Supplemental Digital Content is available in the text
Keywords: hepatitis C, interferon, quality of life, ribavirin, sofosbuvir
Abstract
Patients’ experience during treatment may affect treatment adherence. Our aim was to assess the impact of patient-reported outcomes (PROs) on adherence to different anti-hepatitis C virus (HCV) regimens.
Clinical, demographic, and PRO data (short form-36 [SF-36], chronic liver disease questionnaire-hepatitis C version [CLDQ-HCV], functional assessment of chronic illness therapy-fatigue [FACIT-F], work productivity and activity impairment: specific health problem [WPAI:SHP]) from 13 multinational clinical trials of anti-HCV treatment were available. Treatment adherence was defined as >80% of prescribed doses taken.
Included were 4825 HCV patients. Regimens were grouped into: interferon- and ribavirin (RBV)-containing (±sofosbuvir [SOF]), interferon-free RBV-containing (RBV + SOF ± ledipasvir [LDV]), and interferon-free RBV-free (LDV/SOF). The adherence to these regimens were 77.6%, 84.3%, and 96.2%, respectively (P < 0.0001). Nonadherent patients were more likely to be unemployed and to have a greater PRO impairment at baseline (up to −5.3% lower PRO scores, P < 0.0001). During treatment with interferon- or RBV-based regimens, nonadherent patients experienced lower PROs and had larger decrements from their baseline PRO scores. In contrast, there were no significant declines in PRO scores (all P > 0.05) for the small number of patients who were nonadherent to LDV/SOF. In multivariate analysis, being treatment-naive, longer treatment duration, and receiving an interferon- or RBV-containing regimen were associated with a lower likelihood of adherence (all P < 0.003). Better baseline and on-treatment PRO scores were associated with a higher likelihood of adherence to interferon and RBV.
The use of interferon and/or RBV, longer duration of treatment, and lower baseline and on-treatment PRO scores were linked to a decreased likelihood of being adherent to interferon + RBV-containing or interferon-free RBV-containing antiviral regimens. Interferon- and RBV-free regimens were associated with excellent adherence.
1. Introduction
When treating patients with chronic hepatitis C, adherence to the full treatment regimen is important to optimize the efficacy of the antiviral therapy.[1,2] Although adherence may be assessed differently, it is partly defined as taking all prescribed medications and attending all office visits.[1,2] For some treatment regimens, adherence is defined as completion of at least 80% of treatment and at least 80% receipt of the prescribed medications.[1–4]
Adherence is very important in clinical trials of new medications aiming to achieve high efficacy.[5,6] Despite pretreatment screening and close on-treatment monitoring, adherence in clinical trials may still be limited due to the treatment regimen side effects (ex. depression, fatigue, flu-like symptoms, and anemia), which may alter patients’ ability to remain adherent to their prescribed intervention.[7–9]
Adherence becomes even more critical when drugs are approved and utilized in the real world setting. There are multiple instances where the efficacy rates reported from clinical trials are substantially reduced when drugs are released to clinical practices.[10–13] This substantial drop in treatment success may be due to not only treatment-associated side effects, but also the complexity of a regimen (contains more than 1 drug, requires self-injections, requires modifications of behavior, or last for more than 12 weeks), and patient-related characteristics.[12–14] Patient-related factors that may negatively influence adherence rates include less than 12 years of education, history of incarceration, current alcohol and drug use, and failure to show for follow-up appointments.[1–4,7,12,15–21] Other patient-related factors potentially affecting adherence are associated with their sense of well-being before and during treatment.[15,16,18,21]
The new interferon-free direct-acting antiviral-based regimens reportedly have a substantially higher efficacy rate, an improved side effect profile, and a less complex administration schedule.[22,23] Therefore, these regimens are expected to have an improved adherence rate. The aims of this study then were to: determine the adherence rates for the different hepatitis C virus (HCV) treatment regimens, including the new interferon- and ribavirin (RBV)-free regiments; determine the impact of patient-reported outcomes (PROs) to adherence to different anti-HCV regimens; relate adherence to clinical outcomes and PROs; and determine whether baseline PROs were predictive of adherence to the treatment regimens.
2. Methods
2.1. Study cohort
In this study, we analyzed PROs data collected as exploratory endpoints in 13 multicenter multinational phase 3 clinical trials of sofosbuvir (SOF)-based regimens for treatment of chronic hepatitis C (2012–2015).[22–29] Patients were treated with one of the following regimens: SOF in combination with pegylated interferon (peg-IFN) and RBV, peg-IFN + RBV, SOF + RBV, or a fixed-dose combination of ledipasvir and SOF (LDV/SOF) with or without RBV. Treatment duration ranged from 8 to 24 weeks.
The details and results of the clinical trials have been published elsewhere.[23–29] Briefly, treatment regimens were assigned randomly or selected based on patients’ treatment history and HCV genotype, blinded or not to patients and providers. Enrolled patients were of all HCV genotypes, they were treatment-naive or -experienced, with or without compensated cirrhosis. Patients with history of decompensated cirrhosis were excluded from these studies. In 3 trials, patients with HCV–HIV coinfection were enrolled.[23–29]
From the medical history collected at screening for all trials’ participants, we extracted history of depression or mood disorders, fatigue or asthenia, anxiety or panic disorders, sleep disorders or insomnia, and type 2 diabetes or hyperglycemia. During treatment, adverse events were recorded as previously described[29]; for the purpose of this study, we selected only adverse events which were labeled as treatment related by the investigators. Patients were presumed to have achieved sustained virologic response (SVR-12) if they had undetectable HCV RNA at posttreatment week 12.
2.2. Adherence
Across all trials, treatment adherence data were collected by recording the total number of pills/doses dispensed and the total number of pills/doses returned at monthly visits for each drug for the entire treatment duration for all patients. The number of pills taken was calculated by subtracting the number of pills returned from the number of pills dispensed for each study drug. Adherence was then calculated for each drug separately by dividing the number of pills taken by the number of pills expected to be taken during a specific period. In the current post-hoc analysis, for multidrug regimens, the minimal adherence for a patient was calculated as the minimum across all drugs used. A binary adherence outcome was further introduced; a patient was defined as adherent if their minimal adherence was at least 80%; otherwise, a patient was considered to be nonadherent. A binary adherence outcome for adherence to individual drugs was also calculated similarly using the 80% threshold.
