Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
Hif-1α is dispensable for cell-autonomous HSC survival.
HSCs do not require intrinsic Hif-1α to respond to hematopoietic injury.
Abstract
The hematopoietic stem cell (HSC) pool is maintained under hypoxic conditions within the bone marrow microenvironment. Cellular responses to hypoxia are largely mediated by the hypoxia-inducible factors, Hif-1 and Hif-2. The oxygen-regulated α subunits of Hif-1 and Hif-2 (namely, Hif-1α and Hif-2α) form dimers with their stably expressed β subunits and control the transcription of downstream hypoxia-responsive genes to facilitate adaptation to low oxygen tension. An initial study concluded that Hif-1α is essential for HSC maintenance, whereby Hif-1α–deficient HSCs lost their ability to self-renew in serial transplantation assays. In another study, we demonstrated that Hif-2α is dispensable for cell-autonomous HSC maintenance, both under steady-state conditions and following transplantation. Given these unexpected findings, we set out to revisit the role of Hif-1α in cell-autonomous HSC functions. Here we demonstrate that inducible acute deletion of Hif-1α has no impact on HSC survival. Notably, unstressed HSCs lacking Hif-1α efficiently self-renew and sustain long-term multilineage hematopoiesis upon serial transplantation. Finally, Hif-1α–deficient HSCs recover normally after hematopoietic injury induced by serial administration of 5-fluorouracil. We therefore conclude that despite the hypoxic nature of the bone marrow microenvironment, Hif-1α is dispensable for cell-autonomous HSC maintenance.
Introduction
Hematopoietic stem cells (HSCs) reside in hypoxic bone marrow (BM) niches where they self-renew and sustain life-long multilineage hematopoiesis.1-3 Cellular responses to hypoxia are predominantly mediated by the hypoxia-inducible factors, Hif-1 and Hif-2, which facilitate the transcription of hypoxia-responsive genes. Several studies used a conditional gene knockout strategy to determine the role of Hif-1α and Hif-2α in HSC functions.4,5 An initial study concluded that inducible Hif-1α deletion from the mouse hematopoietic system results in the progressive loss of HSCs upon serial transplantation,4 indicating that Hif-1α is required for HSC maintenance.4 We demonstrated that constitutive or inducible hematopoiesis-specific Hif-2α deletion did not affect HSC maintenance.5 Surprisingly, codeletion of Hif-1α and Hif-2α had no impact on HSC numbers, steady-state hematopoiesis, or reconstitution upon transplantation.5 These unexpected findings gave rise to the hypothesis that Hif-1α may not be as essential for HSC maintenance as previously suggested.4 Here, using serial transplantation assays, we demonstrate that unstressed HSCs do not critically require Hif-1α to survive and self-renew.
Materials and methods
Mice
Hif-1αfl/fl, Mx1-Cre, and Vav-iCre mice have been described previously6-8 and were of C57BL/6 genetic background. Sex-matched 8- to 12-week-old mice were used. Animal experiments were authorized by the UK Home Office.
FACS analysis
Analysis and sorting was done using BD LSRFortessa cell analyzer and BD FACSAriaII cell sorter. Antibodies (see supplemental Table 1 on the Blood Web site) were used as described previously.9-11 Representative gating is shown in supplemental Figure 1.
Transplantation assays
A total of 500 000 CD45.2+ test donor unfractionated BM cells were injected intravenously into lethally irradiated (10 Gy) CD45.1+/CD45.2+ congenic recipients alongside 500 000 CD45.1+ competitor unfractionated BM cells. Conditional gene deletion was achieved by administration of 6 intraperitoneal polyinosinic-polycytidylic acid (pIpC) injections over 10 days, every other day (GE Healthcare; 0.3 mg per dose). For Lin−Sca-1+c-Kit+ (LSK) cell transplantation assays, 3000 LSK cells sorted from donor BM were transplanted together with 200 000 CD45.1+ unfractionated BM cells.
Statistical analysis
Statistical significance was determined using Mann-Whitney or Mantel-Cox tests.
