Abstract
Introduction:
New-type drugs are popular with adolescents and could lead to psychiatry disorders, but no medications have been proven to be effective for these disorders of new-type drug dependence. We aimed to evaluate the efficacy of tryptophan on sleeping disorders and mental symptoms in detoxified individuals with new-type drug dependence.
Methods:
This randomized, placebo-controlled trial included 80 detoxified individuals with new-type drug dependence, recruited successively from a Compulsory Residential Drug Abstinence Institution in Wuhan, China, from April 2012 to November 2012. Eligible participants were randomly allocated to be treated with tryptophan (1000 mg/d, n = 40) or placebo (n = 40) for 2 weeks. The sleeping disorders and mental symptoms were assessed using Athens Insomnia Scale and Symptom Check-List-90 at baseline and 2 weeks. Results were analyzed according to the “intention-to-treat” approach.
Results:
Forty-five participants completed the 2-week study, 24 in the tryptophan group and 21 in the placebo group. There were no statistically significant differences in baseline characteristics between groups and the treatment adherence was similar between groups. The reduction in the Athens Insomnia Scale score in the tryptophan group was significantly greater than that in the placebo group (P = 0.017). However, no significant differences were found in Symptom Check-List-90 scores (either by individual dimension or the overall score) between groups (all P > 0.05). The frequency of adverse events was similar and no serious adverse events were reported during the study.
Conclusion:
Tryptophan was unlikely to be effective for mental symptoms, but could alleviate sleep disorders in short term among detoxified individuals with new-type drug dependence. Future large-scale trials are required to confirm findings from this study.
Keywords: mental symptom, new-type drug dependence, sleep disorders, tryptophan
1. Introduction
Distinguished from traditional drugs (such as opium and heroin), new-type drugs are a category of emerging synthetic drugs, such as methamphetamine, ecstasy, and ketamine.[1,2] In western countries, new-type drugs are often referred as “club drugs,” because of being popular with adolescents and young adults in recreational settings including rave parties and dance clubs.[3,4] Recently, the use of new-type drugs has been increasing,[5,6] and new-type drug dependence has become a major public health problem in many parts of the world.[7,8] In China, amphetamine-type stimulants are the most common new-type drug, and their illegal use is spreading.[9]
Long-term use of new-type drugs can lead to psychological disorders, including anxiety, depression, sleeping disorders, and social problems.[10,11] Previous studies indicated that new-type drugs are associated with psychological disorders through the reward system, such as serotonergic system, dopaminergic system and γ-aminobutyric acid system.[12,13] New-type drugs act on presynaptic neurons, and promote the release of more serotonin (5-HT) and dopamine. Repeated use of new-type drugs will lead to adaptation in 5-HT and dopamine function. Consequently, neurotransmitters such as dopamine and 5-HT will be depleted or much less than needed. Despite knowing this mechanism, however, no medications have been proven to be effective for the treatment of new-type drug dependence.[14,15]
Tryptophan is the precursor of 5-HT, and a high level of tryptophan could increase 5-HT synthesis,[16] which may modulate mood and sleeping disorders.[17–20] Findings from a literature review suggested that tryptophan could improve mood in vulnerable subjects, and improves sleep in adults with sleep disturbances, by increasing brain 5-HT.[21] Moreover, it may also be efficacious for syndromes associated with nicotine and heroin withdrawal.[22,23]
We speculated that the increased 5-HT level with the supplement of tryptophan would attenuate mood and sleeping disorders. This study aimed to test the hypothesis that tryptophan would attenuate mental symptoms and sleeping disorders among individuals with new-type drug dependence.
2. Methods
2.1. Subjects
Eighty participants were recruited successively from a compulsory detoxification center in Wuhan, China from April 2012 to November 2012. The center provides compulsory addiction treatment to patients with drug dependency, where they usually have to stay for 2 years after being held with a positive urinary sample. Investigators provided study information to patients, and invited them to participate in the study. The participation in this study was voluntary, and patients were allowed to stop participation at any time during the study period. Study participants were recruited according to the following inclusion criteria: new-type drug dependence verified by the Structured Clinical Interview for the DSM-IV-TR; being treated in the detoxification center for about a week; aged 18 or above; suffering from mental or sleeping disorders verified by Symptom Check-List-90 (SCL-90) and Athens Insomnia Scale (AIS); and willing and able to comply with the study, and with written informed consent. Patients were excluded according to the following exclusion criteria: substance dependence other than new-type drugs; a current neurological disease (e.g., organic brain disease) or schizophrenia assessed by Structured Clinical Interview for the DSM-IV-TR; the score of AIS < 4[24]; pregnant women; a history of dysfunction of liver, heart, or kidney; serious endocrine and circulatory disorders; currently taking other psychiatric medications; unable to comply with the study procedure; without giving informed consent; and other circumstances that would affect participant's safety.
