Supplemental Digital Content is available in the text
Keywords: atrial fibrillation, cardiac implantable electronic devices, predictor, readmission
Abstract
Objective:
To evaluate predictors and long-term prognosis of atrial fibrillation (AF) following cardiac implantable electronic device (CIED) implantation in patients without history of AF.
Methods:
From May 1994 to April 2014, 1825 patients with CIED were enrolled in a retrospective, single-center registry. A total of 880 patients from the registry without prior documented AF history were included in the final analysis and were placed into either non-detected AF (NDAF) group or CIED-detected AF group according to development of AF over a follow-up period of 7 years. AF development was defined as any paroxysmal atrial tachyarrhythmia (atrial rate ≥ 180 beats/min) lasting at least 5 minutes according to CIED records.
Results:
Overall, 122 (13.8%) of the 880 patients experienced new development of AF during follow-up period. According to multivariate analysis, the independent predictors for development of AF were prior heart failure (hazard ratio [HR], 2.40; 95% confidence interval [CI], 1.50–3.85; P < 0.001), prior sinus node dysfunction (HR, 2.33; 95% CI, 1.62–3.55; P < 0.001), and left atrium volume index of 38.5 mL/m2 or more (HR, 2.01; 95% CI, 1.23–3.30; P = 0.005). In CDAF group, the risk of heart failure readmission (adjusted HR, 3.79; 95% CI, 1.99–7.22; P < 0.001) and stroke readmission (adjusted HR, 5.33; 95% CI, 1.58–17.97; P = 0.007) was higher than in nondetected AF group.
Conclusion:
In patients with CIED, prior history of heart failure, sinus node dysfunction, and LA volume index ≥38.5 mL/m2 were independent predictors of new AF cases. Newly developed AF was significantly associated with increased risk of HF and stroke readmission, according to long-term follow up.
Key points
Newly developed AF frequently occurs following CIED implantation in patients without a history of AF. The rate of development of new AF cases in East Asians over a follow-up period of 7 years was 13.8%.
Prior history of HF, sinus node dysfunction, and large LA volume index are significant predictors for newly developed AF in patients with a CIED.
The predictors for AF development differed between the permanent pacemaker (PPM) subgroup and the implantable cardioverter-defibrillator (ICD) or cardiac resynchronization therapy (CRT) subgroups.
According to the long-term follow-up, the risk of unplanned cardiovascular readmission, HF readmission, and stroke readmission is higher in CDAF patients than in NDAF patients.
1. Introduction
Atrial fibrillation (AF) is the most common cardiac rhythm disorder encountered in clinical practice. It is associated with an increased risk of stroke, hospitalization, and mortality, all of which have a significant impact on health care economic costs.[1] AF is often asymptomatic and is frequently underdiagnosed.[2] In particular, paroxysmal episodes may be missed during clinical evaluation, electrocardiography, and periodic ambulatory telemetry monitoring. Continuous rhythm monitoring with a cardiac implantable electronic device (CIED) is used to diagnose brief episodes of arrhythmia and detection of AF with CIED is common in the absence of clinical evidence of AF.[3,4] With respect to individuals who have never experienced AF, the CIED approach detects newly developed AF more frequently than do conventional diagnostic methods, which is important given the association of AF with a significantly increased risk of future cardiovascular events.[4,5] To date, several clinical conditions have been recognized as risk factors for AF.[6] However, the predictors of AF following CIED implantation in patients without a history of AF remain unclear. Therefore, we investigated the measureable risk factors at the time of device implant that predict development of future AF and evaluated long-term clinical outcomes of newly-developed AF in patients with a CIED.
2. Methods
2.1. Study patients
Between May 1994 and April 2014, 1825 consecutive patients were enrolled in the Samsung Medical Center-CIED registry. CIEDs consisted of permanent pacemakers (PPMs), implantable cardioverter-defibrillators (ICD), and cardiac resynchronization therapy (CRT) with or without defibrillation capability that were available in the domestic market during the study period. Each device was implanted according to the classes I–II recommendations of the current ACCF/AHA/HRS guidelines.[7] The inclusion criteria for the present study were as follows: consecutive patients 18 years of age or older, patients who received 1 of the CIEDs described above, which functioned properly throughout the study, and patients who had at least 1 follow-up visit and device interrogation after the implantation procedure. Patients with reimplantation of devices and patients with a previous history of documented AF/flutter were excluded. A total of 880 patients were included in the final analysis. The patient inclusion process for this study is shown in Fig. 1. For the purpose of analysis, we classified the study population into a non-detected AF (NDAF) group and a CIED-detected AF (CDAF) group according to development of AF over a follow-up period of 7 years.
Figure 1.
