Abstract
Review current treatments of metastatic lesions in the proximal femur.
We reviewed published literature related to diagnosis and surgical treatments and summarized current treatment options.
Surgical management mainly consist of internal fixation, hip replacement, and percutaneous femoroplasty (PFP) which has been newly applied in clinical practice.
An appropriate series of treatments is necessary for patients to avoid the occurrence of paraplegia and prolong survival time.
Keywords: metastases, percutaneous femoroplasty, proximal femur, surgery
1. Introduction
Metastasis accounts for 90% death of cancer patients. In theory, cancer cells can metastasize anywhere in the body but they usually predominate at one site more often than others. Besides lung and liver, bone is the most common site invaded by cancers and actually nearly all types of cancer can metastasize to the bones, particular for breast cancer, prostate cancer, and multiple myeloma.[1] Many factors caused by cancer cells or arisen by the bone marrow microenvironment in response to cancer cells are keys to activation of osteoclastic bone resorption and regulation of osteoblastic activity in bone metastasis.[2–4] Bone metastasize presents a strong correlation with tumor growth in bone which has been referred to the “vicious cycle”, in which tumor cells promote bone metastasize by osteoclasts, and osteoclastic bone destruction causes the activation of growth factors in the microenvironment that further promote tumor progression.[5] In addition to portending a dismal prognosis, bone metastases cause significant morbidity including bone pain, fractures, hypercalcemia, spinal cord compression, and other nerve compression syndromes.[6] The spine, proximal femur, and pelvis are the most common sites of bone metastases.[7,8] Among femur metastatic tumors, 50% of the lesions occur in the femoral neck, 30% occur in the subtrochanteric site, and 20% occur in the intertrochanteric site. These lesions lead to severe bone pain and a high incidence of pathological fractures.[9] The goal of proximal femoral metastasis treatment is to relieve pain, restore weight-bearing abilities, and improve the quality of the patient's remaining life. The treatment of proximal femoral metastases includes nonsurgical and surgical approaches. The nonsurgical approaches include radiation therapy and pharmacologic treatment such as bisphosphonate while the surgical approaches include internal fixation, hip prostheses replacement, and percutaneous femoroplasty (PFP).[10] Because the femur is a major weight-bearing bone with minimal space for surgical errors, the operative procedure must be carefully planned and meticulously executed, with the aim of achieving durable reconstruction. Therefore, all pertinent information must be carefully considered before surgical treatment, such as the location of the metastasis, the extent of bone involvement and the number of metastatic lesions, the perceived effects on other therapies, the type of primary cancer, and the expected life span. PFP represents a promising treatment option for alleviating the pain and weakness associated with metastatic lesions of the femur. PFP resulted in significant pain relief in the affected limbs and significantly improved quality of life. In this review, we have summarized the advances in diagnosis and treatment options for proximal femur metastases.
2. Diagnosis
Proximal femoral metastases can cause severe pain and even pathological fractures, which can negatively affect a patient's quality of life. The evaluation of patients with proximal femoral metastases should be performed according to a systematic method.[11] One should never assume that proximal femoral metastasis need to be treated in a specific manner without a definite diagnosis, particularly when determining whether the patient has multiple metastases or multiple myeloma. x-Ray is the most commonly used method for the diagnosis of bone disease. However, computed tomography (CT) is more advantageous in diagnosis of osteoclastic, osteoblastic, and mixed images of matastases,[12–14] which is frequently used to assess proximal femur stability. Apart from this, CT plays vital roles in operative planning and postoperative assessment, allowing for accurate measurement of the femoral cortices.[15] However, when there is a demand for aprecise image of soft tissues and bone structures, magnetic resonance imaging (MRI) is preferred.[16] Besides accurately detecting bone metastases, MRI offers sensitive and specific detection of early proximal femoral metastases and is able to reveal early pathological changes in the marrow.[17] Other examinations such as emission computed tomography (ECT), single-photon emission tomography (SPECT), and positron emission tomography (PET) have their respective advantages. ECT can detect regions of remodeling that are relevant to bone metastases, infection and bone fractures. SPECT[18,19] provides better sensitivity and specificity for cancer and metastasis diagnoses. PET is also applied to the staging of systemic diseases in tumor patients.[20–22] Additionally, if multiple myeloma is suspected, serum immune electrophoresis and bone marrow aspiration should be performed to obtain a definite diagnosis.
