Abstract
Asia-Pacific Burden of Respiratory Diseases (APBORD) was a cross-sectional, observational study examining the burden of respiratory disease in adults across 6 Asia-Pacific countries.
This article reports symptoms, healthcare resource utilization (HCRU), work impairment and cost burden associated with allergic rhinitis (AR), asthma, chronic obstructive pulmonary disease (COPD), and rhinosinusitis in Thailand.
Consecutive participants aged ≥18 years with a primary diagnosis of AR, asthma, COPD, or rhinosinusitis were enrolled at 4 hospitals in Thailand during October 2012 and October 2013. Participants completed a survey detailing respiratory symptoms, HCRU, work productivity, and activity impairment. Locally sourced unit costs were used in the calculation of total costs.
The study enrolled 1000 patients. The most frequent primary diagnosis was AR (44.2%), followed by rhinosinusitis (24.1%), asthma (23.7%), and COPD (8.0%). Overall, 316 (31.6%) of patients were diagnosed with some combination of the 4 diseases. Blocked nose or congestion (17%) and cough or coughing up phlegm (16%) were the main reasons for the current medical visit. The mean annual cost for patients with a respiratory disease was US$1495 (SD 3133) per patient. Costs associated with work productivity loss were the principal contributor for AR and rhinosinusitis patients while medication costs were the highest contributor for asthma and COPD patients.
The study findings highlight the burden associated with 4 prevalent respiratory diseases in Thailand. Thorough investigation of concomitant conditions and improved disease management may help to reduce the burden of these respiratory diseases.
Keywords: Asia-Pacific, asthma, chronic disease, cost of illness, pulmonary disease, chronic obstructive, rhinitis, allergic, sinusitis
1. Introduction
Chronic respiratory diseases such as asthma, allergic rhinitis (AR), chronic obstructive pulmonary disease (COPD) result in significant morbidity and mortality worldwide.[1,2] The burden of these diseases greatly affects the quality of life of affected individuals.[2] Chronic respiratory diseases have been estimated to account for 4% of the global burden and 8.3% of the burden of chronic diseases.[2] In addition, 4 million deaths annually are attributable to chronic respiratory diseases.[2]
In most parts of the world including Thailand, prevalence of respiratory diseases such as asthma[3] and COPD are increasing.[4] This is leading to increased disease burden among the population as well as increased economic burden for the country. Previous studies of the burden of respiratory disease in Thailand have investigated healthcare-specific costs associated with COPD[5] and asthma.[6,7] However, no study to date has been conducted which comprehensively describes disease characteristics and captures societal costs associated with the most prevalent respiratory conditions in Thailand.
To address the lack of data relating to the burden of care in adults who present to healthcare professionals with chronic respiratory diseases in this region, a cross-sectional, observational study called the Asia-Pacific Burden of Respiratory Diseases (APBORD) study was conducted across Asia-Pacific.[8] This article reports the results from patients in Thailand.
2. Methods
2.1. Study design
This study formed a part of the large multicountry, cross-sectional, observational APBORD study of adult patients receiving care for respiratory diseases across 6 countries in the Asia-Pacific region (India, Korea, Malaysia, Singapore, Taiwan, and Thailand). The study consisted of site-based surveys administered to patients and physicians presenting with a primary diagnosis of respiratory disease during a routine visit to a healthcare provider. In Thailand, subjects were recruited from 4 sites between October 2012 and October 2013. The study was approved by the Khon Kaen University Ethics Committee in human research, the Ethical Review Committee for research involving human subjects in research at Chulalongkorn University, Siriraj Institutional Review Board, Faculty of Medicine Siriraj Hospital at Mahidol University, and the Research Ethics Committee, Faculty of Medicine at Chiang Mai University.
2.2. Patients
Patients presenting consecutively at each study site were assessed by the physician during a routine consultation. The physician ascertained whether the primary reason for the patient's visit was to receive care for a respiratory disease or not. All patients receiving care for a respiratory disease were screened for eligibility to participate in the study. Eligibility criteria included consecutive patients aged ≥18 years, and receiving care for a primary diagnosis of asthma, AR, COPD, or rhinosinusitis. Subjects were excluded if they had participated in any interventional clinical study within the 12 weeks prior to entering the current study. A patient could only participate in the study once and no follow-up visits were recorded. Eligible patients were invited to participate in the study and written informed consent was obtained from each patient.
2.3. Data collection
During the study visit, physicians completed the Screening and Consent Log and the Physician Survey. The Consent Log captured the basic demographics and respiratory diagnosis of each eligible patient, and whether an eligible patient consented to participate in the study. The Physician Survey comprised questions relating to the patient's respiratory diagnosis. Physicians indicated which of the 4 diseases were the primary diagnosis, and whether the patient had any other of the 4 diseases in addition to the primary diagnosis. Physicians also recorded medication use for the 4 weeks prior to the study visit and medication prescribed at the current visit.
