Supplemental Digital Content is available in the text
Keywords: atazanavir, biomarkers, cerebrospinal fluid, CSF escape, monotherapy
Abstract
Background:
Cerebrospinal fluid (CSF) viral escape is a concern in ritonavir-boosted protease inhibitors monotherapy. The aim was to assess HIV-RNA, biomarkers of immune activation and neurodegeneration, and atazanavir concentrations in CSF of patients on successful long-term atazanavir/ritonavir (ATV/r) monotherapy.
Methods:
This is a substudy of the multicentric, randomized, open-label, noninferiority trial monotherapy once a day with atazanavir/ritonavir (NCT01511809), comparing the ongoing ATV/r along with 2 nucleoside retrotranscriptase inhibitors (NRTIs) regimen to a simplified ATV/r monotherapy. Patients with plasma HIV-RNA < 50 copies/mL after at least 96 study weeks were eligible.
We assessed HIV-RNA, soluble (s)CD14, sCD163, CCL2, CXCL10, interleukin-6, and YKL40 by enzyme-linked immunosorbent assay; neopterin, tryptophan, kynurenine, and neurofilament by immunoassays; and ATV concentrations by liquid chromatography–mass spectrometry in paired plasma and CSF samples. Variables were compared with Wilcoxon rank-sum or Fisher exact test, as appropriate.
Results:
HIV-RNA was detected in the CSF of 1/11 patients on ATV/r monotherapy (114 copies/mL), without neurological symptoms, who was successfully reintensified with his previous 2NRTIs, and in none of the 12 patients on ATV/r + 2NRTIs. CSF biomarkers and ATV concentrations did not differ between the 2 arms.
Conclusions:
CSF escape was uncommon in patients on long-term ATV/r monotherapy and was controlled with reintensification.
1. Introduction
Simplification of antiretroviral treatment with ritonavir-boosted protease inhibitors (PI/r) monotherapy is a promising strategy, in selected patients, to reduce nucleoside retrotranscriptase inhibitors (NRTI) side effects, pill burden, and costs and has recently been considered in the Italian and European guidelines for treatment of HIV infection.[1,2] Although virological efficacy of monotherapy was slightly inferior to standard regimen,[3–5] especially in patients with low adherence,[3] low CD4+ cells nadir,[6,7] and hepatitis C virus coinfection,[3,8] development of resistance associated mutations was observed infrequently[9] and prompt reintensification with NRTI backbone at confirmed viral failure was sufficient to resuppress viral replication, thus not compromising future therapeutic options.[7–10]
A reduced efficacy of PI/r monotherapy with atazanavir/ritonavir (ATV/r), lopinavir/ritonavir (LPV/r), and darunavir/ritonavir (DRV/r) in viral sanctuaries like the central nervous system (CNS) is a concern. In clinical trials and isolated case reports, in fact, virological failure to monotherapy was associated with onset of neurological symptoms, higher level of HIV replication in the in the cerebrospinal fluid (CSF) compared with plasma and CSF leukocytosis.[4,6,11] Moreover, CSF viral breakthrough in patients with suppressed systemic viral replication while on monotherapy was observed with or without neurological symptoms.[6,7,11–13] In contrast, PI/r monotherapy was not associated with increased neurocognitive deterioration[7,9,14–16] and CSF levels of immune activation and neurodegeneration biomarkers compared with standard regimen.[17]
Efficacy of ATV/r monotherapy in the CNS was previously assessed with a pilot, single arm study, ATARITMO, where CSF replication despite suppressed plasma HIV-RNA (<50 copies/mL) was observed in 17% of patients.[11] We analyzed virological efficacy and biomarkers of immune activation and neurodegeneration in the CSF of patients enrolled in the randomized, open label study monotherapy once a day with atazanavir/ritonavir (MODAt).[8,18]
2. Methods
2.1. Study design
The MODAt study is a randomized, open-label, multicenter, noninferiority trial, comparing ATV/r (300/100 mg) monotherapy with ATV/r along with 2 NRTIs (NCT01511809). Full study design and patient population of the MODAt trial have been previously described.[8]
After the 96th MODAt study week, participants who did not experience treatment failure, nor drug-related toxicities were allowed to continue study medications and close follow up.
Patients enrolled in 1 of the 11 Italian centers participating in the main study (center 01), with persistently undetectable plasma HIV-RNA (<50 copies/mL) after at least 96 weeks of follow-up, including those who underwent per protocol reintensification for confirmed viral failure, were eligible to this substudy. Those with contraindications to magnetic resonance imaging (MRI) and lumbar puncture were excluded.
Each enrolled patient underwent a single evaluation consisting of an MRI, neuropsychological testing, and lumbar puncture. A second evaluation was warranted whenever CSF escape was detected.
The primary objective was to assess the virological efficacy of ATV/r monotherapy compared with triple therapy in the CSF compartment. Primary endpoint was the proportion of patients, in each treatment group, with CSF escape, defined as CSF HIV-RNA > 37 copies/mL and concomitant plasma HIV-RNA < 37 copies/mL. Secondarily, we aimed to compare the level of immune activation and neurodegeneration biomarkers and ATV concentrations in CSF and plasma, and the neurocognitive performance in patients of each treatment arm.