It is important to note that this definition of adherence does not account for timing and causes of missed doses. Thus, patients for whom the dose of RBV or peg-IFN was intentionally reduced per protocol would still be deemed nonadherent to the regimen per this analysis.
2.3. Patient-reported outcomes
In all but 2 trials, PROs were assessed using 4 separate instruments (short form-36 [SF-36], chronic liver disease questionnaire-hepatitis C version [CLDQ-HCV], functional assessment of chronic illness therapy-fatigue [FACIT-F], and work productivity and activity impairment: specific health problem [WPAI:SHP]); in 2 trials, only SF-36 was administered. Health utility was also measured using the SF-6D metric.[30–34] These validated instruments were self-administered by patients during treatment and follow-up visits prior to initiation of any study-related activities for that visit while blinded to their most recent HCV RNA test.
SF-36, a generic instrument that measures 8 health-related quality of life (HRQL) domains (physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health) and provides 2 summary scores which summarize the physical and mental aspects of HRQL.[30]
The CLDQ-HCV is a disease-specific instrument aimed to assess HRQL in patients with chronic HCV infection by emphasizing the most frequently observed health impairment features typical for these patients. It includes 4 individual HRQL domains (activity/energy, emotional, worry, and systemic) which are averaged to the total CLDQ-HCV score.[31]
The FACIT-F is a fatigue-specific PRO instrument which includes 4 well-being domains (physical, emotional, social, and functional), and a fatigue subscale.[32]
The WPAI:SHP is another PRO instrument which is used to quantify impairment in patients’ daily activities and work productivity. It includes the domains of work productivity impairment (which is a sum of impairment in work productivity due to absenteeism and due to decreased productivity while working, or presenteeism; assessed only in employed patients), and activity impairment (which is impairment in activities other than work; assessed in all patients regardless of employment).[33]
The SF-6D health utility scores, which are preference-based measures for health and are typically used for the calculation of quality-adjusted years of life in economic analyses, were assessed using the SF-36 instrument by a nonparametric Bayesian model as previously reported.[34]
2.4. Statistical analysis
From the original trials, we merged treatment regimens into 3 treatment regimen groups based on the drugs used but regardless of treatment duration: peg-IFN + RBV-containing (peg-IFN + SOF + RBV, peg-IFN + RBV), IFN-free RBV-containing (SOF + RBV, LDV/SOF + RBV), and IFN-free RBV-free (LDV/SOF). Only time points consistently used across all trials were included in this study: baseline (treatment day 1), treatment week 4, end of treatment, and posttreatment follow-up weeks 4, 12, and 24.
All collected demographic and clinical parameters, as well as PRO scores, health utilities, and respective changes (decrements or improvements) from patients’ baseline levels were summarized at all aforementioned study time points. All results were further compared between adherent and nonadherent subjects for the entire cohort as well as separately for the 3 treatment regimen groups. These comparisons were made using Chi-square test (for categorical parameters) or Kruskal–Wallis nonparametric test (for continuous). The changes (decrements or improvements) in HRQL and utilities from each patient's baseline levels were calculated for at each mentioned time point. A sign rank test for matched pairs was used to determine the median changes.
Independent predictors of adherence were assessed using multiple logistic regression with the treatment regimens and treatment duration being tested as potential predictors. Bidirectional stepwise selection of predictors with the significance level of 0.05 for stay was used. The potential adherence predictors used for the selection procedure included patients’ baseline demographic parameters, clinical history, as well as baseline and treatment-emergent PROs. All analyses were run in SAS 9.3 (SAS Institute, Cary, NC).
The original studies were separately approved by each site's Institutional Review Board.
3. Results
3.1. Adherent and nonadherent patients
3.1.1. Patient characteristics
A total of 4825 HCV patients with adherence and PRO data were included. Of all patients, 657 patients received IFN + RBV-containing regimens (peg-IFN + RBV or peg-IFN + RBV + SOF), 3185 received IFN-free RBV-containing (SOF + RBV or LDV/SOF + RBV) regimens, and 1493 received IFN-free RBV-free (LDV/SOF). The average proportion of doses taken across all regimens ranged from 81.5% (peg-IFN + RBV) to 97.5% (LDV/SOF). Furthermore, 87.3% of all patients had the minimal adherence rate of at least 80% (further referred to as adherent patients).
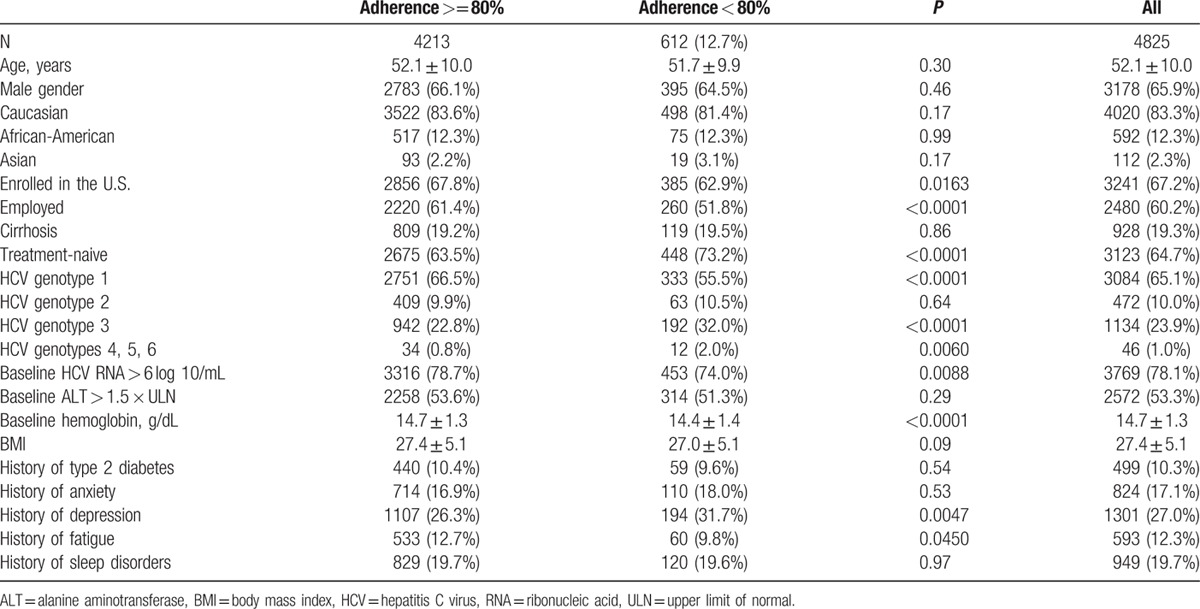
Patients who were adherent to anti-HCV treatment were, on average, more likely to have been enrolled in the U.S., employed at baseline, have HCV genotype 1, had higher baseline hemoglobin, less baseline depression or fatigue, and were less likely to be coinfected with HIV or to be treatment-naive (all P < 0.05). There was no difference in adherence rates based on age, gender, or ethnicity (all P > 0.05) (Table 1).