The diagnosis of respiratory disease was defined by International Statistical Classification of Diseases and Related Health Problems 10th Revision classifications.[9] This excluded some infectious and parasitic diseases that may affect the respiratory system (e.g., tuberculosis) and also excluded neoplasms of the respiratory system. Diagnosis was made by the attending physician using criteria based on international guidelines for asthma, AR, COPD, and rhinosinusitis.[10–13] The physician was required to indicate the clinical criteria for the diagnosis from a list of disease criteria adapted from these clinical practice guidelines for any patients with a new diagnosis of any of the 4 diseases. The patients’ clinical management and physicians’ usual diagnostic practices were not intended to be influenced by participation in the study; however, some patients may have been diagnosed using a more rigorous and standardized approach than may have occurred prior to commencement of the study. No attempt was made to independently verify or confirm the patient's diagnosis and no follow-up diagnostic tests were conducted.
Consenting patients completed the Patient Survey which included questions relating to general demographics, respiratory symptoms, healthcare resource utilization (HCRU), and work productivity. Patients listed their respiratory-related symptoms at the current visit and identified their main symptoms leading to the current visit. HCRU included the number of visits in the previous 4 weeks to a general practitioner (GP), medical specialist, alternative and traditional medicine practitioner, pharmacist, emergency department, and hospital admissions.
Work productivity was assessed using the Work Productivity and Activity Impairment – Specific Health Problem (WPAI-SHP) questionnaire.[14] The WPAI-SHP measures both the amount of absenteeism (work time lost), presenteeism (lost on-the-job productivity), and daily activity impairment attributable to a SHP. The recall period in this questionnaire is 7 days.
2.4. Costing
A broad societal perspective was adopted for the cost analysis and as such costs were collected based on government and patient. Unit costs were sourced from a government hospital. Average costs were calculated using the unit cost of the healthcare resource use item multiplied by reported HCRU in the previous 4 weeks, plus the current visit to the GP or specialist. To cost each medication class, 1 medication, representative of the most commonly prescribed medication for respiratory disease, was identified. Dosing and duration of medication use was according to therapeutic guidelines.[15] Lost productivity costs were calculated by multiplying the overall productivity lost from the WPAI questionnaire by the average monthly wage, estimated to be 10,641 Baht (US$329.75) from International Labor Organisation Global Wage Database 2012. Four-week costs were multiplied by 13 to estimate annual costs, and presented in 2014 United States (US) dollars.
2.5. Sample size
As epidemiological data relating to respiratory diseases in the Asia Pacific region was scarce, sample size calculation was based on an assumption that 4% of patients receiving care for respiratory disease would be diagnosed with asthma, derived from Bettering the Evaluation And Care of Health program, a cross-sectional study of general practice activity in Australia.[16] A sample size of 5000 enrolled patients (providing ± 0.3% precision around the assumed 4%) was selected to examine AR, asthma, COPD, and rhinosinusitis across the 6 Asia-Pacific countries included in APBORD study. To reduce bias, a minimum of 250 enrolled patients were required for each site in Thailand, giving a total sample size of 1000 patients.
2.6. Statistical analysis
Statistical analyses were performed using SAS® for Windows, version 9.3 (SAS Institute Inc., NC). Patient demographics and clinical characteristics were described using mean and standard deviation (SD) for continuous variables and number (percentage) for categorical variables. The percentage and 95% confidence interval (95%CI) of patients with each disease were calculated using the exact (Clopper–Pearson) method.
3. Results
3.1. Patient demographics
A total of 1995 patients were diagnosed with a respiratory disease and screened across 4 study sites in Thailand. Out of the screened patients, 1142 (57.2%) were considered eligible. One thousand eligible patients (87.6%) consented and were enrolled in the study.
