Supplemental Digital Content is available in the text
Keywords: BMI, complications, inpatient, mortality, obesity, racial disparities
Abstract
Importance:
Over one-third of American adults (36%) are obese and more than two-thirds (69%) are overweight. The impact of obesity on hospitalization outcomes is not well understood.
Objective:
To examine the association between body mass index (BMI) and overall, cancer, chronic obstructive pulmonary disease (COPD), asthma, and cardiovascular disease (CVD)-specific in-hospital mortality; postsurgical complications; and hospital length of stay (LOS).
Design:
Cross-sectional study.
Setting:
Representative sample of US hospitals included in the Health Cost and Utilization Project Nationwide Inpatient Sample database.
Participants:
We obtained data for patients admitted with a primary diagnosis of cancer, COPD, asthma, and CVD.
Main Outcome:
In-hospital mortality, postsurgical complications, and hospital LOS.
Results:
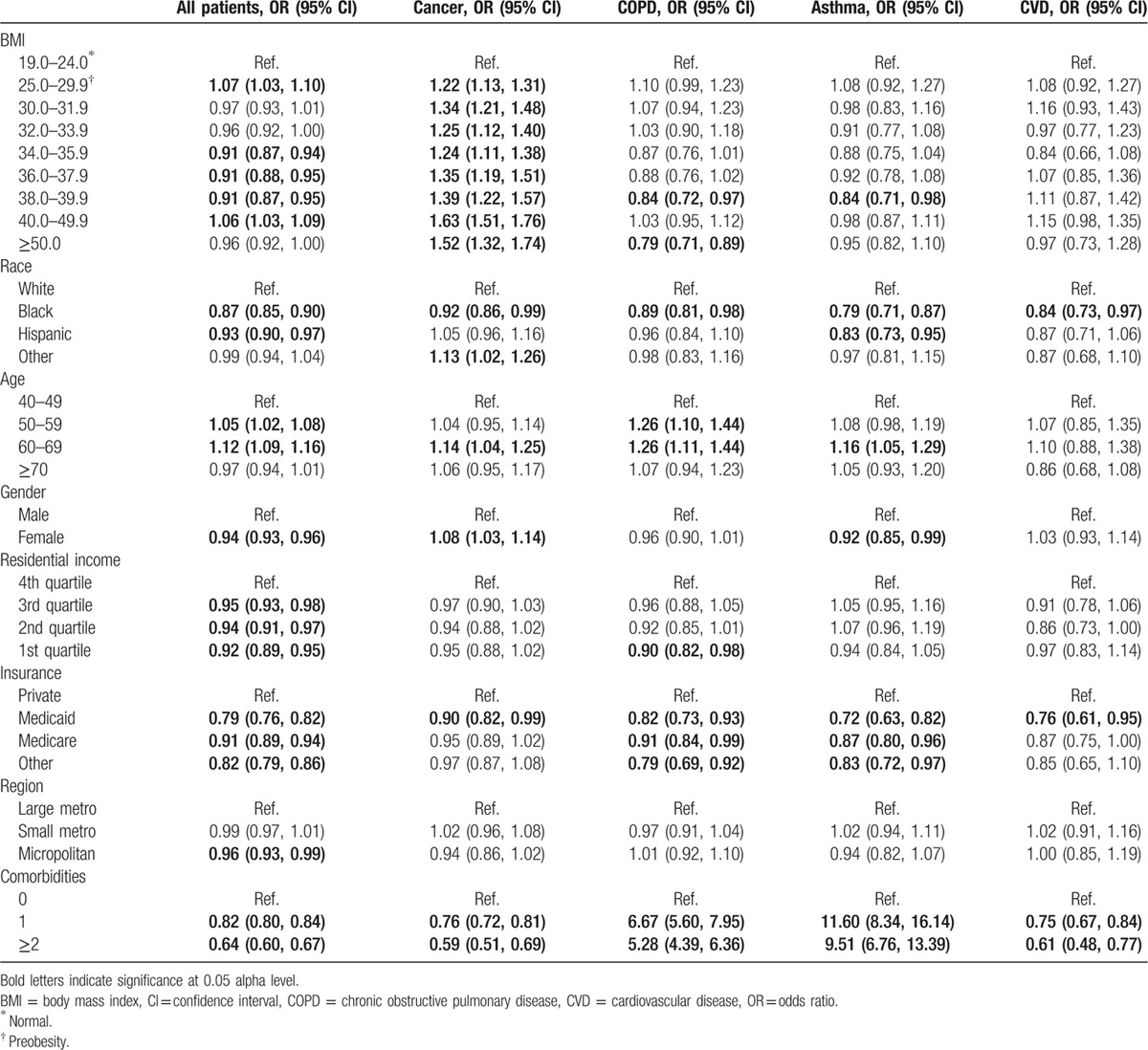
A total of 800,417 patients were included in this analysis. A higher proportion of Blacks (26.8%; 12.5%) and Whites (23.3%; 8.7%) had BMI of 40 to 49.9 and ≥50, respectively, compared with Hispanics (20.4%; 7.3%). Compared with normal BMI patients, the odds of in-hospital mortality increased 3.6-fold (odds ratio [OR] 3.62, 95% confidence interval [CI]: 3.37–3.89) for preobese patients, 6.5-fold (OR: 6.52, 95% CI: 5.79–7.34) for patients with BMI: 30 to 31.9, 7.5-fold (OR: 7.57, 95% CI: 6.67–8.59) for patients with BMI: 34 to 35.9, and 1.6- fold (OR: 1.77, 95% CI: 1.56–1.79) for patients with BMI ≥ 50. Compared with normal BMI patients, preobese and overweight patients had shorter hospital stays (β preobese: −1.58, 95% CI: −1.63, −1.52); however, no clear trends were observed for postsurgical complications.
Conclusions:
The majority of hospitalized patients in this analysis had a BMI > 30, and higher BMI was associated with increased risk of mortality and longer hospital stay.