Rougraff et al[23] recommended that the standard diagnostic strategy for patients without a definite primary site should include an x-ray of the affected limb, a whole-body bone scan, laboratory studies, and CTs of the chest, abdomen, and pelvis. These procedures identified the primary lesion in 85% of patients. If the patient has no history of tumors or a definite diagnosis is needed, a needle biopsy is a better choice for the diagnosis of bone metastasis.
3. Surgical management
The proximal femur is the most common site of mechanical failure in metastatic patients due to its load-bearing function and continuous axial and torsional stresses. Approximately, 10% of patients with metastatic disease will sustain a pathologic fracture, and 65% of all fractures that occur in the femur require surgery.[24] Although the majority of tumor patients with skeletal metastases are in the advanced stages of cancer, invasive surgical treatment is usually performed in patients with proximal femur metastases. The relevant stereoscopic surgical treatments fall into two general categories: internal fixation and endoprosthetic replacement.[25–27] Percutaneous vertebroplasty, which is associated with mild trauma and good analgesic effects, is an effective and widely accepted operational option for patients with vertebral tumors through clinical practice.[28–32] However, the development and evolution of the use of bone cement outside of the spine[8,31] has rendered the injection of bone cement a potential treatment option for proximal femur metastases. The concept of percutaneous vertebroplasty has been developed to PFP after being applied to the treatment of femoral metastases. PFP provides a less invasive therapeutic option that offers pain relief and biomechanical stability to patients with proximal femoral metastases. All patients benefited from the new technique as previous studies shown.[10,33]
3.1. Evaluations prior to surgical treatments
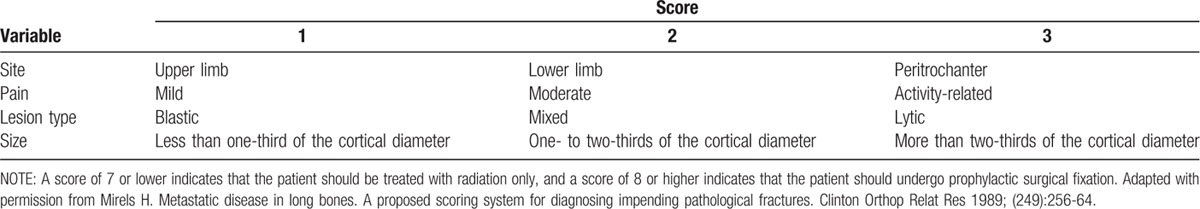
In addition to considering the patient's general systematic conditions, Mirel scoring system, which is the most widely used tool for describing the risk of pathologic fracture, should always be taken into account.[34] These criteria include the location of the lesion, the extent of the lesion, the pain that occurs with walking, and the lytic or blastic nature of the lesion. If the patient has a score >9, the risk of pathologic fracture is >33%, and the patient requires prophylactic surgery, such as internal fixation or prosthetic replacement. Mirel criteria reveal the risk of pathologic fracture and can facilitate initial decisions regarding surgical planning (Table 1). Van der Linden et al[35] argued that axial cortical involvement >30 mm is associated with a higher risk of fracture in cases of proximal femoral metastasis. For these impending fractures, the most appropriate strategies are surgical interventions which can provide mechanical stability before a fracture occurs.
Table 1.
Mirel's scoring system for the risk of pathologic fracture.