Table 1.
Adherent and nonadherent patients: demographics and baseline clinical parameters.
3.1.2. Adherence versus sustained virologic response
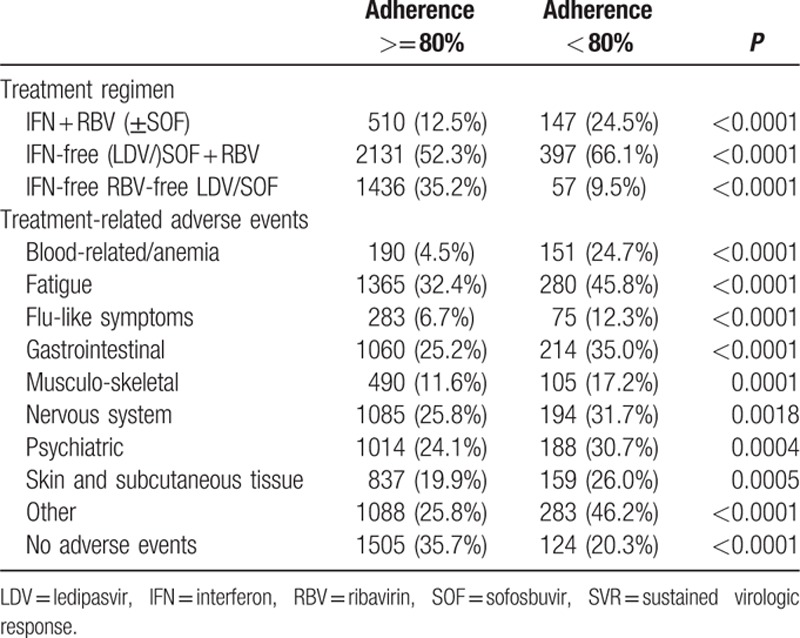
Assessing each regimen separately, adherent patients had higher SVR-12 rates than nonadherent patients. The SVR rates for patients adherent to peg-IFN + RBV-based regimens were 85.9% versus 74.8% in the nonadherent group (P = 0.0015). Furthermore, SVR rates in patients adherent to IFN-free RBV-containing regimens were 85.4% versus 79.1% in the nonadherent group (P = 0.0016). Finally, SVR rates in patients adherent and nonadherent to IFN-free and RBV-free (LDV/SOF) regimens were 97.1% versus 82.5%, respectively (P < 0.0001). Table 2 describes the treatment, side effects, and SVR in adherent and nonadherent patients with significant differences noted between the groups with the nonadherent group experiencing more side effects and were more likely to be treated with PEG-IFN therapy.
Table 2.
Treatment, side effects, and SVR in adherent and nonadherent patients.
3.1.3. Adherence versus baseline patient-reported outcomes
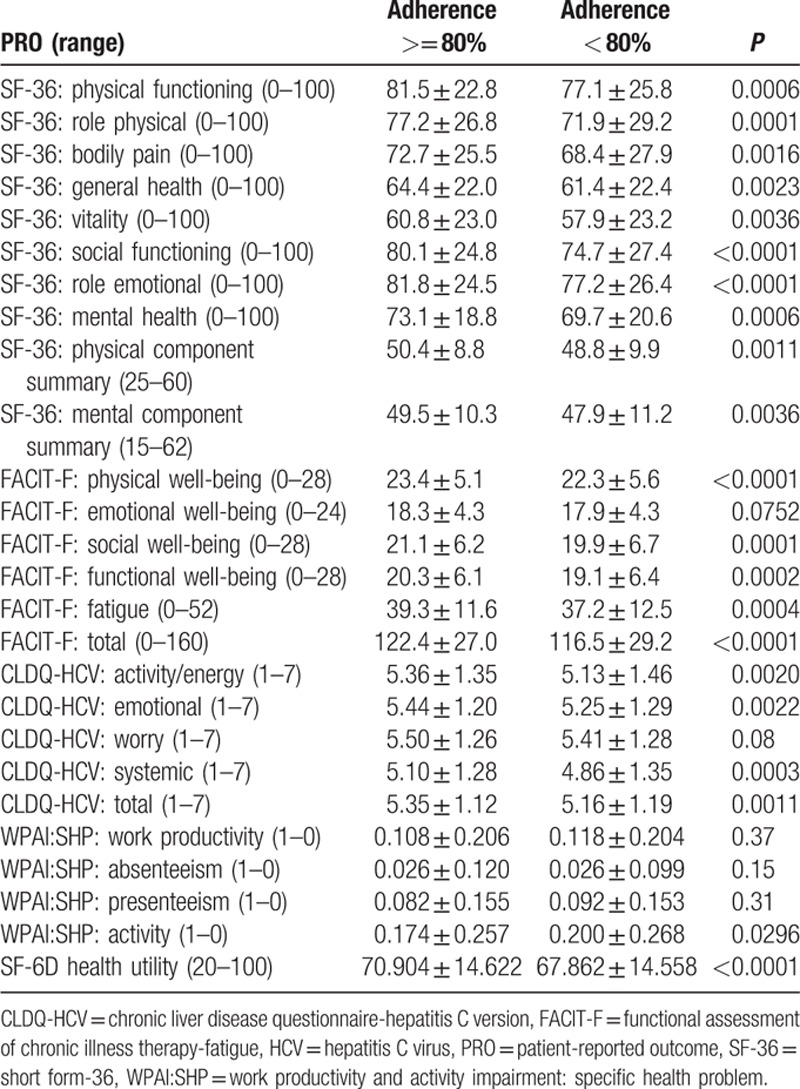
Baseline PRO scores for patients who would be adherent or nonadherent to their treatment are summarized in Table 3. As shown, nearly all PROs were substantially lower in patients who had less than 80% of minimal adherence to the regimen; the average difference was 3.3% on a normalized 0% to 100% scale, the maximum was 5.3% for role physical of SF-36 (P < 0.0001). Only baseline scores for self-reported work productivity, emotional well-being of FACIT-F, and worry of CLDQ-HCV were not different between adherent and nonadherent patients (P > 0.05).