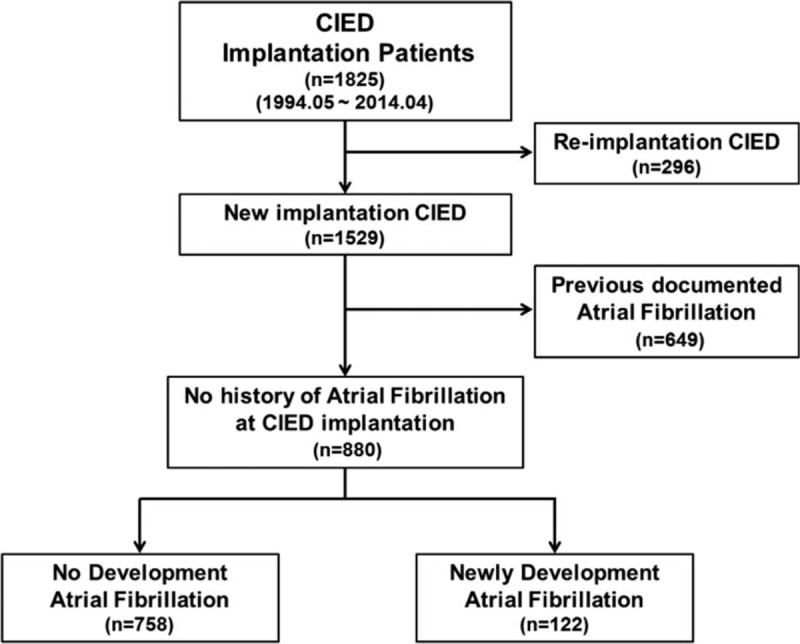
Flow chart of patient inclusion.
2.2. Data collection and detection of AF
All patients underwent a complete baseline history taking and physical examination, 12-lead electrocardiogram (ECG), laboratory exam, and transthoracic echocardiogram prior to CIED implantation. Clinical, laboratory, and patient outcome data were collected by a trained study coordinator. History of AF was defined as documented AF/flutter on surface ECG or ECG-Holter monitoring of collected data. All of the CIEDs used in this study had the capacity to record episodes of atrial tachyarrhythmia. Patients were followed at 3- to 6-month intervals. PPM devices were interrogated at 6-month intervals, whereas ICD or CRT devices were interrogated at 3-month intervals, and diagnostic information on the devices and clinical information was collected at that time. Device interrogations were adjudicated by 2 electrophysiologists blinded to the patients’ clinical events. Intracardiac arrhythmic events were reviewed in each patient during follow-up visits. AF development was defined as any paroxysmal atrial tachyarrhythmia (atrial rate ≥180 beat/min as detected by the device) lasting at least 5 minutes in patients with a dual-chamber device or CRT device.[8] In patients with a single-chamber devices, AF development was defined based on CIED interrogation with device-based diagnostics.[9] Echocardiographic profiles were measured at the echocardiographic core laboratory (Heart Vascular and Stroke Institute, Samsung Medical Center, Seoul, Korea) according to the established protocol of the American Society of Echocardiography.[10] Mitral valve disease was defined as moderate to severe mitral stenosis or mitral insufficiency, and aortic valve disease was defined as moderate to severe aortic stenosis or aortic insufficiency. On the basis of receiver-operating characteristic analysis, the optimal LA volume index cut-off value for new development of AF in patients with CIED was 38.5 mL/m2; LA volume index had an area under the curve of 0.71 (95% confidence interval [CI], 0.66–0.76; P < 0.001), a sensitivity of 69.4% (95% CI, 56.4–80.4 %), and a specificity of 56.7% (95% CI, 52.0–61.5 %). We received approval from the Institutional Review Board of Samsung Medical Center to perform all analyses.
2.3. Clinical outcomes and definitions
The primary outcome was a composite measure of mortality and unplanned cardiovascular readmission during follow-up after device implantation. Secondary outcomes consisted of mortality, heart failure (HF) readmission, stroke readmission, other cardiovascular readmissions, and unplanned cardiovascular readmissions during follow-up. The unplanned cardiovascular readmissions comprised HF readmission, stroke readmission, and other cardiovascular readmissions. HF readmissions were defined as readmissions with a primary diagnosis of HF on the basis of the major and minor clinical criteria described by the Framingham Heart Study.[11] Stroke readmissions were defined as readmissions with a primary diagnosis of cerebral infraction with rapid-onset focal neurologic symptoms lasting at least 24 hours.[5] Other cardiovascular readmissions comprised diagnoses of cardiovascular origin that did not meet the criteria for HF or stroke readmission. The decision for other cardiovascular readmission was left to the physicians’ discretion. With respect to patients with multiple readmissions in the registry, we selected the first instance as the index admission. We classified CDAF as persistent AF or paroxysmal AF. Persistent AF was documented AF that continued for at least one week, and other cases were considered to paroxysmal AF.
2.4. Statistical analysis
Continuous variables were compared using Student's t-test or the Wilcoxon rank-sum test when applicable and are presented as the mean ± standard deviation or median with interquartile range. Categorical data were analyzed using the Chi-square test. The effects of putative risk factors on future AF were analyzed using a Cox proportional hazard model. Crude hazard ratios (HR) were computed by stepwise addition of risk factors into the model. To assess which variables at the time of implantation were independent risk factors for future AF, we built a multivariate Cox proportional hazard model by adding variables that were significant (P < 0.10) into the univariate models. Adjusted hazard rates were compared by a Cox proportional hazard model based on age at the time of the procedure, male sex, prior HF, prior sinus node dysfunction, prior symptomatic atrioventricular (AV) block, an LA volume index ≥38.5 mL/m2, use of angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, and use of antiplatelet therapy, starting with the variable that showed the strongest association first. The Cox proportional model assumption was validated by a Schoenfeld residual plot. With respect to clinical outcomes according to development of AF, cumulative event rates were estimated by the Kaplan–Meier method and were compared using log-rank tests. Receiver-operating characteristic curve analysis was performed to estimate LA volume index for new development of AF based on an estimated area under the curve. Statistical analyses were performed with SAS 9.2 (SAS Institute Inc., Cary, NC, USA). All tests were 2 tailed, and P values < 0.05 were considered statistically significant.
