Abstract
Scope
Fumonisin (FB) occurs in maize and is an inhibitor of ceramide synthase (CerS). We determined the urinary FB1 (UFB1) and sphingoid base 1-phosphate levels in blood from women consuming maize in high and low FB exposure communities in Guatemala.
Methods and results
FB1 intake was estimated using the UFB1. Sphinganine 1-phosphate (Sa 1-P), sphingosine 1-phosphate (So 1-P), and the Sa 1-P/So 1-P ratio were determined in blood spots collected on absorbent paper at the same time as urine collection. In the first study, blood spots and urine were collected every three months (March 2011 to February 2012) from women living in low (Chimaltenango and Escuintla) and high (Jutiapa) FB exposure communities (1240 total recruits). The UFB1, Sa 1-P/So 1-P ratio, and Sa 1-P/ml in blood spots were significantly higher in the high FB1 intake community compared to the low FB1 intake communities. The results were confirmed in a follow-up study (February 2013) involving 299 women living in low (Sacatepéquez) and high (Santa Rosa and Chiquimula) FB exposure communities.
Conclusions
High levels of FB1 intake are correlated with changes in Sa 1-P and the Sa 1-P/So 1-P ratio in human blood in a manner consistent with FB1 inhibition of CerS.
Keywords: Ceramide synthase, Fumonisin, Maize, Sphinganine 1-phosphate, Urinary fumonisin
1 Introduction
Fumonisins (FB) are mycotoxins that contaminate maize and inhibit ceramide synthases (CerS) [1], key enzymes in the biosynthesis of sphingolipids. Numerous studies have shown that when animals consume diets contaminated with FB, the levels of free sphingoid bases and sphingoid base 1-phosphates become elevated in a dose-dependent manner in tissues and blood [2, 3, 4]. For example, in LM/Bc mice, FB1-treatment of pregnant dams resulted in increased levels of sphingoid bases and sphingoid base 1-phosphates in blood and tissues and induced neural tube defects (NTDs) [5]. In mice dosed orally with FB1, the levels of sphinganine 1-phosphate (Sa 1-P), sphingosine 1-phosphate (So 1-P), and the Sa 1-P/So 1-P ratio became elevated in blood and the levels were significantly positively correlated with both the nominal administered dose (estimated mg/kg b.w./day) of FB1 and the urinary FB1 (UFB1) concentration [6]. Thus, the increased level of sphingoid base 1-phosphates in blood is a putative biomarker for FB1 inhibition of CerS. However, even though FB1 inhibition of CerS is a known cause of animal disease via disruption of sphingolipid metabolism (reviewed in [7]), definitive evidence for FB inhibition of CerS in humans or FB as a cause of, or contributing factor in, any human disease has not yet been shown [8]. Nonetheless, it has been hypothesized that in areas of the world where maize is consumed in large amounts and where FB contamination is likely, FB could play a role in the high incidence of NTDs in humans [9, 10], human carcinogenesis (reviewed in [11]), and stunting in children [12, 13]. Human FB1 intake assessment and biomonitoring of mechanism-based biological effects (i.e. disruption of sphingolipid metabolism) are a logical first step to determine if FB1 exposure contributes to any human disease.
UFB1 has been successfully used as an exposure biomarker in several recent studies in countries and regions where maize is potentially contaminated with FB and is consumed in large amounts (reviewed in [8]). Most recently, a study in Tanzanian children found a significant negative correlation between UFB1 and length-for-age z-scores and also reported the concurrent co-exposure to aflatoxins using the serum AFB-albumin biomarker [13].
In Guatemala UFB1 was used to estimate FB intake in a pilot study in two low exposure communities (defined based on the relative difference in FB in the maize) using the results of a study that measured the kinetics of urinary excretion in humans consuming known amounts of FB under controlled conditions in the USA [14]. In the kinetic study it was found that the total UFB1 was less than 1% (0.12% to 0.90%, n=10) of the cumulative dose with a mean of 0.50% [14]. Using 0.5% for the percentage excreted in urine, the estimated average total FB1 intake in the two low exposure communities was 0.45 μg/kg b.w./day [14]. Assuming that FB1 is approximately 70% of the total FB (FB1+FB2+FB3) then the average total FB intake was well below the provisional maximum tolerable daily intake (PMTDI=2 μg/kg b.w./day) proposed by the FAO/WHO Joint Expert Committee on Food Additives (JECFA) [7].
In a subsequent larger study in Guatemala, urine and blood samples were collected from maize consuming women living in high and low exposure communities over the course of one year with sampling at 3 month intervals [15]. A total of 1240 women were recruited for the study. The results showed that UFB1 levels were predictive of the estimated FB intake in a dose-dependent manner and that many women (75%) in the high exposure department of Jutiapa (departments=22 first-level sub-national country governmental subdivisions) exceeded the JECFA PMTDI. The blood samples were collected because it was hypothesized that an increase in the mechanism-based sphingolipid biomarkers in blood spots should correlate with the UFB1, the FB exposure biomarker [16]. Knowing whether or not FB inhibits CerS in humans consuming diets high in FB is important because the inhibition of CerS is the likely proximate cause/key event in all of the downstream biochemical changes leading to FB toxicity in animals. Knowing the FB intake that results in an increased risk for inhibition of CerS will provide an empirical measure to evaluate the effectiveness of the current PMTDI proposed by JECFA [7].
There are at least three important unanswered questions (gaps) in the human FB risk assessment with regards to sphingolipids. First, does FB induce disruption of sphingolipid metabolism in humans consuming maize-based foods contaminated with FB? Second, what level of FB intake will induce disruption of sphingolipid metabolism in humans? Third, does FB-induced disruption of sphingolipid metabolism contribute to adverse effects in populations consuming maize potentially contaminated with high levels of FB? Towards the goal of addressing these questions, we have developed and validated in a mouse model the use of blood spots collected on absorbent paper to assess the accumulation of So 1-P, Sa 1-P and the Sa 1-P/So 1-P ratio as indicators of FB-induced CerS inhibition in vivo. The method has also been used to quantitate Sa 1-P, So 1-P and the Sa 1-P/So 1-P ratio in extracts of human blood spots [6]. We hypothesized that blood spots from Guatemalan women living in areas where FB exposure is high will show higher Sa 1-P/So 1-P ratios when compared to populations with low exposure [16], reflecting the increased sphinganine (Sa) produced in cells and tissues as a consequence of FB-induced CerS inhibition (Fig. 1). This hypothesis is supported by studies in animals. For example, in mice orally dosed with FB1 there is a dose-dependent increase in Sa 1-P and the Sa 1-P/So 1-P ratio in the blood spots [6]. In the mouse model, the levels of Sa 1-P, So 1-P and the Sa 1-P/So 1-P ratio in extracts of blood spots collected on absorbent paper paralleled the levels of Sa seen in liver and kidney and the UFB1 [6].
Figure 1.
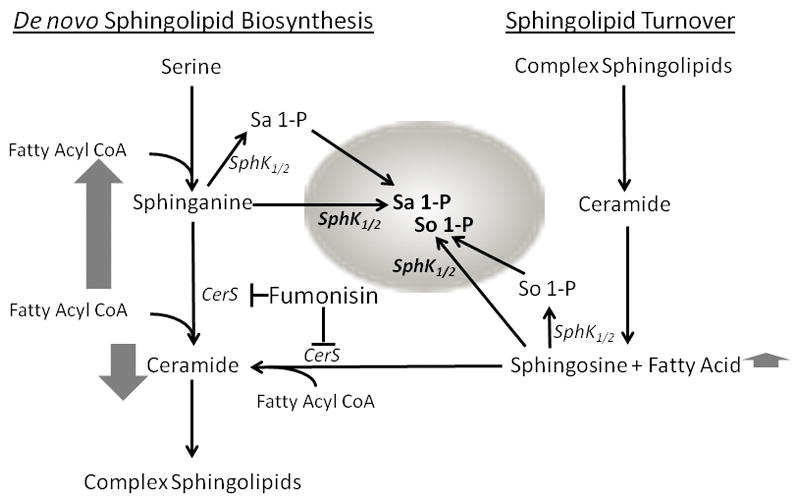
Enzymology of FB-induced sphingoid base 1-phosphate accumulation in RBC. RBC cannot synthesize sphingolipids de novo and lack the ability to de-phosphorylate sphingoid base 1-phosphates. However, RBC have the ability to phosphorylate sphingoid bases via sphingosine kinases (SphK1/2). Thus, the sphingosine (So) and sphinganine (Sa) that is phosphorylated in the RBC is produced outside the RBC. In animals large amounts of So and Sa are produced in liver, kidney, and other tissues following FB inhibition of ceramide synthase (CerS). The So and Sa that is produced in tissues following FB exposure is a likely source of substrates for the SphKs in the RBC following FB intake, however, RBC can also take up and store So 1-P and Sa 1-P from endothelial cells. The vertical arrows next to Sa and So represent the relative magnitude of the accumulation when CerS is inhibited. The downward arrow next to ceramide indicates the decrease in ceramide biosynthesis in tissues.
The specific objectives of the present study were: 1) determine if UFB1 is positively correlated with the Sa 1-P/So 1-P ratio in the extracts of the blood spots collected from Chimaltenango, Escuintla and Jutiapa; 2) if the ratio is increased, determine if the increase is due to increased blood concentrations of Sa 1-P; and 3) validate the results of the study in Chimaltenango (low exposure), Escuintla (low exposure), and Jutiapa (high exposure) by conducting a follow-up study in the departments of Sacatepéquez (low FB exposure), Santa Rosa (high FB exposure), and Chiquimula (high FB exposure). These departments were selected based on the results of a FB survey of the maize across Guatemala conducted in May to October 2012 [17]. The approaches and primary objectives of the three interrelated and sequential studies that will be referred to repeatedly in this manuscript are briefly described in Table 1.
Table 1.
Summary of the approaches and primary objectives of each of the three human studies referred to in this manuscript.
| Study title | Approaches and primary objectives |
|---|---|
| Methods validation and kinetic study 2010 to 2011 | Develop urinary exposure biomarker and blood spot mechanism-based biomarker method and conduct urinary excretion kinetic study in the USA (n=10) and biomarker validation study in Guatemala (n=177) [6, 14] |
| One year human biomarker study 2011 to 2012 | Collect maize, urine, and blood spots from Chimaltenango, Escuintla, and Jutiapa every 3 months for 1 year to determine if the levels of the urinary exposure biomarker [15] and mechanism-based blood spot biomarker are correlated (n=1240). The results of the blood spot analysis are reported herein. |
| Biomarker response validation study 2012 to 2013 | Conduct a survey in 2012 of maize across Guatemala [17] in order to select new sampling sites for validation of the biomarker results from the 2011 to 2012 one year human biomarker study [15]. Collect maize, urine, and blood spots from Sacatepéquez, Santa Rosa, and Chiquimula from February to March 2013 to determine if the relationship between the urinary exposure biomarker and mechanism-based blood spot biomarker observed in the 2011 to 2012 one year human biomarker study could be replicated (n=299). The results of the urinary exposure biomarker and mechanism-base blood spot biomarker analysis are reported herein. |
2. Material and methods
2.1 Chemicals, instrumentation, and analytical methods
All chemicals, external and internal standards, solid-phase extraction cartridges, and instrumentation used for the liquid chromatography/mass spectrometry (LCMS) quantitation of FB and sphingoid base 1-phosphates were the same as described previously [6, 14, 15]. The treatment of the maize, urine samples, and blood spots including extraction and processing, chromatographic conditions and the MS method for quantitation of FB1, FB2, and FB3 and Sa-1-P and So 1-P was also described in detail previously [6, 14] and will only be described briefly below.