The study was approved by the institutional review board at the School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. All study participants signed the written informed consent. The study was prospectively registered online and the Clinical trial registration number was ChiCTR-TRC-12002492.
2.2. Design
This was a randomized, double-blind, placebo-controlled trial. Eighty eligible participants were randomly assigned to a tryptophan (1000 mg/d, n = 40) group or a placebo (glucose 1000 mg/d, n = 40) group. The intervention (and its dose) was based on findings from previous studies.[25,26] A simple randomization schedule was used, and allocation was performed according to a list of computer-generated random numbers by an independent researcher. The medication was provided by Tianjin Tian’an Pharmaceuticals Co., Ltd., Tianjin, P. R. China. The tryptophan and placebo bottles were numbered 3 and 5, respectively, and dispensed by nurses in the detoxification center. Both study participants and nurses were blinded to the treatment provided. Participants took medication orally twice a day for 14 days in total, under a nurse's direct supervision.
The sample size calculation was based on the score of AIS. Previous research indicated a standard deviation of 2.2, and a mean change of AIS scores of 3.9 in the treatment group and 1.7 in the control group, respectively. With a 5% 2-sided alpha and 80% power, a minimum of 74 were required. Considering the possible follow-up loss, 80 subjects were enrolled.
2.3. Measures
The demographic characteristics and new-type drug use variables at baseline were measured using self-devised questionnaire. Sleeping status and mental symptoms of participants were assessed using AIS[27] and SCL-90,[28] respectively.
The AIS is a self-reported instrument consisting of 8 items. The first 4 items are sleep quantitative variables (i.e., sleep induction, night awakenings, early morning awakening, and total sleep time), the fifth item concerns sleep quality, and the remaining ones relate to the next-day performance of insomnia. Each item can be rated from 0 (not at all) to 3 (very serious), so that the total score ranges from 0 to 24. Patients with insomnia usually have an AIS score at least 4. The Cronbach alpha of the scale was 0.81 in Chinese.[29] Despite AIS was initially used to evaluate subjects’ sleep status within the last month, we used it to assess weekly sleep status, according to a previous study of patients with heroin dependence.[23]
The SCL-90 scale consists of 90 items and the following 9 dimensions: somatization, obsessive-compulsive disorder, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, and psychoticism. It is a self-administrated scale based on a 5-point rating method, ranging from 0 (not at all) to 4 (extremely). The Cronbach alpha of the scale was above 0.65, with good reliability and validity for substance abusers.[30]
The mental symptoms and sleeping status were assessed at baseline, 1 week and 2 weeks after randomization. The adherence was measured daily by asking participants if they wanted to continue, and they could drop out of the trial whenever they wanted. Adverse events were assessed daily by asking participants open-ended questions such as “Have you had any problems or side effects (such as nausea, headache or any other problem)”? Nurses recorded both adherence and adverse events. For serious adverse events, nurses could inform a physician for treatment if necessary. Physicians were not blinded with the treatment allocation in order to cope with possible emergencies.
2.4. Data analysis
Data were analyzed using SPSS for Windows (version 12.0). Continuously distributed variables were analyzed using mean (standard deviation or standard error), and categorical variables using frequencies and percentages. Baseline characteristics between groups were compared using Wilcoxon rank sum test for continuous data, and χ2 or Fisher exact test for categorical data. Results were analyzed according to intent-to-treat principle. Differences in changes of AIS and SCL-90 scores from baseline to week 2 between groups were assessed using t test. When outcome data at week 2 were not available due to loss to follow-up, data at week 1 or baseline were used in the analysis. The adherence and adverse events were analyzed using χ2 test. All statistical tests were 2-tailed, with a statistical significance level of <0.05.
3. Results
3.1. Baseline characteristics
A total of 323 individuals were assessed for eligibility and 243 were excluded, of which 230 did not meet the inclusion criteria and 13 refused to participate. We included 80 patients in the study, and randomly allocated them to the treatment (n = 40) and the control group (n = 40; Fig. 1).
Figure 1.