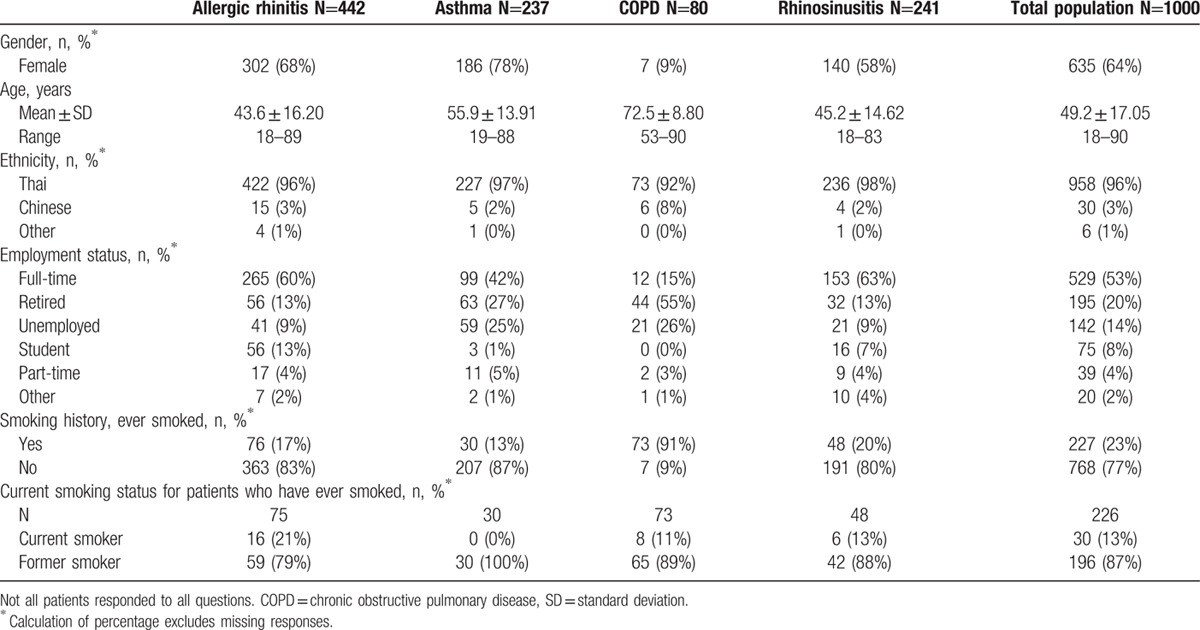
The mean age of enrolled patients was 49.2 years (SD 17.05) and 63.5% were females (Table 1). Fifty-seven percent of the patients were employed, either full-time or part-time. Seventy-seven percent of patients had never smoked. Of the 226 patients to have ever smoked, 13% were current smokers.
Table 1.
Patient demographics by primary diagnosis.
3.2. Frequency of respiratory disease
AR was the most frequent primary diagnosis in enrolled patients (44.2%, 95%CI: 41.1%, 47.3%), followed by rhinosinusitis (24.1%, 95%CI: 21.5%, 26.9%), asthma (23.7%, 95%CI: 21.1%, 26.5%), and COPD (8.0%, 95%CI: 6.4%, 9.9%). Patients were frequently diagnosed with multiple respiratory diseases (Fig. 1). Overall, 316 (31.6%) of patients were diagnosed with a combination of the 4 diseases of interest, with asthma patients having the highest proportion with multiple diagnoses. The most common combinations were AR and asthma (185 patients, 18.5% of total enrolled) and AR and rhinosinusitis (83 patients, 8.3% of total enrolled).
Figure 1.
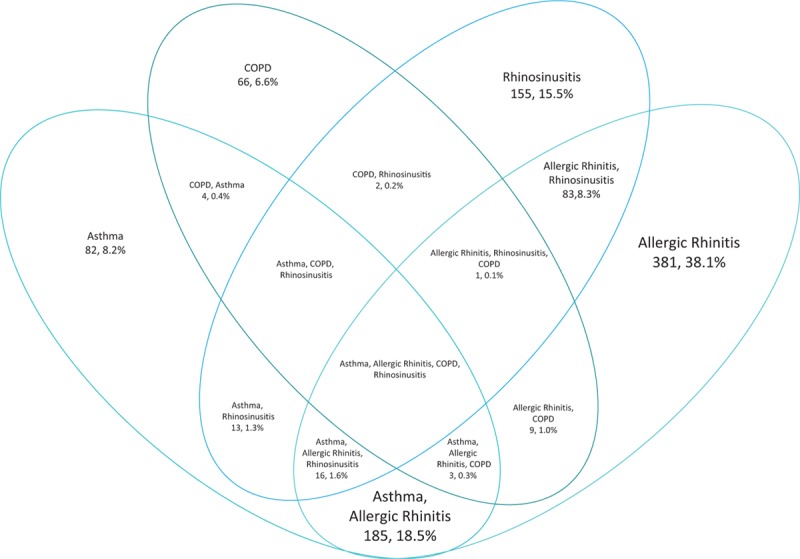
Percentage of enrolled patients (N = 1000) with a combination of diseases. COPD = chronic obstructive pulmonary disease.
