–2.65, p=0.01). There was also a positive interaction on the additive scale, such that of the observed increased risk with >3 HLA mismatches, 42% (AP: 0.42, 95%CI: 0.24–0.60, p=0.01) was attributable to the interaction between HIV-infection and >3 HLA mismatches and exceeded the expected effect of the sum of the individual exposures (RERI: 0.75, 95%CI: 0.22–1.28, p=0.01) (Table 3 & Figure 2).
Figure 2.
Risk of graft loss by exposure category (HIV-infection and HLA mismatches). Models were adjusted for the following: recipient age, race, HCV status, HLA mismatches, PRA, history of diabetes, donor age, and donor race. HCV, hepatitis C virus; HIV, human immunodeficiency virus; HLA, human leukocyte antigen; PRA, panel-reactive antibody.
Among monoinfected HIV+ recipients with ≤3 HLA mismatches, there was no difference in risk of graft loss compared to their counterfactuals from the general population (HIV−/HCV−& ≤3 HLA mismatches) (aHR: 0.95, 95%CI: 0.62–1.47, p=0.82).
Other examined donor and transplant factors, including older donor age (>50 years), donor race, high KDPI (>85%), CIT and development of DGF, did not significantly modify the effect of HIV-infection on the risk of graft loss.
HIV-infected Recipient Subgroup Analysis
High-HIV-risk recipients were defined as being co-infected with HCV (HIV+/HCV+) and having received a kidney with >3 HLA mismatches, and low-HIV-risk recipients were defined as being mono-infected (HIV+/HCV−) and having received a kidney with ≤3 HLA mismatches. Of patients receiving KT during the study period, 16% were low-HIV-risk (mono-infected and ≤3 HLA mismatches). When high-HIV-risk recipients of KT with were compared with low-HIV-risk recipients of KT there was a 3.86-fold increased risk of graft loss (aHR: 3.86, 95%CI: 2.37–6.30, p< 0.001). Moreover, among KT recipients with both exposures, co-infection with HCV and >3 HLA mismatches, 57% of the observed increased risk was attributable to the interaction (AP: 0.57; 95%CI: 0.21–0.92, p=0.02) and exceeded the expected effect of the sum of the individual exposures (RERI: 2.19, 95%CI: 0.33–4.04, p=0.02) (Table 4 & Figure 3).
Table 4.
Modification of the effect of recipient HCV status on likelihood of graft loss by recipient and donor characteristics (adjusted)
| RECIPIENT FACTORS | HIV+/HCV+ | HIV+/HCV− | HR within strata | ||
|---|---|---|---|---|---|
|
| |||||
| N with/without graft loss | N with/without graft loss | ||||
| HR (95% CI)A | HR (95% CI)A | ||||
| HLA mismatch ≤3 A | 6/13 | 1.00 (0.32, 3.15) | 20/64 | Ref | 1.00 (0.32, 3.15) |
| HLA mismatch >3 | 63/52 | 3.86 (2.37, 6.30) | 82/208 | 1.68 (1.08, 2.61) | 2.30 (1.62, 3.28) |
| HIV+*HLA>3 | 2.30 (0.67, 7.97) | ||||
|
| |||||
| AP | 0.57 (0.21, 0.92) | RERI | 2.19 (0.33, 4.04) | p=0.02 | |
adjusted for recipient age, race, HCV status, HLA mismatches, PRA, diabetes, CIT, and KDPI
AP, attributable proportion; CIT, cold ischemia time; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HLA, human leukocyte antigen; HR, hazard ratio; KDPI, Kidney Donor Profile Index; PRA, panel-reactive antibody; RERI, relative excess risk due to interaction.
Figure 3.
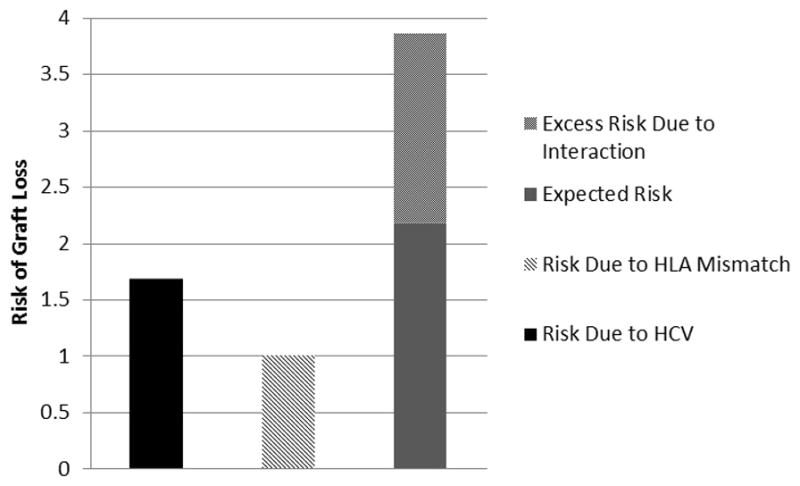
Risk of graft loss by exposure category among HIV+ recipients. Models were adjusted for the following: recipient age, race, HCV status, HLA mismatches, PRA, history of diabetes, donor age, and donor race. HCV, hepatitis C virus; HIV, human immunodeficiency virus; HLA, human leukocyte antigen; PRA, panel-reactive antibody.
