Abstract
Pest species pose major challenges to global economies, ecosystems, and health. Unfortunately, most conventional approaches to pest control remain costly, and temporary in effect. As such, a heritable variant of the Sterile Insect Technique (SIT) was proposed, based on the introduction of mitochondrial DNA mutations into pest populations, which impair male fertility but have no effects on females. Evidence for this “Trojan Female Technique” (TFT) was recently provided, in the form of a mutation in the mitochondrial cytochrome b gene (mt:Cyt-b) of Drosophila melanogaster which reduces male fertility across diverse nuclear backgrounds. However, recent studies have shown that the magnitude of mitochondrial genetic effects on the phenotype can vary greatly across environments, with mtDNA polymorphisms commonly entwined in genotype-by-environment (G × E) interactions. Here we test whether the male-sterilizing effects previously associated with the mt:Cyt-b mutation are consistent across three thermal and three nuclear genomic contexts. The effects of this mutation were indeed moderated by the nuclear background and thermal environment, but crucially the fertility of males carrying the mutation was invariably reduced relative to controls. This mutation thus constitutes a promising candidate for the further development of the TFT.
Pest species pose a significant threat to native biota, and cause major damage to natural environments and economies globally1. Conventional approaches to their control or eradication (for example through poisoning or trapping) can be laborious to implement, economically costly, temporary in nature, and cause undesirable environmental contamination, or detrimental effects on non-target species2,3,4. Much attention has thus focused on the development of control measures that are species-specific and long-lasting in effect, even when applied to pest populations at low density. One approach that meets the criteria of species-specificity is the sterile insect technique (SIT), whereby sterile males are introduced en masse into target populations, reducing offspring production of females that mate with them5. However, the SIT generally requires continuous large-scale production and introduction of sterile males to sustain population suppression5; it would be further improved if sterile males could be produced continually within the populations targeted for control, without requiring manual introductions each generation.
Significant research effort has thus been directed at developing novel SIT variants that promise trans-generational heritability of male sterility, and that ideally require only periodic releases of sterile males to successfully control pest populations5. Such approaches usually employ some form of genome editing to achieve both heritability and reproductive suppression. One such approach is the Release of Insects carrying a Dominant Lethal (RIDL™), whereby transgenic insects homozygous for a dominant repressible lethal gene are introduced into target populations6. A repressor for the lethal gene, e.g. a tetracycline-repressible transcription factor, allows for mass-breeding of insects reared on repressor-containing diets. Once released, and in the absence of the repressor, expression of the lethal gene is triggered by the expression of a female-specific transcription enhancer, killing females during development while leaving males unscathed. The survival of males enables trans-generational heritability, and feasibility of this repressible approach has been demonstrated in laboratory fruit fly populations6,7 and field mosquito populations8.
A challenge to SIT variants that remains is to establish effector genes at sufficiently high frequencies within target populations to achieve sustained control. An interesting and alternative approach to SIT, to help the spread of effector genes through target populations, is to cause their biased inheritance through linkage to site-specific selfish genetic elements (“gene drive”)9,10. This concept is not new, but has recently gained considerable traction with the development of the CRISPR-Cas9 (clustered, regularly interspaced, short palindromic repeats (CRISPR) and CRISPR-associated protein [Cas] system) genome editing technology11,12. Developing and applying a CRISPR-Cas9-based gene drive system, researchers have demonstrated effector gene transmission rates to offspring of over 90% in mosquitoes11. However, while promising very efficient means to control pest populations or disease vectors11,13, the release of genetically engineered animals into nature raises ethical issues, and a debate is currently underway discussing safety and regulatory concerns14,15.
A novel variant of the SIT, which in theory can achieve sustained population suppression over multiple generations in any taxa, and which does not rely on the introduction of novel gene constructs into nature, is the recently proposed Trojan Female Technique (TFT)16. The TFT is based on the use of naturally-occurring mutations in the mtDNA that incur reductions to male fertility while having no deleterious effects on females17,18. Males that inherit these mutations will sire fewer offspring than wild-type counterparts, while females will remain fully fertile. Since mtDNA is generally strictly maternally inherited, this sex-bias in effects means that under most conditions there will be greatly reduced selection pressure against the TFT mutation. Thus a single release of females carrying the TFT mutation into a pest population could potentially cause multi-generational population suppression16.