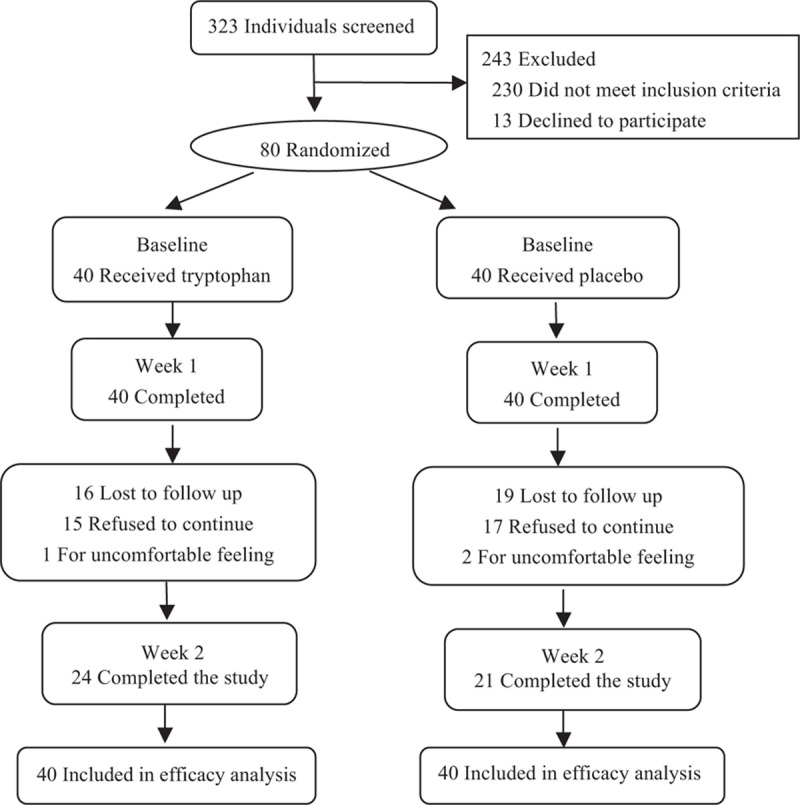
Study participant flow chart.
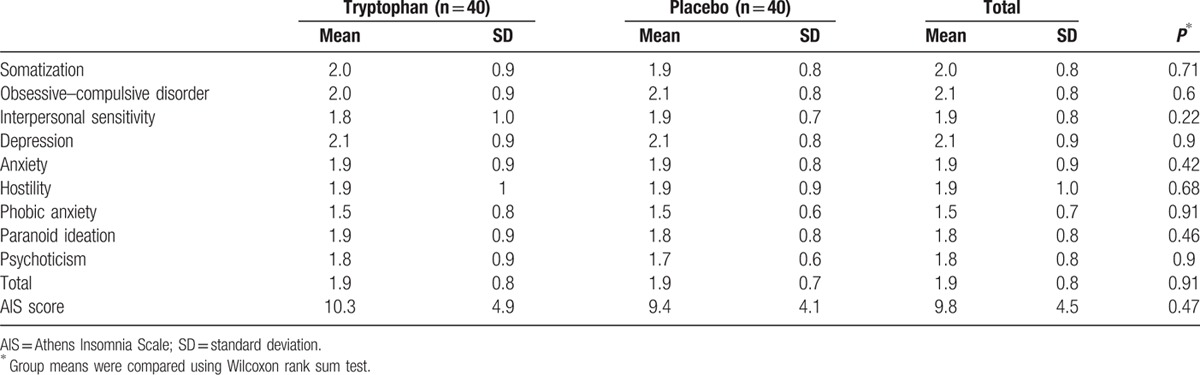
There were no significant differences in participant characteristics at baseline between the 2 groups (Table 1). In addition, there were no significant differences in AIS and SCL-90 scores at baseline between the groups (Table 2). The majority of participants was male (67.5%), unmarried (47.5%), with a mean age of 33.2 (±9.4 years), and low education level. Magu (69.0%) was the most frequently reported new-type drug used, followed by mixed new-type drug (16.3%) and ketamine (11.2%). New-type drugs were mainly smoked, and the mean lifetime years of drug use was 5.2 years (±4.5 years). Moreover, 90.0% of participants were tobacco smokers, and 45.0% alcohol drinkers.
Table 1.
The main characteristics of participants at baseline.
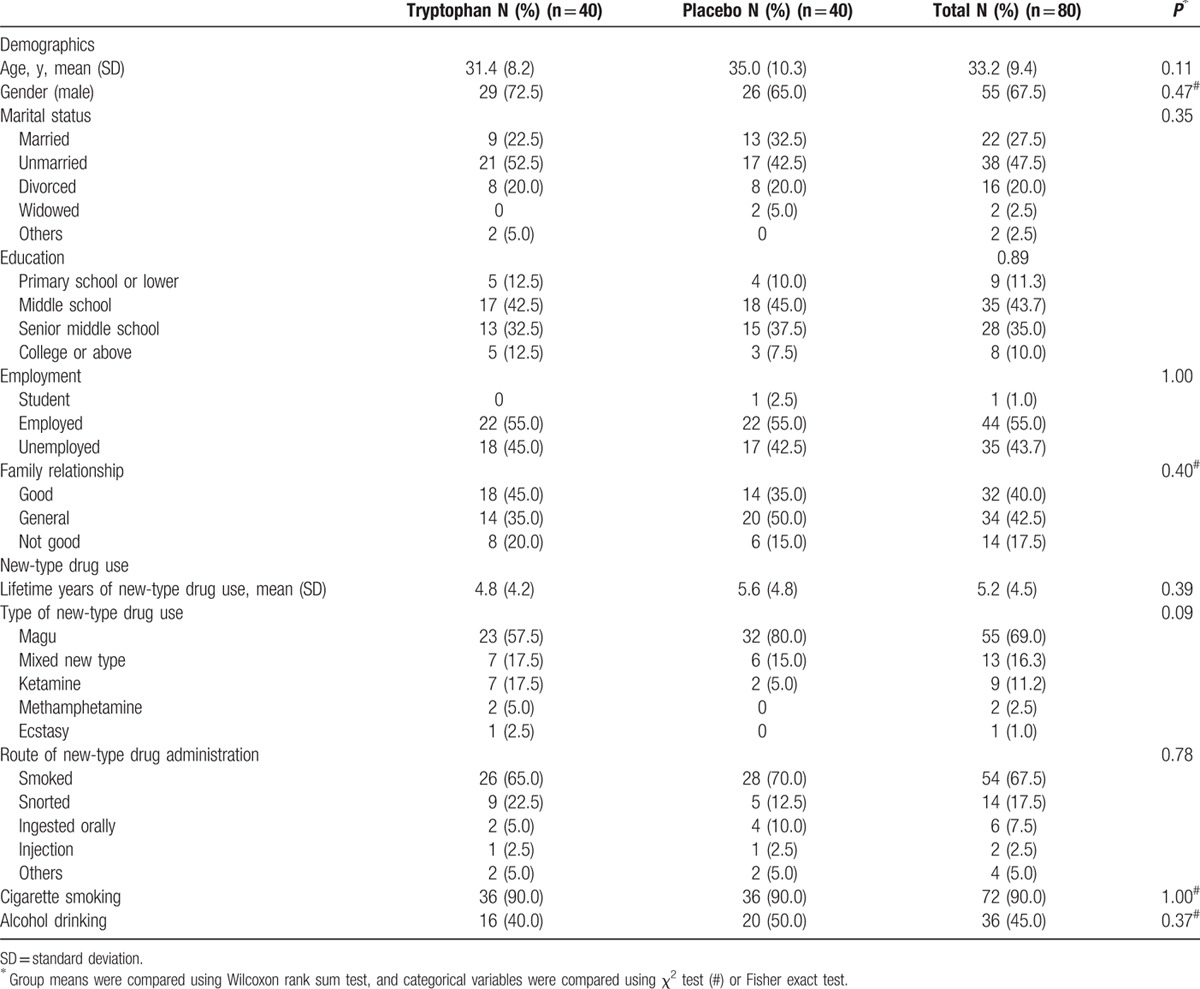
Table 2.
Characteristics of SCL-90 and AIS between groups preintervention.