DISCUSSION
We have compared the characteristics and outcomes of 526 DDKT in HIV-infected recipients with 82,236 DDKT in HIV-negative recipients. Despite clinical judgment and current selection processes, we confirmed previous reports that KT in certain high risk subgroups of HIV-infected recipients was associated with higher risk of graft loss when compared to KT in the HIV-negative general transplant population. However, the adverse consequences of recipient HIV-infection seem to be more pronounced in certain donor-recipient combinations than others. In this study we were able to identify a low-HIV-risk subgroup of recipients (mono-infected and ≤3 HLA mismatches) for whom the risk of graft loss attributable to HIV-infection was minimized and not statistically different from the general population (HIV−/HCV− & <3 HLA mismatches). In contrast, for high-HIV-risk recipients (co-infected and >3 HLA mismatches) the risk of graft loss was 3.86-fold higher. A number of other possible effect modifiers were identified but were not statistically significant, including donor age, race, type, CIT, and DGF.
Increased risk for graft loss among co-infected recipients highlights the negative impact of HCV infection on outcomes after KT. Our findings are consistent with prior studies in both the general HIV-negative and HIV-infected KT populations, which have also demonstrated significantly lower graft survival in the setting of recipient HCV infection.2,3,9,10 Interestingly, the deleterious effects of HCV appear more pronounced among HIV-infected recipients than among their HIV-negative counterparts. Historically, HCV infection was not thought to be a modifiable risk factor. However, the introduction of newer antiviral therapies capable of sustained virologic responses >99% has ushered in a new era in the treatment of HCV, offering the potential for cure and mitigation for this associated increased risk for graft loss. More recently, indications for these antiviral therapies have been extended to include individuals with evidence of renal dysfunction, affording the opportunity to completely mitigate the risk of HCV infection prior to the transplant event.
In addition, HIV-infected KT recipients were more susceptible to the degree of HLA mismatching, such that >3 HLA mismatches significantly amplified the increased risk for graft loss associated with HIV-infection. The amplification of the HIV effect was most pronounced among the high-HIV-risk subgroup, which included coinfected recipients who received a KT with >3HLA mismatches. Traditionally, high degree of HLA mismatching was associated with higher rejection rates and worse graft survival.11,12 In the setting of modern immunosuppressant medications, such as lymphocyte depleting agents and calcineurin inhibitor-based maintenance therapies, the paradigm has shifted, and studies have demonstrated excellent long-term graft outcomes independent of the degree of HLA mismatching.13 Our data suggest, however, that greater HLA mismatching significantly amplifies the risk for graft loss among HIV-infected KT recipients, in particular the subset that are co-infected with HCV. Avoidance of HLA mismatches may serve to mitigate the increased risk for graft loss observed in KT recipients infected with HIV.
It is important to note that the only transplant factor in our analyses that significantly amplified the risk of graft loss in those infected with HIV was >3 HLA mismatches. Intuitively, it would seem that other donor/transplant factors, such as KDPI>85%, donor age, and CIT, would also modify the effect of HIV on outcomes. However, it is likely that the results of our interaction term analyses were impacted by selection bias created by the clinical judgement of transplant physicians, which is not captured in our secondary dataset. Moreover, SRTR’s data lack granularity with regard to important recipient factors known to influence outcomes among HIV-infected individuals, including CD4 counts, viral loads, infections, and malignancies, and as such, it was not possible to assess the ability of those factors to modify the effect of HIV-infection on graft outcomes. SRTR data also lack granularity with regard to anti-retroviral therapy (ART), and as such, it was not possible to assess the impact of potential drug-drug interactions between maintenance immunosuppression and ART type. However, we did perform sensitivity analyses adjusting for transplant era (pre and post introduction of integrase inhibitors), maintenance immunosuppression, and induction type; and inferences were confirmed.