Results and discussion
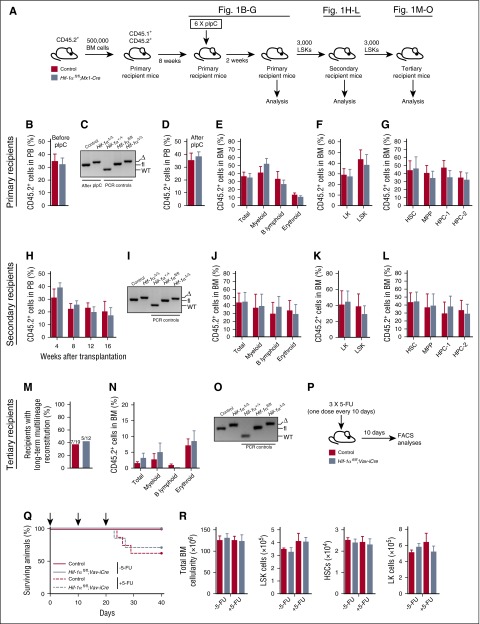
We acutely deleted Hif-1α specifically within the hematopoietic system using Mx1-Cre5,9 (Figure 1A). Given that Mx1-Cre recombines within the hematopoietic system, BM microenvironment, and extramedullary tissues,7,12 we first transplanted unfractionated BM from untreated CD45.2+ Hif-1αfl/fl;Mx1-Cre and control (Hif-1α+/+ without Mx1-Cre or Hif-1αfl/fl without Mx1-Cre) mice into wild-type (WT) lethally irradiated syngeneic recipients (Figure 1A). Following reconstitution (Figure 1B), recipients received pIpC 8 weeks after transplantation and were analyzed 2 weeks later. Peripheral blood (PB) analyses of the pIpC-treated recipients revealed that deletion of Hif-1α (Figure 1C) had no impact on CD45.2+ donor-derived chimerism (Figure 1D) and multilineage hematopoiesis in the BM (Figure 1E) and spleen (supplemental Figure 2). The contribution of donor-derived cells to Lin–Sca-1–c-Kit+ (LK), LSK, LSKCD48−CD150+ HSC, LSKCD48−CD150− multipotent progenitor (MPP), LSKCD48+CD150− hematopoietic progenitor cell-1 (HPC-1), and LSKCD48+CD150+ HPC-2 compartments was also comparable (Figure 1F-G). Therefore, acute deletion of Hif-1α has no immediate impact on HSC survival and multilineage hematopoiesis.
Figure 1.
Hematopoiesis-specific deletion of Hif-1α does not affect HSC survival and maintenance and their ability to respond to 5-FU–induced stress. (A) Experimental design. A total of 500 000 donor-derived (CD45.2+) unfractionated BM cells from untreated Hif-1αfl/fl;Mx1-Cre and control (without Mx1-Cre) mice were transplanted into lethally irradiated syngeneic CD45.1+/CD45.2+ recipient mice (together with 500 000 CD45.1+ competitor BM cells). Two independent donors were used per genotype. Eight weeks posttransplantation, the mice received 6 sequential doses of pIpC over a period of 10 days (every alternate day) and were analyzed 2 weeks after last dose of pIpC. CD45.2+ LSK cells from the primary recipients were serially transplanted into secondary and tertiary recipients. (B) Percentage of CD45.2+ donor-derived cells 8 weeks posttransplantation in the PB of primary recipient mice before pIpC treatment (n = 5-6 per group). (C) Representative gel showing PCR amplification of genomic DNA from donor-derived CD45.2+ fraction of the PB of pIpC-treated recipient mice 2 weeks after last pIpC injection. Δ, excised allele; fl, undeleted conditional allele. For PCR controls, we used genomic DNA from c-Kit+ cells from BM of Hif-1α+/+, Hif-1αfl/fl, and Hif-1αfl/fl;Vav-iCre mice. (D) Percentage of CD45.2+ donor-derived cells in PB 2 weeks after the last pIpC injection (n = 5-6 per group). (E) Percentage of CD45.2+ cells in the unfractionated BM (total BM cells) and myeloid (CD11b+Gr1+), B lymphoid (CD19+B220+), and erythroid (Ter119+) cell compartments of the primary recipient mice. Data are mean ± standard error of the mean (SEM; n = 5-6 per group). (F) Percentage of CD45.2+ donor-derived cells measured in BM LK and LSK cell compartments of the primary recipients. Data are mean ± SEM (n = 5-6 per group). (G) Percentage of CD45.2+ donor-derived cells in LSK CD48−CD150+ HSC, LSK CD48−CD150− MPP, and primitive progenitor (LSK CD48+CD150− HPC-1 and LSK CD48+CD150+ HPC-2) cell compartments of primary recipients. Data are mean ± SEM (n = 5-6 per group). (H) PB chimerism in secondary recipients of control and Hif-1α∆/∆ LSK cells sorted from BM of the primary recipients. Data are mean ± SEM (n = 6 per group). (I) Representative gel showing efficient deletion of the conditional alleles of Hif-1α in the CD45.2+ BM cells of secondary recipients 16 weeks after transplantation. For PCR controls, we used genomic DNA from c-Kit+ cells from BM of Hif-1α+/+, Hif-1αfl/fl, and Hif-1αfl/fl;Vav-iCre mice. (J) Percentage of CD45.2+ cells in total BM, myeloid, B lymphoid, and erythroid cell compartments of