3.2. Treatment with prosthetic replacements
Once a fracture occurs in cases of metastatic lesions, nonsurgical treatments always have inevitable limitations; for example, catagmatic bone cannot be sufficiently immobilized, which prevents recovery and results in sustained pain.[36,37] Because pathologic fractures often exhibit delayed union or even non-union, prosthetic replacements are recommended for patients who have suffered such fractures. Prosthetic replacements not only reconstruct the destroyed lesion but are also used to salvage failed internal fixations or nonsurgical treatments. Standard prostheses are widely used for lesions located in the femoral head and neck. However, if a standard prosthesis is inadequate, particularly when there is substantial bone loss in the peritrochanteric region, tumor prosthesis can be applied. Additionally, if the integrity of the acetabulum is sufficient, bipolar hemiarthroplasty is a better option for patients because the complication rate of this procedure is lower than that of a total hip replacement (4% vs. 22.2%).[38,39] Paul suggested that impending femoral neck lesions should be treated with arthroplasty.[34] In brief, prostheses are an excellent choice for the treatment of proximal femoral metastases in patients with good general fitness and life expectancy. Although the risks of dislocation and infection are high, this method ensures major local tumor control, and the complication and failure rates are not significantly higher than those of osteosynthesis, especially when the latter procedure is associated with curettage and the use of adjuvant cement (Fig. 1).
Figure 1.

Surgical treatment of a proximal femoral metastatic tumor (prosthetic replacement). (a and b) The preoperative hip x-ray shows the metastatic lesions in the right proximal femur; (c) the postoperative hip x-ray shows prosthetic replacements.
3.3. Treatment with internal nail fixation
Pathologic intertrochanteric or subtrochanteric fractures may be treated with a cephalomedullary nail or with a prosthetic replacement.[25–27,34,40] The indications for choosing a prosthetic replacement or a cephalomedullary nail are still not clear. Piatek et al[41] argued that treatment with a gamma nail or a proximal femoral nail may be appropriate for subtrochanteric metastases with a distance to trochanter >20 mm. In general, if the bone in the femoral neck and head is adequate to support the implant, nailing is preferred.[34] Several studies have demonstrated that patients can achieve satisfactory pain relief and marked clinical improvement with improved weight-bearing ability following internal nail fixation operations.[24,42,43] When the surgery is performed, proximal reaming should also be performed as carefully and gently as possible to avoid lateral cortical fracture and embolization. Adequate bone available proximally for fixation is necessary, and distal interlocking is mandatory.[34]
For patients with impending intertrochanteric or subtrochanteric fractures, the bone can be stabilized with cephalomedullary nailing because head and neck fixation can be adequate.[24,34,40,43] The use of intramedullary devices is a traditional treatment method for long bone metastases, particularly those in the proximal femur. These devices have relatively low rates of mechanical failure (2–22%) and incidence of death from cardiopulmonary complications ranges (1–10%).[38,44–46] Considering the abovementioned factors, the treatment method of nailing may be well suited for patients with good proximal bone stock.
3.4. Treatment with PFP
The first application of polymethylmethacrylate (PMMA) bone cement was conducted by Deramond et al.[47] They injected PMMA into C2 vertebra affected by vertebral hemangiomas and observed remarkable chronic pain relief.[48] Gradually, the use of PMMA following an analogous percutaneous and minimally invasive manipulation method with the assistance of radiology guidance has been found to be favorable for vertebral fractures associated with osteoporosis and painful vertebra with broad osteolysis or invasion secondary to a tumor.[49] An effective and minimally invasive surgical technique is desired for the management of many advanced cancers in patients with severe bone pain who cannot tolerate a major surgery and in patients for whom radiotherapy has already been ineffective and therefore only desire pain relief, minimal trauma, and reduced costs. PMMA may possess many advantages for achieving these goals, including bio-inertness, ease of management, relatively high biomechanical strength, and extended sustainability. The injection of bone cement can strengthen the transmission of bone-to-implant and implant-to-bone forces.[50] Previous studies have demonstrated the efficiency of bone cement injection in proximal femoral metastases.[10,33,51] PFP is a minimally invasive therapy that can provide a facile but effective reinforcement of the proximal femur and reduce the risk of fracture[52,53] (Fig. 2).