3.2. Adherence
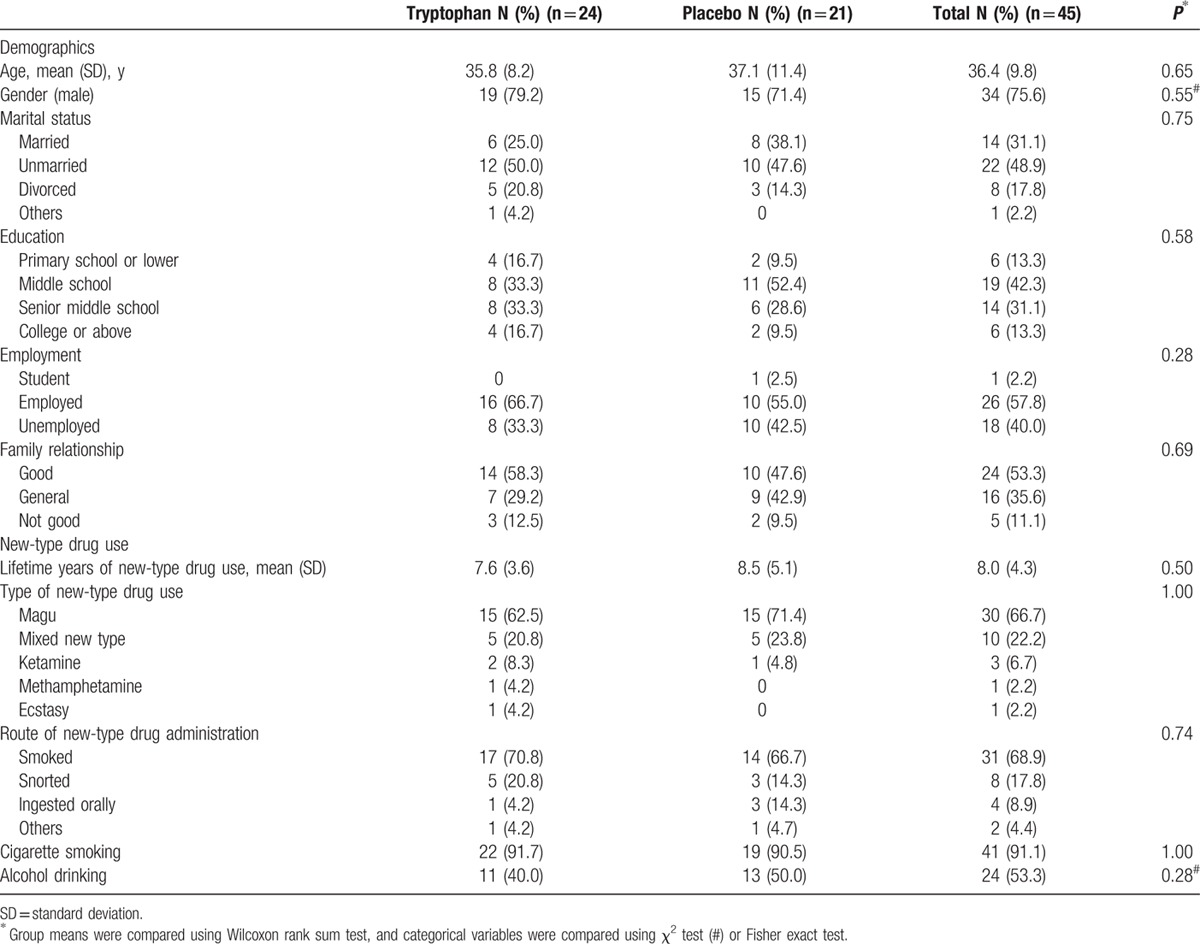
Forty-five participants (56.3%) in total completed the 2-week treatment, 24 in the tryptophan group and 21 in the placebo group (χ2 = 0.46, P = 0.50). There were no significant differences in baseline characteristics between the treatment and control group for the 45 participants who completed the treatment (Table 3). Reasons given for quitting the study were similar between the groups, as the following: side effects (tryptophan 15%, 6/40; placebo 17.5%, 7/40, χ2 = 0.09, P = 0.76), lack of efficacy (tryptophan 12.5%, 5/40; placebo 17.5%, 7/40, χ2 = 0.39, P = 0.53), and being sent to jail (tryptophan 12.5%, 5/40; placebo 12.5%, 5/40, χ2 = 0, P = 1).
Table 3.
Characteristics of the remaining 45 participants.
3.3. Treatment efficacy
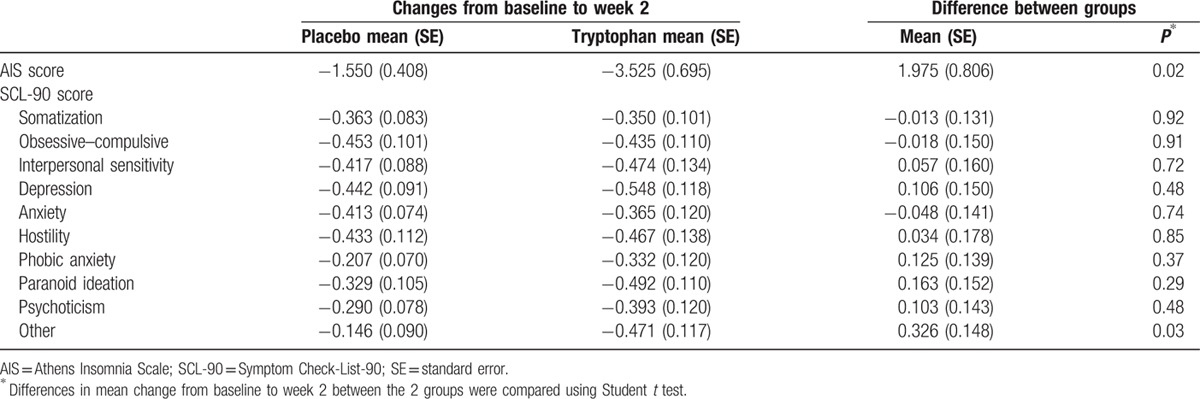