2.2 Human studies in Guatemala
A total of three studies evaluating human exposure in Guatemala to FB and possible effects on sphingolipid metabolism have been conducted by our team and the approaches and objectives are briefly summarized in Table 1. The intent of the methods validation and kinetic study in 2010 to 2011 was to develop methods and the results have been reported in detail elsewhere [6, 14]. The second study in 2011 to 2012 (Table 1) was a one year human biomarker study involving a total of 1240 female consenting volunteers between the ages of 18 and 70 years old recruited in the departments of Chimaltenango ( n=439), Escuintla (n=402), and Jutiapa (n=399) in March (n=341 total), June (n=299 total), and October (n=299 total) of 2011 and again in February (n=301) of 2012. The one year biomarker study was designed to test the hypothesis that FB inhibits CerS in humans consuming maize-based diets contaminated with high levels of FB. Of the total of 1240 women interviewed, spot (random daytime sample) urine samples from 1236 women were analyzed for FB1 and 1233 blood spots were analyzed for sphingoid base 1-phosphates. The study design, rationale, procedures, demographic data, UFB1 concentrations, and the levels of FB contamination in the maize at each sampling time are reported in Torres et al. [15] and the supporting information provided therein [15]. The results of the sphingolipid analysis of blood spots collected as part of the one year human biomarker study are reported here along with the results of the 2012 to 2013 biomarker response validation study (Table 1) which was designed to validate the results of the 2011 to 2012 one year human biomarker study.
Briefly, for the one year human biomarker study in 2011 to 2012 and the 2012 to 2013 biomarker response validation study only females were recruited because the long-term goal of our work is to determine if FB contributes to NTDs. For the one year human biomarker study in 2011 to 2012, the departments of Chimaltenango, Escuintla and Jutiapa were chosen based on FB-maize survey results in 2005 and 2007 [16, 18] and urine, blood spots, and maize samples were collected four times over the course of one year in each department.
The selection of the sampling sites for the 2012 to 2013 biomarker response validation study was based on a survey of FB contamination of the maize from all 22 departments in Guatemala that was conducted from late May to early October 2012 [17]. Three departments were selected for the validation study; Sacatepéquez (low exposure), Santa Rosa (high exposure), and Chiquimula (high exposure). The definition of high and low exposure was based on the relative differences in the total FB (FB1+FB2+FB3) contamination levels in the maize from the 2012 survey (Supporting Information, Table S1). A total of 299 female consenting volunteers between 13 and 82 years of age were recruited in the departments of Sacatepéquez ( n=100), Santa Rosa (n=100), and Chiquimula (n=99) in February to March of 2013. Urine, blood spots, and maize were all collected in the same window of time. A total of 297 urine samples were analyzed and 299 blood spots. For additional information describing the production, marketing, and sourcing of maize and livelihoods within Departments see the Introduction in the Supporting Information.
The human subjects research protocol and informed consent form for all studies were approved by the Comité Institucional de Ética of the Instituto de Nutrición de Centro América y Panamá (Project Number CIE REV003/2010), a U.S. Department of Health and Human Services Office for Human Research Protections registered Independent Ethics Committee. The Comité Nacional de Ética of the Guatemalan Ministry of Health also gave its ethical approval for this project. Consenting women were asked to complete a questionnaire concerning their consumption of maize-based foods. The questionnaire was a modification of a 24 h recall survey developed and validated for use in Guatemala [18] by the Instituto de Nutrición de Centro América y Panamá for the Consejo Nacional de Ciencia y Tecnología (Guatemala City, Guatemala). The modified version of the questionnaire was tested in all three departments with 20 individuals to ensure that the questions asked were understood and were culturally acceptable in the target populations. Those whose self-reported health was “good” or “average” were invited to participate in the study and to provide a spot urine sample (random daytime sample) and two blood spots collected from a finger stick. The height, weight, age and information about maize consumption were recorded as part of the questionnaire. Additional details can be found in Torres et al. [15].
2.3 Processing and analysis of urine, maize, and blood spots
The processing of the urine and maize samples is described in detail elsewhere [14, 15]. Blood spots were collected at the same time as the urine sample. The method of collecting, processing, and quantitating the Sa 1-P and So 1-P in the blood spots was done as described in detail in Riley et al. [6]. Briefly, whole blood from both of the human studies was collected on FTA™ Elute Micro Cards (GE Healthcare Biosciences Corp., Piscataway, NJ, USA, Whatman™ Cat No. WB120401). All blood spots were dried at room temperature overnight and stored desiccated at −20°C. A brief description of the rationale for using FTA™ Elute Micro Cards is contained in the introductory paragraph of the Supplemental Data in Riley et al. [6].
Blood spot discs were cut from the FTA™ Elute Micro Cards with a 6 or 8 mm biopsy punch. The discs and control blanks (discs without blood) were placed in 2.0 ml microcentrifuge tubes and then cut in half, 1.0 ml of extraction mix (1:1 acetonitrile (MeCN): water + 5% formic acid containing C20 So and C17 Sa 1-P internal standards at 60 pmol/ml final concentration) added, and mixed gently. The tubes were sonicated for one hour at 50°C and then gently shaken for 3 hours. Extracts (0.7 ml) were filter centrifuged at 4,500 rcf for approximately 10 minutes using 0.45 μm nylon centrifuge tubes (Costar, Corning Inc., Tewksbury, MA, USA) that had been washed three times with 1.5 ml of 1:1 MeCN: water + 5% formic acid immediately before use to remove compounds which interfere with the LCMS analysis. The extracts were then diluted 1:1 with 1:1 MeCN: water + 1% formic acid, and transferred to sample vials for liquid chromatography-mass spectrometry (LCMS) analysis as described below.
Reversed phase high performance liquid chromatography (HPLC) analysis was conducted using a Finnigan Micro AS or Thermo Scientific Dionex Ultimate 3000 autosampler coupled to either a Finnigan Surveyor MS pump or Thermo Scientific Dionex Ultimate 3000 pump (Thermo Fisher Scientific, Waltham, MA, USA). The column effluent was directly coupled to a Finnigan LTQ XL linear ion trap mass spectrometer (MS version 2.5.0; Thermo Fisher Scientific, Waltham, MA USA). A detailed description of the chromatographic and MS parameters, recovery of sphingoid bases and sphingoid base 1-phosphates by the extraction process, and the stability of sphingoid base 1-phosphates in blood spots collected on FTA™ Elute Micro Cards is reported in Riley et al. [6].
The method of quantitation of Sa 1-P and So 1-P and normalization to blood volume are described in detail in Riley et al. [6]. Briefly, quantitation of So 1-P and Sa 1-P in the extracts was based on the recovery of the C17 So 1-P internal standard which was included in the extraction mixture at 60 pmol/ml. The amount of Sa 1-P and So 1-P in the extracts was normalized to the volume of blood extracted using the linear relationship between blood volume and the UV absorbance at 270 nm in the extracts measured using a Beckman Coulter® DU® 730 spectrophotometer (Beckman Coulter, Inc., Brea, CA USA).
2.4 Statistical analysis
Statistical analysis was performed using SigmaPlot® 12 (Systat Software, Inc., San Jose, CA, USA). When many groups were compared, one-way analysis of variance was used, followed by all pair wise multiple comparison (Dunn’s method). If the data failed normality or were of unequal variance the Kruskal-Wallis One Way Analysis of Variance on Ranks was used. When only two groups were compared, a Student t test was used. The Mann-Whitney rank sum test was used for comparing two groups if data failed normality or was of unequal variance. The Chi-Square Test was used to test for differences in incidence or frequency. Relative risk was calculated using the Relative Risk Test in SigmaPlot® 12. The Pearson product moment and Spearman rank order correlation were used to measure the strength of the association between pairs of variables and to assess the linearity versus monotonic nature of the data. All data are expressed as mean ±SD or the 95% CI and differences among or between means were considered to be significant if the p-value (p) was <0.05.
3 Results
The results of the UFB1 analysis for the 2011 to 2012 one year human biomarker study were reported previously [15]. The sphingolipid analysis of blood spots collected as part of the 2011 to 2012 one year human biomarker study are reported herein followed by the results of both the UFB1 analysis and sphingolipid analysis of the blood spots from the 2012 to 2013 biomarker response validation study. The validation study was conducted in order to see if the results of the one year human biomarker study could be reproduced.
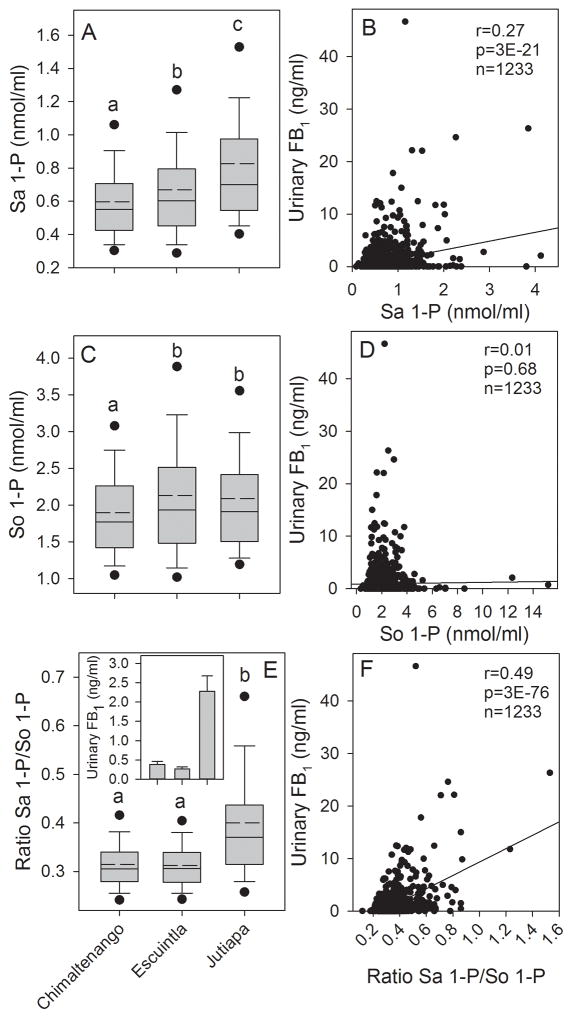
3.1 Urinary FB1, Sa 1-P/So 1-P ratio, and Sa 1-P/ml blood positively correlated in the 2011 to 2012 one year human biomarker study
FB exposure as estimated based on the levels of FB contamination of the maize and FB1 intake based on the levels of UFB1 were found to be significantly higher in Jutiapa compared to Chimaltenango and Escuintla as reported in Torres et al. [15]. Analysis of the blood spot extracts (n=1233) found that the Sa 1-P concentration was significantly higher in Jutiapa compared to Chimaltenango or Escuintla and Escuintla was significantly higher than in Chimaltenango (Fig. 2A). The Sa 1-P concentration in the blood spot extracts was significantly positively correlated with the individually matched UFB1 concentrations (Fig. 2B). The mean So 1-P concentration in the blood spot extracts from Jutiapa and Escuintla were significantly higher than in Chimaltenango but were not significantly different from each other (Fig. 2C) and there was no correlation between the individually matched UFB1 and the So 1-P concentrations (Fig. 2D). The Sa 1-P/So 1-P ratio was significantly higher in Jutiapa compared to either Escuintla or Chimaltenango (Fig. 2E) matching the pattern of the statistical analysis observed for UFB1 (Fig. 2E, inset) as reported in Torres et al. [15]. The Sa 1-P/So 1-P ratio was also significantly positively correlated with the individually matched UFB1 concentrations (Fig. 2F). The best correlations between UFB1 and the Sa 1-P concentrations and Sa 1-P/So 1-P ratio were obtained using the Pearson product moment correlation indicating that the relationship between variables was best fit assuming linearity. Neither the Pearson nor Spearman correlation between UFB1 and So 1-P concentration were statistically significant (p=0.68 and p=0.69 respectively).