The substudy was approved by local ethical committee. Each participant signed written informed consent to participation.
2.2. Biological sample collection and biomarkers assessment
Blood and CSF samples were collected simultaneously (±2 h apart). Lumbar puncture was performed with atraumatic 25 gauge, Sprotte needles. Uncentrifuged CSF and plasma supernatants, obtained after centrifugation at 1500g for 10 min, were aliquoted and kept stored at −80°C until analysis.
HIV-1 viral load was determined by Abbott real-time polymerase chain reaction in plasma and in the CSF (detection limit < 37 copies/mL).
The plasma and CSF concentrations of soluble (s)CD14, CCL2, interleukin (IL)-6, CXCL10, and soluble (s)CD163 were determined by commercial enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN), according to the manufacturer's instructions, at San Raffaele Scientific Institute laboratory in Milan, Italy. Neurofilament light chain (NFL) and YKL-40 were analyzed at Sahlgrenska University Hospital, University of Gothenburg, Sweden. NFL concentrations were determined using a highly sensitive, 2-site enzymatic quantitative immunoassay (lower limit of detection of 50 ng/L) according to the manufacturer's instructions (Uman Diagnostics, Umeå, Sweden). YKL-40 levels were measured with a commercially available enzyme-linked immunosorbent assay according to the manufacturer's instructions (R&D Systems). Tryptophan, kynurenine, and neopterin concentrations were determined using commercially available immunoassays at the Division of Biological Chemistry, Biocenter Medical University Center for Chemistry and Biomedicine, Innsbruck, Austria. Atazanavir concentrations in plasma and CSF were determined at the Unit of Clinical Pharmacology, Luigi Sacco University Hospital, Milan, using liquid chromatography–mass spectrometry methods validated in agreement with the European Medicines Agency Guidelines for Bioanalytical Method validation (lower limit of detection 20 and 1 ng/mL for plasma and CSF, respectively).
2.3. Neuropsychological and radiological evaluation
Formal neuropsychological examination was administered by trained neuropsychologists in patients with at least 96 weeks of follow-up. Attention and concentration were investigated by the revised Wechsler Adult Intelligence Scale Digit Symbol test; learning/memory with Rey Auditory Verbal Learning Test (RAVLT) and Rey Recall (RAVLT rec); psychomotor speed with trail making test—part A (TMTA) and Grooved Pegboard; executive functioning with TMT—part B (TMTB); and language with semantic fluency (SF) and phonemic fluency (PF).
Age, sex, and education adjusted scores were used to obtain normalized zeta scores for each test. A zeta score ≥1 standard deviation below the media of the reference population in at least 1 test of at least 2 different neurocognitive domains was considered pathological.[19] Depressive symptoms were screened with the Center of Epidemiologic Studies Depression (CES-D) scale.
Within 10 days prior to lumbar puncture, all patients underwent a morphologic MRI of the brain without administration of paramagnetic contrast medium, which was reviewed by a neuroradiologist in order to exclude contraindications to the lumbar puncture and detect signs of neurological disease.
2.4. Statistical analysis
Results were expressed as absolute number and percentage or median and interquartile range (IQR). Patients receiving triple therapy after per protocol reintensification were considered in the triple therapy arm for analysis.
Comparisons between the 2 groups of treatment were calculated with Wilcoxon rank-sum and Fisher exact test, as appropriate. Linear correlations between variables were assessed with Spearman correlation coefficients. All the analyses were performed with the SAS Software, release 9.2 (SAS Institute, Cary, NC). Graphs were obtained with Graph pad Prism 6.0 software.
3. Results
3.1. Patients’ inclusion and baseline characteristics
Fourteen of 28 eligible patients randomized to ATV/r monotherapy and 9 of 23 to ATV/r + 2NRTIs were enrolled in the CSF substudy. Three patients of the ATV/r monotherapy arm, who had received per protocol reintensification with their previous 2NRTIs at study week 8, 24, and 48, respectively, were considered in the triple therapy arm for analysis.
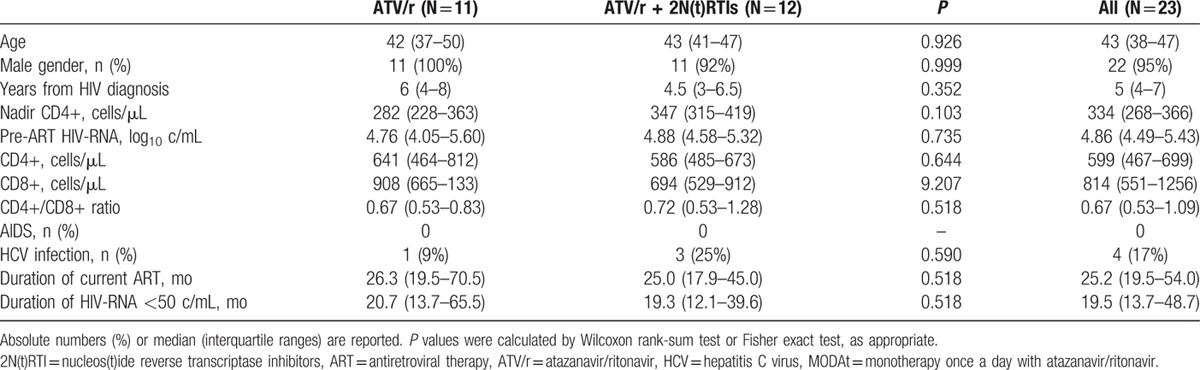
Patients’ demographic and clinical characteristics at the time of randomization for the parent MODAt study were similar between the 2 groups of the CSF substudy (Table 1).