Table 3.
Baseline patient-reported outcomes in adherent and nonadherent HCV patients.
3.2. Adherent and PROs by treatment group
Expectedly, treatment-emergent decrements in PROs were substantially greater in nonadherent subjects (P < 0.0001, Table 3). The magnitudes of these decrements, however, varied greatly depending on the particular regimen.
3.2.1. Peg-IFN + RBV-containing regimens
In subjects who received peg-IFN + RBV-containing regimens (N = 657), the average adherence rate was the lowest of all treatment regimens (87.6%), and thus, only 77.6% were minimally adherent to their regimens. Analyzing the data by treatment duration, adherence also tended to be lower in patients who received 24 weeks of peg-IFN + RBV: 72.1% versus 78.8% in 12 weeks (P = 0.12). However, adherence to the individual drugs did not differ between peg-IFN and RBV (P > 0.05).
Baseline PROs were generally lower in patients who would be nonadherent (Supplementary Table 1). During treatment, some adverse events, including anemia, fatigue, gastrointestinal symptoms, or nervous system disorders, were experienced more frequently by nonadherent subjects, but the overall adverse event rate was high in both groups receiving peg-IFN + RBV (Supplementary Table 1). Further, all patients receiving peg-IFN + RBV experienced substantial declines in their PROs (up to −22.8%, P < 0.0001). However, nonadherent patients showed consistently lower PRO scores during treatment and after treatment than the adherent group (Fig. 1A, Supplementary Figure 1A). These trends continued despite achieving SVR (Fig. 1A).
Figure 1.
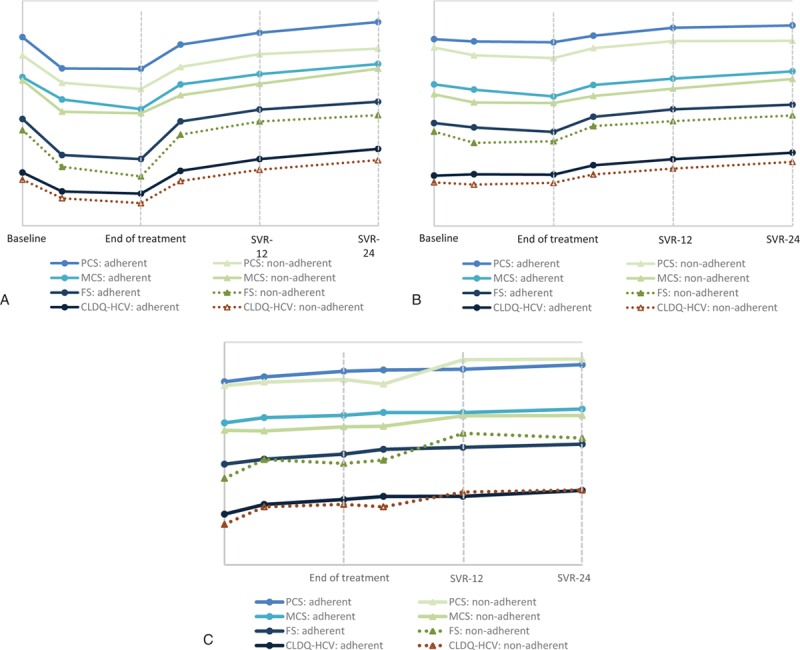
PROs in HCV patients adherent and not adherent to (A) IFN + RBV-containing regimens; (B) IFN-free RBV-containing regimens; and (C) IFN-free RBV-free regimens. CLDQ-HCV = chronic liver disease questionnaire-hepatitis C version (the total score for the instrument), FACIT-F = functional assessment of chronic illness therapy-fatigue, FS = fatigue scale of FACIT-F, HCV = hepatitis C virus, IFN = interferon, MCS = mental component summary of SF-36, PCS = physical component summary of SF-36, PRO = patient-reported outcome, RBV = ribavirin, SF-36 = short form-36.
3.2.2. IFN-free RBV-containing regimens
The minimal adherence rate to the IFN-free RBV-containing regimens was, on average, 91.3%, and, using the 80% threshold, 84.3% were deemed adherent. There was substantial variability between the individual drugs: on average, the adherence rate to SOF or LDV/SOF was 4.1% to 4.4% higher than adherence to RBV (all P < 0.0001). Additionally, there was a notable variability between treatment durations. In particular, subjects who received 24 weeks of SOF + RBV (±LDV) were substantially less adherent than patients who received 12 or 16 week regimens: 79.2% versus 88.8% in 16 weeks, 87.1% in 12 weeks, and 89.3% in 8 weeks (P < 0.0001).