3. Results
3.1. Baseline characteristics
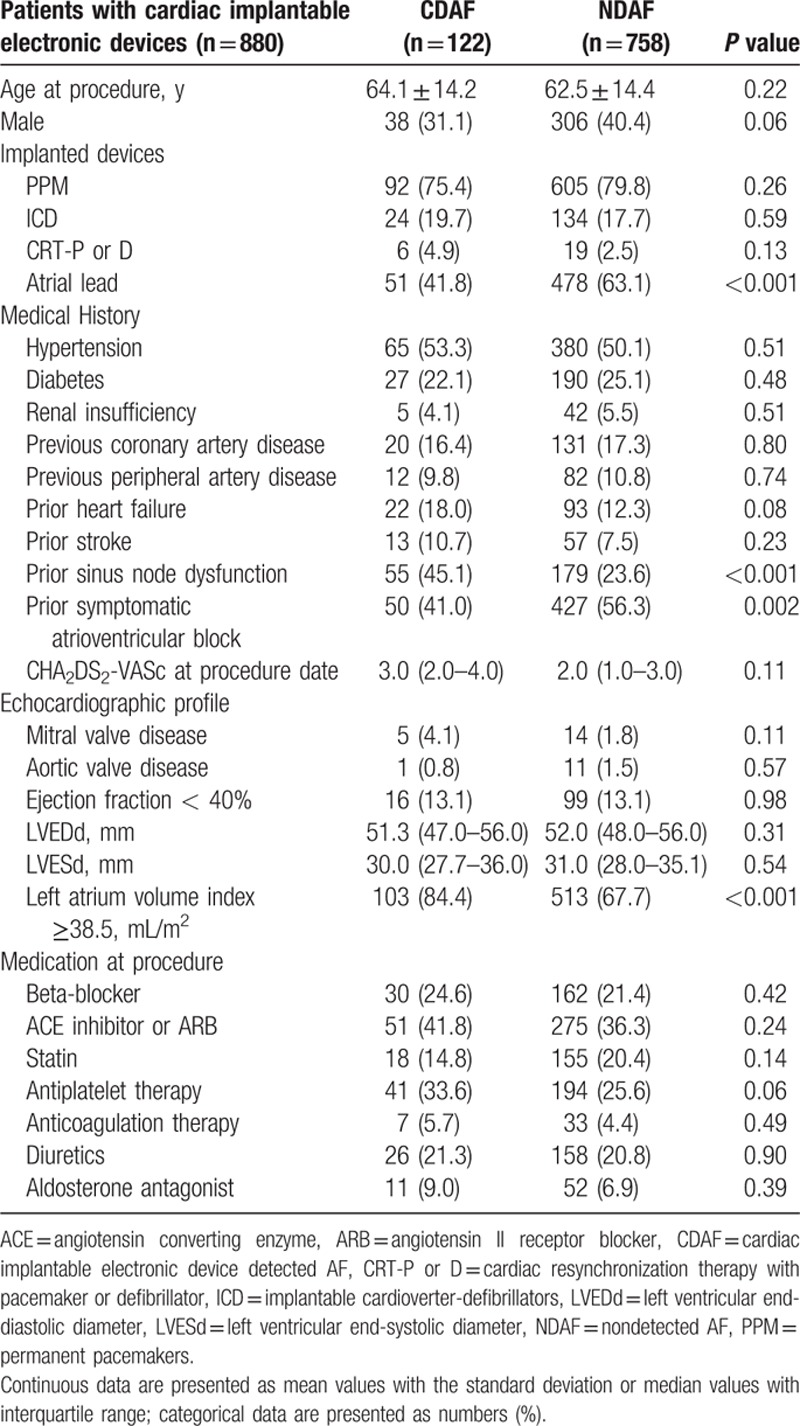
Among the 1825 registered patients, 296 patients with reimplanted CIEDs and 649 patients with previous documented AF/flutter at the time of the CIED implantation procedure were excluded. The remaining 880 patients were classified into 2 groups, namely, the NDAF group (n=758) and the CDAF group (n=112), according to development of AF over a follow-up period of 7 years. The overall median follow-up period was 55.2 (IQR 20.0–90.2) months, 75.0 (IQR 37.1–120.0) months in the CDAF group and 51.1 (IQR 18.1–97.6) months in the NDAF group. The baseline characteristics of the 2 groups at the time of CIED implantation are shown in Table 1. The frequency of CIED with atrial lead was lower in the CDAF group than in the NDAF group (41.8 vs 63.1%, P < 0.001). According to medical history data, the frequency of prior sinus node dysfunction was higher in the CDAF group compared to the NDAF group (45.1 vs 23.6%, P < 0.001), whereas the frequency of prior symptomatic AV block was lower in the CDAF group (41.0 vs 56.3%, P=0.002). Regarding the echocardiographic profile, the frequency of LA volume index ≥38.5 mL/m2was higher in the CDAF group than in the NDAF group (67.7 vs 84.4%, P < 0.001). There were no other significant differences in baseline characteristics between the 2 groups. The baseline characteristics of the CDAF and NDAF groups in the PPM subgroup and ICD or CRT subgroups are shown in Tables S1 and S2 in the Supplementary Appendix.