1. Introduction
In recent decades, the prevalence of obesity has increased substantially in North America reaching epidemic proportions.[1] It is estimated that over one-third of American adults (36%) are obese and more than two-thirds (69%) are overweight.[2] While there are many parameters describing body weight status, the most popular formula is the Quetelet index, also known as body mass index (BMI).[3] The BMI can be calculated easily and quickly and thus it is widely used both in research and clinical areas. BMI of between 25 and 29.9 is considered as overweight, while BMI of 30 or greater is considered obese based on recent National Institute of Health (NIH) classification.[4] Overweight and obesity have long been considered major risk factors for mortality in the general population. Overweight and obesity are associated with a variety of cardiometabolic diseases, such as type 2 diabetes mellitus, hypertension, hyperlipidemia, metabolic syndrome, cardiovascular disease (CVD), and certain types of cancers,[4] and these conditions in turn contribute to increased mortality.[5,6]
Given these associations, we might safely infer that overweight or obese individuals would have more adverse outcomes than healthy weight individuals. Yet, there is increasing evidence that patients, especially elderly with several chronic diseases and elevated BMI, may demonstrate lower all-cause and cardiovascular mortality compared with patients of normal weight. For instance, lower mortality was observed among patients with a BMI range of 20 to 24.9 kg/m2 (for nonsmokers in the American and European populations) while mortality increases below and above this range.[7] This unexpected phenomenon has been termed the “obesity paradox,” and has also been observed in other diseases such as diabetes,[8–10] coronary artery disease,[11,12] heart failure,[13] peripheral arterial disease,[12] hypertension,[14] chronic obstructive pulmonary disease (COPD),[15] and cancer.[16,17] However, other studies have found no evidence supporting this pattern.[18,19]
The inconsistent patterns observed in the obesity–mortality association may be attributable to variations in the cutoff values for BMI categories used in different studies, or in race/ethnicity differences in the pattern of healthy obesity,[20,21] in addition to methodological differences such as source of BMI data (self-reported vs objective measures), residual confounding and/or selection bias. While the obesity paradox is observation of a protective effect in overweight and obese individuals, few studies have examined mortality outcomes in extreme BMI ranges among more severely obese populations, such as those with BMI > 40 kg/m2. Few studies have examined higher ranges of BMI to determine health outcomes. Furthermore little is known about the relationship between obesity, particularly extreme obesity, and chronic disease outcomes particularly among hospitalized individuals. A limitation of prior studies is the lack of detailed data on other health conditions and comorbidities among patients that may strongly influence the obesity–mortality relationship. In addition, differences in baseline demographic characteristics, such as race/ethnicity, social and economic conditions, can contribute to differences in the association between obesity and disease outcomes.[22] For instance, despite the similar low socioeconomic status (SES) of Hispanics and African Americans relative to Whites,[23] and similar propensity for diabetes and obesity, Hispanics experience lower all-cause and cardiovascular mortality than African Americans and Whites.[22,24]
Here, we studied the association between BMI and overall, cancer, COPD, asthma, and CVD-specific in-hospital outcomes in a large racial and socio-economically diverse study population, and determined whether race/ethnicity and SES differences remained after accounting for BMI.
2. Methods
2.1. Study population
Data for this analysis were obtained from the Health Cost and Utilization Project Nationwide Inpatient Sample (HCUP-NIS) inpatient database. The HCUP-NIS is a large all-payer inpatient care database on over 7 million hospitals stays and covers over 1000 hospitals in the United States.[25] The database includes clinical variables on all diagnoses and procedures occurring during each hospital admission, and is widely considered the most valid and reliable source of epidemiological data on in-patient care and outcomes in the United States. Nonclinical variables are also included in the database, including median household income in the patient's zip code, rural/urban residence, and expected payment source. More information on HCUP-NIS can be obtained: https://www.hcup-us.ahrq.gov/nisoverview.jsp. All hospitalized patients ages 40 years and above diagnosed between 2007 and 2011 were included in this analysis. This study was considered institutional review board exempt as it involved analysis of de-identified, publicly available datasets.
2.2. Classifying BMI
The BMI is a measure of body fat based on individual (both men and women) height and weight. It is calculated as a person's weight in kilograms (kg) divided by their height in meters squared (m2). The HCUP-NIS includes specific International Classification of Diseases—9th Revision (ICD-9) codes (V85.XX) for BMI in cases where such measures were obtained during hospital admission. The NIH categorizes BMI < 18.5 as underweight, 18.5 to 24.9 as normal weight, 25 to 29.9 as overweight, and BMI of 30 or greater as obese. Since the purpose of this analysis was to determine the influence of body weight on hospitalization outcomes, we created a wide range of BMI categories: normal (BMI ≤ 24; ICD-9 V85.0–V85.1), preobesity (BMI 25–29; ICD-9 V85.21–V85.25), BMI 30 to 31.9 (ICD-9 V85.30–V85.31), BMI 32 to 33.9 (ICD-9 V85.32–V85.33), BMI 34 to 35.9 (ICD-9 V85.34–V85.35), BMI 36 to 37.9 (ICD-9 V85.36–V85.37), BMI 38 to 39.9 (ICD-9 V85.38–V85.39), BMI 40 to 49.9 (ICD-9 V85.41–V85.42), and BMI ≥ 50 (ICD-9 V85.43–V85.54).
2.3. Clinical variables
We examined the association between BMI categories and overall mortality, disease-specific mortality, postsurgical complications, and hospital length of stay (LOS-defined as number of days between admission and discharge, with single day admissions coded as 0) among patients admitted with a primary diagnosis of cancer (ICD-9: 140–209); COPD (ICD-9: 490, 491, 494, 496); asthma (ICD-9: 493); and CVD (ICD-9: 430–438). In addition, to account for comorbidities that may contribute to risk of mortality, we created a modified Deyo comorbidity index using ICD-9 codes.[26–28] The conditions included diabetes mellitus with or without dementia, rheumatic disease, peptic ulcer disease, mild liver disease, hemiplegia or paraplegia, renal disease, moderate or severe liver disease, and HIV/AIDS. The presence of each condition within each patient was identified, and a single comorbidity score was created as the sum of the number of conditions per patient and was categorized as 0, 1, and ≥2.
