Fig. 5.
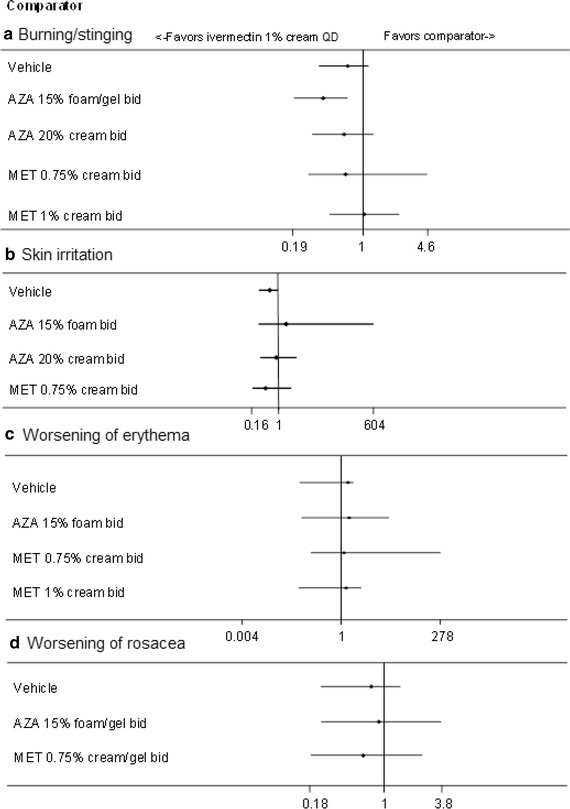
Results of MTC analyses between ivermectin 1 % cream QD and comparators for the incidence of specific adverse events at 12 weeks [burning/stinging (A), skin irritation (B), worsening or erythema (C), and worsening of rosacea (D)]. Risk ratios evaluate the probability of success (relieving rosacea) when using ivermectin 1 % cream QD, compared to other comparator treatments. A risk ratio >1 demonstrates a greater likelihood of success using ivermectin 1 % cream QD, a RR Crl that does not cross 1 demonstrates a significant difference between ivermectin 1 % cream QD and the comparator (positive values indicate superiority, negative values indicate inferiority). Studies contributing to A: six studies (Gold et al. 2014a; Stein et al. 2014; Tan et al. 2002; Galderma 2006; Bjerke et al. 1999; Draelos et al. 2013), B: four studies (Gold et al. 2014a; Stein et al. 2014; Galderma 2006; Bjerke et al. 1999), C: four studies (Tan et al. 2002; Galderma 2006; NCT00617903 2013; Draelos et al. 2013), D: four studies (Gold et al. 2014a; Galderma 2006; NCT00617903 2013; Draelos et al. 2013). MTC results are derived from a fixed effects model. AZA azelaic acid, bid twice daily, Crl credible interval, MET metronidazole, MTC mixed treatment comparison, QD once daily, RR risk ratio
