Abstract
Background—
The clinical profile and arrhythmic outcome of competitive athletes with isolated nonischemic left ventricular (LV) scar as evidenced by contrast-enhanced cardiac magnetic resonance remain to be elucidated.
Methods and Results—
We compared 35 athletes (80% men, age: 14–48 years) with ventricular arrhythmias and isolated LV subepicardial/midmyocardial late gadolinium enhancement (LGE) on contrast-enhanced cardiac magnetic resonance (group A) with 38 athletes with ventricular arrhythmias and no LGE (group B) and 40 healthy control athletes (group C). A stria LGE pattern with subepicardial/midmyocardial distribution, mostly involving the lateral LV wall, was found in 27 (77%) of group A versus 0 controls (group C; P<0.001), whereas a spotty pattern of LGE localized at the junction of the right ventricle to the septum was respectively observed in 11 (31%) versus 10 (25%; P=0.52). All athletes with stria pattern showed ventricular arrhythmias with a predominant right bundle branch block morphology, 13 of 27 (48%) showed ECG repolarization abnormalities, and 5 of 27 (19%) showed echocardiographic hypokinesis of the lateral LV wall. The majority of athletes with no or spotty LGE pattern had ventricular arrhythmias with a predominant left bundle branch block morphology and no ECG or echocardiographic abnormalities. During a follow-up of 38±25 months, 6 of 27 (22%) athletes with stria pattern experienced malignant arrhythmic events such as appropriate implantable cardiac defibrillator shock (n=4), sustained ventricular tachycardia (n=1), or sudden death (n=1), compared with none of athletes with no or LGE spotty pattern and controls.
Conclusions—
Isolated nonischemic LV LGE with a stria pattern may be associated with life-threatening arrhythmias and sudden death in the athlete. Because of its subepicardial/midmyocardial location, LV scar is often not detected by echocardiography.
Keywords: athletes, cardiomyopathy, myocarditis, sport, sudden death
WHAT IS KNOWN
CMR shows subendocardial/midmyocardial LV LGE suggesting scar in a variety of diseases associated with sudden cardiac death, including hypertrophic, dilated, and arrhythmogenic cardiomyopathy.
Isolated LV LGE has been reported in athletes with apparently idiopathic ventricular arrhythmias and/or repolarization abnormalities, but the clinical meaning of this finding remains to be elucidated.
WHAT THE STUDY ADDS
Athletes showing ventricular arrhythmias with a right bundle branch block pattern (suggesting a left ventricular origin) and isolated nonischemic LV LGE at CMR can experience malignant arrhythmia, including sudden cardiac death.
The pattern of LGE can be helpful; subepicardial/midmyocardial stria pattern was associated with life-threatening arrhythmias, whereas the junctional spotty pattern was nonspecific for these arrhythmias.
Because these LV scars can escape detection by echocardiography, athletes presenting with right bundle branch block morphology ventricular arrhythmias should be further investigated by contrast-enhanced CMR.
An underlying structural cardiac abnormality is found in most cases of life-threatening ventricular arrhythmias (VA) and sudden cardiac death (SCD) during sports.1–6 Malignant arrhythmic events may occur in athletes with a structurally normal heart, as a result of a genetic channelopathy.7–9 On the contrary, failure to detect structural heart abnormalities may depend on the unknown or concealed nature of the underlying pathological substrates along with the low sensitivity of available clinical tests. Subtle structural heart conditions potentially at risk of arrhythmic cardiac arrest include focal myocarditis and segmental cardiomyopathies that may remain undetected by standard clinical examination including echocardiography.8,9
Contrast-enhanced cardiac magnetic resonance (CE-CMR) imaging has become in the recent years part of clinical work-up of athletes with VA.10 Besides evaluating the presence of morphofunctional ventricular abnormalities, CE-CMR allows myocardial tissue characterization by late gadolinium enhancement (LGE) technique, which provides information on the presence, morphology, and wall distribution of myocardial scar tissue otherwise overlooked.11,12 A nonischemic left ventricular (LV) scar, which is characteristically localized at the midmyocardial or subepicardial layers of the LV wall, can be found in a broad spectrum of heart muscle diseases at risk of SCD, including myocarditis, sarcoidosis, dilated cardiomyopathy, hypertrophic cardiomyopathy, and arrhythmogenic right ventricular cardiomyopathy (ARVC).13 Isolated, nonischemic LV LGE has been previously reported in anecdotic cases or small series of individuals presenting with VA and/or repolarization abnormalities.14–16 Whether this isolated, segmental LV myocardial lesion may act as a substrate of life-threatening arrhythmias and SCD in the athlete remains to be elucidated. The presence of spotty LGE at the junction of the right ventricle (RV) with the ventricular septum has been consistently reported in a sizeable proportion of endurance athletes and related to the duration and intensity of sports activity.17–26 This morphological pattern of LGE is traditionally deemed a nonclinically relevant manifestation of the structural adaptive changes of the athlete’s heart; however, follow-up studies evaluating its arrhythmic risk are lacking.
The main objective of this study was to characterize the clinical and imaging profile and the arrhythmic outcome of a cohort of competitive athletes showing isolated nonischemic LGE on CE-CMR, which was performed for clinical evaluation of apparently idiopathic VA. The CE-CMR features and outcome of the index athletes were compared with those of athletes with frequent premature ventricular beats (PVB) or complex VA and no LGE and control healthy athletes to assess whether the presence, specific morphological pattern, regional localization, and wall distribution of the LGE are associated with an increased arrhythmic risk.
Methods
The Inherited Arrhythmogenic Cardiomyopathy unit of the Department of Cardiac, Thoracic, and Vascular Sciences is a tertiary center for evaluation of young people and athletes with established or suspected cardiac disease at risk of life-threatening VA. This study enrolled a series of competitive athletes with frequent PVB (>500/d) or complex VA (sustained or nonsustained ventricular tachycardia [VT] or ventricular fibrillation), which occurred in the absence of coronary artery disease, cardiomyopathy, and other clinically overt heart disease and were diagnosed as idiopathic at routine clinical evaluation. All athletes underwent a comprehensive CE-CMR study for further imaging assessment and tissue characterization of myocardial substrate.
Breakdown of the Study Population
During the period 2009 to 2014, a total of 223 competitive athletes who had undergone preparticipation cardiovascular evaluation by a sports medicine physician were referred to our cardiology center for diagnostic evaluation of VA. One hundred thirty-eight athletes were excluded from the study because they did not fulfill the enrollment criteria of frequency and complexity of VA and did not undergo CE-CMR study. Among the other 85 athletes with frequent PVB or complex VA, 8 were excluded because of a diagnosis of structural heart disease including partial anomalous pulmonary venous return (n=1), ARVC either definite (n=1) or borderline (n=2), apical hypertrophic cardiomyopathy (n=1), ventricular preexcitation (n=1), and coronary artery disease (n=2). Four athletes (all with no LGE) were lost to follow-up. The athletic study population comprised the remaining 73 athletes with frequent PVB or complex VA and routine diagnostic work-up negative for overt heart disease who underwent additional CE-CMR study. According to the CE-CMR findings, 2 groups were identified: group A (n=35) including athletes with VA and evidence of isolated LV LGE and group B (n=38) including athletes with VA and a totally negative CE-CMR study.
Controls
A group of 40 competitive athletes (group C) with a negative family history for SCD or cardiomyopathy, normal ECG, and no VA served as controls. They underwent CE-CMR for further imaging assessment of a borderline LV hypertrophy found at preparticipation echocardiography. The CE-CMR study ruled out a pathological ventricular remodeling because of hypertrophic cardiomyopathy or other structural heart diseases in all.
Clinical Investigation
At the time of first evaluation, all athletes underwent a routine clinical evaluation, including family and personal history, physical examination, resting 12-lead ECG, signal-averaged ECG, 12-lead 24-hour Holter monitoring to evaluate the morphology of VA, bicycle exercise testing with a protocol of 25 to 50 W increments every 3 minutes, and 2-dimensional transthoracic echocardiography. Late potentials on signal-averaged ECG were defined according to previously proposed criteria.27
Additional invasive tests such as coronary angiography (n=36), endomyocardial biopsy including molecular pathology investigation for viral genomes (n=6), or programmed ventricular stimulation (n=10) were reserved to selected cases.
All subjects gave written informed consent after counseling in accordance with the ethical standards of the Declaration of Helsinki (2001) and with recommendations given by the institutional ethical committee.
Contrast-Enhanced Cardiovascular Magnetic Resonance
All athletes were evaluated de novo, with repeated CE-CMR if performed in other institutions, to provide independent diagnosis.
Scan Protocol
CMR was performed on a 1.5-Tesla scanner (Magnetom Avanto, Siemens Medical Solutions, Erlangen, Germany) using a comprehensive dedicated protocol.
All patients underwent detailed CE-CMR study protocol including postcontrast sequences.
Images were acquired using a steady-state free precession sequence (true FISP) cine loops in sequential short-axis views (slice thickness 6 mm, gap 2 mm; repetition time 2.5–3.8 ms; echo time 1.1–1.6 ms, average in-plane resolution 1.5×2.4 mm, flip angle 45° to 60°, temporal resolution 40–45 ms) and long- axis views (2-, 3-, and 4-chamber views).