2.4. Individual variables
Other covariates used in the analysis included: race/ethnicity (White, Black, Hispanic, and other), age, gender, residential income, insurance type, and residential region. Age was categorized into 40 to 50, 50 to 60, 60 to 70, and >70 years; residential income was categorized into quartiles ranging from the lowest income to the highest income based on median household income at the zip code level; residential region was categorized into large metropolitan areas (metropolitan areas with 1 million residents or more, reference group), small metropolitan areas (metropolitan areas with <1 million residents), and micropolitan areas (nonmetropolitan areas adjacent to metropolitan areas) using the 2003 version of the Urban Influence Codes.[29] Insurance status was classified into Medicaid, Medicare, private (includes Blue Cross, commercial carriers, private health maintenance organizations and preferred provider organizations, and self-insured, reference group) and others (includes Worker's Compensation, Title V, and other government programs).
2.5. Statistical analysis
We conducted descriptive statistics to examine differences in the distribution of the 9 BMI categories by race/ethnicity and other sociodemographic variables using chi-squared tests and analysis of variance (for continuous variables). Logistic regression analysis was performed to determine the association between each BMI category and overall, cancer, COPD, asthma, and CVD-specific in-hospital mortality adjusting for race/ethnicity; age; gender; residential income; insurance type; residential region; and comorbidities. Multivariable linear regression models were used to examine the association between BMI categories and hospital LOS, and multivariable logistic regression models were used to examine postoperative complication outcomes. All statistical analyses were conducted in SAS 9.4.
3. Results
This study included a total of 800,417 patients, 72% White, 16% Black, 8.4% Hispanic, and 4% other race (Table 1). The distribution of BMI categories by other sociodemographic variables is presented in Table 2. A higher proportion of other race (19.4%) and Whites (13.2%) were of normal weight (BMI 19–24) compared with Blacks (11.8%) and Hispanics (11.5%); and a higher proportion of other race (15.8%) and Hispanics (15.1%) were preobese (BMI 25–29.9) compared with Whites (12.3%) and Blacks (10.9%). However, a higher proportion of Blacks (26.8%; 12.5%) and Whites (23.3%; 8.7%) had BMI of 40 to 49.9 and ≥50, respectively, compared with Hispanics (20.4%; 7.3%) and other race (16.9%; 6.3%). Patients with normal BMI tended to have a higher mean age (71.5 years) at admission, and the mean age tended to decrease as BMI increased. There was a higher proportion of females in the higher BMI categories (BMI > 36) compared with males, and the proportion of residents in high residential income areas tended to decline as BMI increased. A higher proportion of patients with normal BMI had 1 (10.6%) or ≥2 (1.9%) comorbid conditions, compared the highest BMI category with 1 (2.8%) or ≥2 (0.6%) comorbid conditions. Similarly, the proportion of patients with a primary diagnosis of cancer (6.0%), asthma (1.2%), and COPD (7.3%) was the highest among patients of normal weight, except patients with BMI 40 to 49.9, who had a higher prevalence of asthma (2.5%).
Table 1.
Distribution of BMI categories by race/ethnicity, Nationwide Inpatient Sample 2007 to 2011.
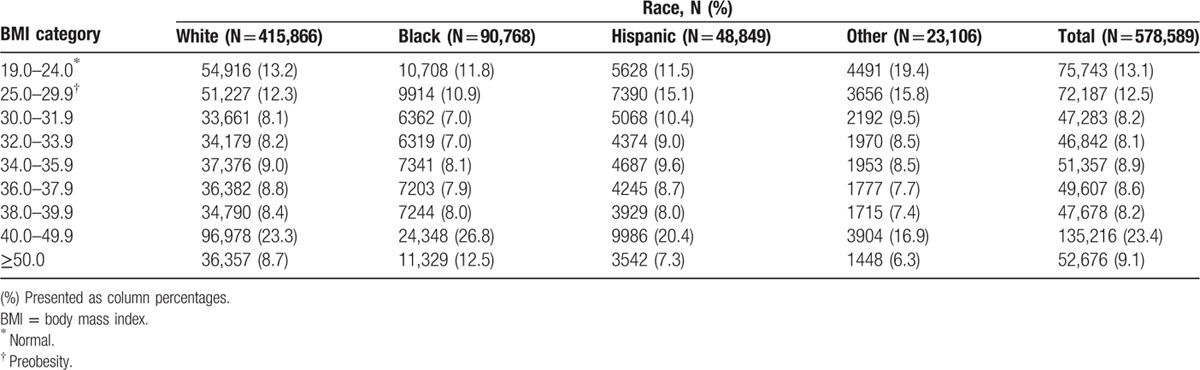
Table 2.
Socio-demographic characteristics by BMI categories, Nationwide Inpatient Sample 2007 to 2011.