Figure 2.

Surgical treatment of a proximal PFP. (a) The preoperative hip x-ray shows the metastatic lesions in the left proximal femur; (b) the postoperative hip x-ray shows bone cement injection in proximal femoral metastases. PFP (femoral metastatic tumor). PEP = percutaneous femoroplasty.
Main indications for the PFP treatment of proximal femoral metastases include the following: patients present with steady vital signs, without severe heart or lung disease, or topical inflammation and ulceration; the lesion site should involve primarily osteolytic damage, and the cortex around lesion should be complete, particularly within the calcarfemorale; pathological fractures must not be present. And these components are recommended for: PFP is not recommended for osteoblastic metastases. Given the large volume of bone cement injected in a topical position, the injection should be performed before the bone cement becomes solidification. During the operation, pulse oximetry should be monitored closely to avoid pulmonary embolism. Bone cement leakage represents a common complication of PFP. In the event of leakage, the needle should be repositioned by fluoroscopy to prevent further leakage. In addition, compression of the osteoclasia or soft tissue lump can cause cancerous cells to transfer into blood, which could accelerate the process of metastasis.
Regarding weight-bearing capacity and biomechanical stabilization after PFP, the injection of bone cement can sufficiently strengthen and reinforce the destroyed bone. Several studies have shown that cement injections performed in different ways can result in different outcomes. Hayashi et al[54] claimed that filling the femoral neck with bone cement could improve the mechanical stability of the hip (when loads are applied to the greater trochanter) and protect against fractures of the proximal femur. Palumbo et al[55] reported that bicortical cement columns spanning from the superior to inferior femoral neck cortices play important roles in restoring the integrity of the femoral neck and improving the load-bearing capacity of the proximal femur. Beckmann et al[56] showed that single central and centrodorsal bone cement filling patterns significantly improve the mechanical stability of the hip, but double craniocaudal augmentation degenerates the skeleton and results in markedly reduced peak loads and decreased energies to the point of failure. Sutter et al[53] reported that 15 mL of cement is inadequate to reinforce the proximal femur and there is little biomechanical advantage to placing the cement in the proximal femur when compared with placing it in the trochanter.
Why is PFP so effective? The chemotoxicity and thermal necrosis during exothermic polymerizationmight account for the possible mechanisms of PMMA in pain relief.[57,58] PFP may alleviate pain via the damage of the sensitive nerve endings in periosteum.[59] The role of methylmethacrylate (MMA), a component of PMMA, in cell toxicity, neurotoxicity, and chemotoxicity remains unclear and opposite opinions have arisen in previous studies.[49,60] After ruling out the “chemical effect” and “thermal effect” as probable causes of pain relief, “mechanical stabilization” can be regarded as the second most likely reason for pain relief. In a previous study, PMMA was found to restore the mechanical stability of the bone by stabilizing the micro-motions of micro-trabecular fractures, which are the most frequently observed histologic change in patients. At present, the most likely mechanism of pain relief is the mechanical stabilization.[10,61] Our previously PFP procedures performed and observed an encouraging curative effect, however, the proposed indications should be strictly followed, as shown Table 2.[10]
Table 2.
Main indications and recommendations for the PFP treatment of proximal femoral metastases.