After intravenous administration of contrast agent (gadobenate dimeglumine, Multihance; Bracco; 0.2 mmol/kg of body weight), 2-dimensional segmented fast low-angle shot inversion recovery sequence after at least 10 minutes were acquired in the same views of cine images, covering the entire ventricles (repetition time 5.4–8.3 ms, echo time 1.3–3.9 ms, average in-plane spatial resolution 1.4–1.5×2.2–2.4 mm, 6- mm slice thickness, 2-mm gap, and flip angle 20° to 25°). Inversion times were adjusted to null normal myocardium using the Look-Locker sequence, and images were repeated in 2 separate phase-encoding directions to exclude artifacts.
Image Analysis
Global ventricular volumes, systolic function, and LV myocardial mass were calculated from the short-axis cine images, excluding papillary muscles from the myocardium, using a dedicated software (CMR42; Circle Cardiovascular Imaging, Inc). The presence, location, and extent of LGE were independently assessed by 2 experienced observers (M.P.M. and B.G.) who were blinded to patient clinical data and outcome; ambiguous cases were reviewed using a third expert (G.D.C). To exclude artifact, LGE was deemed present only if visible in 2 orthogonal views (short-axis and long-axis views). Myocardial LGE was quantified by a semiautomatic detection and LGE mass (in grams) expressed as a percentage of total LV mass, according to previously validated methods. LGE was quantified using a signal intensity threshold of >2 SD above a remote reference region. The pattern of LGE distribution and morphology was characterized as either epicardial/midmyocardial stria or patchy/junctional spotty. If >1 pattern was present, the distribution was characterized on the basis of the predominant pattern.
Follow Up
After the enrollment, patients were followed up for a mean duration of 38±25 months. The primary study end point was the occurrence of any major arrhythmic event defined as SCD, arrhythmic cardiac arrest, sustained VT or appropriate implantable cardiac defibrillators (ICD) intervention on VT/ventricular fibrillation. Routine ICD interrogation and ECG recordings at the time of symptoms were used to document the occurrence of spontaneous VT during follow-up. SCD was defined as any natural death occurring instantaneously or within one hour from symptoms onset. Sustained VT was defined as tachycardia originating in the ventricle with rate >100 beats/min and lasting >30 seconds or requiring an intervention for termination. Appropriate ICD intervention was defined as a device shock or antitachycardia overdrive pacing delivered in response to a ventricular tachyarrhythmia and documented by stored intracardiac ECG data.
Statistical Analysis
Data are expressed as mean±SD or median with 25 to 75 percentiles for normally distributed and skewed variables, respectively. Normal distribution was assessed using Shapiro–Wilk test. Categorical differences among groups were evaluated by the χ2 test or the Fisher exact test as appropriate. Differences among continuous variables were evaluated with the Kruskal–Wallis test. Kaplan–Meier analysis was used to estimate the survival distributions of the arrhythmic end point and to show the differences in survival among groups of patients. Kaplan–Meier curves were compared with the log-rank test. Start of follow-up was defined as the date of the initial CMR. Patients were followed until the time of their first event or until the time of their last clinical follow-up. A value of P<0.05 was considered significant. Statistics were analyzed with SPSS version 19 (SPSS, Inc, Chicago, IL).
Results
Clinical Findings
The clinical characteristics of the 3 study groups are shown in Table 1. There were no statistically significant differences with regard to sex and age among groups.
Table 1.
Clinical Characteristics of the Study Population
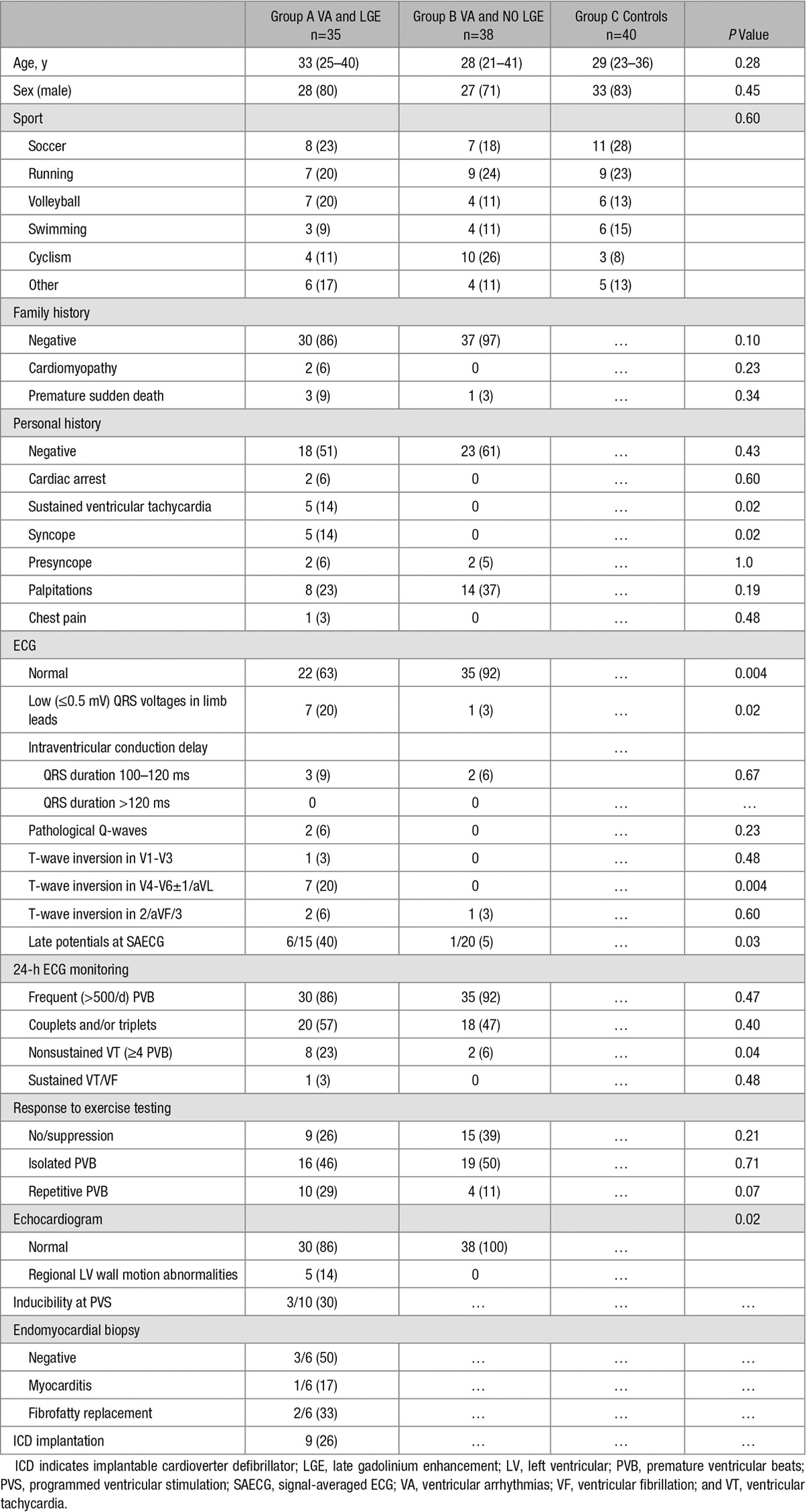
Group A included 35 athletes (80% men; mean age: 33 years; range: 14–48) with frequent PVB or complex VA and LGE at CE-CMR. Thirty (86%) had a negative family history for SCD or cardiomyopathies. Seventeen athletes (49%) had a positive personal history for ≥1 of the following events/symptoms: cardiac arrest (n=2), sustained VT (n=5), syncope (n=5), presyncope (n=5), palpitations (n=8), and/or chest pain (n=1). No patient had a previous diagnosis of acute myocarditis.
The ECG was abnormal in 13 (37%) athletes and the most common abnormalities were low QRS voltages in limb leads (20%) and T-wave inversion in inferolateral leads (20%). Signal-averaged ECG was positive in 6 of 15 (40%) athletes. In 28 athletes (80%), 12-leads, 24-hour ambulatory ECG monitoring recorded frequent PVB and/or complex VA with a predominant right bundle branch block (RBBB) morphology (suggestive of LV origin) with superior axis (n=23), inferior axis (n=3), or intermediate axis (n=2); the remaining 7 (20%) had frequent isolated PVB with a predominant left bundle branch block/inferior axis morphology. On stress testing, VA occurred or worsened at the peak of exercise in 26 athletes (74%). Echocardiography was abnormal in 5 athletes (14%) showing hypokinesis of the inferolateral LV wall. Sustained VT was induced by programmed ventricular stimulation in 3 of 10 athletes (30%).
Endomyocardial biopsy was performed in 6 athletes: histopathologic findings were consistent with focal acute myocarditis in 1, segmental fibrofatty replacement in 2, and were normal in the remaining 3. Molecular pathology investigation by polymerase chain reaction and reverse-transcriptase polymerase chain reaction was negative for viral genomes.