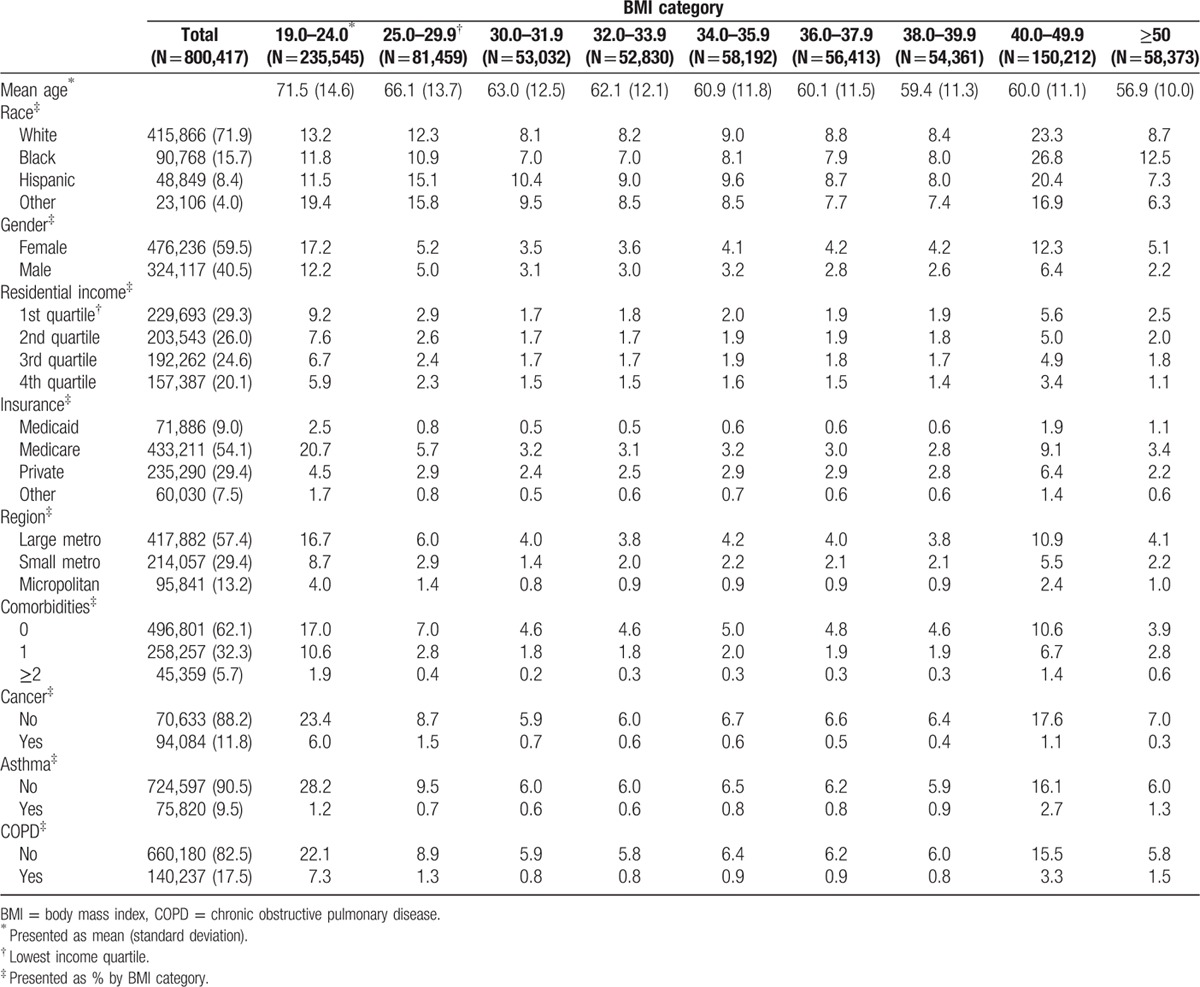
Figure 1 shows the odds of in-hospital mortality in relation to increasing BMI, which appeared to follow a bell-shaped curve, increasing 3.6-fold (odds ratio [OR] 3.62, 95% confidence interval [CI]: 3.37–3.89) for preobese compared with normal BMI patients, 6.5-fold (OR: 6.52, 95% CI: 5.79–7.34) for patients with BMI: 30 to 31.9, 7.5-fold (OR: 7.57, 95% CI: 6.67–8.59) for patients with BMI: 34 to 35.9, 2.5-fold (OR: 2.59, 95% CI: 2.46–2.73) for patients with BMI 40 to 49.9, and 1.6-fold (OR: 1.77, 95% CI: 1.56–1.79) for patients with BMI ≥ 50. There were no racial differences in overall mortality after accounting for BMI and other study covariates, except for patients of other race who experienced significantly lower odds of mortality (OR: 0.88, 95% CI: 0.82–0.95) compared with Whites.
Figure 1.
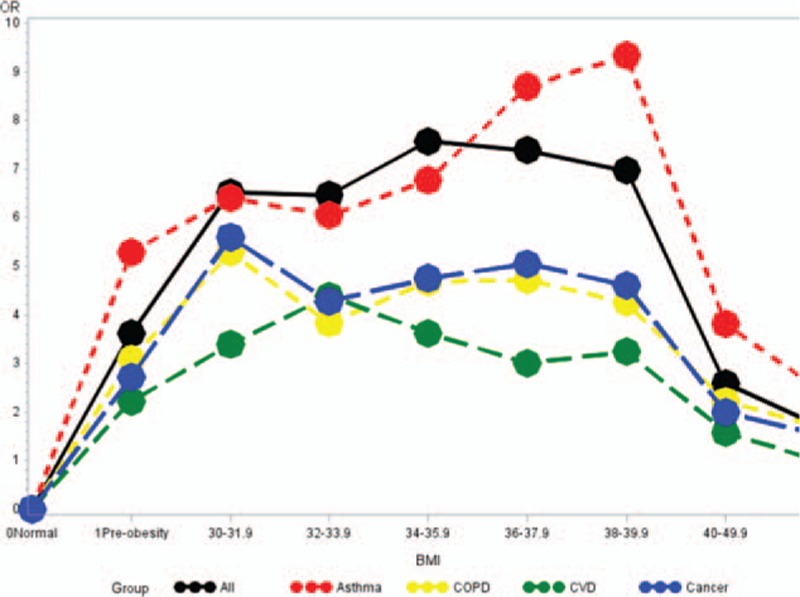
Odds ratio for in-hospital mortality by BMI for major chronic disease. BMI = body mass index.
There were clear trends of decreasing number of days spent in the hospital with increasing BMI and then an attenuation of effect in the highest BMI categories (Fig. 2). Preobese patients spent an average of 1.6 days less (β: −1.58, 95% CI: −1.63, −1.52) in the hospital compared with normal weight patients, while patients with BMI 30 to 39.9 spent an average of 2 days less in the hospital. Blacks (β: 0.38, 95% CI: 0.33, 0.43), Hispanics (β: 0.24, 95% CI: 0.18, 0.31), and other (β: 0.84, 95% CI: 0.76, 0.92) races experienced longer hospital stays compared with Whites as did females, patients residing in higher residential income areas and patients with at least 1 comorbid. Similar trends by BMI category were observed among cancer, COPD, asthma, and CVD patients. OR and estimates from multivariable adjusted analysis of in-hospital mortality and hospital LOS in relation to BMI are presented in Supplementary Tables 1 and 2.
Figure 2.