4. Overview and conclusion
By the time metastatic tumors are diagnosed, patients have advanced disease; thus, even with the recent advances in the treatment of primary cancers, the survival of patients with bone metastases remains poor.[62,63] The goals of treatments of proximal femoral metastatic tumors (particularly surgical treatments) are to maximize pain relief, prevent pathologic fractures, provide mechanical stabilization, and enable weight-bearing. For patients with pathologic fractures and ossified metastatic tumors in the proximal section of the femur, prosthetic replacement following proximal femoral resection is an appropriate operative choice. Internal nail fixation may be suitable for patients with good proximal bone stock. For patients without fractures and with continuous cortical bone metastatic tumors, PFP may be an attractive option when the goal is to simply relieve pain. The importance of careful and appropriate preoperative assessment before the execution of any therapeutic procedure should be emphasized. A carefully considered multidisciplinary approach will provide patients the best opportunity for an improved quality of life and maximal satisfaction. The patient provided written informed consent for the publication of these pictures and the study was approved by the Human Ethics and Research Ethics committees of the Fourth Hospital of Hebei Medical University.
Footnotes
Abbreviations: CT = computed tomography, ECT = emission computed tomography, MRI = magnetic resonance imaging, PET = positron emission tomography, PFN = proximal femoral nail, PFP = percutaneous femoroplasty, PMMA = polymethylmethacrylate, PVP = percutaneous vertebroplasty, SPECT = single-photon emission tomography.
The authors have no conflicts of interest to disclose.
References
- 1.Coleman RE. Bone cancer in 2011: prevention and treatment of bone metastases. Nat Rev Clin Oncol 2012; 9:76–78. [DOI] [PubMed] [Google Scholar]
- 2.Johnson RW, Merkel AR, Page JM, et al. Wnt signaling induces gene expression of factors associated with bone destruction in lung and breast cancer. Clin Exp Metastasis 2014; 31:945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X, Akech J, Browne G, et al. Runx2-Smad signaling impacts the progression of tumor-induced bone disease. Int J Cancer 2015; 136:1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luis-Ravelo D, Anton I, Zandueta C, et al. A gene signature of bone metastatic colonization sensitizes for tumor-induced osteolysis and predicts survival in lung cancer. Oncogene 2014; 33:5090–5099. [DOI] [PubMed] [Google Scholar]
- 5.Clines GA, Guise TA. Molecular mechanisms and treatment of bone metastasis. Expert Rev Mol Med 2008; 10:e7. [DOI] [PubMed] [Google Scholar]
- 6.Clines GA, Guise TA. Molecular mechanisms and treatment of bone metastasis. Expert Rev Mol Med 2008; 10:e7. [DOI] [PubMed] [Google Scholar]
- 7.Laufer I, Sciubba DM, Madera M, et al. Surgical management of metastatic spinal tumors. Cancer Control 2012; 19:122–128. [DOI] [PubMed] [Google Scholar]
- 8.Sun G, Jin P, Liu XW, et al. Cementoplasty for managing painful bone metastases outside the spine. Eur Radiol 2014; 24:731–737. [DOI] [PubMed] [Google Scholar]
- 9.Nazario J, Tam AL. Ablation of bone metastases. Surg Oncol Clin N Am 2011; 20:355–368.ix. [DOI] [PubMed] [Google Scholar]
- 10.Feng H, Feng J, Li Z, et al. Percutaneous femoroplasty for the treatment of proximal femoral metastases. Eur J Surg Oncol 2014; 40:402–405. [DOI] [PubMed] [Google Scholar]
- 11.Badila A, Radulescu R, Sajin M, et al. Immunohistochemistry in diagnosis and surgical treatment of femoral bone metastasis. Rom J Morphol Embryol 2014; 55:135–139. [PubMed] [Google Scholar]