The applicability of the TFT to pest control has been theoretically substantiated16, but its empirical success, and ultimate practicability, hinges on circumvention of several potential obstacles. First, the effects of any given candidate TFT mutation on male fertility must be upheld across a range of nuclear genetic backgrounds. This is salient given previous findings indicating that the effects of polymorphisms within the mtDNA sequence are typically moderated by genetic variation in the nuclear genome19,20. That is, mtDNA haplotypes that might be poorly performing against one nuclear genomic background can often be high performers against others21,22,23. Mitonuclear interactions thus threaten to impede the development of the TFT. However, it has recently been shown that a non-synonymous candidate mutation in the mitochondrial genome of the fruit fly Drosophila melanogaster (D. melanogaster) indeed consistently impairs male fertility across a range of diverse nuclear genomic backgrounds24. The non-synonymous mutation causes a change from Alanine to Threonine at amino acid position 278 of the expressed protein of the mitochondrial cytochrome b gene (mt:Cyt-b), a component of the mitochondrial respiratory complex III25,26. Spermatogenesis of male fruit flies carrying the BRO haplotype is perturbed, inhibiting sperm maturation, which can lead to complete sterility while having no apparent effects on female fertility26. Although the detrimental effect of the mt:Cyt-b mutation on male fertility in D. melanogaster is well established24,27, the underlying mechanism that leads to the suppression of male fertility is yet to be resolved26. However, the potential for use of the mt:Cyt-b mutation for the TFT is particularly interesting given that the same mutation has already been identified in the mt:Cyt-b gene of a number of other species, both vertebrate and invertebrate26.
However, a second obstacle that could plausibly stand in the way of further development of the TFT is the scope for genotype-by-environment (GxE) interactions involving sequence polymorphisms in the mtDNA that alter male-fertility phenotypes (cases of “mtDNA-mediated phenotypic plasticity”). Evidence for such plasticity has accumulated in recent years. In early studies that examined mtDNA transmission patterns following experimentally-enforced heteroplasmy in Drosophila, observed patterns were not only shaped by the nuclear background but also by the temperature at which flies were reared28,29. Furthermore, in seed beetles, the effect of the mitochondrial haplotype on the speed of juvenile development and metabolic rate is contingent not only on the nuclear background, but also on complex interactions involving the mtDNA, nuclear background and thermal environment30,31. Since natural populations invariably exist within heterogeneous environments, and simultaneously face ongoing exposure to climate change, it is thus crucial to screen the effects of candidate TFT mutations across different thermal contexts to assess ongoing practicability. Here we do so for the described Ala278→Thr mt:Cyt-b mutation, assessing whether its fertility outcome for males is modified by interactions with the thermal environment. Because of the importance of the mitonuclear interaction in life-history expression, we further test the thermal interaction across three different non-coevolved nuclear backgrounds.