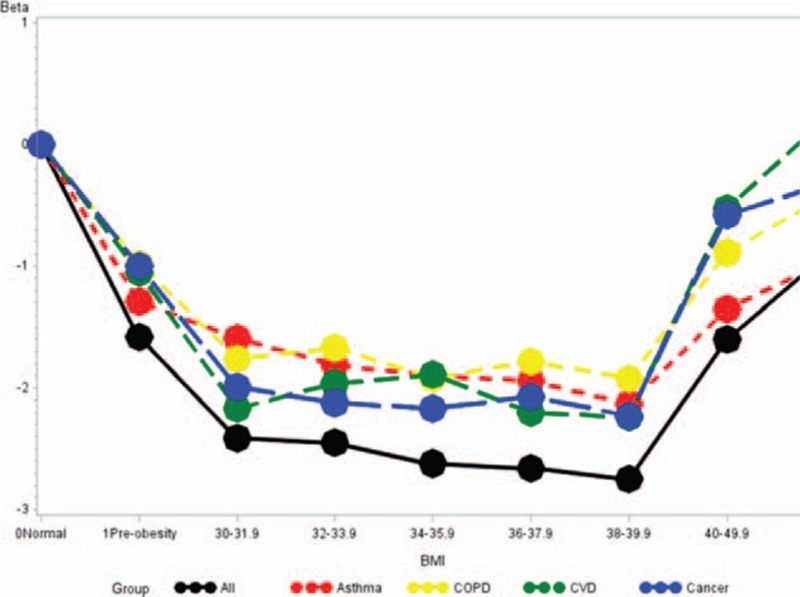
Estimated hospital length of stay by BMI for major chronic diseases. BMI = body mass index.
In multivariable adjusted analysis assessing medical complications overall (Table 3), there were no clear trends across BMI categories, and preobese patients had slightly higher odds of complications (OR: 1.07, 95% CI 1.03–1.10) compared with normal weight patients, while patients with BMI 34 to 39.9 had about 9% lower odds of complications. In addition, Blacks, Hispanics, and females also experienced lower odds of complications, as well as patients residing in higher residential income areas and patients with at least 1 comorbid condition. Among cancer patients, there was a trend of increasing odds of complications with increasing BMI, ranging from 22% higher odds among the preobese to 63% higher odds among patients with BMI 40 to 49.9. Cancer patients with 1 or ≥2 comorbid condition also experienced 5- to 7-fold increased odds of complications (OR for 1 complication: 6.67, 95% CI: 5.60–7.95; OR for ≥2 complications: 5.28, 95% CI: 4.39–6.36). There were no trends by BMI among asthma patients; however, those with comorbidities experienced up to 11-fold increased odds of complications.
Table 3.
Multivariable adjusted associations between BMI and complications.
4. Discussion
Using the HCUP Nationwide Inpatient database, we examined hospitalized patients with a wide-range of BMI values, and determined the association between BMI and overall, cancer, COPD, asthma, and CVD-specific mortality. We observed that 74.5% of hospitalized patients in this analysis had a BMI > 30, and thus were obese; 32.5% of patients were morbidly obese (BMI > 40 kg/m2); and Black patients were least likely to have BMI in the normal range, with 12.5% of Black patients having a BMI > 50. In addition, we observed a striking bell-shaped relationship between BMI and overall, cancer, COPD, Asthma, and CVD-specific in-hospital mortality. Compared with those with normal BMI (BMI: 19.0–24.0 kg/m2), patients with BMI 25 to 29 experienced a 3.6-fold increase in odds of in-hospital mortality, increasing to 7.5-fold among those with BMI 34 to 35.9, then declining to 1.7-fold among those with BMI > 50. Similar trends were observed for patients hospitalized with a primary diagnosis of cancer, COPD, asthma, and CVD, with the largest difference among asthma patients. In contrast, odds of complications increased significantly in a linear fashion with increasing BMI, but only among cancer patients, while a less pronounced but significant U-shaped pattern was observed for hospital LOS with increasing BMI. There were significant Black–White differences in in-hospital mortality but only for COPD and CVD, and while Black patients were less likely to experience complications for all examined conditions, they had significantly longer hospital stay across all conditions compared with Whites.
As we expected, the prevalence of overweight and obese among adults in the HCUP-NIS database was higher compared with national estimates. According to the 2011 National Health and Nutrition Examination Survey (NHANES), the national prevalence estimate of obesity was 34.9% in the general population of US adult's age ≥20 years.[30] The prevalence of extreme obesity (BMI ≥ 40 kg/m2) was 6.4% overall, with a higher prevalence in women (8.3%) compared with men (4.4%), and the highest prevalence observed among non-Hispanic Black adults (12.1%).[30] Since obesity is the most common risk factor across multiple chronic diseases, our results, while striking, were expected and in line with other studies of overweight and obesity among nonhealthy adults, mostly focused on adult outpatients, with prevalence rates of around 80%.[31,32] In extremely obese patients, the overrepresentation of younger, Black, and female patients corresponds to estimates reported in NHANES study, and provides further evidence that these trends are important contributors to worsening race and sex disparities in outcomes of multiple chronic diseases, such as COPD and cancer.[33,34]
In the general population, overweight and obesity are well-known risk factors for several chronic diseases and all-cause mortality. However, among elderly or diseased individuals, the relationship between body weight and mortality has been the subject of some debate. Janssen and Mark[35] performed a meta-analysis examining the association between elevated BMI and all-cause mortality in the elderly, and reported that overweight was not associated with a significantly increased risk of mortality, while moderate obesity was associated with a modest increase in risk. In contrast, another meta-analysis by Flegal et al[36] evaluating the associations of overweight and obesity with all-cause mortality in the elderly showed that overall obesity was associated with significantly higher all-cause mortality, although the association varied depending on BMI.[2] Mixed results have also been reported in studies that examined the association between obesity and mortality among participants with cancer,[37] COPD,[15,38,39] asthma, and CVD.[7,11]
We observed modest associations between BMI categories and complications, except for cancer patients. The relationships between obesity and clinical outcomes have been explored in among adults with severe illness, across various disciplines. Overall, results are conflicting: some studies reported increase association between obesity and complications,[40–47] while other studies did not observe significant associations.[48–51] This suggests that for some chronic diseases, higher BMI may not necessarily translate to more invasive treatment modalities, which are often the proximal causes of complications. Based on the findings of various large-scale studies,[52–54] it is plausible to presume that obese patients who would have longer hospital stays through increased prevalence of comorbidities, complications, and longer recovery. Yet, we observed that patients with higher BMI had shorter hospital stays compared with patients with normal BMI. This trend was consistent across all conditions, and observed even after adjusting for number of comorbidities. This suggests that when differences in comorbid conditions are accounted for, patients with higher BMI tended to be discharged sooner. Hauck and Hollingsworth examined the hospital data for 122 Australian public hospitals during the year 2005/2006 (Victorian Admitted Episodes Data),[52] and observed that surgically managed obese patients spent less time in the hospital (−0.3 day, P = 0.029). The authors suggested that hospitals may shorten the LOS for obese surgical patients by transferring them to another hospital or recovery facilities after surgery. Further studies may focus on assessing whether higher BMI patients were more likely to be discharged to skilled nursing facilities, to hospice care, or to their homes. If patients at the highest extremes of BMI are more likely to be discharged to hospice care, for instance, then health events such as mortality occurring after discharge will be under-estimated in hospital-based studies as those typically only captures in-hospital events. This is a potential limitation of the current analysis that may be further examined in longitudinal studies of patients.