Group B included 38 athletes with frequent PVB or complex VA and no LGE at CE-CMR. They significantly less often showed ECG changes, nonsustained VT at 24-hour ECG monitoring, and echocardiographic LV wall motion abnormalities compared with athletes of group A (Table 1). The majority of athletes (71%) of group B had VA with a predominant left bundle branch block/inferior axis pattern.
By study design, the 40 control athletes (group C) had no VA and a negative routine cardiovascular evaluation.
CMR Features
Findings of cine CMR and tissue characterization CMR sequences are reported in Table 2.
Table 2.
Contrast-Enhanced Cardiac Magnetic Resonance Findings*
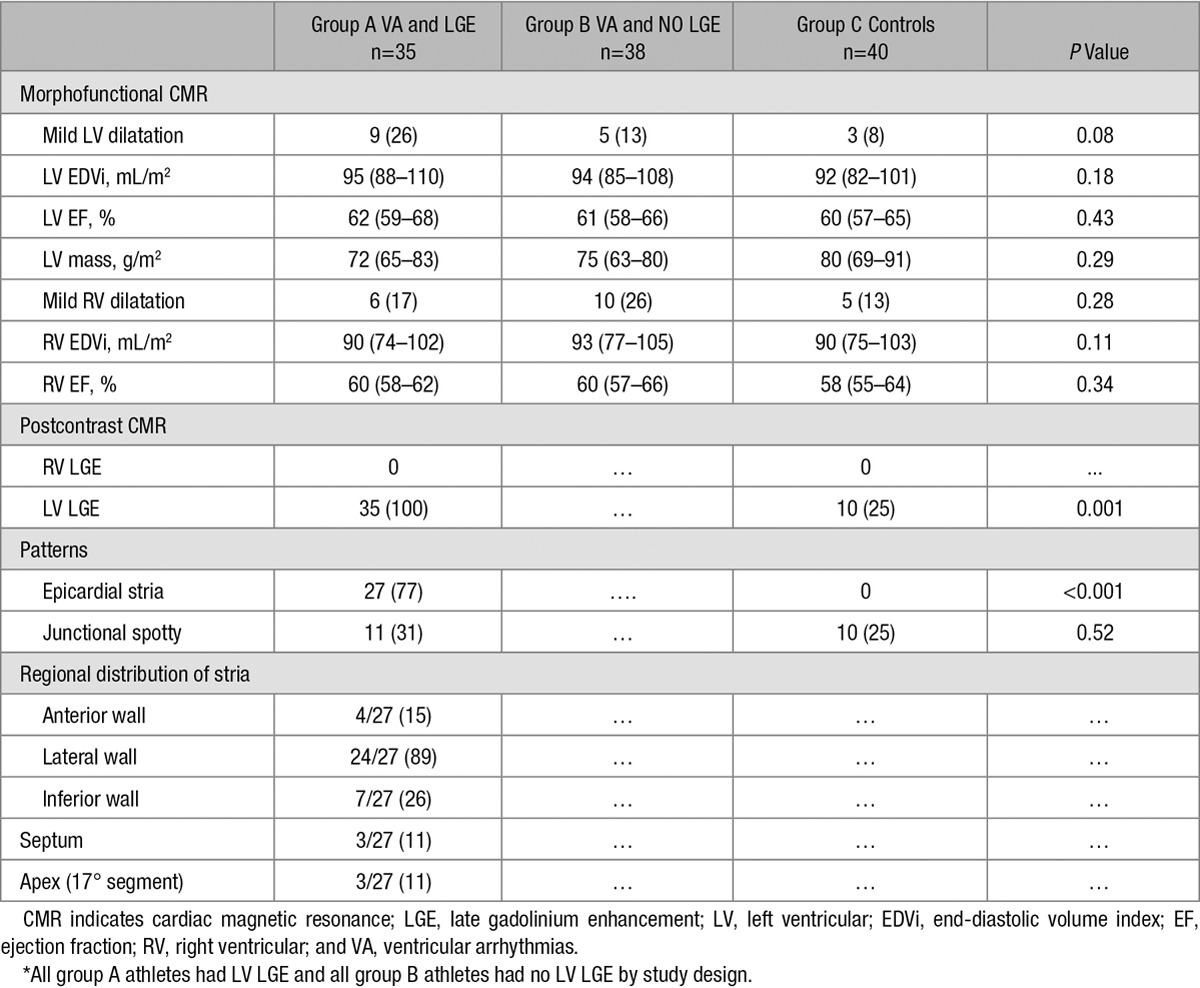
Group A
Mild LV dilation with a preserved LV ejection fraction was detected in 9 (26%) athletes of group A. Mild RV dilation in the absence of RV dysfunction was detected in 6 (17%) cases. At postcontrast sequences, 27 (77%) showed an epicardial/midmyocardial stria pattern of LGE, which was associated with a junctional spotty LGE (ie, LGE spot at the insertion points of the RV free wall to the interventricular septum) in 5 (Figure 1 and 2). The subepicardial/midmyocardial stria was localized in the lateral LV region in 24/27 patients, in isolation or in association with other regions. Three patients showed isolated involvement of the LV apical segments. The remaining 8 (23%) index athletes had isolated junctional spotty LGE at the posterior junction (N=7) or at both the anterior and posterior junctions (N=1). No patients exhibited RV LGE.
Figure 1.
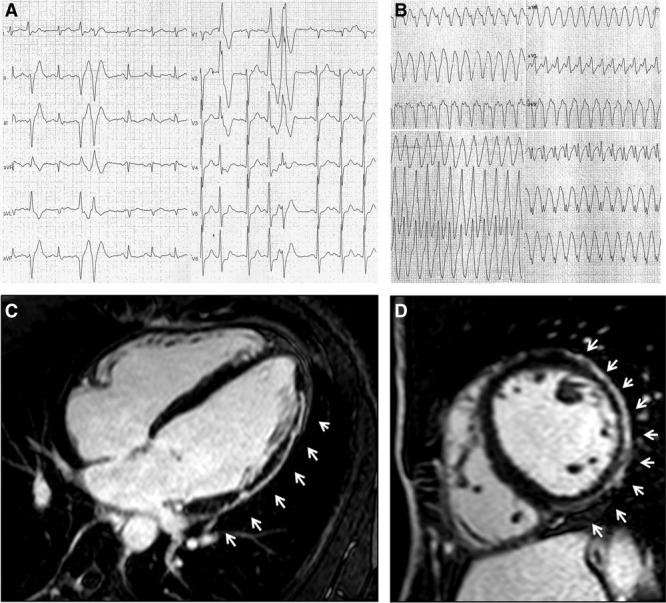
A 42-y-old martial art player presenting with frequent and coupled premature ventricular beats with right bundle branch block/superior axis morphology during exercise testing (A). The athlete experienced sustained ventricular tachycardia during follow-up (B). Contrast-enhanced cardiac magnetic resonance revealed a subepicardial/midmyocardial stria of late gadolinium enhancement involving the anterolateral, lateral, and inferolateral left ventricular wall (white arrows; C, D).
Figure 2.
A 23-y-old soccer player who suffered syncope during a match. Contrast-enhanced cardiac magnetic resonance revealed a subepicardial/midmyocardial stria of late gadolinium enhancement involving the lateral left ventricular wall (A). Twelve-lead ECG showed T-wave inversion in the inferolateral leads and premature ventricular beats (B). The patient received an ICD because of sustained ventricular tachycardia inducibility by programmed ventricular stimulation. After 13 months, he experienced an ICD shock on fast (tachycardia cycle length 200 ms) ventricular tachycardia while he was playing table tennis (C).
Among the 27 athletes with a stria pattern, 5 (19%) had a positive family history, 8 (30%) a history of sustained VT or cardiac arrest, 13 (48%) ECG changes, 5 (19%) echocardiographic abnormalities compared with none of the 8 index athletes with isolated junctional spotty LGE. Moreover, all 27 athletes with a stria pattern had VA with a predominant RBBB morphology compared with 1/8 (13%) of those with an isolated junctional spotty pattern.
Group B
Mild LV and RV dilatation, in the absence of ventricular systolic dysfunction, were found, respectively, in 5 athletes (13%) and 10 athletes (26%) of group B.
Group C
Mild LV dilatation in the absence of LV dysfunction was found in 3 of 40 (8%) controls. Mild RV dilation in the absence of RV dysfunction was detected in 5 (13%) controls. At postcontrast sequences, 10 controls (25%) showed LV LGE, all with a junctional spotty pattern. The junctional LGE spot was localized in the posterior junction, alone (n=8) or associated with anterior junction (n=2). No control exhibited RV LGE.
Comparison Among Groups
At cine CMR, morphofunctional features did not significantly differ among the 3 groups. The epicardial/midmyocardial stria pattern was distinctively observed in athletes with VA (group A), whereas the prevalence of junctional spotty LGE was similar in athletes of group A and controls (Figure 3).
Figure 3.