3.3. Symptoms
Patients were asked to report all their current symptoms and indicate which symptom they considered the main reason for their medical visit (Fig. 2). Sneezing was the most frequently reported symptom (60.5%, Fig. 3) followed by cough or coughing up phlegm (57.0%). Cough or coughing up phlegm was the most frequently reported main reason for the medical visit for patients diagnosed with asthma (22.2%) and COPD (34.9%). Blocked nose or nasal congestion was the most common main reason for the medical visit for patients with a primary diagnosis of AR (21.6%) and rhinosinusitis (27.4%).
Figure 2.
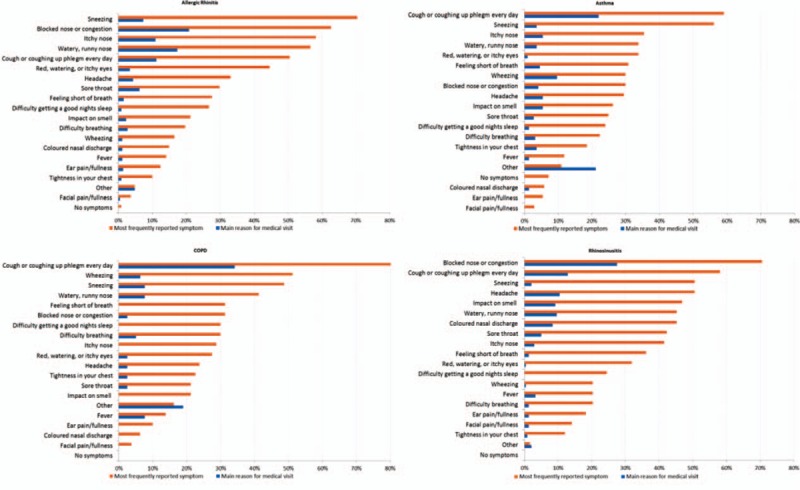
Main reason for the medical visit and symptoms reported by primary diagnosis.
Figure 3.
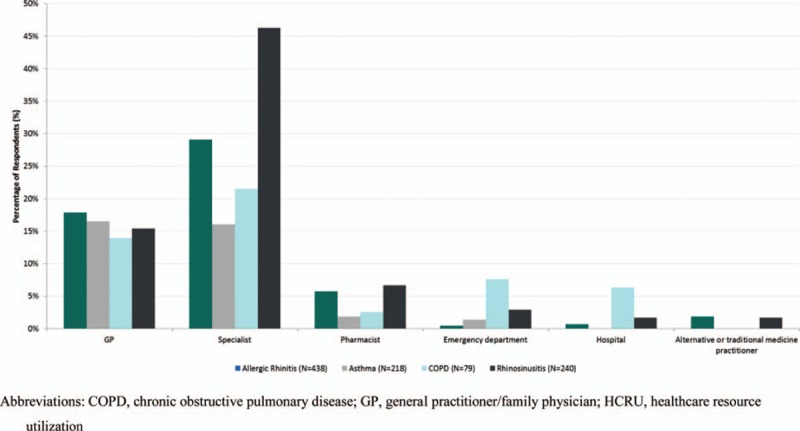
Percentage of patients with healthcare resource utilization (HCRU) in previous four weeks by primary diagnosis.
3.4. Healthcare resource utilization
Patients reported HCRU associated with their main respiratory symptom in the 4 weeks prior to the medical visit (Fig. 3). The percentage of patients reporting specialist visits was higher than those reporting GP visits for patients with a primary diagnosis of rhinosinusitis (46.3% vs 15.4%), AR (29.1% vs 17.8%), and COPD (21.5% vs 13.9%). Pharmacist visits were reported by a greater percentage of patients with AR and rhinosinusitis compared with asthma and COPD. For patients with primary diagnosis of COPD, 7.6% had visited emergency department while 6.3% had been hospitalized in the prior 4 weeks.
Medication use in the 4 weeks prior to the current visit was high, with 88.0% of the patients reporting use of medications (Fig. 4). Previous medication use was highest for patients with a primary diagnosis of asthma (96.6%) and COPD (88.8%), followed by rhinosinusitis (85.9%) and AR (85.7%). Patients with AR and rhinosinusitis most frequently used oral antihistamines whereas fixed-dose combination inhalers had the highest usage among patients with a primary diagnosis of asthma and COPD. A total of 963 patients were prescribed medication at the current visit.
Figure 4.