Figure 2.
(A) Box plot showing the mean (dashed line), median (solid line), 25th and 75th percentile (upper and lower limits of the box), the 10th and 90th percentile (error bars), and 5th and 95th percentile (solid circles) for the Sa 1-P concentration in the blood spot extracts from Chimaltenango, Escuintla, and Jutiapa for all subjects for all sampling periods combined (March, June and October 2011 and February 2012); (B) Scatter plot showing the correlation between the urinary FB1 levels and the Sa 1-P concentration in the matched blood spots for all subjects (Inset is the Pearson product moment correlation coefficient (r), p-value (p), and the total number of matched samples (n)); (C) Same as in (A) but for So 1-P; (D) same as in (B) but for So 1-P; (E) Same as in (A) but for the ratio of Sa 1-P/So 1-P and inset is the mean urinary FB1 and 95% CI from Torres et al. [15]; (F) same as in (B) but for the ratio Sa 1-P/So 1-P. In graphs A, C, and E, bars with differing superscripts are significantly different (p<0.05) based on the Kruskal-Wallis ANOVA on ranks.
3.2 Increase in Sa 1-P/So 1-P ratio due primarily to increased Sa 1-P in blood in the 2011 to 2012 one year human biomarker study
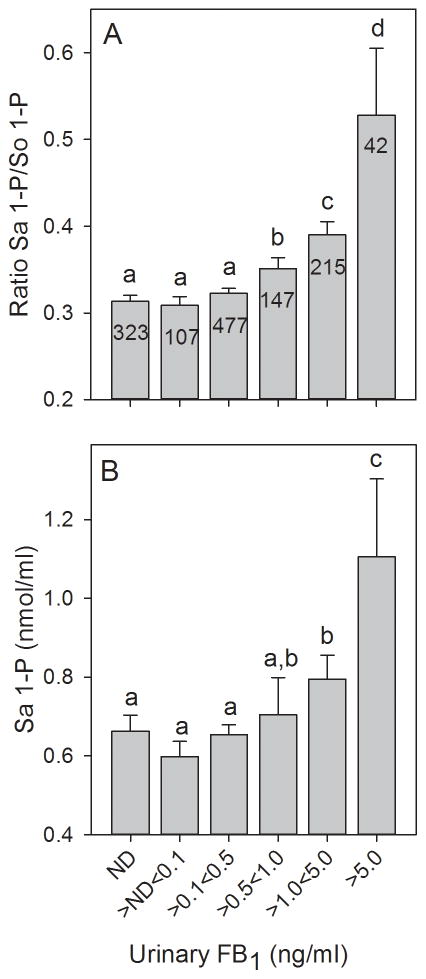
The statistically significant positive correlation between the Sa 1-P/So 1-P ratio in the blood spot extracts and the individually matched UFB1 concentrations was observed at all four sampling times (Supporting Information, Figure S1A–S1D). The correlation between the Sa 1-P concentration and UFB1 was statistically significant at three of the four sampling times (Supporting Information Figure, S1E–S1H). The one sampling time (June 2011) when the correlation between Sa 1-P and UFB1 was not statistically significant was also the sampling time when the mean UFB1 concentration in Jutiapa (and overall) was the lowest (1.22 ng FB1/ml, see Supporting Information, Table S4 in Torres et al. [15]). The So 1-P concentration was only significantly positively correlated with the UFB1 (r=0.14, p=0.02, n=297) at the third sampling time (October 2011) (data not shown). As expected, overall, there was a statistically significant positive correlation between the concentrations of Sa 1-P and So 1-P in the blood spot extracts. The Sa 1-P and the ratio of Sa 1-P/So 1-P in the blood spot extracts were also significantly positively correlated but there was no correlation between the So 1-P concentration and the Sa 1-P/So 1-P ratio (Supporting Information, Figure S2A–S2C); indicating that the increase in the Sa 1-P/So 1-P ratio was caused by the increase in Sa 1-P and not a decrease in So 1-P.
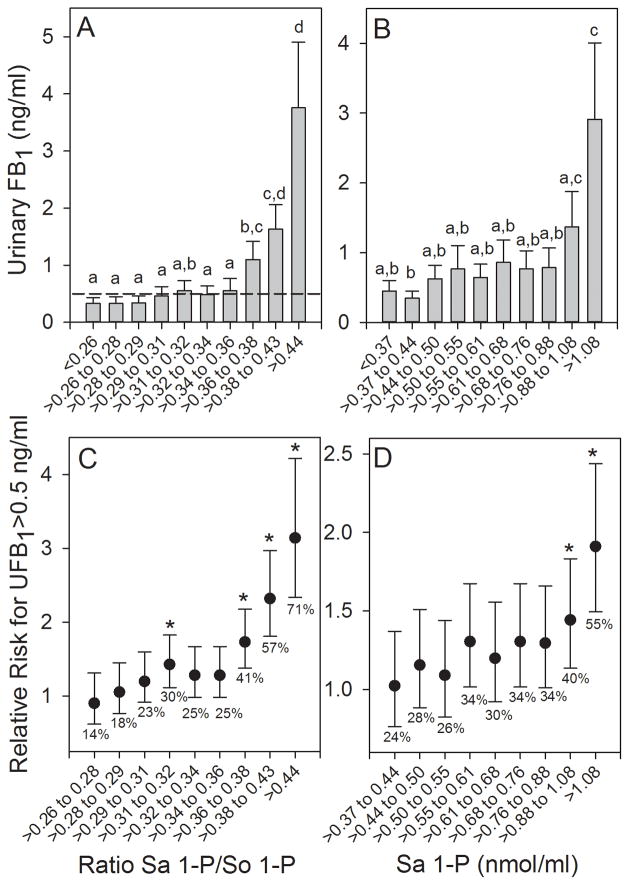
3.3 Urinary FB1 levels and Sa 1-P/So 1-P ratio dose-dependence and relative risk calculations in the 2011 to 2012 one year human biomarker study
The Sa 1-P/So 1-P ratios in blood spot extracts were sorted (ascending) and then divided into 10 approximately equal groups (123 or 124 individuals in each grouping) and the results were plotted versus the mean UFB1 values for each grouping (Fig. 3A). The results show that there was an apparent threshold below which the increase in the Sa 1-P/So 1-P ratio was not associated with a statistically significant increase in the UFB1 concentration relative to the group with the lowest Sa 1-P/So 1-P ratio. The UFB1 concentration at the breakpoint was visually approximated at 0.5 to 1.0 ng FB1/ml (see the 0.5 ng/ml reference line in Fig. 3A). The Sa 1-P concentrations (sorted and grouped as described above) in the blood spot extracts also exhibited an apparent threshold below which the increase in the Sa 1-P was not associated with a statistically significant increase in the UFB1 concentration. A statistically significant increase in the UFB1 concentration was only observed in the highest Sa 1-P grouping (Fig. 3B). There was no relationship between the grouped So 1-P concentrations and UFB1 (Supporting Information, Figure S3); again indicating that the increase in the Sa 1-P/So 1-P ratio was a consequence of the increase in Sa 1-P and not a decrease in So 1-P.
Figure 3.
The relationship between urinary FB1 (UFB1) levels (mean and 95% CI) and the (A) Sa 1-P/So 1-P ratio and (B) concentration (nmol/ml) of Sa 1-P in blood spot extracts for 10 approximately equal groupings (n=123 or 124 individuals/group) of all subjects from Chimaltenango, Escuintla, and Jutiapa over all sampling periods combined (March, June and October 2011 and February 2012). The dashed reference line in (A) marks the UFB1 concentration equal to 0.5 ng/ml. Bars with differing superscripts are significantly different (p<0.05) based on the Kruskal-Wallis ANOVA on ranks. Panels (C) and (D) show the relationship between the increasing Sa 1-P/So 1-P ratio (C) and the concentration of Sa 1-P (D) and the increasing relative risk for exceeding a UFB1 concentration of 0.5 ng/ml. Mean UFB1 values with an asterisk (*) are significantly greater than the UFB1 in the Sa 1-P/So 1-P ratio or Sa 1-P grouping that was ≤ 0.26 or ≤0.37 nmol/ml, respectively. The percentages of individuals exceeding a UFB1 of 0.5 ng/ml are also shown.
Assuming that individuals in the 2011 to 2012 one year human biomarker study with Sa 1-P/So 1-P ratios below 0.26 were at the lowest risk on average for exceeding 0.5 ng FB1/ml, the relative risk for exceeding 0.5 ng FB1/ml was determined (Fig. 3C). The results show that relative to the group with the lowest Sa 1-P/So 1-P ratio the relative risk increased markedly at the breakpoint (>0.36), although there was also a small but statistically significant increased risk at the grouping corresponding to the Sa 1-P/So 1-P ratio of 0.31 to 0.32. The percentage of individuals with UFB1 concentrations greater than 0.5 ng/ml ranged from 14% in the lowest grouping to 71% in the highest grouping (>0.44) (Fig. 3C). A similar increase in the relative risk was seen for the Sa 1-P concentrations (Fig. 3D), however, the increased risk was only statistically significant for the two highest groupings.
The UFB1 were sorted (ascending) and then divided into six windows of increasing UFB1 concentrations and plotted against the mean Sa 1-P/So 1-P ratios (Fig. 4A) and the Sa 1-P concentrations (Fig. 4B) in the blood spot extracts for each UFB1 grouping. The results show that there is a dose-response relationship between the two variables and that there was also an apparent threshold below which the increase in UFB1 was not associated with a statistically significant increase in either the Sa 1-P/So 1-P ratio or the Sa 1-P concentration in the blood spots. For the Sa 1-P/So 1-P ratio the first statistically significant increase occurred at the UFB1 window that was >0.5<1.0 ng FB1/ml (Fig. 4A) and for the Sa 1-P concentration the window was >1.0<5.0 ng FB1/ml (Fig. 4B).