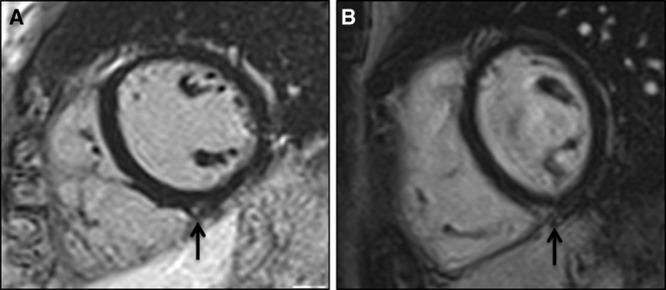
Short-axis postcontrast cardiac magnetic resonance views of a 27-y-old rower with frequent premature ventricular beats with a left bundle branch block/inferior axis morphology (suggestive of right ventricular outflow tract origin) (A) and in a 31-y-old healthy marathon runner without arrhythmias (B) showing late gadolinium enhancement with a spotty pattern at the inferior insertion point of the right ventricular free wall to the interventricular septum (black arrows).
Follow Up
Thirty-two athletes were restricted from competitive sports activity including all 27 index athletes with arrhythmias and a subepicardial/midmyocardial stria pattern of LGE, 1 athlete with complex VA and isolated junctional spotty pattern, and 4 athletes with complex, exercise-induced VA and no LGE. Twenty-five athletes with demonstration of exercise-inducible LV arrhythmias and LV LGE were prescribed β-blockers and 9 group A athletes received an ICD for either secondary prevention (cardiac arrest N=2; spontaneous sustained VT N=5) or primary prevention (syncope and inducible VT/ventricular fibrillation by programmed ventricular stimulation, N=2).
During follow-up, 6 patients (all with a subepicardial/midmyocardial stria of LV LGE) experienced major arrhythmic events including appropriate ICD shocks (n=4), SCD (n=1), and sustained VT (n=1). Five of six events occurred during exercise (Table 3). One patient had a progressive LV dysfunction leading over time to end-stage heart failure requiring heart transplantation 5 years after initial evaluation (Figure 4). Both the athlete who died suddenly and the one who underwent heart transplantation had pathological evidence of biventricular subepicardial and/or midmural fibrofatty replacement, with a distribution in keeping with a diagnosis of left-dominant ARVC.
Table 3.
Characteristics of Patients Who Experienced Clinical Events During Follow-Up
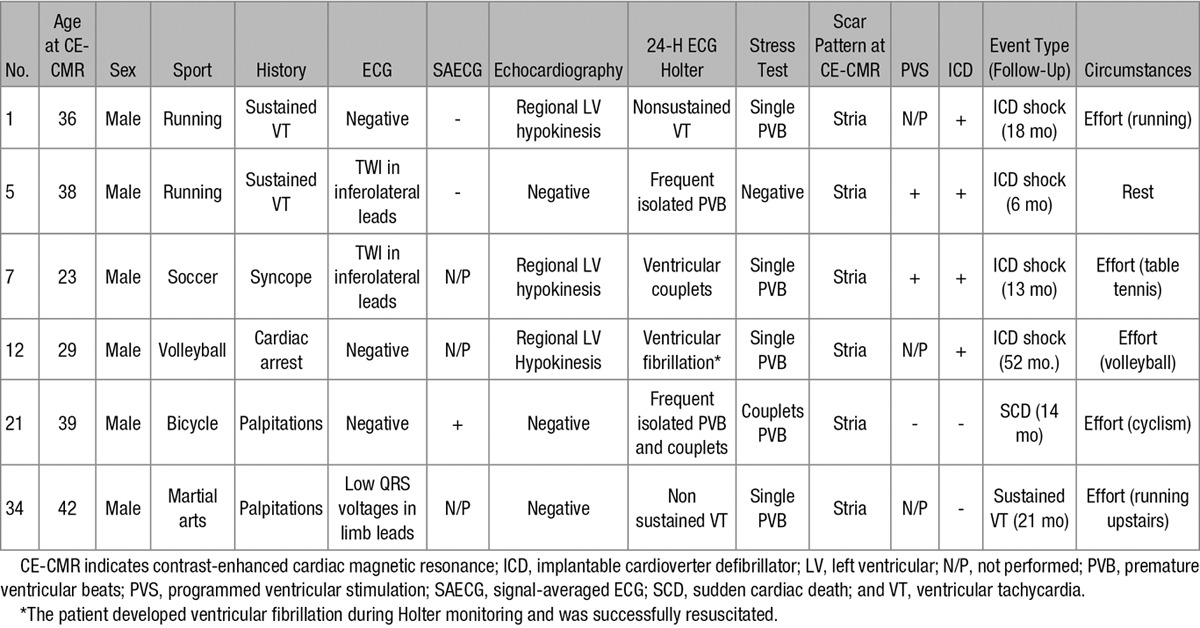
Figure 4.
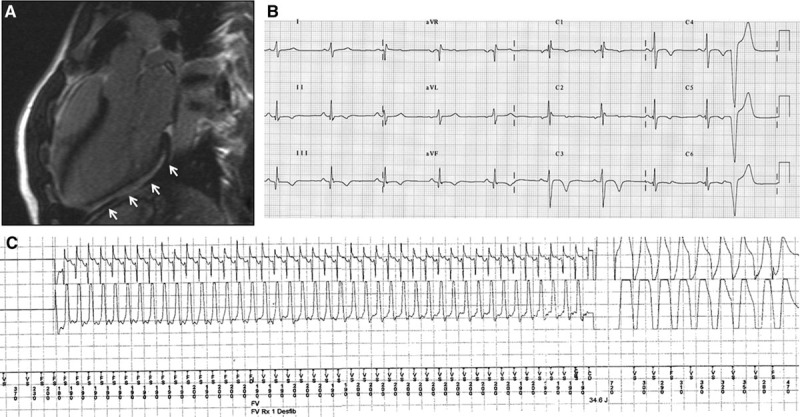
A 18-y-old tennis player who underwent contrast-enhancement cardiac magnetic resonance for inferolateral T-wave inversion at baseline 12-lead ECG (A) and frequent ventricular ectopic beats with a right bundle branch block/superior axis at exercise testing (B). Cardiac magnetic resonance revealed subepicardial/midmyocardial late gadolinium enhancement with a stria pattern involving the inferolateral left ventricular wall (white arrows; C). During follow-up, he developed progressive left ventricular dysfunction that led to refractory heart failure and heart transplantation. Panoramic view of the inferolateral left ventricular wall of the removed heart showed extensive replacement-type fibrosis mostly in the subepicardial and midmural layers, with focal fatty infiltration (trichrome heidenhain stain; D). At higher magnification, the residual cardiomyocytes are hypertrophic and show dysmetric and dysmorphic nuclei, with cytoplasmic vacuolization: note the diffuse fibrosis and patchy fatty infiltration (hematoxylin–eosin stain; E).
A familial recurrence of the LV LGE was ascertained in 2 cases. The first was the cyclist who died suddenly. CE-CMR study demonstrated inferolateral LV LGE stria in his asymptomatic brother who was also a cyclist. The second was a volleyball player who sought medical attention because of frequent PVB. The CE-CMR showed a nonischemic LV LGE with a stria pattern involving both the septum and the inferior LV free wall. The same CE-CMR features were observed in his asymptomatic identical twin brother (Figure 5).
Figure 5.
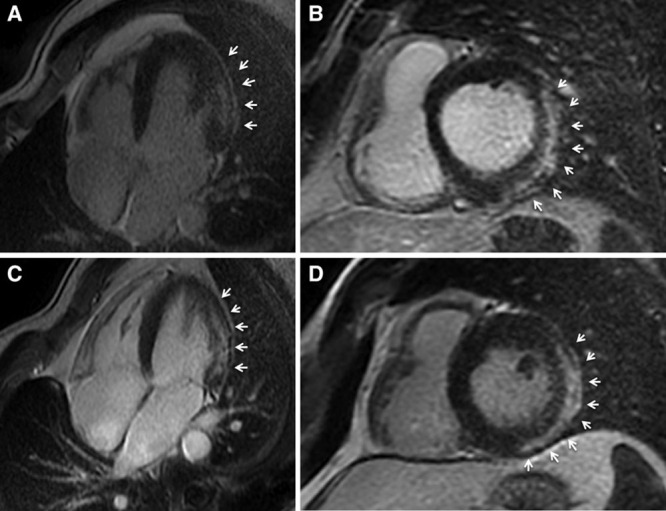
Long-axis (A and C) and short-axis (B and D) postcontrast cardiac magnetic resonance views of two 34-y-old identical twin brothers showing a subepicardial/midmyocardial stria of late gadolinium enhancement involving the lateral and inferolateral left ventricular wall (white arrows).
Kaplan–Meier analysis of freedom from malignant arrhythmias during follow-up showed that major arrhythmic events distinctively occurred in the subset of athletes with VA and a stria pattern of LV LGE at the time of enrollment (Figure 6).
Figure 6.