Results and Discussion
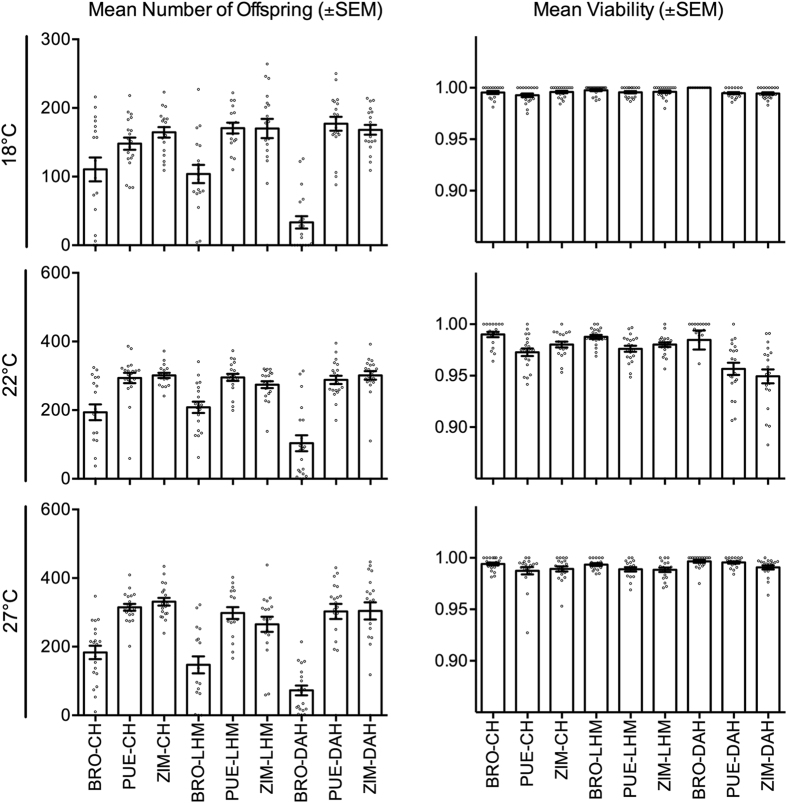
To test whether the male-sterilizing effects previously associated with the mt:Cyt-b mutation are consistent across varying thermal and nuclear genomic contexts, we placed three different mtDNA haplotypes (one which harbours the candidate TFT mutation in the mt:Cyt-b gene [BRO] and two control haplotypes [PUE; ZIM]) alongside three foreign non-coevolved nuclear backgrounds (CH, DAH, or LHM) and measured their effects on male fertility at three different temperatures (for details see Methods; Fig. S1). We then conducted male fertility assays for all nine mitonuclear combinations, and determined offspring number and pupal viability (Fig. 1). We found context-dependent phenotypic effects of mtDNA mutations that hinged on both the nuclear background in which they were expressed (χ2 = 36.6, p < 0.001, Table 1) and the temperature at which they had been reared for three generations (χ2 = 24.9, p < 0.001, Table 1). Crucially, the average fertility of males with the BRO mtDNA haplotype, which carries the candidate TFT mutation, was consistently lower across all nuclear genomic and thermal contexts than that of males with either of the two control haplotypes (Figs 1 and 2). On average, the BRO haplotype conferred an approximate 50% reduction in fertility. Interestingly, the observed mitochondrially-mediated thermal plasticity was directly tied to the BRO haplotype; male fertility for the control haplotypes increased with assay temperature, whereas BRO-linked fertility peaked mid-temperature (22 °C; Figs 1 and 2).
Figure 1. Mean offspring number and mean pupal viability per nuclear and thermal environment (±SEM) of three mitochondrial haplotypes (Brownsville [BRO]; Puerto Montt [PUE]; Zimbabwe [ZIM]) across three nuclear backgrounds (Coffs Harbour [CH], Dahomey [DAH], LHM [LHM]), and three thermal environments (18 °C, 22 °C, 27 °C).
Table 1. Sources of variance affecting male reproductive success.
| Fixed effects | χ2 | df | p |
|---|---|---|---|
| Source | |||
| mtDNA haplotype | 8.94 | 2 | 0.0114 |
| Nuclear background | 45.13 | 2 | <0.001 |
| Temperature | 32.76 | 2 | <0.001 |
| mtDNA × nuclear | 36.56 | 4 | <0.001 |
| mtDNA × temperature | 24.90 | 4 | <0.001 |
| Random effects | Standard deviation | ||
| Vial ID | 26.29 | ||
| mtDNA duplicate | 0 | ||
| Residual | 64.59 |
Figure 2. Mean offspring number per nuclear and thermal environment.
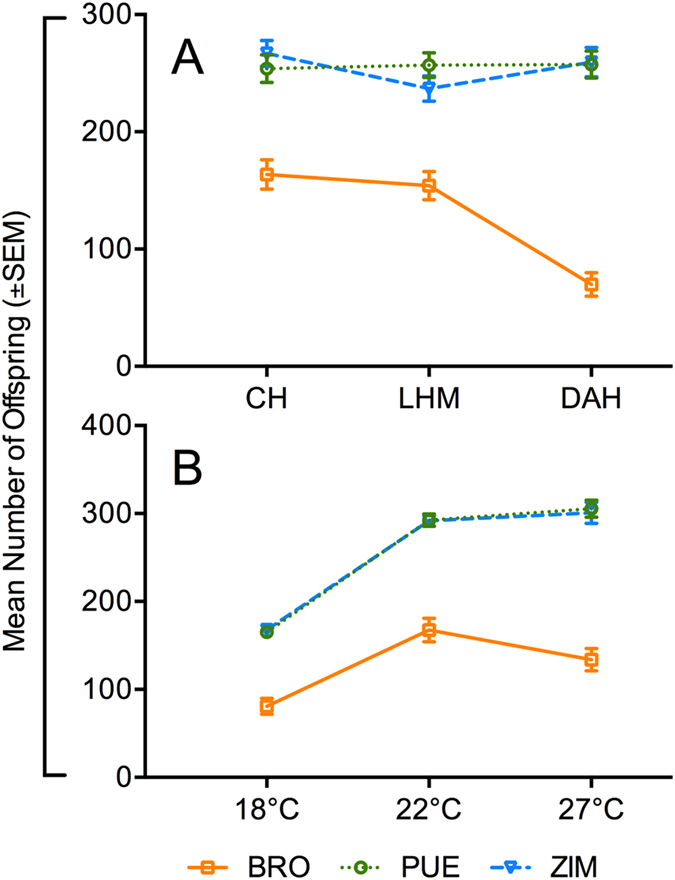
Interaction plots depicting mean offspring number (±SEM) of three mitochondrial haplotypes (Brownsville [BRO]; Puerto Montt [PUE]; Zimbabwe [ZIM]) across (A) three nuclear backgrounds (Coffs Harbour [CH], Dahomey [DAH], LHM [LHM]), and (B) three thermal environments (18 °C, 22 °C, 27 °C).