- 12.Khandekar S, Dive A, Munde P, et al. Chondroblastic osteosarcoma of the left zygomatic bone: rare case report and review of the literature. J Oral Maxillofac Pathol 2014; 18:281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caglar M, Kupik O, Karabulut E, et al. Detection of bone metastases in breast cancer patients in the PET/CT era: do we still need the bone scan. Rev Esp Med Nucl Imagen Mol 2015; 35:3–11. [DOI] [PubMed] [Google Scholar]
- 14.Shi HY, Zhao XS, Miao F. Metastases to the pancreas: computed tomography imaging spectrum and clinical features: a retrospective study of 18 patients with 36 metastases. Medicine (Baltimore) 2015; 94:e913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Treece GM, Gee AH. Independent measurement of femoral cortical thickness and cortical bone density using clinical CT. Med Image Anal 2015; 20:249–264. [DOI] [PubMed] [Google Scholar]
- 16.Subhawong TK, Wilky BA. Value added: functional MR imaging in management of bone and soft tissue sarcomas. Curr Opin Oncol 2015; 27:323–331. [DOI] [PubMed] [Google Scholar]
- 17.Torres C, Hammond I. Computed tomography and magnetic resonance imaging in the differentiation of osteoporotic fractures from neoplastic metastatic fractures. J Clin Densitom 2015; 19:63–69. [DOI] [PubMed] [Google Scholar]
- 18.McLoughlin LC, O’Kelly F, O’Brien C, et al. The improved accuracy of planar bone scintigraphy by adding single photon emission computed tomography (SPECT-CT) to detect skeletal metastases from prostate cancer. Ir J Med Sci 2014; 185:1–5. [DOI] [PubMed] [Google Scholar]
- 19.Miao W, Zheng S, Dai H, et al. Comparison of 99mTc-3PRGD2 integrin receptor imaging with 99mTc-MDP bone scan in diagnosis of bone metastasis in patients with lung cancer: a multicenter study. PLoS One 2014; 9:e111221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humbert O, Cochet A, Coudert B, et al. Role of positron emission tomography for the monitoring of response to therapy in breast cancer. Oncologist 2015; 20:94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sacks A, Peller PJ, Surasi DS, et al. Value of PET/CT in the management of liver metastases, part 1. AJR Am J Roentgenol 2011; 197:W256–W259. [DOI] [PubMed] [Google Scholar]
- 22.Xu GZ, Guan DJ, He ZY. (18)FDG-PET/CT for detecting distant metastases and second primary cancers in patients with head and neck cancer. A meta-analysis. Oral Oncol 2011; 47:560–565. [DOI] [PubMed] [Google Scholar]
- 23.Rougraff BT, Kneisl JS, Simon MA. Skeletal metastases of unknown origin. A prospective study of a diagnostic strategy. J Bone Joint Surg Am 1993; 75:1276–1281. [DOI] [PubMed] [Google Scholar]
- 24.Piccioli A, Rossi B, Scaramuzzo L, et al. Intramedullary nailing for treatment of pathologic femoral fractures due to metastases. Injury 2014; 45:412–417. [DOI] [PubMed] [Google Scholar]
- 25.Dutka J, Sosin P, Libura M. Internal fixation with bone cement in reconstruction of bone defects due to bone metaseses. Ortop Traumatol Rehabil 2006; 8:620–626. [PubMed] [Google Scholar]
- 26.Smith RM, Ng A, Giannoudis PV. Stabilization of metastatic lesions of the proximal femur with the AO solid femoral nail. J Orthop Trauma 2001; 15:307–308. [DOI] [PubMed] [Google Scholar]
- 27.Steensma M, Boland PJ, Morris CD, et al. Endoprosthetic treatment is more durable for pathologic proximal femur fractures. Clin Orthop Relat Res 2012; 470:920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chew C, Craig L, Edwards R, et al. Safety and efficacy of percutaneous vertebroplasty in malignancy: a systematic review. Clin Radiol 2011; 66:63–72. [DOI] [PubMed] [Google Scholar]