Other possible reasons for conflicting results in prior studies might include multiple sources of methodological bias. First, different BMI cut-points are often used in different studies. In some studies, higher BMI categories are collapsed to delineate more severe degrees of obesity (i.e., only ≥30 kg/m2), and most studies show worse prognosis in the group with BMI > 35 kg/m2.[1] For example, a recent pooled analysis of 20 prospective studies found BMI of 40 to 59 kg/m2 was associated with substantially elevated rates of total mortality, with most of the excess deaths due to heart disease, cancer, and diabetes, compared with normal weight.[55] Conversely, some studies include the underweight BMI category (18.5–24.9 kg/m2) as a reference group, seriously underestimating the BMI–mortality association by creating an artifact of reduced mortality among the overweight and moderately obese groups. This is likely because the underweight BMI category contains not only individuals who are lean and active but also heavy smokers, the frail and elderly, and those who are ill with previous weight loss or diminished weight gain due to existing diseases.
Another methodological limitation of some prior studies is the source of BMI data. Self-reported height and weight are the least reliable sources of epidemiologic data, subject to significant bias, which may be differential in relation to disease status. Furthermore, among adult inpatients, previous studies have reported that BMI calculations are seldom performed during hospitalization, and that overweight/obesity is rarely included among discharge diagnoses documentation,[56,57] raising questions about differential misclassification in whose BMI gets recorded and/or included in administrative databases. By assessing a wide range of BMI values with smaller categorical cut-points, using claims-based ICD-9 codes for BMI categories, and assessing outcomes only among patients with a BMI coding, this analysis aimed to address some of these limitations.
The specific biological mechanisms linking obesity and clinical outcomes have not been entirely elucidated. One possible explanation of the negative effect of obesity may be accounted for by malnutrition. While obesity involves excess caloric intake, several studies have shown a high prevalence of micronutrient deficiencies in obese patients across a broad range of both vitamins and minerals.[58] Alternatively, it is possible that BMI is a poor measure of body fat due to the positive correlation between BMI and muscle mass, while lean muscle mass has been shown to exert protective effects on cardiometabolic diseases such as CVD and cancer. Studies among COPD[59] and cancer[60] patients have demonstrated that overweight or obese patients are at increased risk for malnutrition, which contributes to poor outcomes. Malnourished patients have slower healing, more complications, increased LOS, increased hospital costs, and greater mortality.[61] Severely obese patients are also medically more complex in part due to an increased burden of pre-existing illness (comorbidities and acute illness), delayed wound healing and undiagnosed or under-diagnosed conditions such as asthma and COPD. Both asthma and COPD are major chronic obstructive airway diseases that involve airway inflammation, and the coexistence of both conditions in obese patients has been reported,[62] in some studies exceeding half of the patients examined.[63,64] These conditions are also associated with severe morbidity and mortality.[65] Other major health conditions, such as nonalcoholic fatty liver disease, have also been strongly linked with overweight/obesity and diabetes,[66] and in turn may be associated with increased risk of other systemic chronic diseases such as CVD[67] and cancer,[68] further highlighting the multifactorial role of overweight/obesity on negative health outcomes in adults.
In conclusion, the majority of US hospitalized patients are either overweight or obese, and higher levels of obesity are associated with significantly higher risk of mortality and length of hospital stay. The observed attenuation of the association between higher BMI categories and mortality may be due to early discharge of obese patients to external facilities prior to death.
Supplementary Material
Footnotes
Abbreviations: BMI = body mass index, COPD = chronic obstructive pulmonary disease, CVD = cardiovascular disease, HCUP-NIS = Health Cost and Utilization Project Nationwide Inpatient Sample, ICD-9 = International Classification of Diseases—9th Revision, LOS = length of stay, NHANES = National Health and Nutrition Examination Survey, NIH = National Institute of Health, SES = socioeconomic status.