The BRO haplotype was also the mediator of the mitonuclear interactions detected in this study. While male fertility of the two control haplotypes was similar across the three nuclear backgrounds, BRO-linked fertility was much reduced when expressed alongside the DAH nuclear background (reduced by ~72%) relative to CH or LHM (reduced by ~37% for both; Figs 1 and 2). Notably, none of three tested nuclear backgrounds, nor the w1118 nuclear background (alongside which male-sterilizing effects were first observed), have coevolved with the BRO haplotype. This indicates that variably successful mechanisms coded for by the nuclear genome (dependent on both the nuclear background and thermal conditions) already exist within the gene pools of tested nuclear backgrounds, and which may be acting to compensate for mtDNA-linked reductions in male fertility. The existence of such modifying mechanisms within the nuclear background is evolutionary plausible, because they would have been selected for over time by the natural occurrence of male fertility-reducing mtDNA variation in the wild populations19,32. Importantly, however, we note that despite the BRO haplotype being entwined in gene-by-gene (mitonuclear) and gene-by-environment (mito-by-temperature) interactions, it was invariably the inferior performer relative to the controls, reinforcing the promise of harnessing mtDNA mutations, such as the Ala278→Thr in the mt:Cyt-b gene, in the development of the TFT to aide eradication of pest species.
Our analyses of pupal viability showed that while mitonuclear interactions affect viability rates (χ2 = 17.4, p = 0.0011, Table S2), invariably the BRO haplotype, which harbours the candidate TFT mutation, exhibited highest pupal viability (Figs 1 and 3). This indicates that the low male fertility associated with the BRO haplotype is not underpinned by low rates of pupal-to-adult eclosion. It is conceivable that higher pupal viability could aid the BRO haplotype to spread slowly within introduced populations following TFT treatment. However, further empirical testing is required to validate or refute this hypothesis. One approach would be to monitor changes in haplotype frequencies in mixed population cage studies, where the BRO haplotype is put in direct competition to other mtDNA haplotypes. Pupal viability was also affected by an interaction between the nuclear background and temperature (χ2 = 37.7, p < 0.001; Figs 1 and 3; Table S2). While pupal viability was lowest for all nuclear genotypes at 22 °C, this was particularly evident for the DAH background.
Figure 3. Mean pupal viability per nuclear and thermal environment.
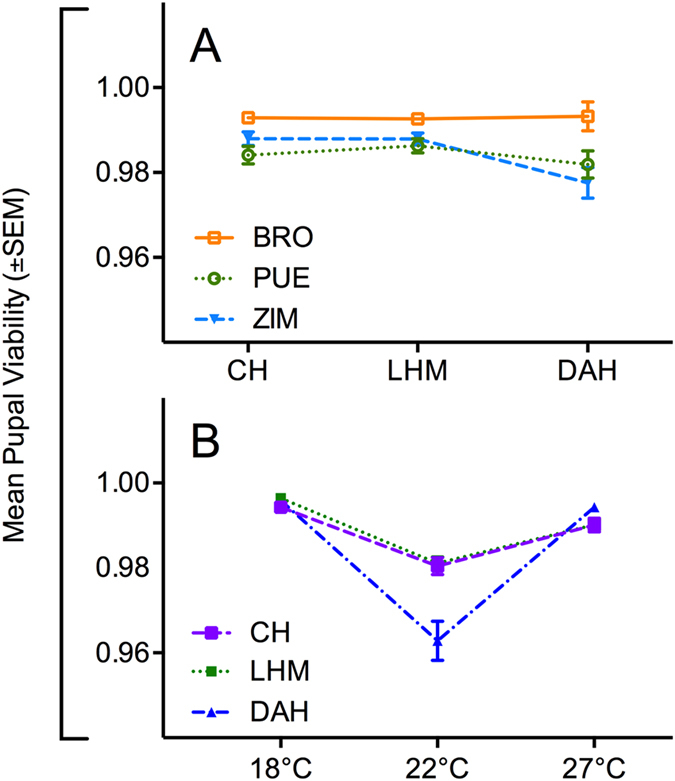
Interaction plots depicting mean pupal viability (±SEM) of three mitochondrial haplotypes (Brownsville [BRO], Puerto Montt [PUE], Zimbabwe [ZIM]) across (A) three nuclear backgrounds (Coffs Harbour [CH], Dahomey [DAH], LHM [LHM]), and (B) three nuclear backgrounds (CH, DAH, LHM) across three temperatures (18 °C, 22 °C, 27 °C).