Table 1.
Clinical and demographic characteristics of patients at time of randomization for the MODAt study.
3.2. Patients’ characteristics at lumbar puncture
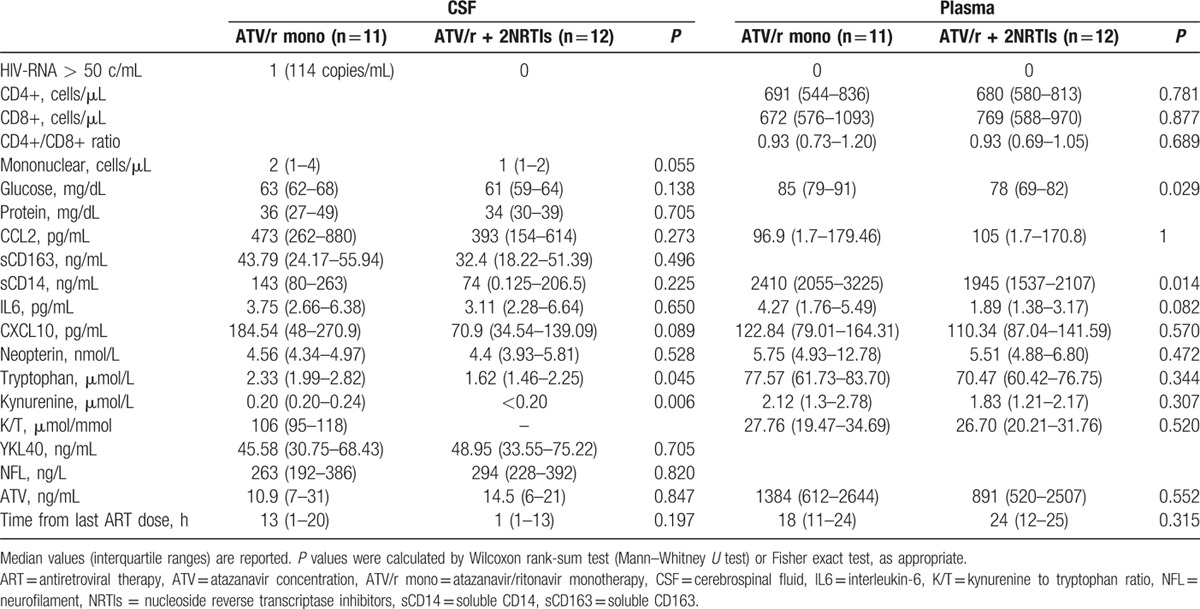
Lumbar puncture was performed at a median (IQR) of 120 (108–132) weeks after randomization. At this time point, all patients were asymptomatic for neurological conditions, had undetectable plasma HIV-RNA (<50 copies/mL), as for inclusion criteria, and there were no differences in the CD4+ and CD8+ cell counts between the 2 groups (Table 2).
Table 2.
Cerebrospinal fluid and plasma biomarkers of viral replication, inflammation and neurodegeneration in patients receiving either atazanavir/ritonavir monotherapy or atazanavir/ritonavir containing triple therapy.
At MRI evaluation, 4 patients in each group had scattered, nonspecific, focal T2-weighted hyperintense, T1-weighted isointense–hypointense microscopic subcortical lesions in the frontal white matter. These findings were considered consistent with microvascular lesions, within the normal range for patients’ age and of no clinical significance.
3.3. Virological efficacy of ART in CSF
CSF escape was found in none of the patients receiving triple therapy with ATV/r + 2NRTIs and in 1 of 11 (9%) on ATV/r monotherapy, with CSF HIV-RNA of 114 copies/mL. The patient with CSF escape was a 39-year-old man, who had just recovered from an upper respiratory tract infection at the time of lumbar puncture. He was otherwise healthy, asymptomatic for neurological diseases and had normal MRI and CSF standard parameters (glucose 63 mg/dL, proteins 37 mg/dL, and mononuclear cells 2/μL). He had been treated with ATV/r monotherapy for 120 weeks, during which his plasma HIV-RNA had been well controlled (<50 copies/mL) except for 2 nonconsecutive viral blips of 94 and 99 copies/mL. His current CD4+ cells were 743/μL (41%) and had been 311/μL at nadir, his CD8+ cells were 576/μL (31%). He had missed 1 dose of his ATV/r therapy the day before the lumbar puncture was performed. Reintensification with his previous NRTI regimen (tenofovir and emtricitabine) led to suppression of CSF HIV replication at subsequent evaluation, 20 weeks afterward. Genotypic analysis of the CSF isolate was not successful (Fig. 1).