- 29.Huang M, Zhu H, Liu T, et al. Comparison of external radiotherapy and percutaneous vertebroplasty for spinal metastasis. Asia Pac J Clin Oncol 2014; 12:201–208. [DOI] [PubMed] [Google Scholar]
- 30.Kushchayev S, Kushchayeva Y, Theodore N, et al. Percutaneous vertebroplasty for thyroid cancer metastases to the spine. Thyroid 2010; 20:555–560. [DOI] [PubMed] [Google Scholar]
- 31.Salapura V, Jeromel M. Minimally invasive (percutaneous) treatment of metastatic spinal and extraspinal disease—a review. Acta Clin Croat 2014; 53:44–54. [PubMed] [Google Scholar]
- 32.Saliou G, Kocheida eM, Lehmann P, et al. Percutaneous vertebroplasty for pain management in malignant fractures of the spine with epidural involvement. Radiology 2010; 254:882–890. [DOI] [PubMed] [Google Scholar]
- 33.Plancarte-Sanchez R, Guajardo-Rosas J, Cerezo-Camacho O, et al. Femoroplasty: a new option for femur metastasis. Pain Pract 2013; 13:409–415. [DOI] [PubMed] [Google Scholar]
- 34.Issack PS, Barker J, Baker M, et al. Surgical management of metastatic disease of the proximal part of the femur. J Bone Joint Surg Am 2014; 96:2091–2098. [DOI] [PubMed] [Google Scholar]
- 35.Van der Linden YM, Dijkstra PD, Kroon HM, et al. Comparative analysis of risk factors for pathological fracture with femoral metastases. J Bone Joint Surg Br 2004; 86:566–573. [PubMed] [Google Scholar]
- 36.Gosling T, Becker-Schiebe M. [Surgical treatment of skeletal metastases]. Unfallchirurg 2015; 118:347–363. [DOI] [PubMed] [Google Scholar]
- 37.Katzer A, Meenen NM, Grabbe F, et al. Surgery of skeletal metastases. Arch Orthop Trauma Surg 2002; 122:251–258. [DOI] [PubMed] [Google Scholar]
- 38.Wedin R, Bauer HC. Surgical treatment of skeletal metastatic lesions of the proximal femur: endoprosthesis or reconstruction nail. J Bone Joint Surg Br 2005; 87:1653–1657. [DOI] [PubMed] [Google Scholar]
- 39.Zheng K, Peng ZX, Zheng PP. Chondrosarcoma of the proximal humerus secondary to ollier disease: an 8-year follow-up of successful resection of the tumor with endoprosthetic replacement of the proximal humerus. J Clin Med Res 2014; 6:218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steensma M, Healey JH. Trends in the surgical treatment of pathologic proximal femur fractures among Musculoskeletal Tumor Society members. Clin Orthop Relat Res 2013; 471:2000–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piatek S, Westphal T, Bischoff J, et al. [Intramedullary stabilisation of metastatic fractures of long bones]. Zentralbl Chir 2003; 128:131–138. [DOI] [PubMed] [Google Scholar]
- 42.Assal M, Zanone X, Peter RE. Osteosynthesis of metastatic lesions of the proximal femur with a solid femoral nail and interlocking spiral blade inserted without reaming. J Orthop Trauma 2000; 14:394–397. [DOI] [PubMed] [Google Scholar]
- 43.Moholkar K, Mohan R, Grigoris P. The Long Gamma Nail for stabilisation of existing and impending pathological fractures of the femur: an analysis of 48 cases. Acta Orthop Belg 2004; 70:429–434. [PubMed] [Google Scholar]
- 44.Dijstra S, Wiggers T, van Geel BN, et al. Impending and actual pathological fractures in patients with bone metastases of the long bones. A retrospective study of 233 surgically treated fractures. Eur J Surg 1994; 160:535–542. [PubMed] [Google Scholar]
- 45.Samsani SR, Panikkar V, Venu KM, et al. Breast cancer bone metastasis in femur: surgical considerations and reconstruction with Long Gamma Nail. Eur J Surg Oncol 2004; 30:993–997. [DOI] [PubMed] [Google Scholar]