The AIS score was reduced on average by 3.53 in the tryptophan group and by 1.55 in the control group. The difference between the groups was statistically significant (mean difference 1.975; P = 0.017). However, there were no statistically significant differences in SCL-90 scores in term of individual items and the overall score (Table 4).
Table 4.
Changes in AIS and SCL-90 scores from baseline to week 2 by treatment groups.
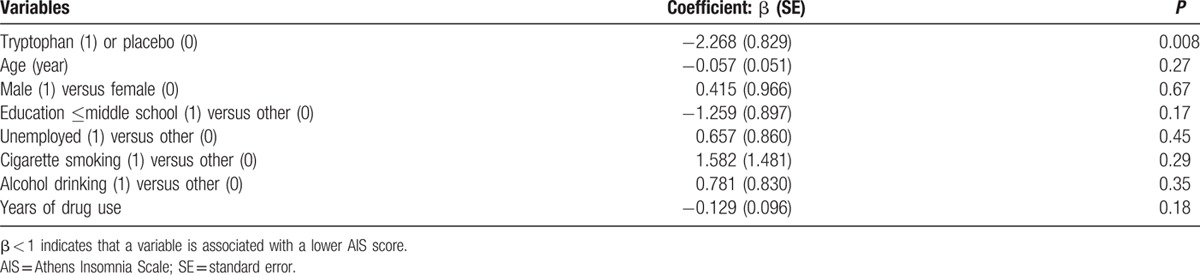
We conducted a linear regression analysis to evaluate the efficacy of tryptophan on sleep disorders (AIS scores) after adjusting for multiple baseline variables, including age, gender, married or not, education level, unemployment, smoking, drinking, and years of drug use. The results of the multiple variable regression analysis found that the treatment with tryptophan was the only variable that was statistically significantly associated with changes in AIS scores, and other baseline variables were not significantly associated with changes in AIS scores (Table 5).