Figure 4.
The UFB1 levels in all spot urine samples from the one year human biomarker study grouped in six windows of increasing concentrations and the corresponding mean Sa 1-P/So 1-P ratio (A) and Sa 1-P concentrations (nmol/ml) (B) in matched blood spot extracts. The bars are the means and 95% CI at each window of UFB1 concentration and the number in each bar is the total number of blood spot extracts falling within each UFB1 concentration window. All UFB1 data for which there was matching FB intake data (n=1233) were used. If UFB1 was not detected (ND) then it was assigned a value of 0 ng/ml. The UFB1 detection limit of the method is 0.03±0.01 ng/ml [13]. All of the UFB1 values < 0.03 ng/ml are included in the first bar (ND). Bars with differing superscripts are significantly different (p<0.05) based on the Kruskal-Wallis ANOVA on ranks.
3.4 Demographics and maize consumption in Sacatepéquez, Santa Rosa, and Chiquimula in the 2012 to 2013 biomarker response validation study
The demographic characteristics of the women in the three departments chosen for the validation study were similar in many respects (Table 2). However, there were some statistically significant differences (p<0.05) among the female volunteers recruited in the three departments. For example, there were significantly more pregnant women in Chiquimula compared to either Sacatepéquez or Santa Rosa. There were also significantly more lactating women in Chiquimula compare to Santa Rosa. Similarities include that approximately 90% of the volunteers identified themselves as rural as opposed to urban. Greater than 99% of the volunteers reported that they ate maize-based foods every day and tortillas comprised on average 91% of the reported maize-based food consumed; similar to what was reported in the 2011 to 2012 one year human biomarker study in Chimaltenango, Escuintla, and Jutiapa [15]. Other maize-based foods consumed were for the most part also prepared from nixtamalized maize (data not shown). In Sacatepéquez, the source of the maize was reported to be primarily from the highlands (85%), whereas, in Santa Rosa it was primarily from the lowlands (86%) and in Chiquimula a majority of the women (53%) did not know the source of the maize, although 35% reported that it was from the lowlands and only 1% identified the highlands as the source of the maize they consumed (Supporting Information, Table S2). It is likely that most of the maize consumed in Chiquimula was from the lowlands and from hot dry regions based on the relatively low incidence of AFB1 positive samples (27%) and high incidence of FB1 positive samples (100%) seen in the survey conducted in 2012 (Torres et al., 2015).
Table 2.
Demographics and maize consumption from all participants sampled from 14 February to 18 March 2013 in the biomarker response validation study.a
| Sacatepéquez | Santa Rosa | Chiquimula | All Departments | |
|---|---|---|---|---|
| Age (yr) | 36.0a (13.4) n=100 |
37.6a (15.4) n=100 |
36.5a (12.9) n=99 |
36.7a (13.9) n=299 |
| Weight (kg) | 58.6a (11.2) n=100 |
57.7b (12.4) n=100 |
51.1c (8.5) n=99 |
55.8a (11.3) n=299 |
| Height (cm) | 147.6a (5.2) n=100 |
150.9b (5.7) n=100 |
145.9c (5.3) n=99 |
148.1a (5.8) n=299 |
| Urban/rural (%/%) | 12/88a n=100 |
12/88a n=100 |
5/95a n=98 |
10/90a n=298 |
| Pregnant/not preg. (%/%) | 4/96a n=100 |
2/98a n=100 |
16/84b n=99 |
7/93a n=299 |
| Lactating/not lact. (%/%) | 30/70a,b n=100 |
22/78a n=100 |
39/61b n=98 |
30/70a n=298 |
| Hours since urination (h) | 2.3a (2.0) n=100 |
3.2b (2.0) n=100 |
4.6c (2.4) n=99 |
3.4a (2.3) n=299 |
| Days/week eat maize (d) | 7a (0.3) n=99 |
7a (0.4) n=100 |
7a (0.0) n=98 |
7a (0.3) n=298 |
| Hours since ate maize (h) | 4.3a (4.2) n=100 |
4.6a (5.1) n=100 |
4.3a (3.0) n=99 |
4.4a (4.2) n=299 |
| Tortillas/day (g) | 348a (236) n=100 |
290a (168) n=100 |
346a (247) n=99 |
328a (221) n=299 |
| Total maize food/day (g) | 381a (246) n=100 |
312a (175) n=100 |
382a (265) n=99 |
358a (233) n=299 |
Where a single value is shown it is the average followed by the (SD) for the responses to that question and below that is the “n”. The statistic used for comparing mean values was the Kruskal-Wallis one way analysis of variance on ranks. Where the data is presented as a percentage or frequency (%/%) the statistical analysis was done using the Chi square test. Values in rows with differing superscripts are significantly different (p<0.05).
The reported number of hours since last consuming maize-based food was on average 4.4 hr before providing the urine and blood spot sample similar to the average length of time since the last urination (3.4 h) (Table 2). For all three departments, the great majority of the women volunteers (>96%) had recently been potentially exposed to FB through consumption of maize-based foods. Only, 2.3% of the women reported having not eaten maize-based food within the previous 24 hr (7/299). Of those who had not eaten maize-based food for 24 hr, there were two from Sacatepéquez, four from Santa Rosa, and one from Chiquimula. Thus, for each department the volunteers had about the same amount of time for absorbing and eliminating the FB consumed prior to providing the urine sample.
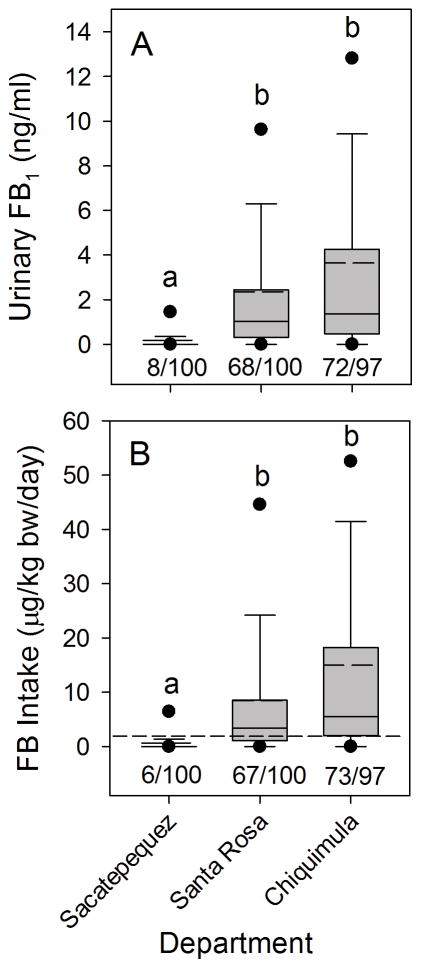
Daily consumption of maize-based foods by the recruits was similar across the three departments and tortillas were the primary source of the maize consumed (Fig. 5A and Table 2). The mean amount of tortillas (g tortillas/day) was not significantly different (p>0.05) for the three departments, however, the mean amount of tortillas consumed in Santa Rosa was 16% to 17% lower than in Sacatepéquez and Chiquimula (Fig. 5A and Table 2) and a similar difference was seen for consumption of total maize-based foods (Table 2).
Figure 5.
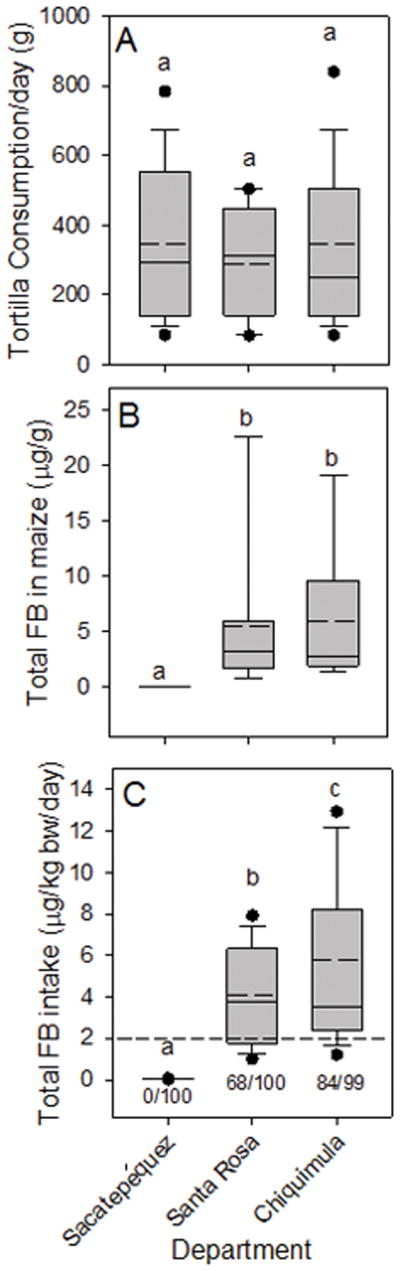
Box plots as described in Figure 2 legend showing (A) the daily maize tortilla consumption (g fresh weight/day) by women in the biomarker response validation study from Sacatepéquez, Santa Rosa, and Chiquimula for all subjects in February to March 2013, (B) total fumonisin (FB1+FB2+FB3) in maize collected from each department at the same time as collecting urine and blood spots, and (C) the estimated total FB intake in each department calculated using the mean FB contamination (Supporting Information, Table S1), the individual tortilla consumption, and body weights corrected for the grams dry weight of maize in tortillas and the reduction of FB by the nixtamalization process (see Torres et al., [15] and Palencia et al. [19] for additional details). The dashed line in (C) is the JECFA PMTDI. In all graphs, bars with differing superscripts within groups are significantly different (p<0.05) based on the Kruskal-Wallis ANOVA on ranks. The ratio of numbers below each box in (C) is the number of individuals with calculated total FB (FB1+FB2+FB3) intake that exceeded the JECFA PMTDI/total subjects.
3.5 Fumonisin contamination of Maize in Sacatepéquez, Santa Rosa, and Chiquimula in the 2012 to 2013 biomarker response validation study
The level of FB in the maize being sold for human consumption, at the time of the sampling of the urine and blood (February to March 2013), was significantly higher in Santa Rosa and Chiquimula compared to Sacatepéquez (Fig. 5B and Supporting Information, Table S1). The relative difference between departments was similar to what was seen in the survey of 2012 (Supporting Information, Table S1). The average amount of FB1 and total FB (FB1+FB2+FB3) in Sacatepéquez maize was 0.03 μg/g and 0.04 μg/g, respectively, compared to 3.61 μg/g and 5.56 μg/g , respectively, in the maize from Santa Rosa and 3.98 μg/g and 5.91 μg/g in maize from Chiquimula, respectively (Fig. 5B and Supporting Information, Table S1). These relative differences were similar to those seen in the 2012 survey [17] and in earlier surveys [16, 18].