Table 1.
Baseline characteristics in patients with cardiac implantable electronic device without prior documented AF.
3.2. Incidence and predictors of AF
Overall, 122 of the 880 (13.8%) patients included in this study experienced newly developed AF during the follow-up period. The median duration from the implantation procedure date to development of AF was 17.2 (IQR 1.8–50.3) months. The mean age at development of AF was 66.3 ± 14.3 years. In the PPM subgroup, 92 of the 697 (13.2%) patients experienced newly developed AF. The median duration to development of AF was 17.2 (IQR 1.9–52.4) months and the mean age at development of was 69.4 ± 13.3 years. In the ICD and CRT subgroups, 30 of the 183 (16.4%) patients experienced newly developed AF. The median duration to development of AF was 16.2 (IQR 1.6–42.3) months and the mean age at development of AF was 57.1 ± 13.2 years. The Kaplan–Meier curves demonstrated that patients in the ICD or CRT subgroup experienced higher rates of new AF development than did patients in the PPM subgroup during the follow-up period (Log rank P = 0.035; Fig. 2).
Figure 2.
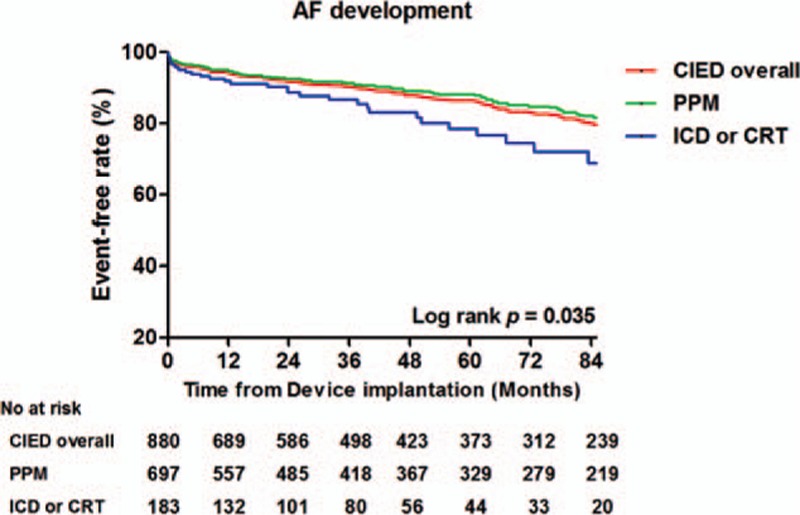
Kaplan–Meier curve for development of atrial fibrillation in patients with CIEDs.
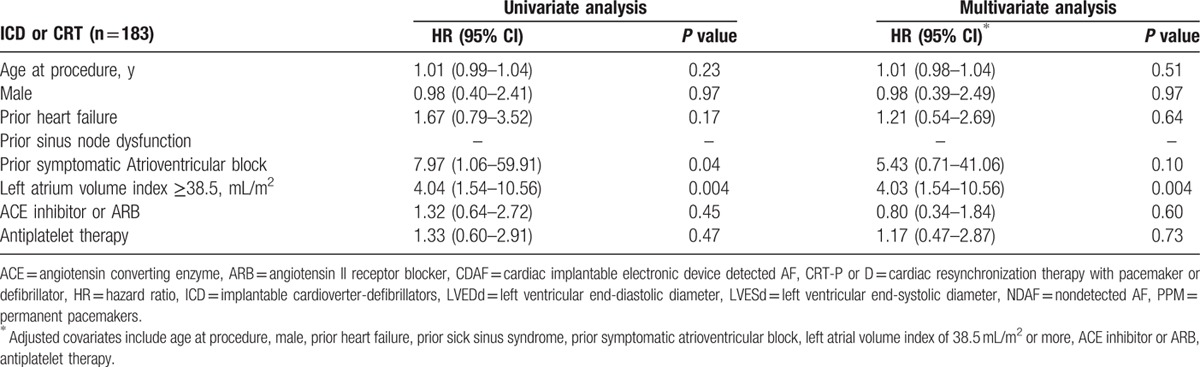
Significant univariate predictors of AF development were prior HF, prior sinus node dysfunction, prior symptomatic AV block, LA volume index ≥38.5 mL/m2, and use of antiplatelet therapy. According to a multivariate Cox regression model, the independent predictors for AF development were prior HF (hazard ratio [HR] 2.40; 95% confidence interval [CI] 1.50–3.85; P < 0.001), prior sinus node dysfunction (HR 2.33; 95% CI 1.62–3.55; P < 0.001), and LA volume index ≥38.5 mL/m2 (HR 2.01; 95% CI 1.23–3.30; P = 0.005) (Table 2; upper). An additional modified univariate model and multivariate Cox regression model, which excluded CRT devices, showed identical independent predictors for AF development (Table 2; lower). In the PPM subgroup, the independent predictors for AF development in the multivariate analysis were age at the time of the procedure (HR 1.02; 95% CI 1.00–1.04; P = 0.04), prior HF (HR 2.05; 95% CI 1.05–4.01; P = 0.03), prior sinus node dysfunction (HR 15.4; 95% CI 7.94–29.86; P < 0.001), and prior symptomatic AV block (HR 5.89; 95% CI 3.08–11.25; P < 0.001) (Table 3). In the ICD and CRT subgroups, the only independent predictor for AF development in the multivariate analysis was LA volume index ≥38.5 mL/m2 (HR 4.03; 95% CI 1.54–10.56; P = 0.004) (Table 4).