Table 5.
Changes in AIS score from baseline to week 2: results of multiple variable linear regression analysis.
3.4. Safety
Adverse events were reported by 13 of the 80 participants (16.3%), 6 in the tryptophan group (15%) and 7 in the placebo group (17.5%). The difference in frequency of adverse events between the 2 groups was not statistically significant (χ2 = 0.09, P = 0.76). The common adverse events included: nausea (7 in the treatment and 6 in the placebo group, P = 0.76), headache (4 in the treatment and 3 in the placebo group, P = 0.69), and chest pain (6 in the treatment and 4 in the placebo group, P = 0.50). All these adverse events were mild and no serious events reported during the study.
4. Discussion
To our knowledge, the current study was the first to empirically evaluate the efficacy of tryptophan on sleeping disorders and mental symptoms in patients with new-type drug dependence in China. In our study, the AIS score was reduced by 5.5 in the tryptophan group, and it was only 1.6 in the placebo group, and the difference between the groups was statistically significant. Therefore, tryptophan may mitigate sleeping disorders of detoxified individuals with new-type drug dependence. This finding was similar to previous studies in heroin addicts[23] and adults with sleep disturbances.[21] It has great significance for patients with new-type drug dependence, as sleeping disorders are usual withdrawal symptoms for individuals with new-type drug dependence.[31,32]
Findings from this study indicated that treatment with tryptophan for 2 weeks had no effect on mental symptoms of detoxified individuals with new-type drug dependence, in terms of all dimensions of the SCL-90 scale, which was similar to previous studies of medications for methamphetamine dependence.[33,34] Furthermore, it revealed that mental symptoms may not change simultaneously with changes in sleep disorders. It is possible that mental symptoms and sleep disorders involve different neurobiological mechanisms or additional pathways.[35,36]
Tryptophan appeared to be safe and well tolerated. There were no serious adverse events reported. However, the adherence in the tryptophan and placebo group was similarly low, which was consistent with other clinical trials of methamphetamine users.[37,38] Adherence was usually an issue in follow-up studies, and our study was no exception, even though participants of the study were from a compulsory detoxification center. Low adherence may be associated with suspicious attitudes, which was a typical psychiatric symptom among individuals with new-type drug dependence.[39]
In terms of the participant characteristics at baseline, most new-type drug users were male and relatively young, which was similar to the 2010 drug users reported by the State Food and Drug Administration and a Chinese study.[9] Magu was the most frequently used new-type drug, as an emerging drug in China, and its ingredients include methamphetamine and caffeine. This was different from other studies of methamphetamine,[40,41] which may be due to different geographic areas or reflect a real change over time.
The current study had some limitations. Firstly, patients in the current study were treated for only 2 weeks. A previous study showed that amphetamine users experienced a long period of insomnia after ceasing amphetamine use for 3 days.[42] Another study revealed that the sleep time of amphetamine users was shorter than controls after using drugs.[43] Moreover, the treatment adherence in medication trials was usually low. Considering all the above reasons, we treated patients in the current study for 2 weeks. Secondly, other confounding factors, which could affect sleep in such subjects may still not be included in our study, though a multiple variable regression analysis found that the observed treatment effect of tryptophan on sleep disorders was not due to imbalanced distribution of baseline characteristics between the groups (Table 5). Thirdly, our study just focuses on the effect of tryptophan on the sleeping disorder and mental symptom in patients with new-type drug dependence, not the treatment of addiction to these substances, which needs to be confirmed in further studies. Finally, the current study was very small, with a total of 80 participants. Thus, a larger, multisite, and longer-term trial needs to be conducted to confirm the present results.
In summary, the current study indicated that tryptophan was unlikely effective in reducing mental symptoms, but might be effective for alleviating sleep disorders in individuals with new-type drug dependence in short term, although this needs to be confirmed in a future definitive trial. This might provide a new insight to the treatment of sleeping disorders of patients with new-type drug dependence. Further well-designed large-scale randomized controlled trials are required to confirm the results.
Acknowledgments
The authors thank the support of Yuanxuan College Foundation in HongKong, and also thank all the study participants and the staff and management in Wuhan City Compulsory Residential Drug Abstinence Institution for assistance in this study.