Together, our findings demonstrate that the thermal environment can play a role in male fertility outcomes of the Ala278→Thr mt:Cyt-b mutation in fruit fly. The observed mtDNA-mediated thermal plasticity for male fertility is a novel finding, and shows that trait expression reliant on genes encoded within the evolutionary-conserved mitochondrial genome is modified across environments. The underlying mechanisms remain to be resolved, but it is plausible that haplotype-specific changes in mitonuclear protein complexes alter efficiencies across environments of what are temperature-sensitive processes in the mitochondrial electron transport chain. Alternatively, it is also possible that observed thermal plasticity for male fertility is driven by changes in gene expression in response to mitochondrial genomic variation and temperature, both of which are known to alter gene expression33,34.
While it is possible that the context-dependency of the BRO haplotype might be traced directly to the Ala278→Thr mt:Cyt-b mutation, this requires further testing given that the BRO and control haplotypes are delineated by additional SNPs within the coding and non-coding region25. Such tests require placement of this candidate mutation inside other mitochondrial haplotype backgrounds, to disentangle direct effects of the SNP from that of the hosting haplotype. Also, the general applicability across species of this mutation for use as the basis for TFT pest control has yet to be demonstrated; the effects of this mutation must be tested in other taxa, most importantly those that represent economic pests. Although the mutation has already been identified in a range of other invertebrates and vertebrates26, the phenotypic consequences are yet to be quantified. If consistency of effect is demonstrated, and if population suppression can ultimately be achieved, the potential applicability of this mutation is widespread. This is especially so given the rapid progress being made in gene editing technologies based on CRISPR/Cas935, suggesting that it could soon be technically feasible to directly place it into target pest species rather than needing to screen for its natural occurrence.
In summary, we have provided empirical support for the TFT, comprising consistent reductions in male fertility linked to a candidate mutation in the mt:Cyt-b gene of the BRO haplotype of the fruit fly. Mathematical modelling predicts that even the lowest reduction in male fertility observed in the current study (i.e. a 35% reduction in fertility for BRO compared to PM in the CH background) could still theoretically cause marked suppression of natural populations (up to 38%) as its frequency increases (see Supplementary Information). In the longer term, TFT development will be facilitated by the identification of other mtDNA mutations exhibiting male-biased fertility effects in natural populations. While evolutionary theory suggests such mutations should be common17,18, very few studies to date have looked for them. A next step is thus to employ approaches that screen for such mutations, with the potential for multiple mutations to be coupled, both with each other and the already identified Ala278→Thr mutation of the BRO haplotype, for enhanced male fertility reduction and resultant population suppression effects.
Ultimately, however, the feasibility of the TFT requires testing at the population level by introducing the Ala278→Thr mt:Cyt-b mutation into test populations of D. melanogaster. This is particularly warranted considering that the conceptual framework and previously-published modeling underpinning the TFT to date assumes complete sterility of affected males36. While our experiments here document consistently-strong male-sterilizing effects of the mutation across thermal and nuclear genomic contexts, it did not confer complete male sterility. Rather, the mutation appears to be compensated for, at least in part, by modifier alleles present within the nuclear genomic variation of the outbred lines. This limited the observed reduction in male fertility to approximately 50% of wild-type. Trials in which laboratory populations are seeded with varying proportions of females harboring the BRO haplotype would thus provide both a first test for numerical suppression at a population scale, and insights into the maintenance of the haplotype across generations. If successful, we note that the development of the TFT could have profound benefits that extend far beyond those of pest control, to the control of vectors that carry human diseases such as Dengue and Zika virus.