Figure 1.
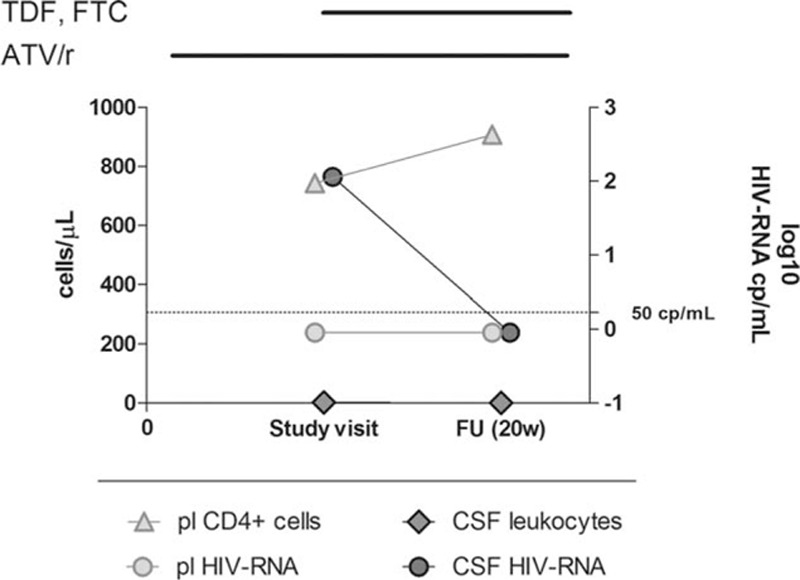
Plasma CD4+ cells, CSF leukocyte number, and plasma and CSF HIV-RNA level in the patient with CSF escape. Bars above the diagram indicate duration of antiretroviral treatments. ATV/r = ritonavir-boosted atazanavir/r, CSF = cerebrospinal fluid, FTC = emtricitabine, FU = follow-up, pl = plasma, TDF = tenofovir.
3.4. Soluble biomarkers in CSF and plasma
CSF glucose and protein levels were within the normal range in all CSF samples and did not differ between treatment arms. There was a trend of higher CSF mononuclear cell counts in patients on monotherapy compared with those on standard treatment, but, still, they all were below the upper normal limit of 5 cells/μL (Table 2). CSF levels of neopterin, IL6, sCD14, CCL2, sCD163, CXCL10, NFL, and YKL-40 were not different between the 2 groups (Table 2). Levels of tryptophan and kynurenine in the CSF were significantly higher in the monotherapy group compared with the triple therapy (P = 0.045 and P = 0.006, respectively). We were not able to calculate the kynurenine to tryptophan ratio, an index of indoleamine 2,3-dioxygenase (IDO) activity, in most of the patients receiving triple therapy, because CSF kynurenine was undetectable.
In plasma, sCD14 was significantly higher in the monotherapy arm than in the triple therapy arm (P = 0.014). There were no differences in the level of all the other biomarkers between the 2 arms (Table 2). In the patient with asymptomatic CSF escape, CSF neopterin and CXCL10 levels were higher than the 75th percentile of all patients (Fig. 2).
Figure 2.
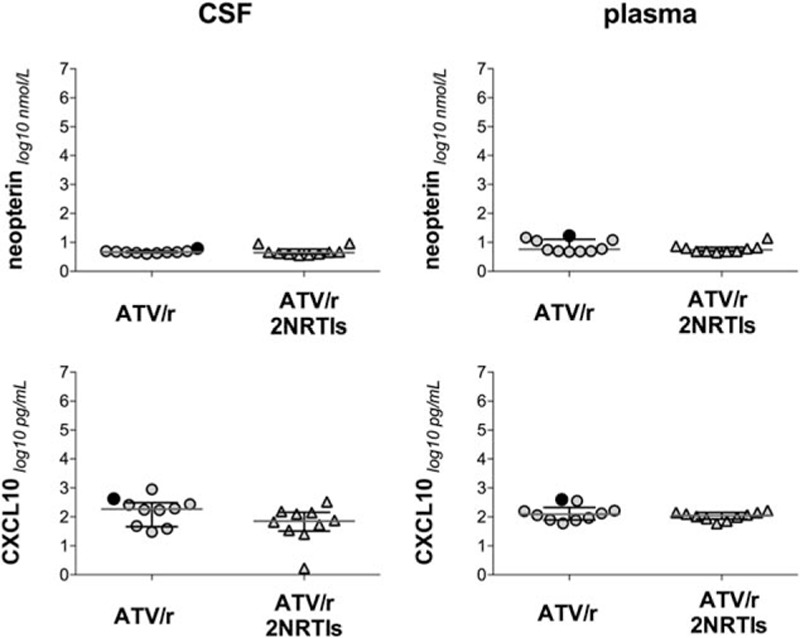
CSF and plasma biomarkers of immune activation and neurodegeneration in patients receiving either atazanavir/ritonavir monotherapy or atazanavir/ritonavir containing triple therapy. Full dots represent the patient with CSF escape. P values were calculated by Wilcoxon rank-sum test. ATV/r = atazanavir/ritonavir, CSF = cerebrospinal fluid, NRTIs = nucleoside reverse transcriptase inhibitors.