Table 2.
Independent predictors of newly developed AF following CIED implantation overall and when excluding patients with CRT devices.

Table 3.
Independent predictors of newly developed AF following implantation of permanent pacemaker.
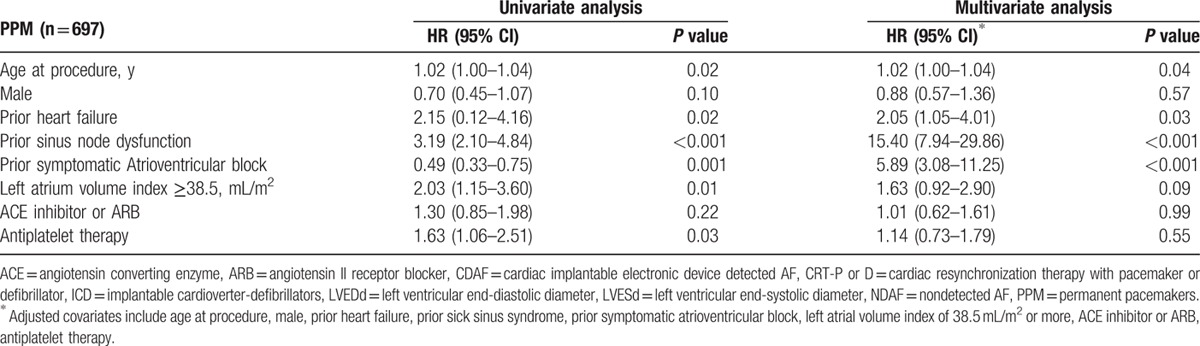
Table 4.
Independent predictors of newly developed AF following implantation of implantable cardioverter-defibrillators and cardiac resynchronization therapy device.
3.3. Long-term clinical outcomes according to AF development
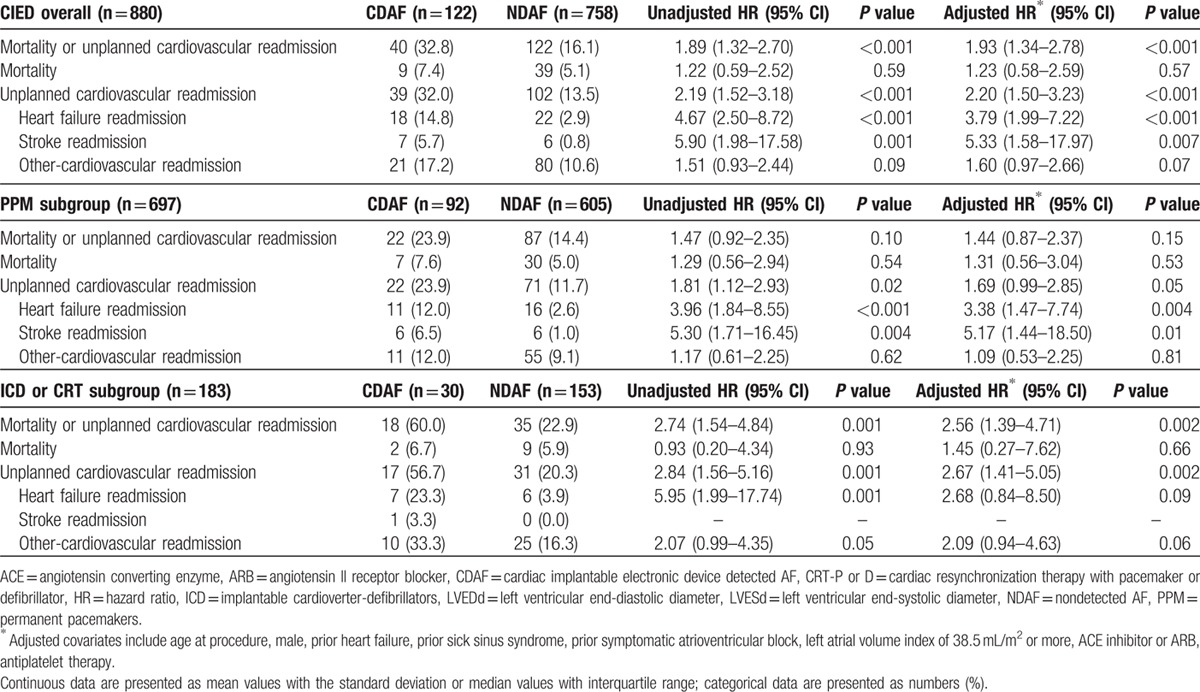
During the follow-up period, a primary endpoint event, defined as a composite outcome of mortality and unplanned cardiovascular readmission, occurred in 40 patients (32.8%) in the CDAF group and 122 patients (16.1%) in the NDAF group (Log rank P < 0.001) (Fig. 3A). Unplanned cardiovascular readmission occurred in 39 patients (32.0%) in the CDAF group and 102 patients (13.5%) in the NDAF group (Log rank P < 0.001) (Fig. 3B). HF readmission occurred in 18 patients (14.8%) in the CDAF group and 22 patients (2.9%) in the NDAF group (Log rank P < 0.001) (Fig. 3C). Stroke readmission occurred in 7 patients (5.7%) in the CDAF group and 6 patients (0.8%) in the NDAF group (Log rank P < 0.001) (Fig. 3D). Table 5 represents the clinical outcomes of the study population and a comparison of the unadjusted and adjusted hazard ratios between the CDAF and NDAF groups in patients with any type of CIED and in each device subgroup. Overall, the CDAF group, compared to the NDAF group, had a higher risk of unplanned cardiovascular readmission (adjusted HR 2.20; 95% CI 1.50–3.23; P < 0.001), HF readmission (adjusted HR 3.79; 95% CI 1.99–7.22; P < 0.001), stoke readmission (adjusted HR 5.33; 95% CI 1.58–17.97; P = 0.007), and composite outcome of mortality and unplanned cardiovascular readmission (adjusted HR 1.93; 95% CI 1.34–2.78; P < 0.001). There was no statistical difference with respect to the incidence of mortality and other cardiovascular readmissions between the CDAF and NDAF groups. In the PPM subgroup, the CDAF group had a higher risk of HF readmission (adjusted HR 3.38; 95% CI 1.47–7.74; P = 0.004) and stroke readmission (adjusted HR 5.17; 95% CI 1.44–18.50; P = 0.01). In the ICD or CRT subgroups, the CDAF group had a higher risk of HF readmission (adjusted HR 2.67; 95% CI 1.41–5.05; P = 0.002) and composite outcome of mortality and unplanned cardiovascular readmission (adjusted HR 2.56; 95% CI 1.39–4.71; P = 0.002).