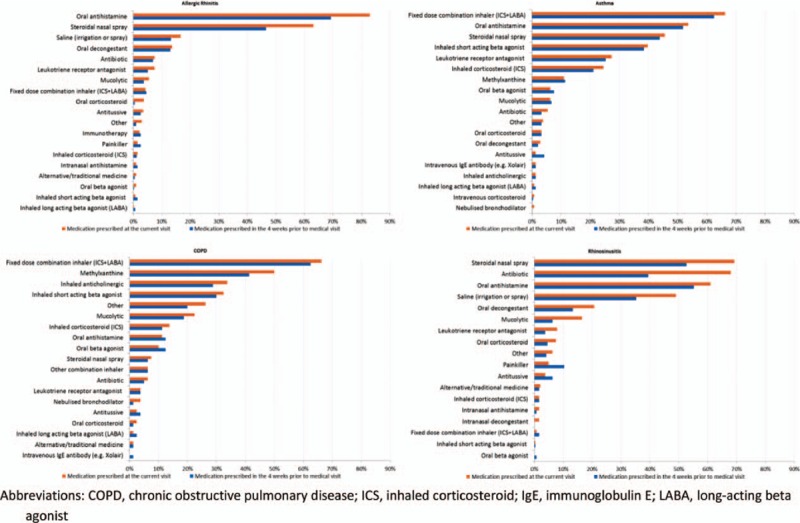
History of medication use for respiratory disease and medications prescribed at the medical visit by primary diagnosis.
3.5. Work productivity and activity impairment
Patients completed the WPAI questionnaire to assess the impact of their respiratory diseases on activity impairment and work productivity loss (Fig. 5). Presenteeism was the main contributing factor to the high productivity loss reported. On average, patients with AR (32.0%, SD 25.74) and rhinosinusitis (43.5%, SD 30.24) reported higher productivity loss than patients with asthma (19.6%, SD 25.23) and COPD (17.1%, SD 26.67). Activity impairment was similarly impacted across all conditions.
Figure 5.
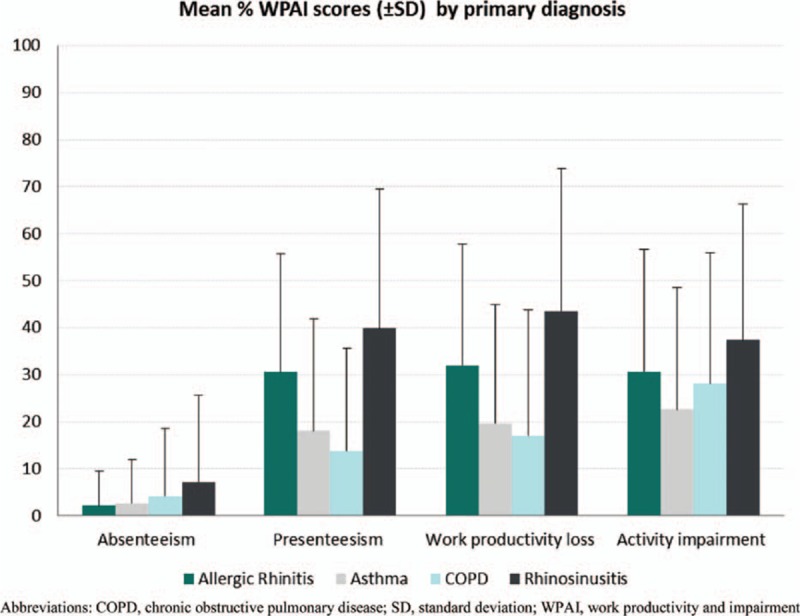
Mean Work Productivity and Activity Impairment (WPAI) scores by primary diagnosis.
3.6. Costs
The annual direct and indirect costs by primary diagnosis are presented in Fig. 6. The mean overall annual cost for patients with a respiratory disease was US$1495 (SD 3133) per patient. For employed patients, the mean annual cost was US$1885 (SD 2340) with productivity loss the highest cost component at US$1391 (SD 1209). Medication costs comprised 58.9% and 41.1% of the overall costs for patients with a primary diagnosis of asthma (US$1287, SD 4654) and COPD (US$1235, SD 4550). Hospitalization costs were also high (25.1%) for patients with COPD (US$754, SD 3367). Costs associated with work productivity loss were the main contributor for patients with a primary diagnosis of AR at 82.8% of overall costs (US$1378, SD 1105) and rhinosinusitis at 80.4% (US$1872, SD 1300).
Figure 6.