Methods
Fly strains
The experiment utilised three naturally-occurring mitochondrial haplotypes, sourced from Brownsville USA [BRO], Puerto Montt Chile [PUE], and Zimbabwe [ZIM]25. Each of these haplotypes had been placed alongside an isogenic nuclear background w1118 22, creating distinct “mitochondrial strains”. The BRO haplotype harbours the candidate TFT mutation (Ala278→Thr) in the mitochondrial cytochrome b gene (mt:Cyt-b), conferring complete male sterility but normal female fertility when expressed alongside the w1118 background26. Both sexes of the PUE and ZIM haplotypes are putatively healthy27,36; these haplotypes thus acted as controls. All mitochondrial strains have been maintained in independent duplicates since 2007 (hereafter “mitochondrial strain duplicates”), which were thereafter propagated as separate entities, with virgin daughters of each strain duplicate back-crossed to males of the w1118 strain for a further 60 generations to ensure that all four chromosomes in the w1118 nuclear background were isogenic across all mitochondrial strains. The w1118 strain itself is propagated by one pair of full-siblings. Each generation, virgin females of each mitochondrial strain duplicate are backcrossed to males of w1118, thus maintaining nuclear isogenicity across strains. Nuclear isogenicity across mitochondrial strains, coupled with the use of independent duplicates per strain, allowed us to partition true mitochondrial effects from nuclear effects and other sources of variance.
We also created a strain of flies that generated ‘tester’ females of standardized genotype for the male fertility assays. This strain was created by non-reciprocally crossing two near-isogenic lines together, each derived from the same laboratory population37, to create F1 females that exhibited high genome-wide heterozygosity. All vials were density-controlled throughout the experiment, at 10 pairs of adults and then 80 eggs per vial following ovipositioning, and maintained under constant environmental conditions. All crosses described involved flies of standard-age (3 days), and maintained on a potato-dextrose-agar medium at constant temperature (25.0 ± 0.1 °C), and diurnal cycle (12 h:12 h light:dark).
Experimental design
Fly strains were reared at each of three temperatures (18 °C, 22 °C, and 27 °C) for two generations prior to the assay of male fertility (i.e. the grandparents and parents of all flies used in the experiment were reared at the appropriate temperature). The chosen temperature range reflects thermal conditions under which D. melanogaster maintains complete adult fertility38. For each of the three temperature treatments, virgin females of each mitochondrial strain duplicate were crossed to males from each of three distinct outbred laboratory populations: Coffs Harbour Australia [CH]39, Dahomey Benin [DAH],40, and California USA [LHM]37, (Table S1, Fig. S1). Three outbred nuclear lines from three different continents were chosen to maximise the nuclear allelic variation that mitochondrial haplotypes were expressed against, with the aim of testing the robustness of male-sterilizing effects and thus the general utility of the candidate TFT mutation. The F1 sons, each of whom possessed one of the three mitochondrial haplotypes (BRO, PUE, or ZIM), a haploid nuclear copy of the w1118 strain (inherited from the mother), and a paternally-contributed haploid nuclear copy derived from one of the three laboratory populations (CH, DAH, or LHM), were collected as virgins and used as the focal males in the fertility assays (Table S1, Fig. S1).
Fertility assay
For each of the three temperature treatments, each focal male was maintained with two virgin ‘tester’ females for 24 h. Males were then removed, and females left to oviposit for another 24 h. Each pair of females was then transferred to a new vial every 24 h, for another two days, enabling further ovipositing. Male fertility was scored as the number of offspring eclosing from the four vials associated with each focal male. We also counted the number of pupae that did not result in adult eclosion, and were thus able to estimate pupal-to-adult viability across mtDNA haplotypes, nuclear backgrounds and temperatures.
Statistical analysis
The structure of the data is outlined in Fig. S1. We fitted general linear mixed models to the fertility data. We also fitted generalized linear mixed models to the pupal viability data, with a binomial distribution, using the lme4 package41 in R 3.0.342. The response variable was a binomial vector consisting of the number of pupae that resulted in viable offspring eclosing, and the number of pupae that did not result in viable eclosion, per male. For both analyses we treated mtDNA haplotype (BRO, PUE, ZIM), nuclear background (CH, DAH, LHM), and temperature as fixed effects, and mitochondrial strain duplicate and the vial identity in which the focal males were reared from egg to adults, as random effects. Final models of fixed effects were deduced by sequentially removing terms that did not change the deviance of the model; starting with the highest-order interactions. Log-likelihood ratio tests (and an α of 0.05) were used to assess the change in deviance in the reduced model relative to the previous model. Significance of fixed effects in the final model was then assessed using Type III sums-of-squares, χ2 tests, and maximum likelihood estimation in the car package43. Random effect estimates were calculated using REML.