At baseline, patients who would adhere to treatment with an IFN-free RBV-containing regimens were more likely to be Caucasian, enrolled in the U.S., employed, and had a higher baseline hemoglobin. They were also less likely to have a history of depression or be treatment-naive (Supplementary Table 1). From all the treatment-emergent adverse events, only anemia and fatigue were significantly different between adherent and nonadherent patients: 6.1% versus 23.7% and 36.1% versus 43.1%, respectively (both P < 0.01) (Supplementary Table 1). Also, despite similarity of baseline clinical variables, the baseline PROs, as well as on-treatment and post-SVR PRO scores were consistently lower in nonadherent patients (Fig. 1B, Supplementary Figure 1B).
3.2.3. IFN-free RBV-free regimens
The minimal adherence rate to IFN-free RBV-free regimen (LDV/SOF) was the highest of all the regimens: 97.5% on average, and 96.2% of patients were adherent above 80%. Furthermore, adherence rates did not vary between treatment durations with LDV/SOF (8, 12, or 24 weeks: P = 0.18).
Unlike other regimens, in patients receiving IFN- and RBV-free regimens (LDV/SOF), there was very little difference in the clinical parameters and PRO scores between the adherent and nonadherent groups (Supplementary Table 1). The only adverse event which was significantly higher in the nonadherent group was flu-like symptoms: 5.3% versus 1.2% (P = 0.0086).
During treatment, patients experienced some improvement in their PRO scores (Fig. 1C, Supplementary Figure 1C). Surprizingly, these improvements were slightly higher in nonadherent subjects: average +4.1%, max +10.9% in adherent versus average +7.4%, and max +15.3% in nonadherent (P = 0.0033 for the max improvement in activity of WPAI:SHP). Furthermore, greater improvements in nonadherent subjects were observed starting as early as treatment week 4: average +3.9%, max +10.7% versus average +2.1%, and max +7.9% (P up to 0.0035). Some of these differences persisted after achieving SVR: average +5.4%, max +12.8% in adherent versus average +7.5%, and max +17.0% in nonadherent (P < 0.05 for vitality, emotional well-being, and fatigue scale).
3.2.4. Predictors of adherence to anti-HCV treatment
Independent predictors of being minimally adherent were assessed using different pools of candidates, for all patients and by treatment regimens separately (Table 4).
Table 4.
Independent predictors of adherence to anti-HCV treatment.
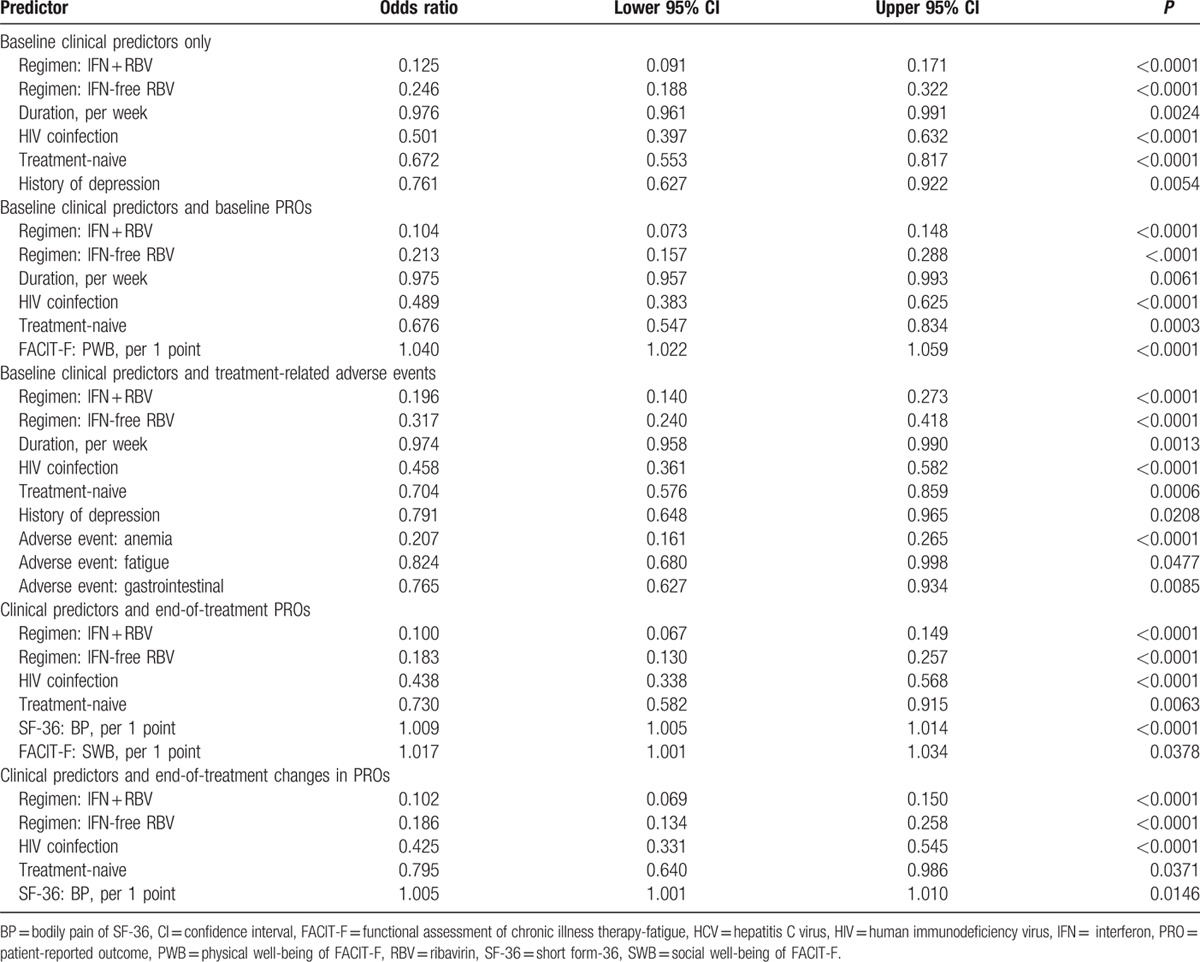
Of baseline clinical parameters, being treatment-naive, and having a history of depression were consistently associated with lower adherence (Table 4). On the other hand, treatment-related factors driving lower adherence were receiving an IFN-containing (odds ratio [OR] = 0.125) or an RBV-containing (OR = 0.25) regimens as well as longer treatment duration (OR = 0.976 per each additional week) (all P < 0.003) (Table 4). The treatment-related adverse events that were independently associated with lower adherence included treatment-induced anemia (OR = 0.21), clinically-overt fatigue (OR = 0.82), and having an episode of gastrointestinal system disorder such as nausea and vomiting (OR = 0.76) (Table 4).