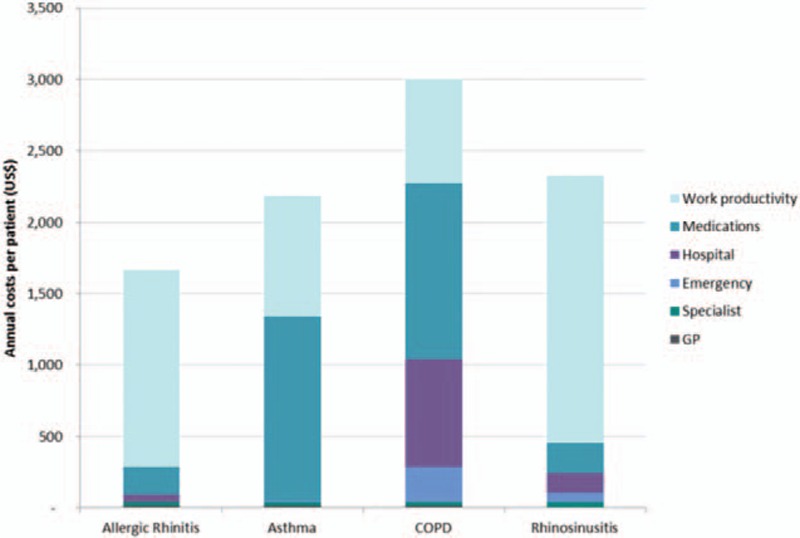
Annual direct and indirect costs for study population by primary diagnosis. COPD = chronic obstructive pulmonary disease, GP = general practitioner.
4. Discussion
This is the first study to comprehensively investigate the disease characteristics and economic burden of 4 highly prevalent respiratory diseases in Thailand. Nearly a 3rd of the patients were diagnosed with multiple respiratory diseases, with asthma and AR the most common combination. The mean annual cost associated with respiratory diseases was US$1495 (SD 3133) per patient with work productivity loss and medication costs the main contributors.
The results presented in this study were part of a larger, APBORD study, which investigated burden of care relating to the selected respiratory diseases in 5 additional countries in the Asia-Pacific (India, Korea, Malaysia, Singapore, and Taiwan).[8,15] In the overall population, the frequency of patients receiving care with the primary diagnosis of AR (14.0%, 95%CI: 13.4%, 14.6%) and rhinosinusitis (5.4%, 95%CI: 4.6%, 5.3%) was lower[8] compared to Thailand. Cough was the main reason for seeking medical care among patients.[8] The mean annual cost for patients with a respiratory disease was US$4191 (SD 8489) per patient with productivity loss the highest cost component for all 4 diseases.[15] The mean annual cost per patient in the overall study was understandably higher due to inclusion of countries with higher healthcare costs than Thailand. Majority of the other outcomes are comparable between Thailand and the overall study.
Few studies have investigated economic burden of respiratory diseases in Thailand. A study on cost of asthma conducted in Thai cities of Chiang Mai and Lumphun reported average total per person annual cost of 16,288 Baht (US$525), with direct costs contributing 93.9% to the total.[7] A cross-sectional study in a regional Thai hospital reported that the average asthma-related hospitalization cost was 5809 Baht (US$135) per patient.[6] The authors also reported that higher total hospital costs associated significantly with older patients (≥60 years) and significant comorbidities.[6] Another study that used a prevalence-based, disease-specific approach, estimated the average direct out-of-pocket treatment cost for COPD in Thailand to be 12,357 Baht (US$383) per patient.[5] In the present study, the annual per patient costs were considerable higher at US$1716 for asthma patients and US$2353 for COPD patients. For the calculation of annual costs, a broader societal perspective was used compared with previous studies; consisting of direct healthcare costs (incorporating both government and patient out-of-pocket costs) and indirect costs captured by productivity loss.
In the current study, 96.6% of asthma patients and 88.8% of COPD patients were taking medications in the 4 weeks prior to the healthcare professionals visit. Medication costs contributed significantly to the mean total annual costs for both asthma and COPD patients. The high medication cost for asthma and COPD patients was mostly attributed to high cost of maintenance medications (e.g., immunoglobulin antibody, fixed-dose combinations, and leukotriene medications). The costs associated with asthma and COPD medications would consume approximately 30% and 29% of an average Thai income, and could be considered unaffordable for low to average income earners. It should be noted that asthma and COPD are chronic conditions; hence, the need for medications is a long-term matter, and high medicine prices may be a cause for concern. Furthermore, maintenance medications are important in reducing downstream costs associated with acute exacerbations.[17]
Among the COPD patients, a noticeable proportion had visited the emergency department (7.6%) or been hospitalized (6.3%). A 3rd of the total annual cost for COPD patients could be attributed to these 2 factors. Generally, acute exacerbations have been found to be the most common cause of COPD-related hospitalizations.[18] Regular use of medications such as combinations of inhaled corticosteroids and long-acting beta agonists have been shown to reduce COPD-related exacerbations significantly, including those requiring hospitalizations.[19] This may point to COPD patients being undertreated in Thailand and could be related to high cost of COPD medications.
Lost productivity was significant for all the patients, in particular patients with a primary diagnosis of AR and rhinosinusitis. Presenteeism contributed a greater proportion to costs associated with lost productivity, compared with absenteeism. Presenteeism is defined as the lost productivity that arises from continuing to work when unwell.[20] Studies investigating specific risk factors for future health problems or reduced activity in the workforce have observed that presenteeism, especially when frequent, has negative health consequences at follow-up.[21,22] Strategies to limit presenteeism should start with promoting better health; hence, improving management of chronic respiratory conditions could reduce economic burden as well as improve patient quality of life.