3.6 Estimates of FB intake from maize contamination in Sacatepéquez, Santa Rosa, and Chiquimula in the 2012 to 2013 biomarker response validation study
Total FB (FB1+FB2+FB3) intake from tortillas for each department in the validation study was estimated using the mean total FB (μg/g maize) in maize samples collected from February to March 2013 (Fig. 5B and Supporting Information, Table S1), each individual’s reported tortilla consumption (g/day) from the questionnaires, and each individual’s body weight. The FB intake from tortillas alone underestimates total FB intake by approximately 9% since tortillas were 91% of the total maize-based food consumed (Table 2). The resulting total FB intake estimate was then corrected as in the 2011 to 2012 one year human biomarker study [15] for the amount of dry maize equivalents per gram of tortilla (57%) and the reduction in total FB in tortillas by the process of nixtamalization as practiced in Guatemala [19].
The overall estimated mean and median FB intake in Sacatepéquez was less than the JECFA PMTDI of 2 μg/kg b.w./day and none of the female volunteers exceeded the PMTDI (Fig. 5C). The estimated mean and median intake in Santa Rosa and Chiquimula exceeded the JECFA PMTDI and 68% and 85% of the volunteers, respectively, had estimated FB1 intake that exceeded the PMTDI (Fig. 5C).
3.7 Estimated FB intake based on UFB1 levels in Sacatepéquez, Santa Rosa, and Chiquimula in the 2012 to 2013 biomarker response validation study
An alternative approach for estimating individual FB intake is to use the UFB1 for individual women. In the 2012 to 2013 biomarker response validation study, the women in Sacatepéquez had significantly lower levels of UFB1 compared to the women in Santa Rosa and Chiquimula (Fig. 6A) and the number of women with UFB1 levels greater than 0.5 ng/ml or 0.1 ng/ml was also significantly less (Fig. 6A; Supporting Information, Table S3). This is similar to what was seen in the 2011 to 2012 one year human biomarker study where the first UFB1 window where there was a statistically significant increase in the relative risk for exceeding the JECFA PMTDI was > 0.1 ng/ml < 0.5 ng/ml (as documented in Fig. 3A of Torres et al. [15]).
Figure 6.
Box plots as described in Figure 2 legend showing (A) UFB1 (ng /ml) in spot urine samples collected from women in the biomarker response validation study from Sacatépequez, Santa Rosa, and Chiquimula in February to March 2013, and (B) the FB intake calculated based on the assumption that urinary excretion of FB1 is 0.5% of the total FB1 consumed [14]. The ratio of numbers below each box in (A) is the number of individuals with UFB1 concentrations >0.5 ng/ml/total subjects. The ratio of numbers below each box in (B) is the number of individuals with calculated FB1 intake that exceeded the JECFA PMTDI/total subjects. The dashed line in (B) is the JECFA PMTDI. Bars with differing superscripts within groups are significantly different (p<0.05) based on the Kruskal-Wallis ANOVA on ranks.
The FB intake was calculated using the individual UFB1 concentrations, body weights and the assumptions that 0.5% of the FB consumed was excreted in the urine [14] and total urine output was 1000 ml/day. The calculated FB1 intake using UFB1 was significantly lower in Sacatepéquez compared to either Santa Rosa or Chiquimula (Fig. 6B) and the number of women with calculated FB1 intake greater than the JECFA PMTDI (2 μg/kg b.w./day) was similar to the number of women exceeding the PMTDI based on the calculation using the level of total FB in the maize and individual maize tortilla consumption (Fig. 5C). However, it should be noted that using the UFB1 does not take into account the intake of FB2 and FB3 which were only detected in a few of the urine samples with the highest levels of FB1 in agreement with what was reported previously for the 2011 to 2012 one year human biomarker study [15] and the 2010 to 2011 methods validation and kinetic study [14]. Nonetheless, Chiquimula had the highest estimated FB intake based on either the level of maize contamination and tortilla consumption (Fig. 5C) or the UFB1 (Fig. 6B). The estimated FB intake by women in Chiquimula based on the UFB1 was not significantly different (ANOVA, p>0.05) from the calculated intake for women in Santa Rosa (Fig. 6B). However, a t-test comparing the FB intake calculated from the UFB1 for only Santa Rosa and Chiquimula was significantly different (p=0.027).
There was a statistically significant, albeit weak, Pearson product moment correlation between the estimated daily FB intake calculated using the individual maize intake (Fig. 4C; r=0.15, p=0.01, n=297) or estimated using the individual UFB1 levels (Fig. 5B; r=0.15, p=0.008, n=297). When the UFB1 values were divided into six groups of increasing UFB1 there was a positive dose-dependent relationship between UFB1 and FB intake based on maize consumption and levels of FB contamination (Fig. 7). This is similar to what was seen in the 2011 to 2012 one year human biomarker study as documented in Fig. 3A of Torres et al. [15]. All of the individual UFB1 values for which there were matching FB intake values (n=297) were used in Fig. 7. The UFB1 level that bracketed the JECFA PMTDI (2 μg/kg b.w./day) was > 0.1 and <0.5 ng/ml (Fig. 7). The percentage of individuals exceeding the PMTDI within each grouping of UFB1 increased in a dose-dependent manner up to the >1<5 ng/ml group (Fig. 7). Compared to the group with no detectable UFB1, the relative risk for exceeding the JECFA PMTDI was first statistically significant for the grouping that was > 0.1 ng/ml < 0.5 ng/ml (Supporting Information, Table S4); similar to what was seen in the 2011 to 2012 one year human biomarker study [15].
Figure 7.
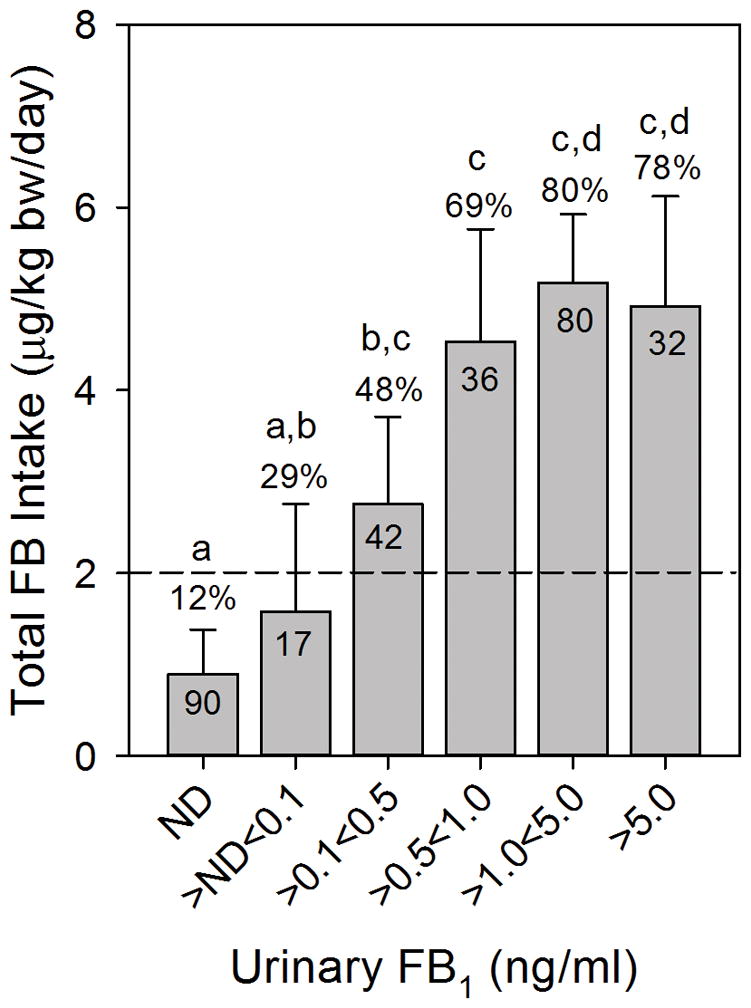
Estimated total FB intake (data from Fig. 5C) grouped in six windows of increasing UFB1 concentrations from spot urine samples. The bars are the mean μg/kg b.w./day and 95% CI at each window of UFB1 concentration and the number in each bar is the total falling within each UFB1 concentration window and the percentage above each bar is the number exceeding the JECFA PMTDI. All UFB1 data for which there was matching FB intake data (n=297) was used. If UFB1 was not detected then it was assigned a value of 0 ng/ml. The UFB1 detection limit of the method is 0.03±0.01 ng/ml [13]. All of the UFB1 values < 0.03 ng/ml are included in the first bar. The dashed line is the JECFA PMTDI. Bars with differing superscripts are significantly different (p<0.05) based on the Kruskal-Wallis ANOVA on ranks.
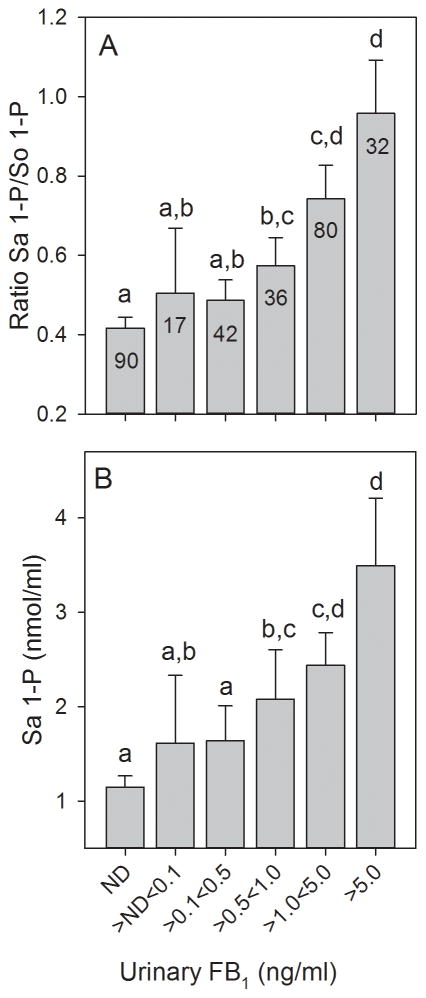
3.8 Urinary FB1, Sa 1-P/So 1-P ratio, and Sa 1-P/ml blood positively correlated in the 2012 to 2013 biomarker response validation study
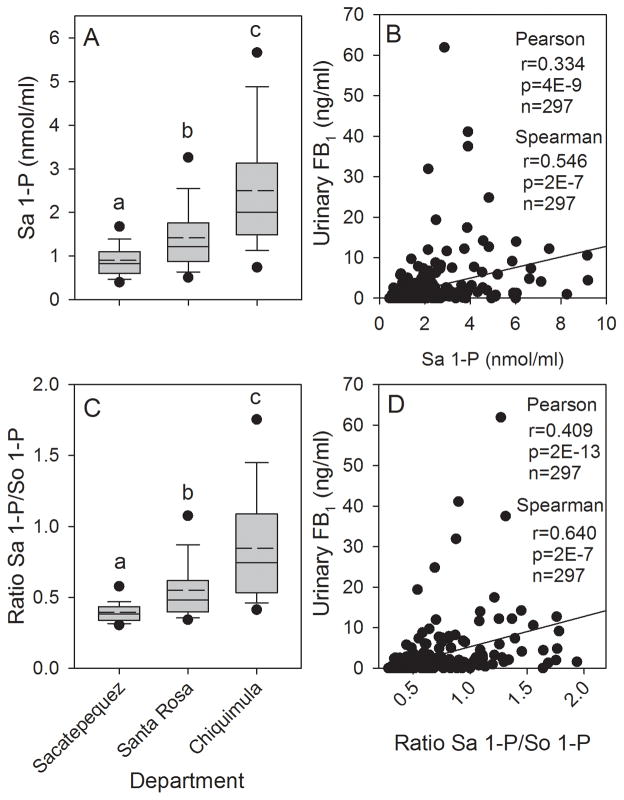
Analysis of the blood spot extracts (n=299) found that the Sa 1-P concentration was significantly higher in Chiquimula and Santa Rosa compared to Sacatepéquez, and Chiquimula was significantly higher than Santa Rosa (Fig. 8A). The Sa 1-P concentration was also significantly correlated with the individually matched UFB1 concentrations (Fig. 8B). The mean So 1-P concentration in the blood spot extracts from Santa Rosa and Chiquimula were significantly higher than in Sacatepéquez but were not significantly different from each other (data not shown) and there was no significant correlation (Pearson product moment) between the individually matched UFB1 and So 1-P concentrations (r=0.106, p=0.07, n=297). The Sa 1-P/So 1-P ratio was significantly higher in Santa Rosa and Chiquimula compared to Sacatepéquez (Fig. 8C), matching the pattern of the statistical analysis observed for UFB1 (Fig. 6A). The Sa 1-P/So 1-P ratio was also significantly correlated with the individually matched UFB1 concentrations (Fig. 8D). The best correlations between UFB1 and the Sa 1-P concentrations and Sa 1-P/So 1-P ratio were obtained using the Spearman rank correlation, however, the p-values were much lower using the Pearson product moment correlation (Fig. 8B and 8D, inset).