Additional Information
How to cite this article: Wolff, J. N. et al. Mitonuclear interactions, mtDNA-mediated thermal plasticity, and implications for the Trojan Female Technique for pest control. Sci. Rep. 6, 30016; doi: 10.1038/srep30016 (2016).
Supplementary Material
Acknowledgments
We thank Belinda Williams for her contribution to data collection, and David Clancy for providing Drosophila melanogaster mitochondrial populations in 2007. Funding was provided by a Smart Ideas grant from the New Zealand Ministry of Business, Innovation and Employment (MBIE).
Footnotes
Author Contributions J.N.W. carried out experiments and data collection, participated in data analysis, and drafted the manuscript; D.K.D., D.M.T. and N.J.G. conceived the study, and drafted the manuscript. D.K.D. carried out statistical analyses and coordinated the study. All authors gave final approval for publication.
References
- Pimentel D., Zuniga R. & Morrison D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 52, 273–288 (2005). [Google Scholar]
- Tompkins D. M. & Veltman C. J. Unexpected consequences of vertebrate pest control: Predictions from a four-species community model. Ecol. Appl. 16, 1050–1061 (2006). [DOI] [PubMed] [Google Scholar]
- Tabashnik B. E., Gassmann A. J., Crowder D. W. & Carriere Y. Insect resistance to Bt crops: Evidence versus theory. Nat. Biotechnol. 26, 199–202 (2008). [DOI] [PubMed] [Google Scholar]
- Bergstrom D. M. et al. Indirect effects of invasive species removal devastate World Heritage Island. J. Appl. Ecol. 46, 73–81 (2009). [Google Scholar]
- Alphey L. et al. Sterile-insect methods for control of mosquito-borne diseases: An analysis. Vector Borne Zoonot. Dis. 10, 295–311 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Donnelly C. A., Wood R. J. & Alphey L. S. Insect population control using a dominant, repressible, lethal genetic system. Science 287, 2474–2476 (2000). [DOI] [PubMed] [Google Scholar]
- Heinrich J. C. & Scott M. J. A repressible female-specific lethal genetic system for making transgenic insect strains suitable for a sterile-release program. Proc. Natl. Acad. Sci. USA 97, 8229–8232 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. F. et al. Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat. Biotechnol. 30, 828–830 (2012). [DOI] [PubMed] [Google Scholar]
- Burt A. Site-specific selfish genes as tools for the control and genetic engineering of natural populations. Proc. R. Soc. Biol. Sci. Ser. B 270, 921–928 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champer J., Buchman A. & Akbari O. S. Cheating evolution: engineering gene drives to manipulate the fate of wild populations. Nat. Rev. Genet. 17, 146–159 (2016). [DOI] [PubMed] [Google Scholar]
- Hammond A. et al. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat. Biotechnol. 34, 78–83 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M. et al. A programmable dual-RNA–guided dna endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz V. M. et al. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc. Natl. Acad. Sci. USA 112, E6736–E6743 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oye K. A. et al. Regulating gene drives. Science 345, 626–628 (2014). [DOI] [PubMed] [Google Scholar]
- Lunshof J. Regulate gene editing in wild animals. Nature 521, 127 (2015). [DOI] [PubMed] [Google Scholar]
- Gemmell N. J., Jalilzadeh A., Didham R. K., Soboleva T. & Tompkins D. M. The Trojan Female Technique: A novel, effective and humane approach for pest population control. Proc. R. Soc. Biol. Sci. Ser. B 280 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S. A. & Hurst L. D. Mitochondria and male disease. Nature 383, 224–225 (1996). [DOI] [PubMed] [Google Scholar]
- Gemmell N. J., Metcalf V. J. & Allendorf F. W. Mother’s curse: The effect of mtDNA on individual fitness and population viability. Trends Ecol. Evol. 19, 238–244 (2004). [DOI] [PubMed] [Google Scholar]
- Wolff J. N., Ladoukakis E. D., Enriquez J. A. & Dowling D. K. Mitonuclear interactions: Evolutionary consequences over multiple biological scales. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 10.1098/rstb.2013.0443 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobler R., Rogell B., Budar F. & Dowling D. K. A meta-analysis of the strength and nature of cytoplasmic genetic effects. J. Evol. Biol. 27, 2021–2034 (2014). [DOI] [PubMed] [Google Scholar]