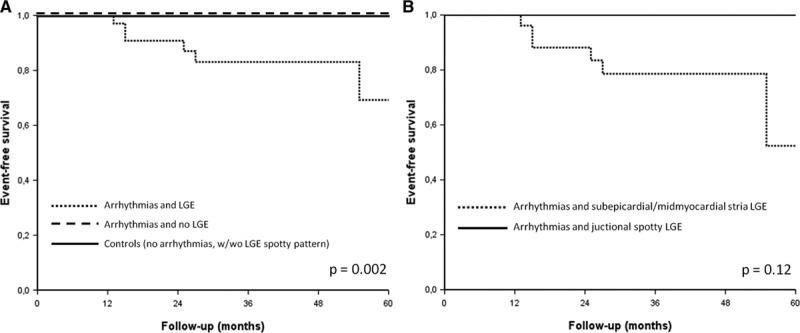
A, Kaplan–Meier analysis for survival from major arrhythmic events (sudden death, cardiac arrest because of ventricular fibrillation, sustained ventricular tachycardia, or appropriate implantable cardiac defibrillator shock) in athletes with ventricular arrhythmias and late gadolinium enhancement (LGE), in athletes with ventricular arrhythmias and no LGE, and in controls (with or without spotty LGE). B, Kaplan–Meyer analysis for survival from major arrhythmic events in the subgroup of athletes with ventricular arrhythmias and LGE according to specific LGE patterns (stria vs spotty).
Discussion
The study was designed to characterize the clinical profile and arrhythmic outcome of a cohort of elite athletes with isolated nonischemic LGE at CE-CMR, which was performed for clinical evaluation of apparently idiopathic VA. The main study results were that: (1) an isolated nonischemic LV LGE suggesting myocardial scar was associated with malignant arrhythmic events including SCD in the athlete; (2) the LGE stria pattern with a subepicardial/midmyocardial location was distinctively found among athletes with VA, at variance with the junctional LGE spotty pattern whose prevalence did not differ in athletes with and without arrhythmias; (3) during follow-up, athletes with a stria LGE pattern experienced life-threatening arrhythmic events and SCD, whereas athletes with no LGE or junctional spotty LGE pattern in isolation had an uneventful outcome, (4) because of its peculiar focal and/or nontransmural wall distribution, which spares the subendocardial layer, the LV scar as evidenced by CE-CMR may be undetectable by echocardiography.
Previous Studies
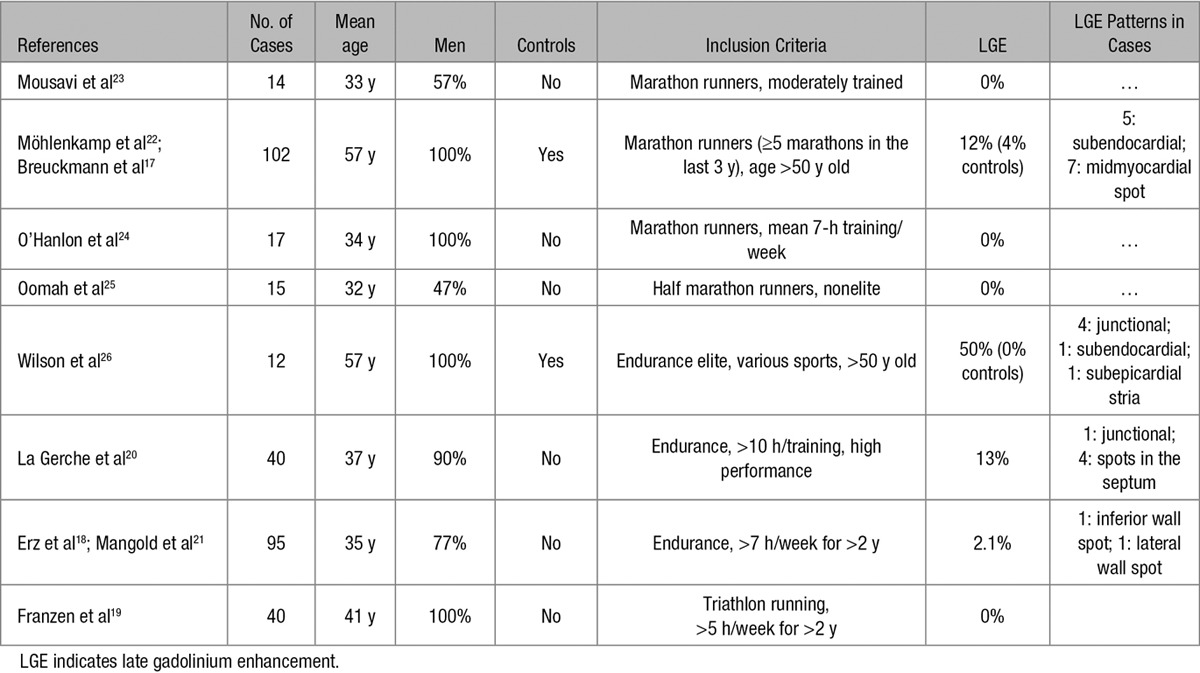
Idiopathic myocardial fibrosis, either interstitial or replacement-type, with a predilection for the inferolateral LV wall, has been occasionally reported at postmortem examination of athletes who died suddenly.28–30 The increasing use in the clinical practice of CE-CMR has offered the potential to identify in vivo potentially arrhythmogenic LV scar tissue. Previous CE-CMR studies in asymptomatic athletes reported a prevalence of LV LGE ranging from 0% to 50%17–26 (Table 4). The previous studies predominantly comprised veteran endurance athletes in whom a variety of LGE patterns were identified, either ischemic or nonischemic. Moreover, the correlation between LGE and clinical manifestation such as ECG and echocardiographic features, arrhythmias, and outcome was not systematically evaluated. The recent study by Schnell et al16 reported the clinical follow-up of 7 athletes with subepicardial LGE and arrhythmias or ECG abnormalities. However, there was no comparison with control athletes and by design, athletes with junctional patchy LGE pattern were excluded based on the presumption that this pattern is part of physiological adaptive changes of the athlete’s heart. The limited and incomplete data of previous studies prompted us to investigate the clinical characteristics and outcome of a larger cohort of competitive athletes with different patterns of nonischemic LV LGE, with or without associated VA.
Table 4.
Prevalence of LGE in Previous Cardiac Magnetic Resonance Studies on Healthy Athletes
Clinical Meaning of Different Late-Enhancement Patterns
The analysis of morphology, regional location, and wall distribution of LV LGE, both in the athletes presenting for VA and in control healthy athletes, allowed to identify 2 different patterns of nonischemic LV LGE scar: stria and spotty.
The stria LGE pattern had a subepicardial/midmyocardial wall distribution, mostly involved (89%) the inferolateral wall segments and was distinctively found in the group of athletes with VA. It was often associated with a history of sustained VT or cardiac arrest and ECG changes such as low QRS voltages in limb leads and T-wave inversion in inferolateral leads and predicted a malignant arrhythmic outcome. Of note, 85% of athletes with a LGE stria pattern showed VA with a RBBB/superior axis configuration. This morphology is consistent with the origin of the arrhythmia from the inferolateral LV segments, which was the region most frequently affected by LGE. Myocardial fibrosis could act as a substrate of VA through a variety of mechanisms. Structural discontinuity of myocardial tissue induced by fibrosis could generate conduction slowing and block predisposing to reentry. Myocyte uncoupling could favor arrhythmias based on abnormal impulse initiation such as automaticity or triggered activity. Fibrosis could also be a marker of abnormal and proarrhythmic myocyte electrophysiology as suggested by the histopathologic evidences in one of our athletes of myocytes hypertrophy, which is a recognized arrhythmogenic condition caused by action potential prolongation, heterogeneous repolarization, and/or calcium overload.31
The junctional spotty LGE pattern was typically localized in the basal segment of the inferior ventricular septum and the junction with the RV free wall and not associated with abnormal history or ECG findings and arrhythmic events during follow-up. Moreover, the majority of patients with isolated junctional spotty LGE exhibited a left bundle branch block/inferior axis arrhythmia configuration, suggesting that the arrhythmia originated from the RV outflow tract and was unrelated to the LGE. Similarly, group B athletes with no LV LGE showed PVB/VA with a predominant left bundle branch block morphology and an uneventful follow-up. Pooled together, these findings suggest that the combination of VA with a predominant RBBB morphology and LV LGE with a stria pattern predicted an increased risk of malignant arrhythmic events during follow-up.
Echocardiography
In our population of athletes with LV arrhythmias and nonischemic LV LGE with a stria pattern, echocardiographic hypokinesis of the inferolateral LV segments involved by the subepicardial/midmyocardial scar as evidenced by CE-CMR was found in 5/27 (29%) patients. This finding is in keeping with the results of a previous study showing that 5 of 7 with subepicardial LGE had a normal echocardiography.16 That nonischemic LV scar is undetectable by echocardiography may be explained by the segmental nature of myocardial fibrosis confined to small myocardial area and involving outer wall layers without reaching the subendocardium, which is the part of the LV that contributes the most to myocardial thickening.32 It is noteworthy that nonischemic LV scar as evidenced by CE-CMR accounted for life-threatening arrhythmic events including SCD during follow-up, despite the largely preserved global LV systolic function.
