Abstract
Introduction:
The World Health Organisation recognizes confusing drug names as one of the most common causes of medication errors. Other factors include spelling, phonetic, or packaging similarities.
Case presentation:
We presented a case report of an inadvertent administration of a non-ocular pharmaceutical product (Novasone® lotion) into the eye of an octogenarian individual, and briefly reviewed the relevant literature.
Discussion:
We discussed prevention strategies to avoid similar ophthalmic medication errors.
Keywords: eye, eye drop, medication error, nonocular pharmaceutical product, novasone
1. Introduction
Medication errors refer to a failure during the treatment process which may lead to patient harm.[1] The World Health Organisation identified that confusing drug names is one of the most common causes of medication errors.[2] Other factors that contribute to potential confusion between drug names include spelling, phonetic, or packaging similarities.[3] Medication errors have been well-described in the medical literature, particularly in the hospital settings.[4] Nursing staff were identified as major contributors to medication administration errors, as they are largely responsible for administering medication.[4] At present, there is limited evidence available regarding medication administration errors that occur in the community setting.[5] The accidental ophthalmic administration of nonocular pharmaceutical products appears to be both under-reported and unrecognized in the international medical literature.[5]
2. Ethical considerations
The authors of this report referred to a deceased individual. However, all identifiable personal details have been properly anonymised to protect the patient's identity. There is no identifiable data presented in the manuscript.
3. Case presentation
An octogenarian woman in a nursing home was mistakenly administered a Novasone scalp lotion in her left eye. The Novasone lotion was mistaken for a lubricating eye drop, which was similar in shape and size (Fig. 1). The mistake was identified by the resident as she felt pain, burning sensation, and discomfort in her eye immediately after the administration. The resident indicated that the wrong eye drop must have been given to her. Within a few minutes, her eye was washed with saline with immediate good response. Nursing staff contacted the general practitioner and received advice to continue cleansing with normal saline and report if there is any concern. No ophthalmology review was conducted. The supplier pharmacy was contacted and the bottle was re-labeled. In this particular case, there was no major harm suffered by the patient as a result of this medication error, other than self-limiting pain and discomfort in the eye.
Figure 1.

Similarities in packaging. Please note that both products are manufactured in dropper bottles.
4. Discussion
To our knowledge, this is the first clinical case in the international medical literature, reporting an inadvertent instillation of mometasone scalp lotion into the eye. However, of concern, this error does not appear to be uncommon. A recent retrospective review of calls made to an Australian Poisons Information Centre from 2004 to 2011 identified ∼1290 cases involving accidental eye administration of pharmaceutical products not intended for ophthalmic use during the 7-year period[5] (Fig. 2). The most common pharmaceutical product accidently instilled into the eye was mometasone (Elocon/Novasone lotion); other products included antiseptic, antifungal, antibacterial, and ear wax removal preparations.[5] Notably, the majority of the products incorrectly instilled into the eye were in dropper bottles. Out of the total number of cases reported to Australian Poisons Information Centre, >75 percent involved adults. Twenty-seven percent of these affected individuals received medical assistance.[5] Additionally, 3 individuals had suffered corneal ulceration, including 1 case which resulted from the application of mometasone lotion.[5]
Figure 2.
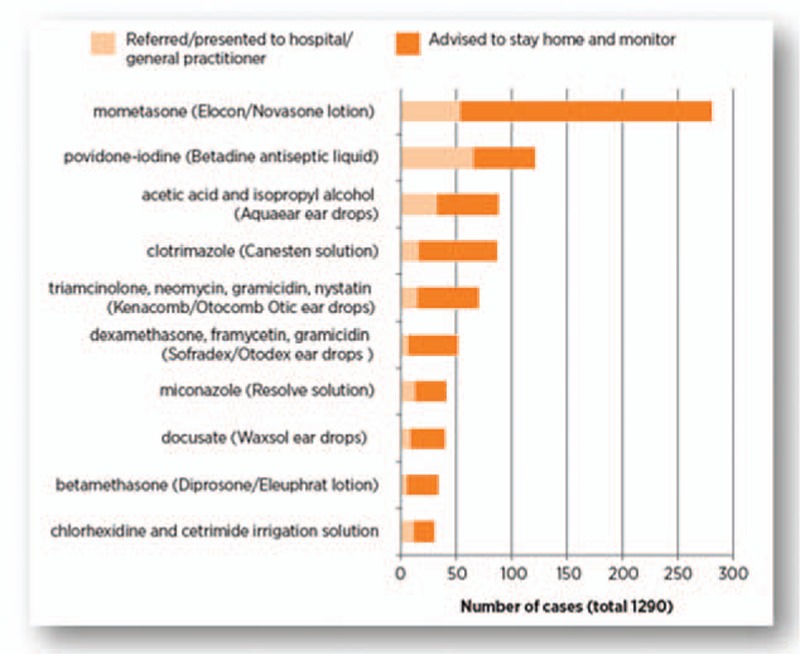
Most common pharmaceuticals accidentally administered into the eye and reported to the New South Wales Poisons Information Centre, Australia; 2004–11 (republished with permission).[5]
Novasone and Elocon lotion contains mometasone furoate (1 mg/g active ingredient), isopropyl alcohol, propylene glycol, hydroxyl-propylcellulose, and water, with small quantities of phosphoric acid and sodium phosphate monobasic dihydrate as a buffer to maintain the pH of the formulation.[6,7]
Given the low concentration of mometasone (1 mg/g), the active ingredient is unlikely to cause the observed acute eye irritation. The buffering agents are usually incorporated at low concentrations and are unlikely to induce the observed irritation. Hydroxy-propylcellulose is an emulsifier, stabilizer and thickener which in the form of a slow release insert is used in the treatment of dry eye which is an unlikely cause of eye irritation in the current context.[8] Therefore, the most likely irritants present in mometasone lotion, which led to the observed eye irritation are propylene glycol and isopropyl alcohol.