- Rand D. M., Fry A. & Sheldahl L. Nuclear-mitochondrial epistasis and Drosophila aging: introgression of Drosophila simulans mtDNA modifies longevity in D. melanogaster nuclear backgrounds. Genetics 172, 329–341 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy D. J. Variation in mitochondrial genotype has substantial lifespan effects which may be modulated by nuclear background. Aging Cell 7, 795–804 (2008). [DOI] [PubMed] [Google Scholar]
- Dowling D. K., Friberg U., Hailer F. & Arnqvist G. Intergenomic epistasis for fitness: within-population interactions between cytoplasmic and nuclear genes in Drosophila melanogaster. Genetics 175, 235–244 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling D. K., Tompkins D. M. & Gemmell N. J. The Trojan Female Technique for pest control: A candidate mitochondrial mutation confers low male fertility across diverse nuclear backgrounds in Drosophila melanogaster. Evol. Appl. 8, 871–880 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J. N., Camus M. F., Clancy D. J. & Dowling D. K. Complete mitochondrial genome sequences of thirteen globally sourced strains of fruit fly (Drosophila melanogaster) form a powerful model for mitochondrial research. Mitochondrial DNA, 1–3 (2015). [DOI] [PubMed] [Google Scholar]
- Clancy D. J., Hime G. R. & Shirras A. D. Cytoplasmic male sterility in Drosophila melanogaster associated with a mitochondrial CYTB variant. Heredity 107, 374–376 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee W. K., Sutton K. L. & Dowling D. K. In vivo male fertility is affected by naturally occurring mitochondrial haplotypes. Curr. Biol. 23, R55–R56 (2013). [DOI] [PubMed] [Google Scholar]
- Matsuura E. T., Tanaka Y. T. & Yamamoto N. Effects of the nuclear genome on selective transmission of mitochondrial DNA in Drosophila. Genes Genet. Syst. 72, 119–123 (1997). [DOI] [PubMed] [Google Scholar]
- Doi A., Suzuki H. & Matsuura E. T. Genetic analysis of temperature-dependent transmission of mitochondrial DNA in Drosophila. Heredity 82, 555–560 (1999). [DOI] [PubMed] [Google Scholar]
- Dowling D. K., Abiega K. C. & Arnqvist G. Temperature-specific outcomes of cytoplasmic-nuclear interactions on egg-to-adult development time in seed beetles. Evolution 61, 194–201 (2007). [DOI] [PubMed] [Google Scholar]
- Arnqvist G. et al. Genetic architecture of metabolic rate: Environment specific epistasis between mitochondrial and nuclear genes in an insect. Evolution 64, 3354–3363 (2010). [DOI] [PubMed] [Google Scholar]
- Gabay-Laughnan S., Kuzmin E. V., Monroe J., Roark L. & Newton K. J. Characterization of a novel thermosensitive restorer of fertility for cytoplasmic male sterility in maize. Genetics 182, 91–103 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. D. et al. Mitochondrial DNA haplotypes define gene expression patterns in pluripotent and differentiating embryonic stem cells. Stem Cells 31, 703–716 (2013). [DOI] [PubMed] [Google Scholar]
- Laayouni H. et al. Thermal evolution of gene expression profiles in Drosophila subobscura. BMC Evol. Biol. 7, 1–15 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo A. et al. Efficient mitochondrial genome editing by CRISPR/Cas9. BioMed. Res. Int. 2015, 1–10 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camus M. F., Clancy D. J. & Dowling D. K. Mitochondria, maternal inheritance, and male aging. Curr. Biol. 22, 1–5 (2012). [DOI] [PubMed] [Google Scholar]
- Friberg U. & Dowling D. K. No evidence of mitochondrial genetic variation for sperm competition within a population of Drosophila melanogaster. J. Evol. Biol. 21, 1798–1807 (2008). [DOI] [PubMed] [Google Scholar]
- Hoffmann A. A. Physiological climatic limits in Drosophila: patterns and implications. J. Exp. Biol. 213, 870–880 (2010). [DOI] [PubMed] [Google Scholar]
- Williams B. R., van Heerwaarden B., Dowling D. K. & Sgro C. M. A multivariate test of evolutionary constraints for thermal tolerance in Drosophila melanogaster. J. Evol. Biol. 25, 1415–1426 (2012). [DOI] [PubMed] [Google Scholar]
- Partridge L. & Andrews R. The effect of reproductive activity on the longevity of male Drosophila melanogaster is not caused by an acceleration of ageing. J. Insect Physiol. 31, 393–395 (1985). [Google Scholar]
- Bates D., Maechler M., Bolker B. & Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015). [Google Scholar]
- R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria, 2013).
- Fox J. & Weisberg S. An R Companion to Applied Regression. 2nd edn (Sage Publishing, 2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.