Considering all patients, levels were significantly higher in plasma than in CSF for IL6 (P = 0.0394), sCD14 (P < 0.0001), neopterin (P = 0.0002), tryptophan (P < 0.0001), and kynurenine (P < 0.0001), while CCL2 was higher in CSF (P < 0.0001) and CXCL10 was not different between the 2 compartments.
Several statistically significant correlations were observed between CSF but not plasma levels of different biomarkers of immune activation. In particular, in the CSF, there was a significant correlation between sCD163 and sCD14 (r = 0.932, P < 0.0001), sCD163 and CXCL10 (r = 0.531, P = 0.016), sCD163 and CCL2 (r = 0.468, P = 0.037), sCD163 and IL6 (r = 0.517, P = 0.019), sCD14 and CXCL10 (r = 0.558, P = 0.01), and sCD14 and IL6 (r = 0.639, P = 0.0024) (Supplementary material). CSF and plasma levels of IL6 (r = 0.657, P < 0.0022) and sCD14 (r = 0.542, P = 0.016) were also positively correlated.
3.5. Pharmacokinetics
Measured ATV concentrations in plasma and CSF (Table 2) and estimated ATV concentrations in plasma at 24 h after the last dose (P = 0.47) were similar between the 2 groups. CSF ATV concentrations were in median (IQR) 0.75% (0.68–1.39) of plasma concentrations, without differences between the 2 arms. CSF ATV concentrations were below the previously reported inhibitory concentration (IC)-95% value for wild type virus (6.5 ng/mL)[20] in 7/22 (31%) patients (5 on triple therapy and 2 on ATV/r monotherapy, including the patient with CSF escape, who had ATV concentrations of 2.9 ng/mL in CSF and 383 ng/mL in plasma). There was a correlation between plasma and CSF levels of ATV (r = 0.876, P < 0.0001). Plasma, but not CSF concentrations of ATV were inversely correlated to the number of hours elapsing between last dose of antiretroviral therapy (ART) and sample collection (plasma, r = −0.639, P = 0.003; CSF, r = 0.175, P = 0.46).
3.6. Neuropsychological evaluation
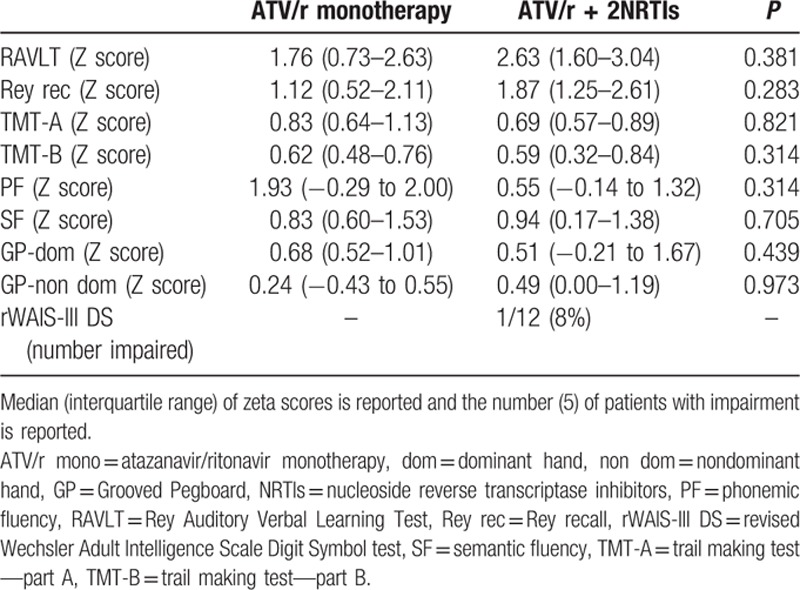
Formal neuropsychological evaluation was normal in all participants and zeta scores did not significantly differ between the 2 groups (Table 3). Screening for depression detected severe symptoms in 4 patients (CES-D score ≥ 23) and mild symptoms in 2 additional patients (score ≥16 and <23), without frequency or score differences between the 2 groups.
Table 3.
Neuropsychological evaluation in patients receiving either atazanavir/ritonavir monotherapy or atazanavir/ritonavir containing triple therapy.
4. Discussion
In this pilot substudy of the randomized controlled trial MODAt, CSF HIV-RNA was suppressed in all but 1 patient receiving effective ATV/r monotherapy (9%) and in all patients receiving effective ATV/r-based triple therapy for at least 96 weeks. CSF escape was successfully controlled with reintensification with 2NRTIs. CSF immune activation and neurodegeneration biomarkers and ATV concentrations were not different in patients receiving ATV/r monotherapy compared with those receiving ATV/r along with 2NRTIs.
There was no evidence of neurocognitive impairment in either of the treatment arms.