Pathogenetic Hypotheses
The distribution of LGE to subepicardial and midmyocardial wall layers indicates a nonischemic nature of the LV lesion and suggests myocarditis as the most likely underlying cause.33,34 However, myocardial inflammation is found in association with myocyte necrosis and replacement fibrosis in a variety of nonischemic heart muscle disorders such as infective or autoimmune myocarditis (including sarcoidosis),35,36 dilated cardiomyopathy, and ARVC,37–39 in which myocardial cell death is mediated or accompanied by reactive myocarditis.
Traditionally, the presence of nonischemic LV fibrosis with an epicardial/midmyocardial distribution and preferential involvement of the inferolateral LV regions is interpreted as the consequence of a previous myocarditis.33,34 Although in this study no athletes had a history of clinical myocarditis, we cannot exclude this cause because of a possible clinically silent course35 and a greater vulnerability to infective diseases related to the sports-induced depression of the immune system, which in turn might be favored by the undeclared use of performance-enhancing drugs such as steroids.40,41 Moreover, in the subset of 6 athletes undergoing endomyocardial biopsy at the time of cardiac catheterization, diagnostic histopathologic features of acute myocarditis, characterized by inflammatory infiltrates associated with myocyte necrosis, were observed in one case only. Noteworthy, viral genomes were not detected and inflammation was later found to be reactive to ARVC myocardial lesion in the removed native heart at the time of cardiac transplantation. However, the low sensitivity of endomyocardial biopsy may reflect the mismatch between the location of myocardial LGE (ie, the epicardial lateral LV wall) and the site of the biopsy (ie, the endocardial RV septal apex).
The LV scar as evidenced by CE-CMR may reflect a segmental inherited cardiomyopathy, namely, a left-dominant ARVC.42 This ARVC variant is characterized by fibrous or fibrofatty replacement of the LV, with possible focal abnormalities of the RV that can be detected only by histological examination (as was the case of 2 athletes who underwent endomyocardial biopsy). The clinical features of left-dominant ARVC mirror those of the classic right-dominant counterpart, with ECG repolarization abnormalities in the left precordial leads V4-V6 and VA with a RBBB configuration.43,44 In our study, a family history of SCD was ascertained in 3 index athletes; moreover, 2 athletes had a clinical evidence of familial recurrence of the disease, a finding that supports the hypothesis of the inherited cardiomyopathy nature of the LV LGE at least in some cases.
A previous study by Wilson et al26 in veteran athletes demonstrated a link between lifelong endurance exercise and LGE (typically with a junctional spotty pattern), suggesting a cause–effective relationship. In our study, among 40 healthy control athletes with no VA, 28% exhibited LGE with a distinctive junctional spotty pattern and none showed a stria pattern despite their training-induced augmented LV mass. This finding is in agreement with previous studies demonstrating that junctional spotty LGE pattern is a common finding in highly trained athletes, whereas the subepicardial/midmyocardial stria pattern is exceptional17–26 (Table 4). Our results extended previous observations by showing that unlike the stria LGE pattern, the spotty LGE pattern was characterized by an uneventful outcome during follow-up. The spotty LGE pattern resembles that described in patients with pulmonary hypertension, in whom the increased interventricular wall stress caused by RV pressure overload may induce a myocardial injury at this site.45 A significant increase in pulmonary pressures has been demonstrated in trained athletes during intense exercise which suggests a similar pathogenesis.20,46 Of interest, myocardial areas located at the attachment of RV wall to the septum, where spotty LGE characteristically localizes, showed at histological examination a loss of compact myocardium and markedly expanded extracellular space with interstitial fibrosis,47 morphological features that may suggest a different pathogenesis and explain the lack of associated arrhythmias compared with the stria LGE pattern. According to our study results, the subepicardial/midmyocardial stria is not part of the physiological remodeling of the athlete’s heart, as indicated by its absence among our highly trained control athletes with an augmented LV mass. However, there remains a possibility that strenuous and sustained physical exercise may have worsened myocardial inflammatory lesions41 or favored the development of myocardial damage and fibrous repair in those athletes with a predisposing genetic background.48,49
Finally, performance-enhancing drugs may induce a variety of rhythm disturbances in the athletes, including VA.40 However, none of our athletes admitted the use of any drugs, and at present, there is no evidence that illicit substances may cause nonischemic LV scar.
Study Limitations
Although to our knowledge this study reported on the largest cohort of athletes with VA and LV LGE, a relatively small number of athletes and outcomes were analyzed, and this limited the power to detect associations. In particular, the small number of athletes with VA and junctional spotty LGE did not allow us to confidently conclude that this LGE pattern is associated with a benign outcome. Moreover, because the decision to perform additional CE-CMR and/or to refer the athlete to our center for further evaluation was at discretion of the sports medicine physicians, we were unable to assess in the general athletic population either the prevalence of LV LGE in athletes with VA or the prevalence of VA in athletes with LV LGE. However, the primary objective of our case–control study was to evaluate the prognostic value of the presence, pattern, and distribution of LV LGE in athletes with VA. The investigation of the pathogenesis of isolated LV scar as evidence by CE-CMR, with particular reference to relationship with left- and/or right-dominant ARVC, by systematic molecular genetic analysis and family screening was beyond the scope of this study.
Conclusions and Clinical Implications
The results of this study showed that nonischemic LV LGE with a stria morphological pattern and subepicardial/midmyocardial distribution was associated with malignant VA and SCD in competitive athletes. Hence, it deserves proper clinical attention and cannot be simply dismissed as a benign sign of a healed remote inflammatory process. This condition may represent the result of a healing process common to a variety of nonischemic myocardial lesions including myocarditis and cardiomyopathies.
The most appropriate management strategy of affected athletes remains to be established. By extrapolation from other arrhythmogenic disorders at risk of SCD, ICD implantation is indicated in affected athletes who survived cardiac arrest because of ventricular fibrillation, experienced sustained VT, or had exercise-induced syncope. Successful mapping/catheter ablation of recurrent sustained VT have been preliminary reported in patients with inferolateral scar using an epicardial approach because the reentry circuit is located in the outer layer of LV wall.50 Prophylactic β-blocker therapy seems to be justified on the basis of the exercise test inducibility of VA in the majority of our cases. However, such a therapy may not confer complete protection against life-threatening VA as shown by the significant incidence of appropriate ICD intervention on fast VT during follow-up observed in our study population despite β-blockers. Of note, because the scar may be inheritable, a cascade family screening and clinical follow-up is warranted.
The results of our study raise some challenges regarding (1) the identification of asymptomatic athletes with potentially at-risk LV scar on cardiovascular evaluation before participation in sports and (2) the eligibility to competitive sports activity of asymptomatic individuals with nonischemic LV scar as evidenced by CE-CMR. The finding of a ≈50% prevalence of ECG abnormalities, either T-wave inversion in the inferolateral leads or low QRS voltages in limb leads, in our athletes with a stria LGE pattern suggests the possibility that the disease may be suspected at a presymptomatic stage by ECG screening. However, because of its peculiar nontransmural distribution that spares the subendocardial wall layer, the presence of LV scar was missed by echocardiographic examination in the majority of our cases. As a corollary, in athletes presenting with frequent PVB or complex VA with a RBBB morphology (suggesting a LV origin) and showing normal ECG and echocardiographic findings, the presence of this potentially malignant arrhythmogenic substrate cannot be excluded and should be further investigated by a CE-CMR study. An isolated nonischemic LV LGE is not listed by the current recommendations among the cardiac diseases at risk of SCD and requiring restriction or disqualification from competitive sports activity. Our findings indicate that affected athletes should be accurately evaluated for the arrhythmogenic potential inherent to the LV scar as evidenced by CE-CMR. In the presence of VA, especially if worsened by exercise, athletes should prudentially refrain from practicing sports activity and physical exercise with high cardiovascular demand. Because our data are preliminary, retrospective, and limited to a relatively small cohort of index athletes, further prospective studies on larger athletic population showing a nonischemic LV LGE, with and without arrhythmias, should be designed to provide definitive guidelines for risk stratification and sport eligibility.
Sources of Funding
This study was funded by the TRANSAC Strategic Research Grant CPDA133979/13, University of Padua, Italy; the Registry of Cardio-Cerebro-Vascular Pathology, Veneto Region, Venice, Italy; Target Projects 331/12, RP 2014-00000394, Regional Health System, Venice, Italy. Dr Zorzi research fellowship is supported by F.I.G.C., Rome, Italy.
Disclosures
None.
Footnotes
Drs Zorzi and Perazzolo Marra contributed equally as first authors.