For the baseline PROs, having a higher physical well-being score (FACIT-F) was independently associated with having a higher adherence rate after adjustment for clinical and treatment-related factors: OR = 1.040 (1.022–1.059) (P < 0.0001) per each additional point for the PRO which ranges from 0 to 28 (Table 4). On the other hand, for the end-of-treatment PROs, adherence was associated with a greater improvement in the bodily pain (higher score equals less pain) and an increased social well-being score (Table 4).
The PRO-based adherence predictors for the IFN + RBV-containing regimens studied separately included higher baseline role physical score and lower worry score (more worry), as well as higher end of treatment role physical and a greater posttreatment increase in the worry score. Treatment duration was, in fact, associated with lower adherence to IFN-free RBV-containing regimens only (OR = 0.976 [0.955–0.997] per week), and the PRO-based predictors were limited to baseline and end of treatment social well-being, end of treatment physical well-being and bodily pain, and treatment-emergent increase in vitality. No consistent PRO-based predictors of adherence to IFN-free RBV-free LDV/SOF were found.
Finally, of all the studied predictive models, the greatest concordance was observed for adherence being predicted by a combination of baseline clinical factors, treatment-related factors (drugs, duration), and treatment-related adverse events: C = 74.3%. Concordance for prediction of adherence by clinical and treatment-related factors accompanied by PROs ranged from 70.3% to 73.4% in all patients, from 55.3% to 62.8% in the IFN + RBV-containing study arm, from 60.4% to 65.4% in the IFN-free RBV-containing arm, and not available in IFN-free RBV-free arm due to limited sample size.
3.2.5. Adherence to SOF and LDV/SOF used in different regimens
In addition to the minimal-across-drugs adherence in different multidrug anti-HCV regimens which was the primary study outcome, we also studied adherence to the individual DAAs which were used as parts of different regimens, with the aim to assess whether the regimen would affect the adherence to the studied DAAs. As a result, after adjustment for the duration of treatment, the proportion of patients adherent to SOF while receiving peg-IFN + RBV + SOF and receiving SOF + RBV was not significantly different: 96.5% versus 94.6% (P = 0.09). There were also no treatment-related adverse events associated with adherence to SOF (all P > 0.05). Finally, the adherence rates to LDV/SOF were also identical regardless of the regimen (P = 0.86 between RBV-free LDV/SOF and LDV/SOF + RBV).
4. Discussion
The purpose of the study was to assess factors associated with adherence to the different anti-HCV treatment regimens available on the market today to include the new IFN- and RBV-free regimens. We also aimed to assess the relationship between PROs and adherence to these regimens.
Our study showed that the best adherence profile was seen with the treatment regimens that were free of both IFN and RBV (LDV/SOF). The regimens with the worst adherence profile and the highest number of side effects were those containing both IFN and RBV. Indeed, receiving IFN + RBV or RBV only was consistently associated with lower adherence rates with ORs ranging from 0.10 to 0.13 and from 0.18 to 0.25, respectively. Our findings support the belief that adherence is adversely affected as the number of side effects, the complexity of the treatment regimen, and the duration of treatment increase. In fact, we have found that it was treatment-induced anemia and the resulting fatigue which were the most consistent predictors of nonadherence to treatment.
Additionally, we identified several baseline patient-related factors associated with lower adherence to treatment. These included having a history of depression and being treatment-naive. These findings reaffirm previous research indicating that patients with significant psychiatric issues are less adherent to treatment regimens especially if they receive regimens (IFN and/or RBV) associated with significant psychiatric side effects.[9,13,35–37] Other studies have found treatment-naive patients are less likely to be adherent to treatment with IFN and RBV due to their inability to cope with the treatment side effects, especially fatigue, given the required duration of treatment.[38,39] Findings such as these have important clinical implications, as RBV-containing regimens may remain the preferred treatment for the most difficult to treat patients, such as those who are treatment-experienced with advanced fibrosis.
The primary aim of this study was to assess the relationship between adherence and PROs at baseline as well as the effect of treatment induced PRO changes. We showed that for patients who received PEG-IFN + RBV-containing regimens, the major drivers of nonadherence were lower baseline and on-treatment physical health-related scores. In particular, lower Role Physical scores were independently associated a higher chance of being nonadherent. For patients who received the IFN-free but RBV-containing regimens, a higher baseline and on-treatment social well-being scores were associated with a greater chance of being adherent, suggesting the importance of having a good social network available when undergoing treatment.
A surprizing but intriguing finding was that PROs affect nonadherence differently in those who received LDV/SOF compared to the IFN- and/or RBV-containing regimens. Although patients who were nonadherent to the IFN- and/or RBV-containing regimens experienced lower PRO scores in comparison to adherent patients, patients who were nonadherent to LDV/SOF experienced earlier and better PRO improvement. One can hypothesize that regimens which lack side effects and quickly improve patients’ experience as seen with these regimens may give patients a false sense of security so that they either stop treatment early or not take as much of their prescribed medication. These data support the need for practitioners to emphasize the importance of completing the prescribed regimens regardless of how well their patients may be feeling to avoid the building of resistance to the new drug regimens or relapsing.[40]
In addition to adherence to a regimen, we also have studied adherence to individual drugs when used in different regimens. It is interesting that adherence to SOF and LDV/SOF was not affected when these drugs were used in combination with peg-interferon or RBV; however, adherence to SOF was still lower in longer regimens. In fact, what did vary in terms of adherence was adherence to different drugs (IFN vs RBV vs SOF ± LDV), and, as a result, to regimens that contained those drugs, but not adherence to the same DAA drugs used in different regimens.