Despite differences in study design, limiting comparison among studies, the proportion of CSF escape in the monotherapy arm of the MODAt study was lower than that previously reported during PI/r monotherapy with ATV/r, of 17%[11] and similar to the frequency described in more recent studies with agents with much higher predicted neuropenetration like DRV/r or LPV/r, of around 10%.[6,7,14,17,21–23] This low frequency was observed despite ATV/r concentrations in the CSF were about 1% of those in plasma, similarly to what previously reported,[11,24,25] and were below the estimated IC-90 for the wild type virus in 31% of cases.
CSF escape in patients receiving LPV/r,[6,14] ATV/r,[7,11] or DRV/r monotherapy has previously been associated with low CD4+ cells nadir,[6,7] poor adherence,[4,11] and discordant patterns of resistance associated mutations in plasma and CSF.[26–31] Moreover, in the ATARITMO study, 1 patient presenting with high-level compartmentalized CSF replication after 24 weeks of ATV/r monotherapy was retrospectively found to had low level asymptomatic CSF replication, despite suppression in plasma, at baseline CSF evaluation.[11] These observations suggest that, during profound immunosuppression, HIV might evolve independently within the CNS and, after having been suppressed by effective antiretroviral treatment, it may reemerge when treatment pressure is weakened.[32,33] Participants to our substudy were free from some of the above-mentioned risk factors, such as low CD4+ cell nadir and archived antiretroviral drugs resistances in plasma, as by the inclusion criteria to the MODAt study. In addition, they were selected among those in whom ATV/r monotherapy had been effective for at least 2 years, which might have contributed to the very low frequency of CSF escape, since CSF escape often occurred in the context of plasma failure in patients receiving ritonavir-boosted PI monotherapy.[3,6,8,11]
The rapid suppression of CSF replication in the patient with CSF escape after reintensification with 2NRTI backbone suggests that the escape was probably not sustained by a viral population with compartmentalized resistance associated mutations. Indeed, the patient with CSF escape had at least 2 peculiarities that could explain the loss of virological control in the CSF compartment. First, reduced adherence to ART was suspected on the basis of the 2 previous viral blips, the missed dose just before the lumbar puncture and the low concentrations of ATV both in CSF and in plasma. Second, the CSF escape was preceded by a mild upper respiratory tract infection, similarly to what described in a patient with systemic virological failure to DRV/r monotherapy,[3] suggesting that a systemic inflammatory stimulus could temporarily impair the control of HIV replication.
We did not observe increased CSF levels of biomarkers of immune activation (sCD14, sCD163, CXCL10, IL6, neopterin, and IDO activity), neurodegeneration (NFL), and extracellular matrix remodeling/microglial activation (YKL-40) in patients receiving monotherapy compared with those on triple therapy. This was consistent with a recent observational study that analyzed several immune activation and CNS cell damage biomarkers, including adenosine deaminase, beta2 microglobulin, S-100, neuron-specific enolase, and myelin basic protein, but somehow in disagreement with the findings in the PROTEA study, where CSF neopterin level increased after switch to DRV/r monotherapy.[9,17] While in our study we only included patients on stable and effective therapy for at least 96 weeks and in the study published by Estebanez et al.[17] the median time on monotherapy was of about 2 years, in the PROTEA study CSF examination was repeated after 48 weeks. Therefore, further studies are needed to assess whether biomarkers of immune activation normalize in patients receiving long-term monotherapy, after an initial increase.
In the patient with asymptomatic CSF escape, the levels of CXCL10, which have been shown to directly correlated to CSF viral load and cell count,[34] and of neopterin, a sensitive inflammatory marker,[35] were above the 75% percentile of both the monotherapy and whole study groups while all the other biomarkers were included between the 25th and 75th percentiles. This finding is consistent with previous studies that observed increased neopterin levels in asymptomatic patients with detectable CSF HIV RNA, despite long-term successful ART in plasma,[35] but also suggests that there was no evidence of an overt inflammatory or neurodegenerative process in the patient with asymptomatic CSF escape.
Monotherapy was not associated with pathological neuropsychological evaluation, consistently with data from larger cohorts.[9,14,15] A beneficial effect of reduction of potentially neurotoxic antiretroviral drugs in patients receiving monotherapy has been proposed, but we did not observe any difference between the 2 treatment strategies.
In conclusion, CSF escape was uncommon in neuroasymptomatic patients on long-term ATV/r monotherapy with persistently suppressed systemic viral replication. Although ATV concentrations were low in the CSF, as expected, its efficacy was comparable to that of other, more neuropenetrating, antiretroviral agents. Reduced adherence to ART was probably the most important risk factor for the development of 1 case of CSF escape, which could also have been triggered by the concomitant systemic infection. CSF escape was not associated with overt neurological disease and was successfully treated with reintensification, like observed after systemic failure to monotherapy regimen. Finally, ATV/r monotherapy was not associated with increased CSF levels of immune activation and neurodegeneration biomarkers.
Supplementary Material
Acknowledgment
We thank the patients, investigators, study coordinators, and nurses for their contribution.