A high proportion (31.6%) of patients presented with more than 1 respiratory disease during their visit to HCPs. Asthmatic patients most frequently had comorbid conditions, with the highest proportion presenting with AR (63%). High level of comorbidity of asthma and AR has been reported previously with prevalence of concomitant AR in asthmatics found to be in excess of 50%, with lifetime prevalence estimated to be up to 100% in the US and Europe.[23,24] Evidence from Thailand comes from a survey of school children which found that 55% to 76% of asthmatic children also had concomitant AR.[25] Presence of concomitant AR has been reported to result in higher rates of asthma-related medical resource utilization compared with asthma patients without AR[24,26,27] and may be a marker of more difficult to control asthma as well as worse asthma-related outcomes.[28,29] Therefore, early detection and effective management of comorbid AR could produce better health outcomes for asthmatic patients. In addition, previous research has shown that AR is a risk factor for subsequent development of asthma.[30] The majority of asthma patients in this study presented with cough as their main reason for the medical visit. Therefore, it may be prudent when AR patients present with cough to investigate for comorbid respiratory conditions, especially asthma.
The methodology used in this study introduces some limitations which should be considered when interpreting the results. Patients were recruited from primary health physicians and specialists in urban centers. As a result, they may not be representative of the rural population in Thailand. Hospitalizations and some medications were used infrequently but were associated with high costs. As such, there may be considerable variability around the estimates provided. Despite these limitations, this study is the first to present comprehensive description of the burden of the respiratory diseases with a broad societal perspective for the economic burden.
This study highlights the burden associated with 4 highly prevalent respiratory diseases in Thailand. The economic costs borne by patients with these conditions are significant and higher than previously reported. Work productivity loss and high medication prices were important contributors to the economic costs. Thorough investigation of concomitant conditions and improved disease management may help to reduce the burden of these respiratory diseases.
Acknowledgments
The authors thank Merck and Co for the support. The authors also thank the following colleagues for their nonauthor contributions: Assoc Prof Watchara Boonsawat, Assoc Prof Kowit Chaisiwamongkol, Assoc Prof Wisoot Reechaipichitkul, and Assoc Prof Seksun Chainansamitr from Srinakarind Hospital, Khon Kaen University; Assoc Prof Chanchai Sittipunt and Assoc Prof Surin Assawawitoontip from King Chulalongkorn Memorial Hospital, Chulalongkorn University; Assoc Prof Supranee Fooanant and Assoc Prof Peerasak Lerttrakarnnon from Chiang Mai University; and Assoc Prof Pongsakorn Tantilipikorn, Jariya Kunmong Konwutti, Nuttapol Rittayamai, Triphoom Suwanwech, and Anchalee Tantawiwat from Siriraj Hospital, Mahidol University.
Footnotes
Abbreviations: APBORD = Asia Pacific Burden of Respiratory Diseases, AR = allergic rhinitis, 95%CI = 95% confidence interval, COPD = chronic obstructive pulmonary disease, GP = general practitioner, HCRU = healthcare resource utilization, SD = standard deviation, US = United States, WPAI-SHP = Work Productivity and Activity Impairment – Specific Health Problem.
Authorship: Study conception and design, analysis and interpretation of data, drafting of manuscript, and critical revision: all authors; acquisition of data: ST, CP, BC, and SA.
Funding/support: This work was supported by Merck and Co. SB, SS, and RF were employees of Merck & Co. at the time of the study. SB (Optum) provided consulting services for conduct of this research and manuscript preparation.
The authors have no conflicts of interest to disclose.