It is important to note that adherence has clinically significant consequences. In fact, regardless of the regimen used, those who were nonadherent had lower SVR rates. This indicates that even in the era of new DAA regimens, adherence will remain of utmost importance. This will be especially significant, as more of the new regimens are dispersed in community-based clinical practices. Given that, we believe that making sure that practitioners are aware that even the newer drugs will require strict adherence and therefore, practitioners’ oversight, is indeed critical. Nevertheless, we have to emphasize that the actual rate of nonadherence with the IFN- and RBV-free regimen was very low (<4%) which continues to attest to the superiority of such regimens.
Our study is also the first to provide adherence rates across several different treatment groups to include the new 2nd generation DAAs. In particular, our findings highlight the difficulties patients face when treated for HCV to include the many treatment-induced issues such as depression. These difficulties emphasize that patients be evaluated to receive the shortest and most easily administered treatment regimen if a cure is the goal of treatment. The availability of new IFN-free and RBV-free treatments may offer patients a less complex and more easily administered regimen which, as noted in this and prior studies, improves PROs as early as 4 weeks into treatment.
The major limitation of the study is the source of the data. Indeed, our data were from clinical trials which had strict enrollment criteria, exclusion of patients with comorbidities, and close monitoring during treatment and in follow-up. Therefore, we most likely over-estimated the adherence rates, although we did not necessarily introduce any bias to its drivers such as clinical predictors, baseline, and on-treatment PROs. Another limitation is that different treatment regimens were purposefully tested in different HCV populations, which may have resulted in an additional bias. In particular, IFN-free RBV-containing regimens were, by design, administered to subjects who had a higher rate of cirrhosis or had already failed an IFN-based or a 1st generation DAA-based regimen. Further, we did not have access to information related to timing and reasons for nonadherence in nonadherent subjects which may have resulted in patients who were received protocol-driven dose reduction of RBV and PEG-IFN being labeled as nonadherent. One can argue that this approach is still accurate, since reduced dose regimens were associated with a decrease in SVR rates. Finally, we did not have access to a number of potentially important socio-economic factors which are known to influence adherence to any long-term treatment, such as the level of education, social history, occupation, or marital status.
In conclusion, we found that patients who received interferon- and RBV-free SOF-based HCV treatments had high adherence rates and consequently higher SVR rates regardless of the duration of treatment. These findings are very encouraging as they provide further evidence that these regimens are well-tolerated, carry high adherence rates compared to the older treatment regimens, demonstrate improved PROs, and result in high cure rates. Pragmatic trials of the new anti-HCV regimens are urgently needed to confirm the adherence rates in real world practices and in all HCV subpopulations.
Supplementary Material
Footnotes
Abbreviations: CLDQ-HCV = chronic liver disease questionnaire-hepatitis C version, FACIT-F = functional assessment of chronic illness therapy-fatigue, HCV = hepatitis C virus, HRQL = health-related quality of life, LDV = ledipasvir, OR = odds ratio, peg-IFN = pegylated interferon, PRO = patient-reported outcome, RBV = ribavirin, SF-36 = short form-36, SOF = sofosbuvir, SVR = sustained virologic response, WPAI:SHP = work productivity and activity impairment: specific health problem.
Authorship: ZY designed and supervised the study, and prepared the final version of the manuscript; MS collected the data, performed the statistical analysis, and revised the first draft; LH prepared the first draft, YY, SH, and FN contributed to the study design and critically reviewed the manuscript.
Funding/support: This study was funded by Gilead Sciences. ZY is a consultant to Gilead Sciences, Abbvie, BMS, GSK, and Intercept.
The remaining authors have no conflict of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Patel K, McHutchison JG. Initial treatment for chronic hepatitis C: current therapies and their optimal dosing and duration. Cleve Clin J Med 2004; 71 suppl 3:S8–12. [DOI] [PubMed] [Google Scholar]
- 2.Shehab TM, Fontana RJ, Oberhelman K, et al. Effectiveness of interferon alpha-2b and ribavirin combination therapy in the treatment of naive chronic hepatitis C patients in clinical practice. Clin Gastroenterol Hepatol 2004; 2:425–431. [DOI] [PubMed] [Google Scholar]
- 3.Mravcík V, Strada L, Stolfa J, et al. Factors associated with uptake, adherence, and efficacy of hepatitis C treatment in people who inject drugs: a literature review. Patient Prefer Adherence 2013; 7:1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LaFleur J, Hoop R, Morgan T, et al. High rates of early treatment discontinuation in hepatitis C-infected US veterans. BMC Res Notes 2014; 7:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butt AA, Wagener M, Shakil AO, et al. Reasons for non-treatment of hepatitis C in veterans in care. J Viral Hepat 2005; 12:81–85. [DOI] [PubMed] [Google Scholar]
- 6.Jürgen K. Rockstroh M.D. Summary from EASL 2013 for Hepatitis C – New HCV DAAs on their way soon: what do the phase III studies tell us? EASL 48th Annual Meeting. April 24th–28th, 2013 Amsterdam, ND. [Google Scholar]
- 7.Quelhas R, Lopes A. Psychiatric problems in patients infected with hepatitis C before and during antiviral treatment with interferon-alpha: a review. J Psychiatr Pract 2009; 15:262–281. [DOI] [PubMed] [Google Scholar]
- 8.Bacon BR, McHutchison JG. Treatment issues with chronic hepatitis C: special populations and pharmacy strategies. Am J Manag Care 2005; 11 (10 Suppl):S296–S306. [PubMed] [Google Scholar]