Footnotes
Abbreviations: ART = antiretroviral therapy, ATV/r = atazanavir/ritonavir, CES-D = Center of Epidemiologic Studies Depression, CNS = central nervous system, CSF = cerebrospinal fluid, DRV/r = darunavir/ritonavir, GP = Grooved Pegboard test, IDO = indoleamine 2,3-dioxygenase, IL = interleukin, LPV/r = lopinavir/ritonavir, MODAt = monotherapy once a day with atazanavir/ritonavir, MRI = magnetic resonance imaging, NFL = neurofilament light chain, NRTI(s) = nucleoside retrotranscriptase inhibitor(s), PF = phonemic fluency, PI/r = ritonavir-boosted protease inhibitors, RAVLT = Rey Auditory Verbal Learning Test, RAVLT rec = Rey Auditory Verbal Learning Test Recall, r-WAIS III DS = revised Wechsler Adult Intelligence Scale Digit Symbol test, s = soluble, SF = semantic fluency, TMT-A = trail making test—part A, TMT-B = TMT—part B.
AB, LP, LG, VL, MG, HZ, DF, DC, GC, AL, and AC have none to declare. FF is receiving a fellowship from “Società Italiana Malattie Infettive e Tropicali (SIMIT).” SG has received funding for travel or speaker honoraria from Biogen Idec and Serono Foundation. SG reports personal fees from Bristol Myer Squibb, Gilead Sciences, and MSD. PC reports personal fees from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck, and Viiv. AL has received consultancy payments and speaking fee from Bristol-Myers Squibb, Gilead Sciences, ViiV Health Care, Merck Sharp & Dohme, ABBvie, and Janssen-Cilag. AC has received consultancy payments and speaking fee from Bristol-Myers Squibb, Gilead, ViiV Health Care, Merck Sharp & Dohme, ABBvie, and Janssen-Cilag.
Preliminary results of this work have been presented at the Annual Conference on Retroviruses and Opportunistic Infections CROI, February 23–26, 2015, Seattle, Washington.
Bristol-Myers Squibb provided financial support and ATV/r for the MODAt trial.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.EACS. European guidelines for treatment of HIV-infected adults in Europe 8.0; 2015. http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html Accessed November 25, 2015. [Google Scholar]
- 2.Italian expert panel. Linee Guida Italiane 2014 sull’utilizzo dei farmaci antiretrovirali e sulla gestione diagnostico-clinica delle persone con infezione da HIV-1; 2014. http://www.salute.gov.it/imgs/C_17_pubblicazioni_2261_allegato.pdf Accessed November 25, 2015. [Google Scholar]
- 3.Arribas JR, Horban A, Gerstoft J, et al. The MONET trial: darunavir/ritonavir with or without nucleoside analogues, for patients with HIV RNA below 50 copies/ml. AIDS 2010; 24:223–230. [DOI] [PubMed] [Google Scholar]
- 4.Katlama C, Valantin MA, Algarte-Genin M, et al. Efficacy of darunavir/ritonavir maintenance monotherapy in patients with HIV-1 viral suppression: a randomized open-label, noninferiority trial, MONOI-ANRS 136. AIDS 2010; 24:2365–2374. [DOI] [PubMed] [Google Scholar]
- 5.Arribas JR, Pulido F, Delgado R, et al. Lopinavir/ritonavir as single-drug therapy for maintenance of HIV-1 viral suppression: 48-week results of a randomized, controlled, open-label, proof-of-concept pilot clinical trial (OK study). J Acquir Immune Defic Syndr 2005; 40:280–287. [DOI] [PubMed] [Google Scholar]
- 6.Gutmann C, Cusini A, Gunthard HF, et al. Randomized controlled study demonstrating failure of LPV/r monotherapy in HIV: the role of compartment and CD4-nadir. AIDS 2010; 24:2347–2354. [DOI] [PubMed] [Google Scholar]
- 7.Antinori A, Clarke A, Svedhem-Johansson V, et al. Week 48 efficacy and central nervous system analysis of darunavir/ritonavir monotherapy versus darunavir/ritonavir with two nucleoside analogues. AIDS 2015; 29:1811–1820. [DOI] [PubMed] [Google Scholar]
- 8.Castagna A, Spagnuolo V, Galli L, et al. Simplification to atazanavir/ritonavir monotherapy for HIV-1 treated individuals on virological suppression: 48-week efficacy and safety results. AIDS 2014; 28:2269–2279. [DOI] [PubMed] [Google Scholar]
- 9.Paton NI, Stohr W, Arenas-Pinto A, et al. Protease inhibitor monotherapy for long-term management of HIV infection: a randomised, controlled, open-label, non-inferiority trial. Lancet HIV 2015; 2:e417–e426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pulido F, Arribas JR, Delgado R, et al. Lopinavir-ritonavir monotherapy versus lopinavir-ritonavir and two nucleosides for maintenance therapy of HIV. AIDS 2008; 22:F1–F9. [DOI] [PubMed] [Google Scholar]
- 11.Vernazza P, Daneel S, Schiffer V, et al. The role of compartment penetration in PI-monotherapy: the atazanavir-ritonavir monomaintenance (ATARITMO) trial. AIDS 2007; 21:1309–1315. [DOI] [PubMed] [Google Scholar]
- 12.Gisslen M, Fuchs D, Hagberg L, et al. Cerebrospinal fluid viral breakthrough in two HIV-infected subjects on darunavir/ritonavir monotherapy. Scand J Infect Dis 2012; 44:997–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bunupuradah T, Chetchotisakd P, Ananworanich J, et al. A randomized comparison of second-line lopinavir/ritonavir monotherapy versus tenofovir/lamivudine/lopinavir/ritonavir in patients failing NNRTI regimens: the HIV STAR study. Antivir Ther 2012; 17:1351–1361. [DOI] [PubMed] [Google Scholar]