In conclusion, we have shown that HCV infection and >3 HLA mismatches significantly amplify the risk of graft loss associated with HIV-infection; and in contrast, monoinfected HIV+ recipients (HIV+/HCV−) with ≤3 HLA mismatches have risk of graft loss that is no different from their uninfected counterfactuals (HIV−/HCV− & ≤3 HLA mismatches). These findings suggest that aggressive treatment to eradicate HCV infection prior to KT and avoidance of high degrees of HLA mismatching may serve to mitigate increased risk of graft loss among HIV+ KT recipients.
Acknowledgments
This research was supported in part by the National Institute of Health grant numbers K24-DK101828 (PI: Segev) and K23-DK103918 (PI: Locke), and the US Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation contract number HHSH250201000018C (Segev).
Abbreviations
- HIV
human immunodeficiency virus
- KT
kidney transplantation
- HLA
human leukocyte antigen
- MM
mismatches
- aHR
adjusted hazard ratio
- HCV
hepatitis C
- SRTR
Scientific Registry of Transplant Recipients
- OPTN
Organ Procurement and Transplantation Network
- CIT
cold ischemia time
- PRA
panel reactive antibody
- RERI
relative excess risk due to the interaction
- AP
attributable proportion of risk due to the interaction
Footnotes
DISCLAIMER
The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or US Government.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Roland ME, Barin B, Carlson L, et al. HIV-infected liver and kidney transplant recipients: 1- and 3-year outcomes. Am J Transplant. 2008 Feb;8(2):355–365. doi: 10.1111/j.1600-6143.2007.02061.x. [DOI] [PubMed] [Google Scholar]
- 2.Stock PG, Barin B, Murphy B, et al. Outcomes of kidney transplantation in HIV-infected recipients. N Engl J Med. 2010 Nov 18;363(21):2004–2014. doi: 10.1056/NEJMoa1001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Locke JE, Mehta S, Reed RD, et al. A National Study of Outcomes among HIV-Infected Kidney Transplant Recipients. J Am Soc Nephrol. 2015 Sep;26(9):2222–2229. doi: 10.1681/ASN.2014070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locke JE, James NT, Mannon RB, et al. Immunosuppression regimen and the risk of acute rejection in HIV-infected kidney transplant recipients. Transplantation. 2014 Feb 27;97(4):446–450. doi: 10.1097/01.TP.0000436905.54640.8c. [DOI] [PubMed] [Google Scholar]
- 5.Stock PG, Roland ME, Carlson L, et al. Kidney and liver transplantation in human immunodeficiency virus-infected patients: a pilot safety and efficacy study. Transplantation. 2003 Jul 27;76(2):370–375. doi: 10.1097/01.TP.0000075973.73064.A6. [DOI] [PubMed] [Google Scholar]
- 6.Gasser O, Bihl F, Sanghavi S, et al. Treatment-dependent loss of polyfunctional CD8+ T-cell responses in HIV-infected kidney transplant recipients is associated with herpesvirus reactivation. Am J Transplant. 2009 Apr;9(4):794–803. doi: 10.1111/j.1600-6143.2008.02539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol. 2007 Mar;17(3):227–236. doi: 10.1016/j.annepidem.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 8.VanderWeele TJ, Knol MJ. A Tutorial on Interaction. Epidemiol Methods. 2014;3(1):33–72. [Google Scholar]
- 9.Xia Y, Friedmann P, Yaffe H, et al. Effect of HCV, HIV and coinfection in kidney transplant recipients: mate kidney analyses. Am J Transplant. 2014 Sep;14(9):2037–2047. doi: 10.1111/ajt.12847. [DOI] [PubMed] [Google Scholar]
- 10.Mazuecos A, Fernandez A, Andres A, et al. HIV infection and renal transplantation. Nephrol Dial Transplant. 2011 Apr;26(4):1401–1407. doi: 10.1093/ndt/gfq592. [DOI] [PubMed] [Google Scholar]
- 11.Persijn GG, Cohen B, Lansbergen Q, et al. Effect of HLA-A and HLA-B matching on survival of grafts and recipients after renal transplantation. N Engl J Med. 1982 Oct 7;307(15):905–908. doi: 10.1056/NEJM198210073071501. [DOI] [PubMed] [Google Scholar]
- 12.Beckingham IJ, Dennis MJ, Bishop MC, et al. Effect of human leucocyte antigen matching on the incidence of acute rejection in renal transplantation. Br J Surg. 1994 Apr;81(4):574–577. doi: 10.1002/bjs.1800810432. [DOI] [PubMed] [Google Scholar]
- 13.Martins L, Fonseca I, Sousa S, et al. The influence of HLA mismatches and immunosuppression on kidney graft survival: an analysis of more than 1300 patients. Transplant Proc. 2007 Oct;39(8):2489–2493. doi: 10.1016/j.transproceed.2007.07.033. [DOI] [PubMed] [Google Scholar]