Isopropyl alcohol is known to cause eye irritation and after prolonged exposure can cause temporary changes in the corneal epithelium. Animal tests of Isopropyl alcohol also confirm its irritation potential.[9] In humans where the cornea has become exposed to 70% isopropyl alcohol to cleanse the eye lids prior to ocular surgery, it has caused pain but no ocular damage if removed promptly.[9] Applying propylene glycol into the eye causes eye stinging, involuntary twitching (blepharospams), and increased tears (lacrimation) and mild hyperaemia (reddening of the eye), but without damage to the ocular tissues.[10]
In general, errors are presumed to occur due to human and underlying system failures rather than being random events.[11] The term “the Swiss cheese model” was proposed by James Reason[12] to describe accident causation as depicted in Fig. 3. The holes in the cheese form as a result of both active and latent failures and the majority of adverse events occur when these 2 factors come together.[12] The holes present in the Swiss cheese model are not individually responsible for the errors or do not normally cause an adverse outcome, rather when these holes are in alignment, they are more likely to lead to an error.[12]
Figure 3.

The Swiss cheese model of how defenses, barriers, and safeguards may be penetrated by an accident trajectory (republished with permission).[12]
In the present case, there were several system failures that preceded the medication administration error itself, including storage of the Novasone lotion in the same place as the eye drop. Because the packaging of both products were similar in size and shape, by placing them closely to each other, it increased the likelihood of administering the incorrect formulation to the patient and this is a classic example of a latent failure as “accident waiting to happen.”[11] Additionally, the Novasone lotion was not clearly labeled by the dispensing pharmacy “apply to area as directed by the doctor,” unfortunately, the exact area of application was not specified at all, and this was coupled with the absence of checking procedures by the care staff (active failure). The above factors in combination might have contributed to this particular medication administration error. Interestingly, the care staff member was doing her usual shift and the incident occurred during the morning shift; therefore, fatigue did not appear to be a factor that contributed to this error.
4.1. Recommendations for preventing ophthalmic errors
Below suggested are some of the recommendations to avoid ophthalmic medication errors proposed by the Institute for Safe Medication Practices[13]:
keep eye drops in the original box;
separate eye drops from other types of drops by storing them in different locations;
always discard any leftover eye drops; and
read the label carefully to confirm the correct medication has been selected for administering.
Additionally, dispensary staff in the pharmacy must label nonocular medicines that come in dropper bottles clearly and thoroughly to avoid any potential confusion leading to medication errors.[5] Similarly, at the time of dispensing, it may be worthwhile to remind the patients that nonocular medicine in dropper bottles have the potential to be accidently mistaken for eye drops.[5]
5. Conclusion
This is the first case in the international literature reporting an inadvertent instillation of mometasone scalp lotion into the eye; however, this appears to be a commonly reported error in Australia. Additional efforts to reduce the occurrence of this error are warranted.
Footnotes
Declaration: this paper has not been previously presented or published elsewhere.
Authorship: All authors have contributed to the design and writing of the manuscript.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Aronson JK. Medication errors: what they are, how they happen, and how to avoid them. QJM 2009; 102:513–521. [DOI] [PubMed] [Google Scholar]
- 2.Centre for Patient Safety Solutions, WH.O.C. Look-alike sound-alike medication names. Patient Safety Solutions. Volume 1. 2007. [Google Scholar]
- 3.Emmerton LM, Rizk MF. Look-alike and sound-alike medicines: risks and ‘solutions’. Int J Clin Pharm 2012; 34:4–8. [DOI] [PubMed] [Google Scholar]
- 4.Hughes RG, Blegen MA. Medication administration safety. Patient safety and quality: An evidence-based Handbook for nurses. Rockville (MD) 2008. [Google Scholar]
- 5.Brown JA. Medicinal mishap: Incorrectly dropped in the eye. Australian Prescriber 2013; 36:56–57. [Google Scholar]
- 6.Merck Sharp & Dohme (Australia) Inc. Novasone Cream, Ointment and Lotion Product Information. Available at: http://secure.healthlinks.net.au/content/msd/pi.cfm?product=mkpnovas (accessed October 9, 2015). [Google Scholar]
- 7.Merck Sharp & Dohme Corp. Full Prescribing Information: ELOCON® Lotion. Available at: http://www.merck.com/product/usa/pi_circulars/e/elocon_lotion/elocon_lotion_pi.pdf (accessed October 9, 2015). [Google Scholar]
- 8.Martindale W, Reynolds JEF. Martindale The Extra Pharmacopoeia. 31st ed.London: The Royal Pharmaceutical Society; 1996. [Google Scholar]
- 9.POISINDEX System (electronic version). In: Micromedex 2.0 [Internet]. Greenwood Village (CO): Truven Health Analytics; Available from: http://www.thomsonhc.com (accessed October 9, 2015). [Google Scholar]
- 10.Grant M, Schuman JS. Toxicology of the Eye. 4th ed.Illinois, USA: Charles C Thomas Pub Inc; 1993. [Google Scholar]
- 11.Fry MM, Dacey C. Factors contributing to incidents in medicine administration. Part 1. Br J Nurs 2007; 16:556–558. [DOI] [PubMed] [Google Scholar]
- 12.Reason J. Human error: models and management. Br Med J 2000; 320:768–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Institute for Safe Medication Practices. Safe Medicine Newsletter. Ear drops in eyes: a painful mistake. Volume 5(1). Jan/Feb 2007; Available at: http://www.med.navy.mil/sites/nhbeaufort/Patients/Documents/Safe%20Medicine.pdf (accessed October 9, 2015). [Google Scholar]