Figure 8.
(A) Box plot showing the mean (dashed line), median (solid line), 25th and 75th percentile (upper and lower limits of the box), the 10th and 90th percentile (error bars), and 5th and 95th percentile (solid circles) for the Sa 1-P concentration in the blood spot extracts from Sacatepéquez, Santa Rosa, and Chiquimula for all subjects in February to March 2013; (B) Scatter plot showing the correlation (Pearson and Spearman procedures) between the urinary FB1 levels and the Sa 1-P concentration in the matched blood spots for all subjects (Inset is the correlation coefficient (r), p-value (p), and the total number of matched samples (n)); (C) same as in (A) but for the ratio of Sa 1-P/So 1-P, and (D) same as in (B) but for the ratio Sa 1-P/So 1-P. In graphs A and C bars with differing superscripts are significantly different (p<0.05) based on the Kruskal-Wallis ANOVA on ranks.
3.9 Increase in Sa 1-P/So 1-P ratio due primarily to increased Sa 1-P in blood in the 2012 to 2013 biomarker response validation study
Overall, there was a statistically significant positive correlation between the concentrations of So 1-P and the Sa 1-P/So 1-P ratio using the Pearson product moment procedure (r=0.229, p=7E-5, n=299). However, the correlation between Sa 1-P and the Sa 1-P/So 1-P ratio was much stronger (r=0.830, p=6E-77, n=299); indicating that, as in the 2011 to 2012 one year human biomarker study, the increase in the Sa 1-P/So 1-P ratio was caused primarily by the increase in Sa 1-P and not a decrease in So 1-P. The same relative difference was seen when using the Spearman rank order procedure (data not shown).
3.10 Urinary FB1 levels and Sa 1-P/So 1-P ratio dose-dependence and relative risk calculations in the 2012 to 2013 biomarker response validation study
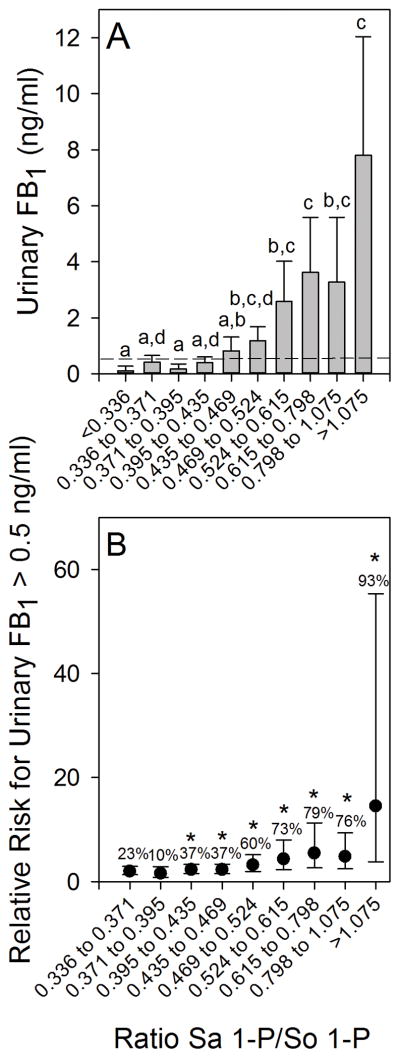
For comparison to the results of the 2011 to 2012 one year human biomarker study (Fig. 3), the Sa 1-P/So 1-P ratios in blood spot extracts from the 2012 to 2013 biomarker response validation study were sorted (ascending) and then divided into 10 approximately equal groups (29 or 30 individuals in each grouping) and the results were plotted versus the mean UFB1 values for each grouping (Fig. 9A). The results show that there was an apparent threshold below which the increase in the Sa 1-P/So 1-P ratio was not associated with a statistically significant increase in the UFB1 concentration, similar to what was seen in the 2011 to 2012 one year human biomarker study (Fig. 3A). The UFB1 concentration at the breakpoint was visually approximated at 0.5 to 1.0 ng FB1/ml (see the 0.5 ng/ml reference line in Fig 9A). The Sa 1-P concentrations (sorted and grouped as described above) in the blood spot extracts also exhibited an apparent threshold below which the increase in the Sa 1-P was not associated with a statistically significant increase in the UFB1 concentration. A statistically significant increase in the UFB1 concentration at the Sa 1-P concentration, relative to the lowest grouping, was observed in the four highest groupings (≥1.81 nmol/ml) (Supplemental Information, Fig. S4A).
Figure 9.
(A) The relationship between urinary FB1 (UFB1) levels (mean and 95% CI) and the Sa 1-P/So 1-P ratio in blood spot extracts for 10 approximately equal groupings (n=29 or 30 individuals/group) for all subjects from Sacatepéquez, Santa Rosa, and Chiquimula in February to March 2013; The dashed reference line in (A) marks the UFB1 concentration equal to 0.5 ng/ml. Bars with differing superscripts are significantly different (p<0.05) based on the Kruskal-Wallis ANOVA on ranks. Panel (B) shows the relationship between the increasing Sa 1-P/So 1-P ratio and the increasing relative risk for exceeding a UFB1 concentration of 0.5 ng/ml. Mean UFB1 values with an asterisk (*) are significantly greater than the UFB1 in the Sa 1-P/So 1-P ratio that was ≤0.336. The percentage of individuals exceeding a UFB1 of 0.5 ng/ml is also shown.
Assuming that individuals with Sa 1-P/So 1-P ratios below 0.336 were at the lowest risk on average for exceeding 0.5 ng FB1/ml, the relative risk for exceeding 0.5 ng FB1/ml was determined (Fig. 9B). The results show that relative to the group with the lowest Sa 1-P/So 1-P ratio (<0.336, n=30) the relative risk increased significantly in the grouping of 0.395 to 0.435 nmol/ml (Fig. 9B). The percentage of individuals with UFB1 concentrations greater than 0.5 ng/ml ranged from 10% in the next to lowest grouping (>0.371 to 0.395) to 93% in the highest grouping (>1.075) (Fig. 9B). A similar increase in the relative risk was seen for the Sa 1-P concentrations (Supplemental Information, Fig. S4B). Increased relative risk was statistically significant for the four highest groupings (>1.81 nmol/ml). The percentage of UFB1 concentrations greater than 0.5 ng/ml ranged from 20% in the lowest grouping to 87% in the highest grouping.
As was done with the 2011 to 2012 one year human biomarker study data (Fig. 4), the UFB1 concentrations were sorted (ascending) and then divided into six windows of increasing UFB1 concentrations and plotted against the mean Sa 1-P/So 1-P ratios (Fig. 10A) and the Sa 1-P concentrations (Fig. 10B) in the blood spot extracts for each UFB1 grouping. The results show that there is a dose-response relationship between the two variables and that there was also an apparent threshold below which the increase in UFB1 was not associated with a statistically significant increase in either the Sa 1-P/So 1-P ratio or the Sa 1-P concentration in the blood spots. For both the Sa 1-P/So 1-P ratio and the Sa 1-P concentration the first statistically significant increase occurred in the UFB1 window that was >0.5<1.0 ng FB1/ml (Fig. 10A and 10B).
Figure 10.
The UFB1 levels in all spot urine samples from the 2012–2013 biomarker response validation study grouped in six windows of increasing concentrations and the corresponding mean Sa 1-P/So 1-P ratio (A) and Sa 1-P concentrations (nmol/ml) (B) in matched blood spot extracts. The bars are the means and 95% CI at each window of UFB1 concentration and the number in each bar is the total blood spot extracts falling within each UFB1 concentration window. All UFB1 data for which there was matching FB intake data (n=297) were used. If UFB1 was not detected (ND) then it was assigned a value of 0 ng/ml. The UFB1 detection limit of the method is 0.03±0.01 ng/ml [13]. All of the UFB1 values < 0.03 ng/ml are included in the first bar (ND). Bars with differing superscripts are significantly different (p<0.05) based on the Kruskal-Wallis ANOVA on ranks.
4 Discussion
Human FB1 intake assessment and biomonitoring of mechanism-based biological effects (i.e. disruption of sphingolipid metabolism) are a necessary first step to determine if FB1 exposure contributes to any of the human diseases that have been associated with FB1 including the high incidence of NTDs in maize consumers in Guatemala [9, 10], stunting in children [12, 13], and chronic liver disease [17]. We have developed and used a solid-phase-extraction cartridge method to quantify UFB1 as an exposure biomarker [14]. The method has been validated in an animal model [6] and used successfully in Guatemalan women to assess FB intake in three human studies (Table 1). With regards to mechanism-based biomarkers, numerous animal studies have shown FB inhibition of CerS induces the accumulation of Sa and to a much lesser extent sphingosine (So) and consequently an increase in the Sa/So ratio [reviewed in 7 and 20]. Several studies have attempted to determine if FB exposure is associated with elevated Sa and the Sa/So ratio in humans, with little success [8]. The present study provides an alternative to using the Sa level and Sa/So ratio in serum or urine as a biomarker of effect, namely to use the level of Sa 1-P and the ratio of Sa 1-P/So 1-P in human blood spots collected at the same time as the matched urine samples for UFB1 analysis. A method for quantifying So 1-P and Sa 1-P in blood spots has also been developed and validated in a mouse model [6] and used to measure So 1-P, Sa 1-P and the Sa 1-P/So 1-P ratio in human studies (Table 1). Assuming that FB1 contamination and consumption is relatively constant over time in the high FB1 exposure areas, once elevated the blood levels of Sa 1-P should be less variable than the levels of urinary FB1 since in our mouse study [6] there was a lag-time between FB1 exposure and the maximal elevation in sphingoid base 1-phosphates in the blood. If FB exposure is temporally variable then there could be a greater chance of having an elevated Sa 1-P level that does not correlate with an elevated urinary FB1.