Figure 3.
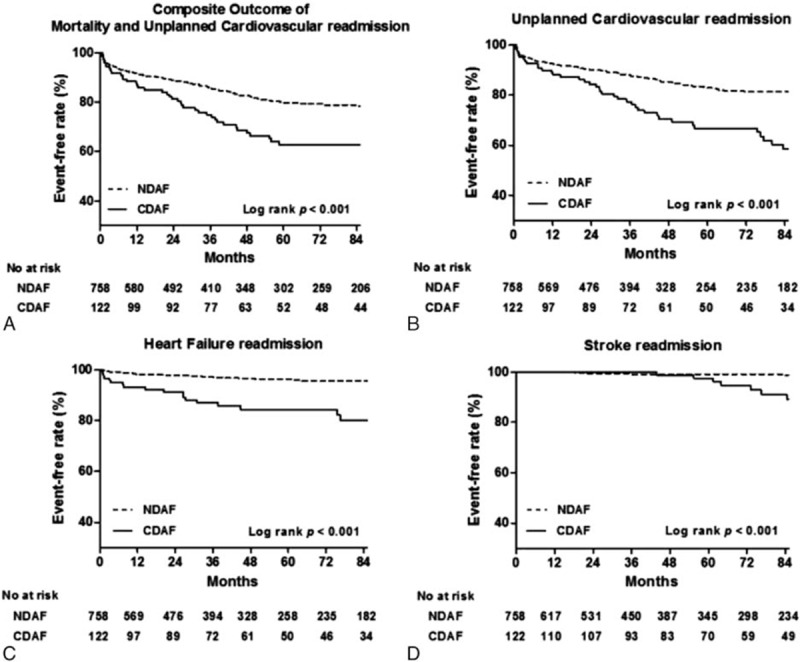
Kaplan–Meier analysis for (A) composite outcomes of mortality and unplanned cardiovascular readmission, (B) unplanned cardiovascular readmission, (C) heart failure readmission, and (D) stroke readmission according to the development of AF based on continuous monitoring by CIEDs.
Table 5.
Cumulative clinical outcomes at 7 years according to development of AF based on continuous monitoring by cardiac implantable electronic devices.
3.4. Clinical outcomes according to type of AF
In patients with newly developed AF, 46 patients (37.7%) had persistent AF and 76 patients (62.3%) had paroxysmal AF. Table 6 shows the clinical outcomes according to type of AF. During the follow-up period, there were no significant differences in the clinical outcomes between patients with persistent AF and those with paroxysmal AF.
Table 6.
Cumulative clinical outcomes at 7 years according to type of AF in continuous monitoring with cardiac implantable electronic devices.

4. Discussion
The results of this study can be summarized as follows: In patients without previously documented AF with CIEDs, the rate of newly developed AF was 13.8% over a 7-year follow-up period; prior history of HF, sinus node dysfunction, and large left atrium (LA) volume index were significant predictors for new development of AF in patients with CIED, and the predictors of AF development were different between the PPM subgroup and the ICD and CRT subgroups; and newly developed AF in patients with CIED was significantly associated with an increased risk of HF and stroke readmission according to long-term follow-up data.
Despite a lack of any associated clinical symptoms, AF is frequently noticed during continuous rhythm monitoring in patients with implanted CIED,[4,5,8,12] and patients with CIEDs constitute a growing and unique population. Thus, the information in this study regarding newly developed AF in patients with CIED may present a useful opportunity to gain a better understanding of the natural time course of AF, of which little is presently known.