the secondary recipient mice 16 weeks posttransplantation. Data are mean ± SEM (n = 6 per group). (K-L) Percentage of CD45.2+ cells in BM stem and progenitor cell compartments of the secondary recipient mice 16 weeks posttransplantation. Data are mean ± SEM (n = 6 per group). (M) Results of the tertiary transplantation assay. The graph shows the percentage of tertiary recipients with long-term multilineage reconstitution (>0.5% of donor-derived myeloid and lymphoid cells in BM) 16 weeks after tertiary transplantation of Hif-1α∆/∆ and control LSK cells sorted from BM of the secondary recipients. Data are mean ± SEM (n = 12-19 per group). (N) Percentage of CD45.2+ cells in the BM hematopoietic compartments of tertiary recipient mice 16 weeks after transplantation (n = 12-19 per group). (O) Representative gel showing efficient deletion of the conditional alleles of Hif-1α in the CD45.2+ BM cells of tertiary recipients 16 weeks after transplantation. For PCR controls, we used genomic DNA from c-Kit+ cells from BM of Hif-1α+/+, Hif-1αfl/fl, and Hif-1αfl/fl;Vav-iCre mice. (P) Experimental design. Hif-1αfl/fl;Vav-iCre and control mice received 3 sequential doses of 5-FU (150 mg/kg; 10 days apart) and were analyzed 10 days after the last 5-FU administration. In parallel, untreated Hif-1αfl/fl;Vav-iCre and control mice that did not receive 5-FU were also analyzed. (Q) Kaplan-Meier survival curve of Hif-1αfl/fl;Vav-iCre and control mice treated with 5-FU (ie, +5-FU) (n = 7-8 per group) or those that were not treated with 5-FU (ie, −5-FU) (n = 4-6 per group). Arrows in the graph indicate 5-FU administration. (R) Total numbers (per 2 femurs and 2 tibias) of BM white blood cells, LSK cells, HSCs, and LK cells from the mice described in panels P and Q. Data are mean ± SEM (n = 4-6 per group). At least 2 independent experiments were performed for all analyses.
To test the self-renewal capacity of Hif-1α–deficient (Hif-1αΔ/Δ) HSCs, we performed competitive serial transplantation assays with sorted CD45.2+ LSK cells from primary recipients 2 weeks after pIpC treatment (Figure 1A). LSK cells from primary recipients efficiently reconstituted short- and long-term hematopoiesis in secondary recipients (Figure 1H). Sixteen weeks after transplantation, sustained Hif-1α deletion was confirmed (Figure 1I), and Hif-1αΔ/Δ LSK cells efficiently contributed to all differentiated lineages in the BM, spleen (Figure 1J; supplemental Figure 3), and LK, LSK, HSC, MPP, and HPC BM compartments of the secondary recipient mice (Figure 1K-L). Finally, sorted Hif-1αΔ/Δ LSK cells from secondary recipients (16 weeks after secondary transplantation) successfully reconstituted long-term multilineage hematopoiesis in tertiary recipients (Figure 1M-N). Complete Hif-1α deletion was maintained for the duration of these experiments (Figure 1O). Therefore, self-renewing long-term HSCs do not critically require Hif-1α to maintain their pool upon the stress of serial transplantation.
To test the cell-autonomous role of Hif-1α in HSC stress responses, we used Hif-1αfl/fl;Vav-iCre mice in which Hif-1α is conditionally deleted specifically from hematopoietic cells using a codon-improved Cre (iCre).5,8 Hif-1αfl/fl;Vav-iCre and control mice received 3 doses of 5-fluorouracil (5-FU) and were analyzed 10 days after the last 5-FU administration (Figure 1P). We also analyzed untreated Hif-1αfl/fl;Vav-iCre and control mice that did not receive 5-FU. Although 5-FU-treated mice had decreased survival compared with untreated mice, we observed no differences in survival between 5-FU–treated Hif-1αfl/fl;Vav-iCre and control mice (Figure 1Q). The 5-FU–treated Hif-1αfl/fl;Vav-iCre and control mice had comparable total BM cellularity and BM LSK, HSC, and LK cell numbers (Figure 1R); thus, Hif-1α is not essential for cell-autonomous HSC maintenance following serial 5-FU administration.