- 9.Guadagnino V, Trotta MP, Carioti J, et al. Does depression symptomatology affect medication compliance during the first weeks of anti-HCV therapy in intravenous drug users? Dig Liver Dis 2006; 38:119–124. [DOI] [PubMed] [Google Scholar]
- 10.Price JC, Murphy RC, Shvachko VA, et al. Effectiveness of telaprevir and boceprevir triple therapy for patients with hepatitis C virus infection in a large integrated care setting. Dig Dis Sci 2014; 59:3043–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ioannou GN, Beste LA, Green PK. Similar effectiveness of boceprevir and telaprevir treatment regimens for hepatitis C virus infection on the basis of a nationwide study of veterans. Clin Gastroenterol Hepatol 2014; 12:1371–1380. [DOI] [PubMed] [Google Scholar]
- 12.McHutchison JG, Manns M, Patel K, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology 2002; 123:1061–1069. [DOI] [PubMed] [Google Scholar]
- 13.Niederau C, Mauss S, Schober A, et al. Predictive factors for sustained virological response after treatment with pegylated interferon a-2a and ribavirin in patients infected with HCV genotypes 2 and 3. PLoS One 2014; 9:e107592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCombs J, Matsuda T, Tonnu-Mihara I, et al. The risk of long-term morbidity and mortality in patients with chronic hepatitis C: results from an analysis of data from a Department of Veterans Affairs Clinical Registry. JAMA Intern Med 2014; 174:204–212. [DOI] [PubMed] [Google Scholar]
- 15.Raptopoulou M, Tsantoulas D, Vafiadi I, et al. The effect of adherence to therapy on sustained response in daily or three times a week interferon alpha-2b plus ribavirin treatment of naive and nonresponder chronic hepatitis C patients. J Viral Hepat 2005; 12:91–95. [DOI] [PubMed] [Google Scholar]
- 16.Les I, Doval E, Flavià M, et al. Quality of life in cirrhosis is related to potentially treatable factors. Eur J Gastroenterol Hepatol 2010; 22:221–227. [DOI] [PubMed] [Google Scholar]
- 17.Rodis JL, Kibbe P. Evaluation of medication adherence and quality of life in patients with hepatitis c virus receiving combination therapy. Gastro Nurs 2010; 33:368–373. [DOI] [PubMed] [Google Scholar]
- 18.Poordad F, Lawitz E, Kowdley KV, et al. Exploratory study of oral combination antiviral therapy for hepatitis C. N Engl J Med 2013; 368:45–53. [DOI] [PubMed] [Google Scholar]
- 19.Bonner JE, Esserman DA, Golin CE, et al. Self-efficacy and adherence to antiviral treatment for chronic hepatitis C. J Clin Gastroenterol 2015; 49:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McHutchison JG, Ware JE, Jr, Bayliss MS, et al. The effects of interferon alpha-2b in combination with ribavirin on health related quality of life and work productivity. J Hepatol 2001; 34:140–147. [DOI] [PubMed] [Google Scholar]
- 21.Sublette VA, Hopwood M, George J, et al. Instrumental support to facilitate hepatitis C treatment adherence: working around shortfalls in shared-care. Psychol Health Med 2015; 20:186–197. [DOI] [PubMed] [Google Scholar]
- 22.Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013; 368:1878–1887. [DOI] [PubMed] [Google Scholar]
- 23.Poordad F, Lawitz E, Kowdley KV, et al. Exploratory study of oral combination antiviral therapy for hepatitis C. N Engl J Med 2013; 368:45–53. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med 2013; 368:1867–1877. [DOI] [PubMed] [Google Scholar]
- 25.Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370:1889–1898. [DOI] [PubMed] [Google Scholar]
- 26.Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 2014; 370:1483–1493. [DOI] [PubMed] [Google Scholar]
- 27.Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med 2014; 370:1993–2001. [DOI] [PubMed] [Google Scholar]
- 28.Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014; 370:1879–1888. [DOI] [PubMed] [Google Scholar]
- 29.Younossi ZM, Stepanova M, Zeuzem S, et al. Patient-reported outcomes assessment in chronic hepatitis C treated with sofosbuvir and ribavirin: the VALENCE study. J Hepatol 2014; 61:228–234. [DOI] [PubMed] [Google Scholar]
- 30.Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res 2001; 10:405–413. [DOI] [PubMed] [Google Scholar]
- 31.Younossi ZM, Guyatt G, Kiwi M, et al. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut 1999; 45:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Webster K, Odom L, Peterman A, et al. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: validation of version 4 of the core questionnaire. Qual Life Res 1999; 8:604. [Google Scholar]
- 33.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993; 4:353–365. [DOI] [PubMed] [Google Scholar]
- 34.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002; 21:271–292. [DOI] [PubMed] [Google Scholar]
- 35.Hassanein T, Cooksley G, Sulkowski M, et al. The impact of peginterferon alfa-2a plus ribavirin combination therapy on health-related quality of life in chronic hepatitis C. J Hepatol 2004; 40:675–681. [DOI] [PubMed] [Google Scholar]
- 36.Spiegel BM, Younossi ZM, Hays RD, et al. Impact of hepatitis C on health related quality of life: a systematic review and quantitative assessment. Hepatology 2005; 41:790–800. [DOI] [PubMed] [Google Scholar]
- 37.Jerkeman A, Norkrans G, Lidman C, et al. Treatment for chronic hepatitis C in a cohort of opiate substitution therapy recipients in three Swedish cities – completion rates and efficacy. Eur J Gastroenterol Hepatol 2014; 26:523–531. [DOI] [PubMed] [Google Scholar]
- 38.Kallman J, O’Neil MM, Larive B, et al. Fatigue and health-related quality of life (HRQOL) in chronic hepatitis C virus infection. Dig Dis Sci 2007; 52:2531–2539. [DOI] [PubMed] [Google Scholar]
- 39.Boscarino JA, Lu M, Moorman AC, et al. Predictors of poor mental and physical health status among patients with chronic hepatitis C infection: The chronic hepatitis cohort study (CHeCS). Hepatology 2015; 61:802–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terrault N, Monto A, Stinchon MR, et al. New therapies, evidence, and guidance in hepatitis C management: expert practices and insights from an Educational Symposium at the AMCP 27th Annual Meeting Expo. J Manag Care Spec Pharm 2015; 21:S1–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