Several studies of patients with pacemakers have reported that the incidence of AF and the predictive value of AF vary considerably over a given range of atrial rates and/or event durations.[12] A recent randomized trial, the Asymptomatic AF and Stroke Evaluation in Pacemaker Patients and the AF Reduction Atrial Pacing Trial (ASSERT), reported that subclinical AF (more than 190 beat/min lasting more than 6 minutes) can be detected in 35% of patients with a newly implanted pacemaker or ICD over a mean 2.5 years of follow-up.[5] In our study, AF development was defined as paroxysmal atrial tachyarrhythmia (atrial rate ≥180 beat/min detected on the device) lasting at least 5 minutes, the rate of which was 13.8% over a 7-year follow-up. The rate detected in the present study was lower than in other study,[12] which may have been because of the age difference and baseline characteristics of the patient population. Patients enrolled in the present study were much younger than patients in the ASSERT trial and consisted entirely of East Asians (Koreans). Aging is a well-known risk factor for the development of AF,[13,14] and ethnic differences in AF development have been reported in observational studies. Indeed, a large-cohort study showed that Asians have a significantly lower incidence of AF than Whites do.[15] A systematic review of worldwide population-based studies also revealed that the age-adjusted AF incidence rate is lowest among individuals from the Asia Pacific region.[16]
The risk factors for new development of AF in patients with CIEDs that we found were consistent with those reported by previous studies. Prior HF is an established risk factor for the development of AF in cross-sectional and cohort population studies using conventional detection methods.[6] Likewise, the ASSERT study and Canadian Trial of Physiologic Pacing both demonstrated that sinus node dysfunction is associated with an increased risk of AF.[5,17] The clinical association of these 2 diseases has long been recognized, suggesting that both diseases are associated with atrial structural remodeling.[18] Even before the development of atrial arrhythmia, patients with other conditions associated with atrial remodeling and atrial stretch may demonstrate significant sinus node dysfunction.[19] In several previous cohort studies, enlarged LA was identified as a significant risk factor for AF,[20] and another cross-sectional study found a higher prevalence of AF among individuals with a dilated LA compared with those with a normal-sized LA.[21] Our data also demonstrated that a large LA volume index is an independent predictor for newly detected AF in patients with CIEDs. In the subgroup analysis of the present study, the risk factors for new development of AF were different between the PPM subgroup and the ICD and CRT subgroups, which may have been due to different baseline characteristics of the patient population between the 2 groups (See Table S3 in the Supplementary Appendix).
With respect to long-term clinical outcomes, our data demonstrated an increased risk of unplanned cardiovascular readmission and composite mortality and unplanned cardiovascular readmission in the CDAF group, which were driven by higher rates of HF readmission and stroke readmission; however, there was no difference in the risks of mortality and other cardiovascular readmissions compared to the NDAF group. Previous studies have reported an increased risk of stroke events associated with device-detected atrial tachyarrhythmia,[4,5] and a similar tendency was noted in our results. By extending the follow-up duration, we found that the risk of unplanned cardiovascular readmissions was higher in the CDAF group compared to the NDAF group. In particular, a higher risk of HF readmission was associated with a higher risk of unplanned cardiovascular readmission over a follow-up period of 7 years. In the present study, clinical outcomes according to type of AF were not significantly different. This result is not consistent with post hoc analysis of a recent randomized trial, which demonstrated that patients with persistent AF have a higher risk of thromboembolic events and a worse survival rate compared with those with paroxysmal AF.[22] Our population was too small to evaluate clinical outcomes according to type of AF, even though AF detection was based on CIEDs.
These data suggest that more CDAF data can be collected from CIEDs for the purpose of risk stratification. Further studies will be required to determine if appropriate CDAF management can significantly affect morbidity and mortality.
5. Limitations
There were some limitations to the present study. First, the study design was nonrandomized, retrospective, and observational, which may have significantly affected the results owing to confounding factors. Although we performed multivariate analysis to adjust for these potential confounding factors, we were not able to correct for unmeasured variables. In particular, because of limitations in the patient database, we did not have information on several important factors, such as thyroid disease, cancer, and chronic pulmonary disease. A relatively small number of variables were included in the multivariate Cox proportional hazard model, which may have reduced the detection power and possibly influenced the validity of interactions. Second, this study was performed in a Korean population, the particular features of which most likely influenced the rate of AF and patient outcomes, as different races and populations have different levels of absolute risk, so the results are not generalizable.[16,23,24] Third, a measure of the ventricular pacing burden was not available in our study. In previous studies on pacemakers, ventricular pacing was shown to be correlated with increased rates of AF and HF readmission.[25,26] Because of the retrospective nature of our registry, we could not fully evaluate clinical outcomes according to the cumulative percentage of ventricular pacing burden.
6. Conclusions
Continuous rhythm monitoring using CIEDs revealed the frequent occurrence of AF after device implantation in patients without a previous history of AF. A prior history of HF, sinus node dysfunction, and large LA volume index were identified as significant predictors of new development of AF in patients with CIEDs. In addition, newly developed AF was significantly associated with an increased risk of HF and stroke readmission. These data suggest that more CDAF data from patients with CIEDs should be collected and used for risk stratification.