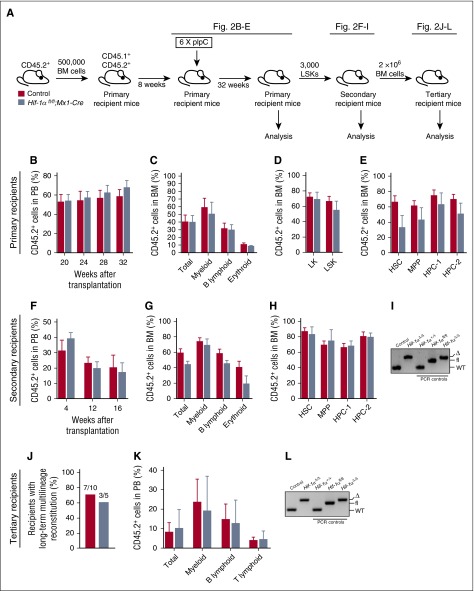
We next determined the long-term consequences of Hif-1α deletion from the hematopoietic system. We transplanted unfractionated BM cells from untreated Hif-1αfl/fl;Mx1-Cre or control mice, administered pIpC, and analyzed the primary recipients 32 weeks after pIpC treatment (Figure 2A). PB and BM analyses indicated that loss of Hif-1α did not affect long-term multilineage hematopoiesis (Figure 2B-C) or donor-derived chimerism in the stem and progenitor cell compartments of the BM (Figure 2D-E) and spleens (data not shown) of the recipient mice 32 weeks after pIpC treatment. Moreover, LSK cells of both genotypes equally reconstituted long-term multilineage hematopoiesis in secondary recipients (Figure 2F-G) and evenly contributed to the stem and progenitor cell compartments of the recipients (Figure 2H). Efficient Hif-1α deletion was confirmed by polymerase chain reaction (PCR) on genomic DNA from donor-derived cells (Figure 2I). Given that donor-derived HSCs in this experiment have undergone long-term stress and are very rare in secondary recipients, to perform tertiary transplantation assays instead of retransplanting purified LSK cells, we transplanted unfractionated BM cells harvested from secondary recipients 16 weeks posttransplantation. BM cells of both genotypes equally generated multilineage hematopoiesis in tertiary recipients (Figure 2J-K). Efficient Hif-1α gene deletion was maintained for the duration of the serial transplantation experiment (Figure 2L). Thus, HSCs with chronic Hif-1α deficiency sustain normal steady-state multilineage hematopoiesis and display normal regenerative capacity upon serial transplantation.
Figure 2.
Prolonged Hif-1α deficiency does not affect the steady-state maintenance of HSCs and their regenerative capacity. (A) Experimental design. Primary recipient mice were transplanted with 500 000 donor-derived unfractionated BM cells from untreated Hif-1αfl/fl;Mx1-Cre and control mice (together with 500 000 CD45.1+ competitor BM cells). Eight weeks posttransplantation, the mice received 6 doses of pIpC. The mice were analyzed 32 weeks after the last dose of pIpC. CD45.2+ LSK cells from the primary recipients were transplanted into secondary recipients. A total of 2 × 106 total BM cells from secondary recipients were transplanted into tertiary recipients together with 200 000 CD45.1+ unfractionated BM cells. (B) CD45.2+ donor-derived PB chimerism 20, 24, 28, and 32 weeks after the last dose of pIpC. Mean ± SEM (n = 5-7 per group). (C) Percentage of CD45.2+ cells in total BM, myeloid, B lymphoid, and erythroid cell compartments of primary recipients 32 weeks after pIpC treatment. Data are mean ± SEM (n = 5-7 per group). (D-E) Percentage of CD45.2+ cells in the indicated hematopoietic compartments of the primary recipients. Data are mean ± SEM (n = 5-7 per group). (F) LSK cells of indicated genotypes were pooled from primary recipients 32 weeks after pIpC treatment and transplanted into secondary recipients. The graph shows CD45.2+ donor-derived PB chimerism in secondary recipient mice 4, 12 and 16 weeks after transplantation. Data are mean ± SEM (n = 4-7 per group). (G) CD45.2+ donor-derived chimerism within the total BM cell fraction, and myeloid, B lymphoid, and erythroid cell compartments of secondary recipient mice 16 weeks after transplantation. Data are mean ± SEM (n = 4-7 per group). (H) Percentage of CD45.2+ cells in the indicated hematopoietic stem and progenitor cell compartments of the secondary recipients. Mean ± SEM (n = 4-7 per group). (I) PCR amplification of genomic DNA from donor-derived CD45.2+ cell fraction of the PB of secondary recipient mice 12 weeks posttransplantation. For PCR controls, we used genomic DNA from c-Kit+ cells from BM of Hif-1α+/+, Hif-1αfl/fl, and Hif-1αfl/fl;Vav-iCre mice. (J) Results of tertiary transplantation assays. The graph depicts