References
- 1.Corrado D, Basso C, Rizzoli G, Schiavon M, Thiene G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J Am Coll Cardiol. 2003;42:1959–1963. doi: 10.1016/j.jacc.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Corrado D, Basso C, Pavei A, Michieli P, Schiavon M, Thiene G. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA. 2006;296:1593–1601. doi: 10.1001/jama.296.13.1593. doi: 10.1001/jama.296.13.1593. [DOI] [PubMed] [Google Scholar]
- 3.Thiene G, Nava A, Corrado D, Rossi L, Pennelli N. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med. 1988;318:129–133. doi: 10.1056/NEJM198801213180301. doi: 10.1056/NEJM198801213180301. [DOI] [PubMed] [Google Scholar]
- 4.Maron BJ. Hypertrophic cardiomyopathy and other causes of sudden cardiac death in young competitive athletes, with considerations for preparticipation screening and criteria for disqualification. Cardiol Clin. 2007;25:399–414, vi. doi: 10.1016/j.ccl.2007.07.006. doi: 10.1016/j.ccl.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 5.de Noronha SV, Sharma S, Papadakis M, Desai S, Whyte G, Sheppard MN. Aetiology of sudden cardiac death in athletes in the United Kingdom: a pathological study. Heart. 2009;95:1409–1414. doi: 10.1136/hrt.2009.168369. doi: 10.1136/hrt.2009.168369. [DOI] [PubMed] [Google Scholar]
- 6.Harmon KG, Asif IM, Maleszewski JJ, Owens DS, Prutkin JM, Salerno JC, Zigman ML, Ellenbogen R, Rao AL, Ackerman MJ, Drezner JA. Incidence, cause, and comparative frequency of sudden cardiac death in national collegiate athletic association athletes: a decade in review. Circulation. 2015;132:10–19. doi: 10.1161/CIRCULATIONAHA.115.015431. doi: 10.1161/CIRCULATIONAHA.115.015431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brugada J, Brugada R, Brugada P. Channelopathies: a new category of diseases causing sudden death. Herz. 2007;32:185–191. doi: 10.1007/s00059-007-2976-1. doi: 10.1007/s00059-007-2976-1. [DOI] [PubMed] [Google Scholar]
- 8.Corrado D, Basso C, Thiene G. Sudden cardiac death in young people with apparently normal heart. Cardiovasc Res. 2001;50:399–408. doi: 10.1016/s0008-6363(01)00254-1. [DOI] [PubMed] [Google Scholar]
- 9.Basso C, Carturan E, Pilichou K, Rizzo S, Corrado D, Thiene G. Sudden cardiac death with normal heart: molecular autopsy. Cardiovasc Pathol. 2010;19:321–325. doi: 10.1016/j.carpath.2010.02.003. doi: 10.1016/j.carpath.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Galderisi M, Cardim N, D’Andrea A, Bruder O, Cosyns B, Davin L, Donal E, Edvardsen T, Freitas A, Habib G, Kitsiou A, Plein S, Petersen SE, Popescu BA, Schroeder S, Burgstahler C, Lancellotti P. The multi-modality cardiac imaging approach to the Athlete’s heart: an expert consensus of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:353. doi: 10.1093/ehjci/jeu323. doi: 10.1093/ehjci/jeu323. [DOI] [PubMed] [Google Scholar]
- 11.Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26:1461–1474. doi: 10.1093/eurheartj/ehi258. doi: 10.1093/eurheartj/ehi258. [DOI] [PubMed] [Google Scholar]
- 12.Perazzolo Marra M, Lima JA, Iliceto S. MRI in acute myocardial infarction. Eur Heart J. 2011;32:284–293. doi: 10.1093/eurheartj/ehq409. doi: 10.1093/eurheartj/ehq409. [DOI] [PubMed] [Google Scholar]
- 13.Vermes E, Carbone I, Friedrich MG, Merchant N. Patterns of myocardial late enhancement: typical and atypical features. Arch Cardiovasc Dis. 2012;105:300–308. doi: 10.1016/j.acvd.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Smaldone C, Pieroni M, Pelargonio G, Dello Russo A, Palmieri V, Bianco M, Gentile M, Crea F, Bellocci F, Zeppilli P. Left-dominant arrhythmogenic cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4:e29–e32. doi: 10.1161/CIRCEP.111.962779. doi: 10.1161/CIRCEP.111.962779. [DOI] [PubMed] [Google Scholar]
- 15.Nucifora G, Muser D, Masci PG, Barison A, Rebellato L, Piccoli G, Daleffe E, Toniolo M, Zanuttini D, Facchin D, Lombardi M, Proclemer A. Prevalence and prognostic value of concealed structural abnormalities in patients with apparently idiopathic ventricular arrhythmias of left versus right ventricular origin: a magnetic resonance imaging study. Circ Arrhythm Electrophysiol. 2014;7:456–462. doi: 10.1161/CIRCEP.113.001172. doi: 10.1161/CIRCEP.113.001172. [DOI] [PubMed] [Google Scholar]
- 16.Schnell F, Claessen G, La Gerche A, Bogaert J, Lentz PA, Claus P, Mabo P, Carre F, Heidbuchel H. Subepicardial delayed gadolinium enhancement in asymptomatic athletes: Let sleeping dogs lie? Br J Sports Med. 2016;50:111–117. doi: 10.1136/bjsports-2014-094546. [DOI] [PubMed] [Google Scholar]
- 17.Breuckmann F, Möhlenkamp S, Nassenstein K, Lehmann N, Ladd S, Schmermund A, Sievers B, Schlosser T, Jöckel KH, Heusch G, Erbel R, Barkhausen J. Myocardial late gadolinium enhancement: prevalence, pattern, and prognostic relevance in marathon runners. Radiology. 2009;251:50–57. doi: 10.1148/radiol.2511081118. doi: 10.1148/radiol.2511081118. [DOI] [PubMed] [Google Scholar]
- 18.Erz G, Mangold S, Franzen E, Claussen CD, Niess AM, Burgstahler C, Kramer U. Correlation between ECG abnormalities and cardiac parameters in highly trained asymptomatic male endurance athletes: evaluation using cardiac magnetic resonance imaging. Int J Cardiovasc Imaging. 2013;29:325–334. doi: 10.1007/s10554-012-0082-9. doi: 10.1007/s10554-012-0082-9. [DOI] [PubMed] [Google Scholar]
- 19.Franzen E, Mangold S, Erz G, Claussen CD, Niess AM, Kramer U, Burgstahler C. Comparison of morphological and functional adaptations of the heart in highly trained triathletes and long-distance runners using cardiac magnetic resonance imaging. Heart Vessels. 2013;28:626–631. doi: 10.1007/s00380-012-0289-7. doi: 10.1007/s00380-012-0289-7. [DOI] [PubMed] [Google Scholar]
- 20.La Gerche A, Burns AT, Mooney DJ, Inder WJ, Taylor AJ, Bogaert J, Macisaac AI, Heidbuchel H, Prior DL. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J. 2012;33:998–1006. doi: 10.1093/eurheartj/ehr397. [DOI] [PubMed] [Google Scholar]
- 21.Mangold S, Kramer U, Franzen E, Erz G, Bretschneider C, Seeger A, Claussen CD, Niess AM, Burgstahler C. Detection of cardiovascular disease in elite athletes using cardiac magnetic resonance imaging. Rofo. 2013;185:1167–1174. doi: 10.1055/s-0033-1350130. doi: 10.1055/s-0033-1350130. [DOI] [PubMed] [Google Scholar]
- 22.Möhlenkamp S, Lehmann N, Breuckmann F, Bröcker-Preuss M, Nassenstein K, Halle M, Budde T, Mann K, Barkhausen J, Heusch G, Jöckel KH, Erbel R Marathon Study Investigators; Heinz Nixdorf Recall Study Investigators. Running: the risk of coronary events: prevalence and prognostic relevance of coronary atherosclerosis in marathon runners. Eur Heart J. 2008;29:1903–1910. doi: 10.1093/eurheartj/ehn163. doi: 10.1093/eurheartj/ehn163. [DOI] [PubMed] [Google Scholar]
- 23.Mousavi N, Czarnecki A, Kumar K, Fallah-Rad N, Lytwyn M, Han SY, Francis A, Walker JR, Kirkpatrick ID, Neilan TG, Sharma S, Jassal DS. Relation of biomarkers and cardiac magnetic resonance imaging after marathon running. Am J Cardiol. 2009;103:1467–1472. doi: 10.1016/j.amjcard.2009.01.294. doi: 10.1016/j.amjcard.2009.01.294. [DOI] [PubMed] [Google Scholar]