Dr TA was supported by grant U54 CA118948 from the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010; 303:235–241. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012; 307:491–497. [DOI] [PubMed] [Google Scholar]
- 3.Eknoyan G. Adolphe Quetelet (1796–1874)—the average man and indices of obesity. Nephrol Dial Transplant 2008; 23:47–51. [DOI] [PubMed] [Google Scholar]
- 4.National Institutes of Health. Classification of overweight and obesity by BMI, waist circumference, and associated disease risks. https://www.nhlbi.nih.gov/health/educational/lose_wt/BMI/bmi_dis.htm Accessed May 9, 2016. [Google Scholar]
- 5.Finkelstein EA, Brown DS, Wrage LA, et al. Individual and aggregate years-of-life-lost associated with overweight and obesity. Obesity (Silver Spring) 2010; 18:333–339. [DOI] [PubMed] [Google Scholar]
- 6.Bastien M, Poirier P, Lemieux I, et al. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis 2014; 56:369–381. [DOI] [PubMed] [Google Scholar]
- 7.Niedziela J, Hudzik B, Niedziela N, et al. The obesity paradox in acute coronary syndrome: a meta-analysis. Eur J Epidemiol 2014; 29:801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doehner W, Erdmann E, Cairns R, et al. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co-morbidity: an analysis of the PROactive study population. Int J Cardiol 2012; 162:20–26. [DOI] [PubMed] [Google Scholar]
- 9.Carnethon MR, De Chavez PJ, Biggs ML, et al. Association of weight status with mortality in adults with incident diabetes. JAMA 2012; 308:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tobias DK, Pan A, Jackson CL, et al. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med 2014; 370:233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet 2006; 368:666–678. [DOI] [PubMed] [Google Scholar]
- 12.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol 2009; 53:1925–1932. [DOI] [PubMed] [Google Scholar]
- 13.Oreopoulos A, Padwal R, Kalantar-Zadeh K, et al. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J 2008; 156:13–22. [DOI] [PubMed] [Google Scholar]
- 14.Carman WJ, Barrett-Connor E, Sowers M, et al. Higher risk of cardiovascular mortality among lean hypertensive individuals in Tecumseh, Michigan. Circulation 1994; 89:703–711. [DOI] [PubMed] [Google Scholar]
- 15.Landbo C, Prescott E, Lange P, et al. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 160:1856–1861. [DOI] [PubMed] [Google Scholar]
- 16.Yoon HH, Lewis MA, Shi Q, et al. Prognostic impact of body mass index stratified by smoking status in patients with esophageal adenocarcinoma. J Clin Oncol 2011; 29:4561–4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Attaran S, McShane J, Whittle I, et al. A propensity-matched comparison of survival after lung resection in patients with a high versus low body mass index. Eur J Cardiothorac Surg 2012; 42:653–658. [DOI] [PubMed] [Google Scholar]
- 18.McEwen LN, Karter AJ, Waitzfelder BE, et al. Predictors of mortality over 8 years in type 2 diabetic patients: Translating Research Into Action for Diabetes (TRIAD). Diabetes Care 2012; 35:1301–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaturvedi N, Fuller JH. Mortality risk by body weight and weight change in people with NIDDM. The WHO multinational study of vascular disease in diabetes. Diabetes Care 1995; 18:766–774. [DOI] [PubMed] [Google Scholar]
- 20.Despres JP. Excess visceral adipose tissue/ectopic fat the missing link in the obesity paradox? J Am Coll Cardiol 2011; 57:1887–1889. [DOI] [PubMed] [Google Scholar]
- 21.Nazare JA, Smith J, Borel AL, et al. Usefulness of measuring both body mass index and waist circumference for the estimation of visceral adiposity and related cardiometabolic risk profile (from the INSPIRE ME IAA study). Am J Cardiol 2015; 115:307–315. [DOI] [PubMed] [Google Scholar]
- 22.Goran MI. Ethnic-specific pathways to obesity-related disease: the Hispanic vs. African-American paradox. Obesity (Silver Spring) 2008; 16:2561–2565. [DOI] [PubMed] [Google Scholar]
- 23.Markides KS, Eschbach K. Rogers GR, Crimmins ME. Hispanic paradox in adult mortality in the United States. International Handbook of Adult Mortality. Dordrecht, The Netherlands: Springer; 2011. 227–240. [Google Scholar]
- 24.Forbang NI, Hughes-Austin JM, Allison MA, et al. Peripheral artery disease and non-coronary atherosclerosis in Hispanics: another paradox? Prog Cardiovasc Dis 2014; 57:237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville (MD): Agency for Health Care Policy and Research (US) http://www.ncbi.nlm.nih.gov/books/NBK52651/ Accessed May 15, 2016. [PubMed] [Google Scholar]
- 26.Dehal A, Abbas A, Johna S. Comorbidity and outcomes after surgery among women with breast cancer: analysis of nationwide in-patient sample database. Breast Cancer Res Treat 2013; 139:469–476. [DOI] [PubMed] [Google Scholar]
- 27.Akinyemiju TF, Vin-Raviv N, Chavez-Yenter D, et al. Race/ethnicity and socio-economic differences in breast cancer surgery outcomes. Cancer Epidemiol 2015; 39:745–751. [DOI] [PubMed] [Google Scholar]
- 28.Vin-Raviv N, Akinyemiju TF, Galea S, et al. Depression and anxiety disorders among hospitalized women with breast cancer. PLoS ONE 2015; 10:e0129169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.United States Department of Agricultural Economic Research Service. Urban Influence Code. Washington, DC: United States Department of Agricultural Economic Research Service; 2013. [Google Scholar]
- 30.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014; 311:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang J, Marin E, Yu H, et al. Prevalence of overweight, obesity, and associated diseases among outpatients in a public hospital. South Med J 2003; 96:558–562. [DOI] [PubMed] [Google Scholar]
- 32.Lebrun LA, Chowdhury J, Sripipatana A, et al. Overweight/obesity and weight-related treatment among patients in U.S. federally supported health centers. Obes Res Clin Pract 2013; 7:e377–e390. [DOI] [PubMed] [Google Scholar]
- 33.Mannino DM. Women and chronic obstructive pulmonary disease: does sex influence survival? Am J Respir Crit Care Med 2006; 174:488–489. [DOI] [PubMed] [Google Scholar]
- 34.Patel AV, Hildebrand JS, Gapstur SM. Body mass index and all-cause mortality in a large prospective cohort of white and black U.S. adults. PLoS ONE 2014; 9:e109153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janssen I, Mark AE. Elevated body mass index and mortality risk in the elderly. Obes Rev 2007; 8:41–59. [DOI] [PubMed] [Google Scholar]