Footnotes
Abbreviations: 5-HT = serotonin, AIS = Athens Insomnia Scale, SCL-90 = Symptom Checklist-90.
DW participated in study design and the conduct of the study, collected the data, performed the statistical analysis and interpretation of data, and wrote the first draft of the manuscript. YX participated in study design and the conduct of the study, and collected the data. WH participated in the conduct of the study. WW, LY, and JY participated in the conduct of the study and collected the data. FS and WL contributed to data analysis and manuscript preparation. ZW participated in study design and the interpretation of data. All authors participated in the revision of the manuscript and have approved the final manuscript.
Funding/support: The study was supported by Joint School on Education, Sociology, Technology and Medicine Research Paper Award in China, Medical Planning Project No. YX12005 (URL: http://www.compe.cn).
The authors have no conflicts of interest to disclose.
References
- 1.Yang X, Xia G. Causes and consequences of increasing club drug use in China: a descriptive assessment. Subst Use Misuse 2010; 45:224–239. [DOI] [PubMed] [Google Scholar]
- 2.Drugs UNOo, Crime, Ctr, V.I., Austria. Patterns and Trends of Amphetamine-Type Stimulants and Other Drugs. Asia and the Pacific; 2010: 12–17. [Google Scholar]
- 3.Guerreiro DF, Carmo AL, da Silva JA, et al. Club drugs. Acta Med Port 2011; 24:739–756. [PubMed] [Google Scholar]
- 4.Gahlinger PM. Club drugs: MDMA, gamma-hydroxybutyrate (GHB), Rohypnol, and ketamine. Am Fam Physician 2004; 69:2619–2626. [PubMed] [Google Scholar]
- 5.Maxwell JC, Rutkowski BA. The prevalence of methamphetamine and amphetamine abuse in North America: a review of the indicators, 1992-2007. Drug Alcohol Rev 2008; 27:229–235. [DOI] [PubMed] [Google Scholar]
- 6.Lea T, Reynolds R, de Wit J. Alcohol and other drug use, club drug dependence and treatment seeking among lesbian, gay and bisexual young people in Sydney. Drug Alcohol Rev 2013; 32:303–311. [DOI] [PubMed] [Google Scholar]
- 7.McKetin R, Kozel N, Douglas J, et al. The rise of methamphetamine in Southeast and East Asia. Drug Alcohol Rev 2008; 27:220–228. [DOI] [PubMed] [Google Scholar]
- 8.Zuckerman MD, Boyer EW. HIV and club drugs in emerging adulthood. Curr Opin Pediatr 2012; 24:219–224. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Lu C, Zhang J, et al. Gender differences in abusers of amphetamine-type stimulants and ketamine in southwestern China. Addict Behav 2013; 38:1424–1430. [DOI] [PubMed] [Google Scholar]
- 10.Rao U. Links between depression and substance abuse in adolescents: neurobiological mechanisms. Am J Prev Med 2006; 31:S161–S174. [DOI] [PubMed] [Google Scholar]
- 11.Darke S, Kaye S, McKetin R, Duflou J. Major physical and psychological harms of methamphetamine use. Drug Alcohol Rev 2008; 27:253–262. [DOI] [PubMed] [Google Scholar]
- 12.Tomkins DM, Sellers EM. Addiction and the brain: the role of neurotransmitters in the cause and treatment of drug dependence. Can Med Assoc J 2001; 164:817–821. [PMC free article] [PubMed] [Google Scholar]
- 13.Sharp T, Cowen PJ. 5-HT and depression: is the glass half-full? Curr Opin Pharmacol 2011; 11:45–51. [DOI] [PubMed] [Google Scholar]
- 14.Anderson AL, Li SH, Biswas K, et al. Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend 2012; 120:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coffin PO, Santos GM, Das M, et al. Aripiprazole for the treatment of methamphetamine dependence: a randomized, double-blind, placebo-controlled trial. Addiction (Abingdon, England) 2013; 108:751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young SN, Gauthier S. Tryptophan availability and the control of 5-hydroxytryptamine and tryptamine synthesis in human CNS. Adv Exp Med Biol 1981; 133:221–230. [DOI] [PubMed] [Google Scholar]
- 17.Yuwiler A, Brammer GL, Morley JE, et al. Short-term and repetitive administration of oral tryptophan in normal men. Effects on blood tryptophan, serotonin, and kynurenine concentrations. Arch Gen Psychiatry 1981; 38:619–626. [DOI] [PubMed] [Google Scholar]
- 18.Attenburrow MJ, Williams C, Odontiadis J, et al. Acute administration of nutritionally sourced tryptophan increases fear recognition. Psychopharmacology 2003; 169:104–107. [DOI] [PubMed] [Google Scholar]
- 19.Minet-Ringuet J, Le Ruyet PM, Tome D, et al. A tryptophan-rich protein diet efficiently restores sleep after food deprivation in the rat. Behav Brain Res 2004; 152:335–340. [DOI] [PubMed] [Google Scholar]