the percentage of tertiary recipients with long-term multilineage reconstitution (>0.5% of donor-derived myeloid and lymphoid cells in PB) 16 weeks after tertiary transplantation of 2 × 106 total BM cells obtained from the secondary recipients (n = 5-10 per genotype). (K) Percentage of CD45.2+ cells in white blood cell (total) compartment, and myeloid, B lymphoid, and T lymphoid cell compartments of PB in tertiary recipients 16 weeks after transplantation. Data are mean ± SEM (n = 5-10 per group). (L) Hif-1α gene deletion was confirmed by PCR on genomic DNA from donor-derived CD45.2+ cell fraction of the BM of tertiary recipients 16 weeks posttransplantation. For PCR controls, we used genomic DNA from c-Kit+ cells from BM of Hif-1α+/+, Hif-1αfl/fl, and Hif-1αfl/fl;Vav-iCre mice. At least 2 independent experiments were performed for all analyses.
Finally, we set out to delete Hif-1α more broadly from the BM using Mx1-Cre, which, in addition to hematopoietic cells, also recombines in the BM microenvironment.13 Hif-1αfl/fl;Mx1-Cre and control mice received 6 doses of pIpC and were analyzed 30 days after the last injection (supplemental Figure 4A). pIpC-treated Hif-1αfl/fl;Mx1-Cre and control mice had comparable numbers of nucleated BM cells, LSK cells, and HSC, MPP, HPC-1, and HPC-2 populations (supplemental Figure 4B). We next treated Hif-1αfl/fl;Mx1-Cre and control mice with 6 doses of pIpC followed by 2 doses of 5-FU (supplemental Figure 4C). We found that a combined stress of pIpC treatment and subsequent 5-FU administration resulted in substantially decreased survival of these mice (supplemental Figure 4D). Importantly, however, there was no difference in survival of pIpC- and 5-FU–treated Hif-1αfl/fl;Mx1-Cre and control mice; therefore, Mx1-Cre–mediated deletion of Hif-1α had no impact on HSC numbers or their ability to respond to 5-FU.
HSCs are constantly exposed to local hypoxia within the BM14,15 and the low oxygen tension has been proposed to protect HSCs.16 Takubo et al4 found a defect in HSC self-renewal upon Hif-1α deletion and suggested that Hif-1α is an important mediator of HSC functions. Here we set out to acutely induce deletion of Hif-1α in the hematopoietic system and determine its short- and long-term impact on HSC maintenance. We found that inactivation of Hif-1α did not compromise survival of HSCs 2 weeks after gene ablation and unstressed Hif-1αΔ/Δ HSCs self-renewed equally efficiently compared with control counterparts upon serial transplantation. Furthermore, deletion of Hif-1α had no long-term consequences for maintenance of unstressed HSCs within 32 weeks after pIpC administration and did not compromise their ability to repopulate recipient mice upon serial transplantation. Different conclusions from our study and that of Takubo et al may result from discrepancies in experimental designs. Although they serially transplanted LSK cells from pIpC-treated Hif-1αfl/fl;Mx1-Cre mice, we conducted our serial transplantation assays using Hif-1αΔ/Δ LSK cells sorted from pIpC-treated WT chimeric recipient mice harboring Hif-1αfl/fl;Mx1-Cre BM cells. Given that Mx1-Cre recombines also within the BM microenvironment12 and that Hif-1α is required for BM mesenchymal progenitor cell functions,17 Hif-1α deletion from the BM microenvironment may have affected HSC functions, thus contributing to the phenotypes observed by Takubo et al. Finally, to test the cell-autonomous role of Hif-1α in ageing, Takubo et al transplanted Hif-1αfl/fl;Mx1-Cre BM cells into recipient mice and administered pIpC 4 months later. Although they did not observe any differences 4 months after pIpC treatment, 11 months after pIpC treatment, they found that the level of repopulation by Hif-1α–deficient BM cells was decreased. This raises a possibility that the cell-autonomous Hif-1α deficiency causes HSC defects only when exposed to an ageing BM microenvironment.