- 24.O’Hanlon R, Wilson M, Wage R, Smith G, Alpendurada FD, Wong J, Dahl A, Oxborough D, Godfrey R, Sharma S, Roughton M, George K, Pennell DJ, Whyte G, Prasad SK. Troponin release following endurance exercise: is inflammation the cause? a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2010;12:38. doi: 10.1186/1532-429X-12-38. doi: 10.1186/1532-429X-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oomah SR, Mousavi N, Bhullar N, Kumar K, Walker JR, Lytwyn M, Colish J, Wassef A, Kirkpatrick ID, Sharma S, Jassal DS. The role of three-dimensional echocardiography in the assessment of right ventricular dysfunction after a half marathon: comparison with cardiac magnetic resonance imaging. J Am Soc Echocardiogr. 2011;24:207–213. doi: 10.1016/j.echo.2010.10.012. doi: 10.1016/j.echo.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Wilson M, O’Hanlon R, Prasad S, Deighan A, Macmillan P, Oxborough D, Godfrey R, Smith G, Maceira A, Sharma S, George K, Whyte G. Diverse patterns of myocardial fibrosis in lifelong, veteran endurance athletes. J Appl Physiol (1985) 2011;110:1622–1626. doi: 10.1152/japplphysiol.01280.2010. doi: 10.1152/japplphysiol.01280.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breithardt G, Cain ME, el-Sherif N, Flowers NC, Hombach V, Janse M, Simson MB, Steinbeck G. Standards for analysis of ventricular late potentials using high-resolution or signal-averaged electrocardiography. A statement by a Task Force Committee of the European Society of Cardiology, the American Heart Association, and the American College of Cardiology. Circulation. 1991;83:1481–1488. doi: 10.1161/01.cir.83.4.1481. [DOI] [PubMed] [Google Scholar]
- 28.Whyte G, Sheppard M, George K, Shave R, Wilson M, Prasad S, O’Hanlon R, Sharma S. Post-mortem evidence of idiopathic left ventricular hypertrophy and idiopathic interstitial myocardial fibrosis: is exercise the cause? Br J Sports Med. 2008;42:304–305. doi: 10.1136/bjsm.2007.038158. doi: 10.1136/bjsm.2007.038158. [DOI] [PubMed] [Google Scholar]
- 29.Pilichou K, Mancini M, Rigato I, Lazzarini E, Giorgi B, Carturan E, Bauce B, d’Amati G, Marra MP, Basso C. Nonischemic left ventricular scar: sporadic or familial? Screen the genes, scan the mutation carriers. Circulation. 2014;130:e180–e182. doi: 10.1161/CIRCULATIONAHA.114.012515. doi: 10.1161/CIRCULATIONAHA.114.012515. [DOI] [PubMed] [Google Scholar]
- 30.d’Amati G, De Caterina R, Basso C. Sudden cardiac death in an Italian competitive athlete: pre-participation screening and cardiovascular emergency care are both essential. Int J Cardiol. 2016;206:84–86. doi: 10.1016/j.ijcard.2016.01.011. doi: 10.1016/j.ijcard.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 31.de Jong S, van Veen TA, van Rijen HV, de Bakker JM. Fibrosis and cardiac arrhythmias. J Cardiovasc Pharmacol. 2011;57:630–638. doi: 10.1097/FJC.0b013e318207a35f. doi: 10.1097/FJC.0b013e318207a35f. [DOI] [PubMed] [Google Scholar]
- 32.Rademakers FE, Rogers WJ, Guier WH, Hutchins GM, Siu CO, Weisfeldt ML, Weiss JL, Shapiro EP. Relation of regional cross-fiber shortening to wall thickening in the intact heart. Three-dimensional strain analysis by NMR tagging. Circulation. 1994;89:1174–1182. doi: 10.1161/01.cir.89.3.1174. [DOI] [PubMed] [Google Scholar]
- 33.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedrich MG, Marcotte F. Cardiac magnetic resonance assessment of myocarditis. Circ Cardiovasc Imaging. 2013;6:833–839. doi: 10.1161/CIRCIMAGING.113.000416. [DOI] [PubMed] [Google Scholar]
- 35.Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–48, 2648a. doi: 10.1093/eurheartj/eht210. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 36.Froehlich W, Bogun FM, Crawford TC. Cardiac sarcoidosis. Circulation. 2015;132:e137–e138. doi: 10.1161/CIRCULATIONAHA.114.013308. doi: 10.1161/CIRCULATIONAHA.114.013308. [DOI] [PubMed] [Google Scholar]
- 37.Kühl U, Noutsias M, Seeberg B, Schultheiss HP. Immunohistological evidence for a chronic intramyocardial inflammatory process in dilated cardiomyopathy. Heart. 1996;75:295–300. doi: 10.1136/hrt.75.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basso C, Thiene G, Corrado D, Angelini A, Nava A, Valente M. Arrhythmogenic right ventricular cardiomyopathy. Dysplasia, dystrophy, or myocarditis? Circulation. 1996;94:983–991. doi: 10.1161/01.cir.94.5.983. [DOI] [PubMed] [Google Scholar]
- 39.Thiene G, Corrado D, Nava A, Rossi L, Poletti A, Boffa GM, Daliento L, Pennelli N. Right ventricular cardiomyopathy: is there evidence of an inflammatory aetiology? Eur Heart J. 1991;12(suppl D):22–25. doi: 10.1093/eurheartj/12.suppl_d.22. [DOI] [PubMed] [Google Scholar]
- 40.Deligiannis A, Björnstad H, Carre F, Heidbüchel H, Kouidi E, Panhuyzen-Goedkoop NM, Pigozzi F, Schänzer W, Vanhees L ESC Study Group of Sports Cardiology. ESC study group of sports cardiology position paper on adverse cardiovascular effects of doping in athletes. Eur J Cardiovasc Prev Rehabil. 2006;13:687–694. doi: 10.1097/01.hjr.0000224482.95597.7a. doi: 10.1097/01.hjr.0000224482.95597.7a. [DOI] [PubMed] [Google Scholar]
- 41.Shephard RJ, Shek PN. Potential impact of physical activity and sport on the immune system–a brief review. Br J Sports Med. 1994;28:247–255. doi: 10.1136/bjsm.28.4.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marra MP, Leoni L, Bauce B, Corbetti F, Zorzi A, Migliore F, Silvano M, Rigato I, Tona F, Tarantini G, Cacciavillani L, Basso C, Buja G, Thiene G, Iliceto S, Corrado D. Imaging study of ventricular scar in arrhythmogenic right ventricular cardiomyopathy: comparison of 3D standard electroanatomical voltage mapping and contrast-enhanced cardiac magnetic resonance. Circ Arrhythm Electrophysiol. 2012;5:91–100. doi: 10.1161/CIRCEP.111.964635. doi: 10.1161/CIRCEP.111.964635. [DOI] [PubMed] [Google Scholar]
- 43.Basso C, Corrado D, Marcus FI, Nava A, Thiene G. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009;373:1289–1300. doi: 10.1016/S0140-6736(09)60256-7. doi: 10.1016/S0140-6736(09)60256-7. [DOI] [PubMed] [Google Scholar]
- 44.Sen-Chowdhry S, Syrris P, Prasad SK, Hughes SE, Merrifield R, Ward D, Pennell DJ, McKenna WJ. Left-dominant arrhythmogenic cardiomyopathy: an under-recognized clinical entity. J Am Coll Cardiol. 2008;52:2175–2187. doi: 10.1016/j.jacc.2008.09.019. doi: 10.1016/j.jacc.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 45.Shehata ML, Lossnitzer D, Skrok J, Boyce D, Lechtzin N, Mathai SC, Girgis RE, Osman N, Lima JA, Bluemke DA, Hassoun PM, Vogel-Claussen J. Myocardial delayed enhancement in pulmonary hypertension: pulmonary hemodynamics, right ventricular function, and remodeling. AJR Am J Roentgenol. 2011;196:87–94. doi: 10.2214/AJR.09.4114. doi: 10.2214/ajr.196.5_supplement.0a87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.La Gerche A. Can intense endurance exercise cause myocardial damage and fibrosis? Curr Sports Med Rep. 2013;12:63–69. doi: 10.1249/JSR.0b013e318287488a. doi: 10.1249/JSR.0b013e318287488a. [DOI] [PubMed] [Google Scholar]
- 47.Chan RH, Maron BJ, Olivotto I, Assenza GE, Haas TS, Lesser JR, Gruner C, Crean AM, Rakowski H, Rowin E, Udelson J, Lombardi M, Tomberli B, Spirito P, Formisano F, Marra MP, Biagini E, Autore C, Manning WJ, Appelbaum E, Roberts WC, Basso C, Maron MS. Significance of late gadolinium enhancement at right ventricular attachment to ventricular septum in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2015;116:436–441. doi: 10.1016/j.amjcard.2015.04.060. doi: 10.1016/j.amjcard.2015.04.060. [DOI] [PubMed] [Google Scholar]
- 48.Martherus R, Jain R, Takagi K, Mendsaikhan U, Turdi S, Osinska H, James JF, Kramer K, Purevjav E, Towbin JA. Accelerated cardiac remodeling in desmoplakin transgenic mice in response to endurance exercise is associated with perturbed Wnt/β-catenin signaling. Am J Physiol Heart Circ Physiol. 2016;310:H174–H187. doi: 10.1152/ajpheart.00295.2015. doi: 10.1152/ajpheart.00295.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corrado D, Zorzi A. Arrhythmogenic right ventricular cardiomyopathy and sports activity. Eur Heart J. 2015;36:1708–1710. doi: 10.1093/eurheartj/ehv183. doi: 10.1093/eurheartj/ehv183. [DOI] [PubMed] [Google Scholar]
- 50.Oloriz T, Silberbauer J, Maccabelli G, Mizuno H, Baratto F, Kirubakaran S, Vergara P, Bisceglia C, Santagostino G, Marzi A, Sora N, Roque C, Guarracini F, Tsiachris D, Radinovic A, Cireddu M, Sala S, Gulletta S, Paglino G, Mazzone P, Trevisi N, Della Bella P. Catheter ablation of ventricular arrhythmia in nonischemic cardiomyopathy: anteroseptal versus inferolateral scar sub-types. Circ Arrhythm Electrophysiol. 2014;7:414–423. doi: 10.1161/CIRCEP.114.001568. doi: 10.1161/CIRCEP.114.001568. [DOI] [PubMed] [Google Scholar]