- 46.van Doorn R, Stapert JW. Treatment of impending and actual pathological femoral fractures with the long Gamma nail in The Netherlands. Eur J Surg 2000; 166:247–254. [DOI] [PubMed] [Google Scholar]
- 47.Deramond H, Depriester C, Toussaint P, et al. Percutaneous vertebroplasty. Semin Musculoskelet Radiol 1997; 1:285–296. [DOI] [PubMed] [Google Scholar]
- 48.Galibert P, Deramond H, Rosat P, et al. [Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty]. Neurochirurgie 1987; 33:166–168. [PubMed] [Google Scholar]
- 49.Yimin Y, Zhiwei R, Wei M, et al. Current status of percutaneous vertebroplasty and percutaneous kyphoplasty—a review. Med Sci Monit 2013; 19:826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arora M, Chan EK, Gupta S, et al. Polymethylmethacrylate bone cements and additives: A review of the literature. World J Orthop 2013; 4:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plancarte R, Guajardo J, Meneses-Garcia A, et al. Clinical benefits of femoroplasty: a nonsurgical alternative for the management of femoral metastases. Pain Physician 2014; 17:227–234. [PubMed] [Google Scholar]
- 52.Beckmann J, Ferguson SJ, Gebauer M, et al. Femoroplasty—augmentation of the proximal femur with a composite bone cement—feasibility, biomechanical properties and osteosynthesis potential. Med Eng Phys 2007; 29:755–764. [DOI] [PubMed] [Google Scholar]
- 53.Sutter EG, Wall SJ, Mears SC, et al. The effect of cement placement on augmentation of the osteoporotic proximal femur. Geriatr Orthop Surg Rehabil 2010; 1:22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayashi K, Aono M, Shintani K, et al. Bisphosphonate-related atypical femoral fracture with bone metastasis of breast cancer: case report and review. Anticancer Res 2014; 34:1245–1249. [PubMed] [Google Scholar]
- 55.Palumbo BT, Nalley C, Gaskins RB, 3rd, et al. Biomechanical analysis of impending femoral neck fractures: the role of percutaneous cement augmentation for osteolytic lesions. Clin Biomech (Bristol, Avon) 2014; 29:289–295. [DOI] [PubMed] [Google Scholar]
- 56.Beckmann J, Springorum R, Vettorazzi E, et al. Fracture prevention by femoroplasty—cement augmentation of the proximal femur. J Orthop Res 2011; 29:1753–1758. [DOI] [PubMed] [Google Scholar]
- 57.Anselmetti GC, Manca A, Kanika K, et al. Temperature measurement during polymerization of bone cement in percutaneous vertebroplasty: an in vivo study in humans. Cardiovasc Intervent Radiol 2009; 32:491–498. [DOI] [PubMed] [Google Scholar]
- 58.Urrutia J, Bono CM, Mery P, et al. Early histologic changes following polymethylmethacrylate injection (vertebroplasty) in rabbit lumbar vertebrae. Spine (Phila Pa 1976) 2008; 33:877–882. [DOI] [PubMed] [Google Scholar]
- 59.Smith HS, Barkin RL. Painful boney metastases. Am J Ther 2014; 21:106–130. [DOI] [PubMed] [Google Scholar]
- 60.Nakano M, Hirano N, Ishihara H, et al. Calcium phosphate cement-based vertebroplasty compared with conservative treatment for osteoporotic compression fractures: a matched case-control study. J Neurosurg Spine 2006; 4:110–117. [DOI] [PubMed] [Google Scholar]
- 61.Antonacci MD, Mody DR, Rutz K, et al. A histologic study of fractured human vertebral bodies. J Spinal Disord Tech 2002; 15:118–126. [DOI] [PubMed] [Google Scholar]
- 62.Selek H, Basarir K, Yildiz Y, et al. Cemented endoprosthetic replacement for metastatic bone disease in the proximal femur. J Arthroplasty 2008; 23:112–117. [DOI] [PubMed] [Google Scholar]
- 63.Utzschneider S, Wicherek E, Weber P, et al. Surgical treatment of bone metastases in patients with lung cancer. Int Orthop 2011; 35:731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]