References
- 1.Burney P, Jarvis D, Perez-Padilla R. The global burden of chronic respiratory disease in adults. Int J Tuberc Lung Dis 2015; 19:10–20. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global Surveillance, prevention and control of chronic respiratory diseases: A comprehensive report. 2007. Geneva, World Health Organization. [Google Scholar]
- 3.Trakultivakorn M. Economic burden of asthma in Thailand. Asian Pac J Allergy 2012; 30:1–2. [PubMed] [Google Scholar]
- 4.Ferkol T, Schraufnagel D. The global burden of respiratory disease. Ann Am Thorac Soc 2014; 11:404–406. [DOI] [PubMed] [Google Scholar]
- 5.Leartsakulpanitch J, Nganthavee W, Salole E. The economic burden of smoking-related disease in Thailand: a prevalence-based analysis. J Med Assoc Thai 2007; 90:1925–1929. [PubMed] [Google Scholar]
- 6.Chuesakoolvanich K. Cost of hospitalizing asthma patients in a regional hospital in Thailand. Respirology 2007; 12:433–438. [DOI] [PubMed] [Google Scholar]
- 7.Gypmantasiri S. Costs of illness of asthma in Chiang Mai and Lumphun. Chiang Mai Univ J Econ 2007; 11:1–9. [Google Scholar]
- 8.Cho S-H, Lin H-C, Ghoshal A, et al. Respiratory disease in the Asia-Pacific region: cough as a key symptom. Allergy Asthma Proc 2016; 37:131–140. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10). 2010. [Google Scholar]
- 10.Fokkens W, Lund V, Mullol J. European position paper on rhinosinusitis and nasal polyps 2007. Rhinol Suppl 2007; 1–136. [PubMed] [Google Scholar]
- 11.Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA (2) LEN and AllerGen). Allergy 2008; 63:8. [DOI] [PubMed] [Google Scholar]
- 12.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2010. [Google Scholar]
- 13.Global Initiatives for Asthma. Global Strategy for Asthma Management and Prevention 2011 (update). 2011. [Google Scholar]
- 14.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993; 4:353. [DOI] [PubMed] [Google Scholar]
- 15.Wang D, Ghoshal A, Muttalif A, et al. Quality of life and economic burden of respiratory disease in Asia-Pacific - Asia-Pacific Burden of Respiratory Diseases (APBORD) study. Value Health 2016; 9:72–77. [DOI] [PubMed] [Google Scholar]
- 16.Britt H, Miller G, Charles J, et al. General practice activity in Australia 2009-10. General Practice Series No.27 Cat no. GEP 27. AIHW 2010. [Google Scholar]
- 17.Bahadori K, Doyle-Waters MM, Marra C, et al. Economic burden of asthma: a systematic review. BMC Pulm Med 2009; 9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nowak D, Berger K, Lippert B, et al. Epidemiology and health economics of COPD across Europe: a critical analysis. Treat Respir Med 2005; 4:381–395. [DOI] [PubMed] [Google Scholar]
- 19.Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356:775–789. [DOI] [PubMed] [Google Scholar]
- 20.Sanderson K, Cocker F. Presenteeism. Implications and health risks. Aust Fam Physician 2013; 42:172–175. [PubMed] [Google Scholar]
- 21.Gustafsson K, Marklund S. Consequences of sickness presence and sickness absence on health and work ability: a Swedish prospective cohort study. Int J Occup Med Environ Health 2011; 24:153–165. [DOI] [PubMed] [Google Scholar]
- 22.Bergstrom G, Bodin L, Hagberg J, et al. Does sickness presenteeism have an impact on future general health? Int Arch Occup Environ Health 2009; 82:1179–1190. [DOI] [PubMed] [Google Scholar]
- 23.Pawankar R, Bunnag C, Chen Y, et al. Allergic rhinitis and its impact on asthma update (ARIA 2008) – western and Asian-Pacific perspective. Asian Pac J Allergy Immunol 2009; 27:237–243. [PubMed] [Google Scholar]
- 24.Gaugris S, Sazonov-Kocevar V, Thomas M. Burden of concomitant allergic rhinitis in adults with asthma. J Asthma 2006; 43:1–7. [DOI] [PubMed] [Google Scholar]
- 25.Trakultivakorn M, Sangsupawanich P, Vichyanond P. Time trends of the prevalence of asthma, rhinitis and eczema in Thai children-ISAAC (International Study of Asthma and Allergies in Childhood) Phase Three. J Asthma 2007; 44:609–611. [DOI] [PubMed] [Google Scholar]
- 26.Yawn B, Yunginger J, Wollan P, et al. Allergic rhinitis and its impact on asthma update (ARIA 2008) – western and Asian-Pacific perspective. J Allergy Clin Immunol 1999; 103:54–59. [DOI] [PubMed] [Google Scholar]
- 27.Price D, Zhang Q, Kocevar VS, et al. Effect of a concomitant diagnosis of allergic rhinitis on asthma–related health care use by adults. Clin Exp Allergy 2005; 35:282–287. [DOI] [PubMed] [Google Scholar]
- 28.Thomas M. Allergic rhinitis: evidence for impact on asthma. BMC Pulm Med 2006; 6:S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hadley J, Derebery J, Marple B. Comorbidities and allergic rhinitis: not just a runny nose. J Fam Pract 2012; 61:S11–S15. [PubMed] [Google Scholar]
- 30.Guerra S, Sherrill D, Martinez F, et al. Rhinitis as an independent risk factor for adult-onset asthma. J Allergy Clin Immunol 2002; 109:419–425. [DOI] [PubMed] [Google Scholar]