- 36.Flegal KM, Kit BK, Orpana H, et al. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013; 309:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yasunaga H, Horiguchi H, Matsuda S, et al. Body mass index and outcomes following gastrointestinal cancer surgery in Japan. Br J Surg 2013; 100:1335–1343. [DOI] [PubMed] [Google Scholar]
- 38.Blum A, Simsolo C, Sirchan R, et al. “Obesity paradox” in chronic obstructive pulmonary disease. Isr Med Assoc J 2011; 13:672–675. [PubMed] [Google Scholar]
- 39.Zapatero A, Barba R, Ruiz J, et al. Malnutrition and obesity: influence in mortality and readmissions in chronic obstructive pulmonary disease patients. J Hum Nutr Diet 2013; 26 suppl 1:16–22. [DOI] [PubMed] [Google Scholar]
- 40.Gendall KA, Raniga S, Kennedy R, et al. The impact of obesity on outcome after major colorectal surgery. Dis Colon Rectum 2007; 50:2223–2237. [DOI] [PubMed] [Google Scholar]
- 41.Bamgbade OA, Rutter TW, Nafiu OO, et al. Postoperative complications in obese and nonobese patients. World J Surg 2007; 31:556–560.discussion 561. [DOI] [PubMed] [Google Scholar]
- 42.Mathur AK, Ghaferi AA, Sell K, et al. Influence of body mass index on complications and oncologic outcomes following hepatectomy for malignancy. J Gastrointest Surg 2010; 14:849–857. [DOI] [PubMed] [Google Scholar]
- 43.Lynch RJ, Ranney DN, Shijie C, et al. Obesity, surgical site infection, and outcome following renal transplantation. Ann Surg 2009; 250:1014–1020. [DOI] [PubMed] [Google Scholar]
- 44.Rockx MAJ. Is obesity a predictor of mortality, morbidity and readmission after cardiac surgery? Can J Surg 2004; 47:34–38. [PMC free article] [PubMed] [Google Scholar]
- 45.Pikarsky AJ, Saida Y, Yamaguchi T, et al. Is obesity a high-risk factor for laparoscopic colorectal surgery? Surg Endosc 2002; 16:855–858. [DOI] [PubMed] [Google Scholar]
- 46.Glance LG, Wissler R, Mukamel DB, et al. Perioperative outcomes among patients with the modified metabolic syndrome who are undergoing noncardiac surgery. Anesthesiology 2010; 113:859–872. [DOI] [PubMed] [Google Scholar]
- 47.Mullen JT, Moorman DW, Davenport DL. The obesity paradox: body mass index and outcomes in patients undergoing nonbariatric general surgery. Ann Surg 2009; 250:166–172. [DOI] [PubMed] [Google Scholar]
- 48.Das SR, Alexander KP, Chen AY, et al. Impact of body weight and extreme obesity on the presentation, treatment, and in-hospital outcomes of 50,149 patients with ST-segment elevation myocardial infarction results from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol 2011; 58:2642–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suleiman LI, Ortega G, Ong’uti SK, et al. Does BMI affect perioperative complications following total knee and hip arthroplasty? J Surg Res 2012; 174:7–11. [DOI] [PubMed] [Google Scholar]
- 50.Dindo D, Muller MK, Weber M, et al. Obesity in general elective surgery. Lancet 2003; 361:2032–2035. [DOI] [PubMed] [Google Scholar]
- 51.Chang VW, Asch DA, Werner RM. Quality of care among obese patients. JAMA 2010; 303:1274–1281. [DOI] [PubMed] [Google Scholar]
- 52.Hauck K, Hollingsworth B. The impact of severe obesity on hospital length of stay. Med Care 2010; 48:335–340. [DOI] [PubMed] [Google Scholar]
- 53.Quesenberry CP, Jr, Caan B, Jacobson A. Obesity, health services use, and health care costs among members of a health maintenance organization. Arch Intern Med 1998; 158:466–472. [DOI] [PubMed] [Google Scholar]
- 54.Zizza C, Herring AH, Stevens J, et al. Length of hospital stays among obese individuals. Am J Public Health 2004; 94:1587–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kitahara CM, Flint AJ, de Gonzalez AB, et al. Association between class III obesity (BMI of 40–59 kg/m2) and mortality: a pooled analysis of 20 prospective studies. PLoS Med 2014; 11:e1001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howe EE, Wright SM, Landis R, et al. Addressing obesity in the hospitalized patient: a needs assessment. South Med J 2010; 103:500–504. [DOI] [PubMed] [Google Scholar]
- 57.Darnis S, Fareau N, Corallo CE, et al. Estimation of body weight in hospitalized patients. QJM 2012; 105:769–774. [DOI] [PubMed] [Google Scholar]
- 58.Küpper S, Karvellas CJ, Khadaroo RG, et al. Increased health services use by severely obese patients undergoing emergency surgery: a retrospective cohort study. Can J Surg 2015; 58:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanson C, Rutten EP, Wouters EF, et al. Influence of diet and obesity on COPD development and outcomes. Int J Chron Obstruct Pulmon Dis 2014; 9:723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gioulbasanis I, Martin L, Baracos VE, et al. Nutritional assessment in overweight and obese patients with metastatic cancer: does it make sense? Ann Oncol 2015; 26:217–221. [DOI] [PubMed] [Google Scholar]
- 61.Rasheed S, Woods RT. Malnutrition and associated clinical outcomes in hospitalized patients aged 60 and older: an observational study in rural Wales. J Nutr Gerontol Geriatr 2013; 32:71–80. [DOI] [PubMed] [Google Scholar]
- 62.Soriano JB, Visick GT, Muellerova H, et al. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest 2005; 128:2099–2107. [DOI] [PubMed] [Google Scholar]
- 63.Marsh SE, Travers J, Weatherall M, et al. Proportional classifications of COPD phenotypes. Thorax 2008; 63:761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fu JJ, McDonald VM, Gibson PG, et al. Systemic inflammation in older adults with asthma-COPD overlap syndrome. Allergy Asthma Immunol Res 2014; 6:316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Global Initiative for Asthma. Asthma, COPD and Asthma-COPD Overlap Syndrome (ACOS) Global Initiative for Asthma [Internet]; 2014. http://www.ginasthma.org/documents/14 Accessed May 3, 2016. [Google Scholar]
- 66.Tarantino G. Should nonalcoholic fatty liver disease be regarded as a hepatic illness only? World J Gastroenterol 2007; 13:4669–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hamaguchi M, Kojima T, Takeda N, et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol 2007; 13:1579–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sorensen HT, Mellemkjaer L, Jepsen P, et al. Risk of cancer in patients hospitalized with fatty liver: a Danish cohort study. J Clin Gastroenterol 2003; 36:356–359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