The rationale for collecting blood spots rather than serum or plasma was that in addition to being less invasive and less expensive, RBC contain large amounts of So 1-P and Sa 1-P (Fig. 1). It has been shown that RBC are the main source of the sphingoid base 1-phosphates in the blood [reviewed in 21]. In addition RBC cannot synthesize sphingolipids de novo [22, 23] and lack the ability to de-phosphorylate sphingoid base 1-phosphates [24]. However, RBC retain the ability to phosphorylate Sa and So via sphingosine kinases (SphKs) [25]. Thus, the So and Sa that are phosphorylated in the RBC are produced in non-RBC tissues. In animals large amounts of So and Sa are produced in liver and kidney following FB exposure [2–6]. The So and Sa that is produced in liver and kidney following FB exposure is a likely source of substrates for the SphKs in the RBC of FB-treated mice [6].
Based on the unique functional and metabolic characteristics of So 1-P in blood, and in particular the fact that RBC cannot make Sa or metabolize Sa 1-P, we hypothesized that blood spots from Guatemalan women living in areas where FB exposure is high would show higher Sa 1-P/So 1-P ratios when compared to populations living in low exposure areas, reflecting the increased Sa produced in cells and tissues as a consequence of FB-induced CerS inhibition. Consistent with this hypothesis would be elevated concentrations of Sa 1-P in the blood spots as we have seen in mice [6]. In mice the levels of Sa 1-P in the RBC closely paralleled the levels of Sa seen in liver and kidney and the UFB1 [6]. The results of the one year human biomarker study and the biomarker response validation study show that in Guatemalan women consuming maize-based diets in high and low FB exposure communities there is a significant positive correlation between the FB contamination of the maize, the individual UFB1 levels, and the time-matched Sa 1-P/So 1-P ratios and Sa 1-P concentrations in blood spots.
There are four important questions that must be answered before we can fully understand the toxicological implications of these findings in humans. First, can the UFB1 be used to predict when individuals are at increased risk for exceeding the JECFA PMTDI? Second, are the positive correlations between calculated FB intake, UFB1 levels, and Sa 1-P concentration and the Sa 1-P/So 1-P ratio proof that FB inhibits CerS in humans consuming diets containing high levels of FB? Third, can the positive correlation between UFB1, Sa 1-P concentrations and the Sa 1-P/So 1-P ratio in RBC be used to predict the level of FB1 intake that results in an increased risk of FB disruption of sphingolipid biosynthesis? Fourth, do the changes in Sa 1-P and the Sa 1-P/So 1-P ratio in RBC imply the potential for adverse effects and, if so, what magnitude of change is needed to elevate the risk of an adverse effect? We will attempt to address each of these questions using the results from the three human studies (Table 1).
Can UFB1 be used to predict when individuals are at increased risk for exceeding the JECFA PMTDI?
In the one year human biomarker study (15) and the biomarker response validation study the relative risk for exceeding the JECFA PMTDI was first significantly increased when the UFB1 window was > 0.1 ng/ml < 0.5 ng/ml (Supporting Information, Table S4). Thus, in these studies using the stated assumptions the window of increased risk can be predicted. Likewise, the UFB1 window in the one year human biomarker study where the mean total FB intake first exceeded 2 μg/kg b.w./day was >0.5 ng/ml < 1.0 ng/ml [15] and in the biomarker response validation study the window was >0.1 ng/ml < 0.5 ng/ml (Fig. 7). These FB intake estimates were based on the actual levels of total FB in the maize sampled at the time of the urine collection. In Riley et al. [14] the average UFB1 excretion was reported to be 0.5% of intake. Assuming excretion is 0.5% of intake, total urine output in the Guatemalan women was 1000 ml, and the average weight was 60 kg, then 0.5 ng/ml represents a total intake of 1.67 μg/kg b.w./day. On this basis, it is probably safe to suggest that at 0.1 ng/ml the relative risk for exceeding the JECFA PMTDI of 2 μg/kg b.w./day is significantly increased and Guatemalan women with a UFB1 >0.5 ng/ml are more likely than not to have a total FB intake that exceeds the JECFA PMTDI.
Are the positive correlations between UFB1 levels, calculated FB intake, and Sa 1-P concentration and Sa 1-P/So 1-P ratio proof that FB inhibits CerS in humans consuming diets containing high levels of FB?
Primary cultures of human neural progenitor cells respond to FB1 treatment by accumulating large amounts of Sa and Sa 1-P and much lesser amounts of So and So 1-P [26], similar to what has been reported in numerous animal studies [2, 3, 4, 6, 7] and attributed to de novo CerS inhibition as first demonstrated by Wang et al. [1]. It is probably not possible with the tools available today to prove by direct measurement that CerS is inhibited by FB1 in vivo in humans. However, the positive correlations between UFB1 and the levels of Sa 1-P and the Sa 1-P/So 1-P ratio in blood spots documented in Figures. 2, 4, 8 and 10, and Supporting Information, Fig. S1 are consistent with that conclusion.
There are many physiological processes and conditions that will alter So 1-P levels in humans [reviewed in 27 and 28], however, elevation in Sa in tissues and Sa 1-P in RBC is most likely dependent on decreased CerS activity that occurs in the absence of decreased serine palmitoyltransferase activity (the first and rate-limiting step in de novo ceramide biosynthesis) resulting in the accumulation of Sa produced de novo (Fig. 1). Thus, an important observation that is consistent with FB inhibition of de novo ceramide biosynthesis is elevated ratios of Sa/So or Sa 1-P/So 1-P that is positively correlated with FB intake based on the levels of UFB1. In addition, the increased ratio following FB treatment is due to a disproportionate increase in the molar concentration of Sa or Sa 1-P relative to So or So 1-P, respectively, and is not a result of a decrease in So or So 1-P, a directional change that is not consistent with FB inhibition of CerS. The data showing that the increase in the Sa 1-P/So 1-P ratio is driven by the increase in Sa 1-P (Supplemental Information, Fig. S2) and not a decrease in So 1-P (Supplemental Information, Fig. S3) is consistent with what has been observed in numerous studies using FB in cultured cells in vitro and animals in vivo.
Can the positive correlation between UFB1, Sa 1-P concentrations and the Sa 1-P/So 1-P ratio in RBC be used to predict the level of FB1 intake that results in an increased risk of FB disruption of sphingolipid biosynthesis?
In the one year human biomarker study, a statistically significant increase in the Sa 1-P/So 1-P ratio and the Sa 1-P concentration (compared to volunteers with no detectable UFB1) was first observed in the window of UFB1 that was >0.5<1.0 ng FB1/ml and >1.0<5.0 ng/ml, respectively (Fig. 4A and 4B). In the biomarker response validation study the first statistically significant increase in both the Sa 1-P/So 1-P ratio and Sa 1-P concentration were observed in the window of UFB1 that was >0.5<1.0 ng FB1/ml (Fig. 10A and 10B). Assuming that 0.5 ng/ml corresponds to 1.67 μg/kg b.w./day (see calculation above) then the JECFA PMTDI is at the lower end of the estimated FB intake that corresponded to significantly elevated Sa 1-P/So 1-P ratios and Sa 1-P concentrations in both the one year human biomarker study and the biomarker response validation study.
Do the changes in Sa 1-P and the Sa 1-P/So 1-P ratio in RBC imply the potential for adverse effects?
The concept of FB-induced “disruption of the de novo pathway of sphingolipid biosynthesis” as a mechanism/critical event in FB-induced diseases was first proposed in 1991 by Wang et al. [1]. In addition to decreased ceramide biosynthesis, there was a marked increase in Sa concentration in the primary rat hepatocytes and rat liver [1]. Many studies have shown that the significant increase in Sa precedes indications of adverse effects. For example, in the two year FB1 long-term feeding study conducted by the National Toxicology Program (NTP) in the USA [29], there were statistically significant increases in Sa in both urine and kidney from male rats sampled at various time points at the no observable adverse effect level [dietary 5 ppm=0.22 mg/kg b.w./day]. At a dietary level of 15 ppm (0.67 mg/kg b.w./day) the primary histopathological findings were cytotoxic and/or regenerative lesions seen at 6 weeks and later [20, 29]. We now know that the Sa that accumulates in tissues can be phosphorylated to Sa 1-P which can also accumulate to very high concentrations in tissues [2] and that Sa 1-P accumulates in blood as evidenced by the large amounts detected in extracts of mouse blood spots [5, 6].
The concentration of Sa 1-P and the Sa 1-P/So 1-P ratio in the blood of unexposed animals is most likely determined by the relative activities of the enzymes involved in de novo sphingolipid biosynthesis and turnover in the tissues. There is a great deal of information supporting the hypothesis that the balance between ceramide and sphingoid base 1-phosphates in tissues is a regulatory mechanism balancing the rates of cell survival (proliferation) and cell death [27], the proposed mechanism for FB toxicity and carcinogenicity [7]. In addition So 1-P and Sa 1-P are proven ligands for the class of extracellular G-protein coupled receptors known as S1P1–5 [28]. S1P receptor (S1Pr)-mediated signaling has been implicated in cancer pathogenesis, including a role in modulation of tumor cell survival, proliferation, migration/metastasis, and neovascularization of the tumour microenvironment [28]. Thus it is not unreasonable to think that FB-induced alterations in Sa 1-P levels in tissues and blood could contribute to human diseases, including those that have been associated with populations consuming large amounts of maize potentially contaminated with high levels of FB.
In addition to a possible role for FB-induced elevation in sphingoid base 1-phosphates as a contributing factor in cancer risk [17], altered S1Pr signaling could contribute to the increased risk of birth defects and other diseases in countries where maize is a dietary staple [9, 10]. For example, administration of fumonisin to pregnant LM/Bc mice results in neural tube defects in exposed embryos [5, 10]. Human and mouse neural progenitors express mRNA for S1P1–5 [30, 31, 32], and S1Pr-mediated signaling is involved in regulation of embryonic nervous system development [33]. S1Pr mRNA is expressed in neuroepithelial cells, as well as in migrating neural crest cells and surrounding tissues throughout the window of neurulation in mouse embryos [31, 32], suggesting a role for S1Pr-mediated signaling in normal neural tube closure. It is therefore conceivable that elevation of Sa 1-P in response to chronic fumonisin exposure could result in sustained and/or aberrant activation of S1Pr-mediated signaling pathways and NTDs through disruption of the timing and orchestration of events required for normal neural tube closure.