In closing, our data presented here, taken together with our previous findings,5 indicate that Hif-1 and Hif-2 are not essential for HSC functions. Specifically, we conclude that despite the hypoxic nature of the BM microenvironment, self-renewing HSCs do not critically require intrinsic Hif-1α to maintain their pool and sustain long-term multilineage hematopoiesis.
Acknowledgments
K.R.K. is a Cancer Research UK Senior Fellow. K.R.K.'s laboratory is supported by grants from Cancer Research UK, Bloodwise, Edinburgh Cancer Research UK Centre Development Fund, The Wellcome Trust ISSF award, Medical Research Council, and the Kay Kendall Leukaemia Fund. T.L.H. was supported by Cancer Research UK.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.R.K. designed experiments and wrote the manuscript. P.J.R. and T.L.H. helped with experimental designs. M.V., C. Sepulveda, and C. Subramani performed all experiments. A.V.G., J.M., L.A., T.I.P., J.P., H.L., A.V., A.A.-D., and D.G. helped with experiments.
Conflict-of-interest disclosure: The authors declare no conflict of interest.
Correspondence to: Kamil R. Kranc, MRC Centre for Regenerative Medicine, 5 Little France Dr, University of Edinburgh, Edinburgh, United Kingdom; e-mail: kamil.kranc@ed.ac.uk.
References
- 1.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takubo K, Goda N, Yamada W, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7(3):391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 5.Guitart AV, Subramani C, Armesilla-Diaz A, et al. Hif-2α is not essential for cell-autonomous hematopoietic stem cell maintenance. Blood. 2013;122(10):1741–1745. doi: 10.1182/blood-2013-02-484923. [DOI] [PubMed] [Google Scholar]
- 6.Ryan HE, Poloni M, McNulty W, et al. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 2000;60(15):4010–4015. [PubMed] [Google Scholar]
- 7.Kühn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269(5229):1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 8.de Boer J, Williams A, Skavdis G, et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur J Immunol. 2003;33(2):314–325. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- 9.Kranc KR, Schepers H, Rodrigues NP, et al. Cited2 is an essential regulator of adult hematopoietic stem cells. Cell Stem Cell. 2009;5(6):659–665. doi: 10.1016/j.stem.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mortensen M, Soilleux EJ, Djordjevic G, et al. The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance. J Exp Med. 2011;208(3):455–467. doi: 10.1084/jem.20101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calaminus SD, Guitart AV, Sinclair A, et al. Lineage tracing of Pf4-Cre marks hematopoietic stem cells and their progeny. PLoS One. 2012;7(12):e51361. doi: 10.1371/journal.pone.0051361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walkley CR, Shea JM, Sims NA, Purton LE, Orkin SH. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell. 2007;129(6):1081–1095. doi: 10.1016/j.cell.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joseph C, Quach JM, Walkley CR, Lane SW, Lo Celso C, Purton LE. Deciphering hematopoietic stem cells in their niches: a critical appraisal of genetic models, lineage tracing, and imaging strategies. Cell Stem Cell. 2013;13(5):520–533. doi: 10.1016/j.stem.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Spencer JA, Ferraro F, Roussakis E, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508(7495):269–273. doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA. 2007;104(13):5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantel CR, O’Leary HA, Chitteti BR, et al. Enhancing Hematopoietic Stem Cell Transplantation Efficacy by Mitigating Oxygen Shock. Cell. 2015;161(7):1553–1565. doi: 10.1016/j.cell.2015.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guarnerio J, Coltella N, Ala U, Tonon G, Pandolfi PP, Bernardi R. Bone marrow endosteal mesenchymal progenitors depend on HIF factors for maintenance and regulation of hematopoiesis. Stem Cell Rep. 2014;2(6):794–809. doi: 10.1016/j.stemcr.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]