Acknowledgments
The authors thank Jung Wae Park, RN, and Hye Ran Yim, RN, for their assistance in the preparation of the data on CIEDs.
Supplementary Material
Footnotes
Abbreviations: ACE = angiotensin-converting enzyme, AF = atrial fibrillation, ARB = angiotensin II receptor blocker, AV = atrioventricular, CDAF = cardiac implantable electronic device-detected AF, CI = confidence interval, CIED = cardiac implantable electronic device, CRT = cardiac resynchronization therapy, HF = heart failure, HR = hazard ratio, ICD = implantable cardioverter-defibrillator, LA = left atrium, NDAF = non-detected AF, PPM = permanent pacemaker.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 2013; 34:2746–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Israel CW, Gronefeld G, Ehrlich JR, et al. Long-term risk of recurrent atrial fibrillation as documented by an implantable monitoring device: implications for optimal patient care. J Am Coll Cardiol 2004; 43:47–52. [DOI] [PubMed] [Google Scholar]
- 3.Glotzer TV, Daoud EG, Wyse DG, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol 2009; 2:474–480. [DOI] [PubMed] [Google Scholar]
- 4.Glotzer TV, Hellkamp AS, Zimmerman J, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation 2003; 107:1614–1619. [DOI] [PubMed] [Google Scholar]
- 5.Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. New Engl J Med 2012; 366:120–129. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad Y, Lip GY, Lane DA. Recent developments in understanding epidemiology and risk determinants of atrial fibrillation as a cause of stroke. Can J Cardiol 2013; 29:S4–S13. [DOI] [PubMed] [Google Scholar]
- 7.Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2013; 61:e6–e75. [DOI] [PubMed] [Google Scholar]
- 8.Mittal S, Stein K, Gilliam FR, 3rd, et al. Frequency, duration, and predictors of newly-diagnosed atrial fibrillation following dual-chamber pacemaker implantation in patients without a previous history of atrial fibrillation. Am J Cardiol 2008; 102:450–453. [DOI] [PubMed] [Google Scholar]
- 9.Bertini M, Borleffs CJ, Delgado V, et al. Prediction of atrial fibrillation in patients with an implantable cardioverter-defibrillator and heart failure. Eur J Heart Fail 2010; 12:1101–1110. [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005; 18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 11.Ho KK, Anderson KM, Kannel WB, et al. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 1993; 88:107–115. [DOI] [PubMed] [Google Scholar]
- 12.DeCicco AE, Finkel JB, Greenspon AJ, et al. Clinical significance of atrial fibrillation detected by cardiac implantable electronic devices. Heart Rhythm 2014; 11:719–724. [DOI] [PubMed] [Google Scholar]
- 13.Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994; 271:840–844. [PubMed] [Google Scholar]
- 14.Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet 2009; 373:739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dewland TA, Olgin JE, Vittinghoff E, et al. Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation 2013; 128:2470–2477. [DOI] [PubMed] [Google Scholar]
- 16.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014; 129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skanes AC, Krahn AD, Yee R, et al. Progression to chronic atrial fibrillation after pacing: the Canadian Trial of Physiologic Pacing. CTOPP Investigators. J Am Coll Cardiol 2001; 38:167–172. [DOI] [PubMed] [Google Scholar]
- 18.Chang HY, Lin YJ, Lo LW, et al. Sinus node dysfunction in atrial fibrillation patients: the evidence of regional atrial substrate remodelling. Europace: European pacing, arrhythmias, and cardiac electrophysiology: journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 2013; 15:205–211. [DOI] [PubMed] [Google Scholar]
- 19.Lee JM, Kalman JM. Sinus node dysfunction and atrial fibrillation: two sides of the same coin? Europace 2013; 15:161–162. [DOI] [PubMed] [Google Scholar]
- 20.Tiwari S, Schirmer H, Jacobsen BK, et al. Association between diastolic dysfunction and future atrial fibrillation in the Tromso Study from 1994 to 2010. Heart (British Cardiac Society) 2015; 101:1302–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qureshi W, Soliman EZ, Solomon SD, et al. Risk factors for atrial fibrillation in patients with normal versus dilated left atrium (from the Atherosclerosis Risk in Communities Study). Am J Cardiol 2014; 114:1368–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinberg BA, Hellkamp AS, Lokhnygina Y, et al. Higher risk of death and stroke in patients with persistent vs. paroxysmal atrial fibrillation: results from the ROCKET-AF Trial. Eur Heart J 2015; 36:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conway DS, Lip GY. Ethnicity in relation to atrial fibrillation and stroke (the West Birmingham Stroke Project). Am J Cardiol 2003; 92:1476–1479. [DOI] [PubMed] [Google Scholar]
- 24.Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol 2014; 11:639–654. [DOI] [PubMed] [Google Scholar]
- 25.Sweeney MO, Hellkamp AS, Ellenbogen KA, et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003; 107:2932–2937. [DOI] [PubMed] [Google Scholar]
- 26.Elkayam LU, Koehler JL, Sheldon TJ, et al. The influence of atrial and ventricular pacing on the incidence of atrial fibrillation: a meta-analysis. Pacing Clin Electrophysiol 2011; 34:1593–1599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