Conclusion
In conclusion, the results of both studies (the one year human biomarker study and the biomarker response validation study) conducted at different times in different locations in high and low exposure communities in Guatemala and involving a total of 1539 women (1532 with matching UFB1 and sphingoid base 1-phosphate data), are consistent with the hypothesis that exposure to, and intake of, high levels of FB in the diet results in inhibition of CerS in humans, similar to what has been seen in numerous studies in farm and laboratory animals [7, 20] and primary cultures of human cells treated with FB1 [26].
Supplementary Material
Acknowledgments
The authors thank all the women who participated in this study, the Ministry of Health of Guatemala and the leaders in the communities of Chimaltenango, Escuintla, Jutiapa, Sacatepéquez, Santa Rosa, and Chiquimula without whose cooperation this study could not have been performed. The authors also thank Adela Ruiz, Rosa Chovix and Waldemar González for the field work and sample collection in Guatemala, Marta María Méndez, Cecilia de Mayorga, Luis Rodríguez and Flor Díaz for the urine and maize extraction in Guatemala and Dr. Zaid Abdo (USDA-ARS South Atlantic Area) for assistance with and review of the statistical analysis. This work was supported by USDA-ARS NP108 in house project 6612-42000-012-00D and Award Number RC4HD067971-01 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health. IRB-approved protocols were followed.
Abbreviations
- CerS
ceramide synthase
- FB
fumonisins
- FB1
fumonisin B1
- FB2
fumonisin B2
- FB3
fumonisin B3
- PMTDI
provisional maximum tolerable daily intake
- RBC
red blood cells
- Sa
sphinganine
- Sa 1-P
sphinganine 1-phosphate
- So
sphingosine
- So 1-P
sphingosine 1-phosphate
- UFB1
urinary fumonisin B1
Footnotes
The authors have declared no conflict of interest.
References
- 1.Wang E, Norred WP, Bacon CW, Riley RT, et al. Inhibition of sphingolipid biosynthesis by fumonisins: Implications for diseases associated with Fusarium moniliforme. J Biol Chem. 1991;266:14486–14490. [PubMed] [Google Scholar]
- 2.Riley RT, Voss KA. Differential sensitivity of rat kidney and liver to fumonisin toxicity: organ-specific differences in toxin accumulation and sphingoid base metabolism. Toxicol Sci. 2006;92:335–345. doi: 10.1093/toxsci/kfj198. [DOI] [PubMed] [Google Scholar]
- 3.Bondy GS, Mehta R, Caldwell D, Coady L, et al. Effects of long term exposure to the mycotoxin fumonisin B1 in p53 heterozygous and p53 homozygous transgenic mice. Food Chem Toxicol. 2012;50:3604–3613. doi: 10.1016/j.fct.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Voss KA, Riley RT, Jackson LS, Jablonski JE, et al. Extrusion cooking with glucose supplementation of fumonisin contaminated corn grits protected against nephrotoxicity and disrupted sphingolipid metabolism in rats. Mol Nutr Food Res. 2011;55:S312–S320. doi: 10.1002/mnfr.201100067. [DOI] [PubMed] [Google Scholar]
- 5.Gelineau-van Waes J, Rainey MA, Maddox JR, Voss KA, et al. Increased sphingoid base-1-phosphates and failure of neural tube closure after exposure to fumonisin or FTY720. Birth Defects Res A Clin Mol Teratol. 2012;94:790–803. doi: 10.1002/bdra.23074. [DOI] [PubMed] [Google Scholar]
- 6.Riley RT, Showker JL, Lee CM, Zipperer CE, et al. A blood spot method for detecting fumonisin-induced changes in putative sphingolipid biomarkers in LM/Bc mice and humans. Food Add Contam (part A) 2015;32:934–949. doi: 10.1080/19440049.2015.1027746. [DOI] [PubMed] [Google Scholar]
- 7.Bulder AS, Arcella D, Bolger M, Carrington C, et al. World Health Organization Food Additives Series. Vol. 65. Geneva: World Health Organization; 2012. Fumonisins (addendum), Safety evaluation of certain food additives and contaminants; pp. 325–794. [Google Scholar]
- 8.Van der Westhuizen L, Shephard GS, Gelderblom WCA, Torres O, et al. Fumonisin biomarkers in maize eaters and implications for human disease. World Mycotoxin J. 2013;6:223–232. [Google Scholar]
- 9.Marasas WFO, Riley RT, Hendricks KA, Stevens VL, et al. Fumonisins disrupt sphingolipid metabolism, folate transport and development of neural crest cells in embryo culture and in vivo: A risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize? J Nutr. 2004;134:711–716. doi: 10.1093/jn/134.4.711. [DOI] [PubMed] [Google Scholar]
- 10.Gelineau-van Waes J, Voss KA, Stevens VL, Speer MC, et al. Maternal fumonisin exposure as a risk factor for neural tube defects. In: Taylor S, editor. Advances in Food and Nutrition Research. Vol. 56. 2009. pp. 145–181. [DOI] [PubMed] [Google Scholar]
- 11.Pitt JI, Wild CP, Gelderblom WCA, Miller JD, et al., editors. Management of mycotoxins in foods and feeds for improving public health. Lyon, France: 2012. p. 165. International Agency for Research on Cancer Scientific Publication No 158. [Google Scholar]
- 12.Kimanya ME, De Meulenaer B, Roberfroid D, Lachat C, et al. Fumonisin exposure through maize in complementary foods is inversely associated with linear growth of infants in Tanzania. Mol Nutr Food Res. 2010;54:1659–1667. doi: 10.1002/mnfr.200900483. [DOI] [PubMed] [Google Scholar]
- 13.Shirima C, Kimanya M, Routledge M, Srey C, et al. A prospective study of growth and biomarkers of exposure to aflatoxin and fumonisin during early childhood in Tanzania. Environ Health Persp. 2015;123:173–178. doi: 10.1289/ehp.1408097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riley RT, Torres O, Showker JL, Zitomer NC, et al. The kinetics of urinary fumonisin B1 excretion in humans consuming maize-based diets. Mol Nutr Food Res. 2012;56:1445–1455. doi: 10.1002/mnfr.201200166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres O, Matute J, Gelineau-van Waes J, Maddox JR, et al. Urinary fumonisin B1 and estimated fumonisin intake in women from high- and low-exposure communities in Guatemala. Mol Nutr Food Res. 2014;58:973–983. doi: 10.1002/mnfr.201300481. [DOI] [PubMed] [Google Scholar]
- 16.Riley RT, Voss KA, Showker JL, Torres O, et al. Development of biomarkers to assess fumonisin exposure and birth defects. In: Binder EM, editor. World Nutrition Forum. Anytime Publishing; Leicesterhire, UK: 2012. pp. 249–256. [Google Scholar]
- 17.Torres O, Matute J, Gelineau-van Waes J, Maddox JR, et al. Human health implications from co-exposure to aflatoxins and fumonisins in maize-based foods in the Latin America: Guatemala a case study. World Mycotoxin J. 2015;8:143–159. [Google Scholar]
- 18.Torres O, Palencia E, Lopez de Pratdesaba L, Grajeda R, et al. Estimated fumonisin exposure in Guatemala is greatest in consumers of lowland maize. J Nutr. 2007;137:2723–2729. doi: 10.1093/jn/137.12.2723. [DOI] [PubMed] [Google Scholar]
- 19.Palencia E, Torres O, Hagler W, Meredith FI, et al. Total fumonisins are reduced in tortillas using the traditional nixtamalization method of Mayan communities. J Nutr. 2003;133:3200–3203. doi: 10.1093/jn/133.10.3200. [DOI] [PubMed] [Google Scholar]
- 20.Bolger M, Coker RD, Dinovi M, Gaylor D, et al. Safety evaluation of certain mycotoxins in food. Food and Agriculture Organization of the United Nations, paper 74. World Health Organization Food Additives. 2001. Fumonisins; pp. 103–279. Series 47. [Google Scholar]
- 21.Thuy AV, Reimann CM, Hemdan NY, Gräler MH. Sphingosine 1-phosphate in blood: function, metabolism, and fate. Cell Physiol Biochem. 2014;34:158–171. doi: 10.1159/000362992. [DOI] [PubMed] [Google Scholar]
- 22.Hänel P, Andréani P, Gräler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 23.Bode C, Sensken SC, Peest U, Beutel G, et al. Erythrocytes serve as a reservoir for cellular and extracellular sphingosine 1-phosphate. J Cell Biochem. 2010;109:1232–1243. doi: 10.1002/jcb.22507. [DOI] [PubMed] [Google Scholar]
- 24.Ito K, Anada Y, Tani M, Ikeda M, et al. Lack of sphingosine 1-phosphate-degrading enzymes in erythrocytes. Biochem Biophys Res Commun. 2007;357:212–217. doi: 10.1016/j.bbrc.2007.03.123. [DOI] [PubMed] [Google Scholar]
- 25.Yang L, Yatomi Y, Miura Y, Satoh K, et al. Metabolism and functional effects of sphingolipids in blood cells. Br J Haematol. 1999;107:282–293. doi: 10.1046/j.1365-2141.1999.01697.x. [DOI] [PubMed] [Google Scholar]
- 26.Callihan P, Zitomer N, Kennedy P, Lynch KR, et al. Distinct generation, pharmacology, and distribution of sphingosine 1-phosphate and dihydro-sphingosine 1-phosphate in human neural progenitor cells. Neuropharmacology. 2012;62:988–996. doi: 10.1016/j.neuropharm.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Brocklyn JR, Williams JB. The control of the balance between ceramide and sphingosine-1-phosphate by sphingosine kinase: Oxidative stress and the seesaw of cell survival and death. Comp Biochem Physiol Part B. 2012;163:26–36. doi: 10.1016/j.cbpb.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Blaho VA, Hla T. An update on the biology of sphingosine 1-phosphate receptors. J Lipid Res. 2014;55:1596–1608. doi: 10.1194/jlr.R046300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Toxicology Program. Toxicology and Carcinogenesis Studies of Fumonisin B1 In F344/N Rats and B6C3F1 Mice. 2001. p. 352. NTP TR 496 (NIH Publication No. 01–3955) [PubMed] [Google Scholar]
- 30.Hurst JH, Mumaw J, Machacek DW, Sturkie C, et al. Human neural progenitors express functional lysophospholipid receptors that regulate cell growth and morphology. BMC Neurosci. 2008;11(9):118. doi: 10.1186/1471-2202-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohuchi H, Hamada A, Matsuda H, Takagi A, et al. Expression patterns of the lysophospholipid receptor genes during mouse early development. Dev Dyn. 2008;237:3280–3294. doi: 10.1002/dvdy.21736. [DOI] [PubMed] [Google Scholar]
- 32.Meng H, Lee VM. Differential expression of sphingosine-1-phosphate receptors 1–5 in the developing nervous system. Dev Dyn. 2009;238:487–500. doi: 10.1002/dvdy.21852. [DOI] [PubMed] [Google Scholar]
- 33.Mizugishi K, Yamashita T, Olivera A, Miller GF, et al. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.