- 14.Santos JR, Munoz-Moreno JA, Molto J, et al. Virological efficacy in cerebrospinal fluid and neurocognitive status in patients with long-term monotherapy based on lopinavir/ritonavir: an exploratory study. PLoS ONE 2013; 8:e70201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez-Valero I, Gonzalez-Baeza A, Estebanez M, et al. A prospective cohort study of neurocognitive function in aviremic HIV-infected patients treated with 1 or 3 antiretrovirals. Clin Infect Dis 2014; 59:1627–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bunupuradah T, Chetchotisakd P, Jirajariyavej S, et al. Neurocognitive impairment in patients randomized to second-line lopinavir/ritonavir-based antiretroviral therapy vs. lopinavir/ritonavir monotherapy. J Neurovirol 2012; 18:479–487. [DOI] [PubMed] [Google Scholar]
- 17.Estebanez M, Stella-Ascariz N, Mingorance J, et al. Inflammatory, procoagulant markers and HIV residual viremia in patients receiving protease inhibitor monotherapy or triple drug therapy: a cross-sectional study. BMC Infect Dis 2014; 14:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spagnuolo V, Galli L, Bigoloni A, et al. Atazanavir/ritonavir monotherapy as maintenance strategy in HIV-1 treated subjects with viral suppression: 96-week analysis results of the MODAT study. J Int AIDS Soc 2014; 17 suppl 3:19806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69:1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acosta EP, Limoli KL, Trinh L, et al. Novel method to assess antiretroviral target trough concentrations using in vitro susceptibility data. Antimicrob Agents Chemother 2012; 56:5938–5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eden A, Fuchs D, Hagberg L, et al. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis 2010; 202:1819–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rawson TMD, Mackie NE, Garvey LJ, et al. Factors associated with cerebrospinal fluid HIV RNA in HIV infected subjects undergoing lumbar puncture examination in a clinical setting. J Infect 2014; 65:239–245. [DOI] [PubMed] [Google Scholar]
- 23.Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 2008; 65:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Best BM, Letendre SL, Brigid E, et al. Low atazanavir concentrations in cerebrospinal fluid. AIDS 2009; 23:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delille CA, Pruett ST, Marconi VC, et al. Effect of protein binding on unbound atazanavir and darunavir cerebrospinal fluid concentrations. J Clin Pharmacol 2014; 54:1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imaz A, Cayuela N, Niubo J, et al. Short communication: focal encephalitis related to viral escape and resistance emergence in cerebrospinal fluid in a patient on lopinavir/ritonavir monotherapy with plasma HIV-1 RNA suppression. AIDS Res Hum Retroviruses 2014; 30:984–987. [DOI] [PubMed] [Google Scholar]
- 27.Mdel PT, Quereda C, Gonzalez-Rozas M, et al. HIV type 1 viral encephalitis after development of viral resistance to plasma suppressive antiretroviral therapy. AIDS Res Hum Retroviruses 2012; 28:83–86. [DOI] [PubMed] [Google Scholar]
- 28.Mangioni D, Muscatello A, Sabbatini F, et al. A case of cerebrospinal fluid viral escape on a dual antiretroviral regimen: worth the risk? Clin Infect Dis 2014; 59:1655–1656. [DOI] [PubMed] [Google Scholar]
- 29.Khoury MN, Tan CS, Peaslee M, et al. CSF viral escape in a patient with HIV-associated neurocognitive disorder. J Neurovirol 2013; 19:402–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canestri A, Lescure FX, Jaureguiberry S, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis 2010; 50:773–778. [DOI] [PubMed] [Google Scholar]
- 31.Peluso MJ, Ferretti F, Peterson J, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS 2012; 26:1765–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnell G, Price RW, Swanstrom R, et al. Compartmentalization and clonal amplification of HIV-1 variants in the cerebrospinal fluid during primary infection. J Virol 2010; 84:2395–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schnell G, Spudich S, Harrington P, et al. Compartmentalized human immunodeficiency virus type 1 originates from long-lived cells in some subjects with HIV-1-associated dementia. PLoS Pathog 2009; 5:e1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cinque P, Bestetti A, Marenzi R, et al. Cerebrospinal fluid interferon-gamma-inducible protein 10 (IP-10, CXCL10) in HIV-1 infection. J Neuroimmunol 2005; 168:154–163. [DOI] [PubMed] [Google Scholar]
- 35.Dahl V, Peterson J, Fuchs D, et al. Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. AIDS 2014; 28:2251–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