- 20.van Dalfsen JH, Markus CR. Interaction between 5-HTTLPR genotype and cognitive stress vulnerability on sleep quality: effects of sub-chronic tryptophan administration. Int J Neuropsychopharmacol 2015; 18:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silber BY, Schmitt JA. Effects of tryptophan loading on human cognition, mood, and sleep. Neurosci Biobehav Rev 2010; 34:387–407. [DOI] [PubMed] [Google Scholar]
- 22.Ohmura Y, Jutkiewicz EM, Yoshioka M, et al. 5-Hydroxytryptophan attenuates somatic signs of nicotine withdrawal. J Pharmacol Sci 2011; 117:121–124. [DOI] [PubMed] [Google Scholar]
- 23.Chen D, Liu Y, He W, et al. Neurotransmitter-precursor-supplement intervention for detoxified heroin addicts. J Huazhong Univ Science Technolog Med Sci 2012; 32:422–427. [DOI] [PubMed] [Google Scholar]
- 24.Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res 2000; 48:555–560. [DOI] [PubMed] [Google Scholar]
- 25.Sterling Publishing Company, Inc, Braverman ER. The Edge Effect: Achieve Total Health and Longevity with the Balanced Brain Advantage. 2005; 154–167. [Google Scholar]
- 26.Meyers S. Use of neurotransmitter precursors for treatment of depression. Altern Med Rev 2000; 5:64–71. [PubMed] [Google Scholar]
- 27.Rounsaville BJ, Glazer W, Wilber CH, et al. Short-term interpersonal psychotherapy in methadone-maintained opiate addicts. Arch Gen Psychiatry 1983; 40:629–636. [DOI] [PubMed] [Google Scholar]
- 28.Tan H, Lan XM, Yu NL, et al. Reliability and validity assessment of the revised Symptom Checklist 90 for alopecia areata patients in China. J Dermatol 2015; 42:975–980. [DOI] [PubMed] [Google Scholar]
- 29.Chung KF, Kan KK, Yeung WF. Assessing insomnia in adolescents: comparison of Insomnia Severity Index, Athens Insomnia Scale and Sleep Quality Index. Sleep Med 2011; 12:463–470. [DOI] [PubMed] [Google Scholar]
- 30.Haver B. Screening for psychiatric comorbidity among female alcoholics: the use of a questionnaire (SCL-90) among women early in their treatment programme. Alcohol Alcohol 1997; 32:725–730. [DOI] [PubMed] [Google Scholar]
- 31.Vincent N, Schoobridge J, Ask A, et al. Physical and mental health problems in amphetamine users from metropolitan Adelaide, Australia. Drug Alcohol Rev 1998; 17:187–195. [DOI] [PubMed] [Google Scholar]
- 32.Zweben JE, Cohen JB, Christian D, et al. Psychiatric symptoms in methamphetamine users. Am J Addict 2004; 13:181–190. [DOI] [PubMed] [Google Scholar]
- 33.Heinzerling KG, Swanson AN, Kim S, et al. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend 2010; 109:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sulaiman AH, Gill JS, Said MA, et al. A randomized, placebo-controlled trial of aripiprazole for the treatment of methamphetamine dependence and associated psychosis. Int J Psychiatry Clin Pract 2013; 17:131–138. [DOI] [PubMed] [Google Scholar]
- 35.Benca RM, Peterson MJ. Insomnia and depression. Sleep Med 2008; 9:S3–S9. [DOI] [PubMed] [Google Scholar]
- 36.Volkow ND, Wang GJ, Fowler JS, et al. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A 2011; 108:15037–15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology 2008; 33:1162–1170. [DOI] [PubMed] [Google Scholar]
- 38.Longo M, Wickes W, Smout M, et al. Randomized controlled trial of dexamphetamine maintenance for the treatment of methamphetamine dependence. Addiction (Abingdon, England) 2010; 105:146–154. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Xu Z, Zhang S, et al. Profiles of psychiatric symptoms among amphetamine type stimulant and ketamine using inpatients in Wuhan, China. J Psychiatr Res 2014; 53:99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Wang Y, Zeng H. Analysis of four new drugs abuse in Beijing. Chin J Drug Depend 2008; 5:367–372. [Google Scholar]
- 41.Ding Y, He N, Detels R. Circumstances of initiation into new-type drug use among adults in Shanghai: are there differences by types of first new-type drug used? Drug Alcohol Depend 2013; 131:278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGregor C, Srisurapanont M, Jittiwutikarn J, et al. The nature, time course and severity of methamphetamine withdrawal. Addiction (Abingdon, England) 2005; 100:1320–1329. [DOI] [PubMed] [Google Scholar]
- 43.Gossop MR, Bradley BP, Brewis RK. Amphetamine withdrawal and sleep disturbance. Drug Alcohol Depend 1982; 10:177–183. [DOI] [PubMed] [Google Scholar]


