Abstract
Several retrospective analyses on patients who underwent gastric cancer (GC) surgery revealed different survival outcomes between Eastern (Korean, Japanese) and Western (USA, Europe) countries due to potential ethnical and biological differences. This study investigates treatment outcomes between specialized institution for GC in Korea and Germany.
The prospectively documented databases of the Gastric Cancer Center of the National Cancer Center, Korea (NCCK) and the Department of Surgery of the Technische Universitaet Muenchen (TUM), Germany were screened for patients who underwent primary surgical resection for GC between 2002 and 2008. Baseline characteristics were compared using χ2 testing, and 2 cohorts were matched using a propensity score matching (PSM) method. Patients’ survival was estimated using Kaplan–Meier method, and multivariable Cox proportional hazard model was used for comparison.
Three thousand seven hundred ninety-five patients were included in the final analysis, 3542 from Korea and 253 from Germany. Baseline characteristics revealed statistically significant differences for age, tumor location, pT stage, grading, lymphatic vessel infiltration (LVI), comorbidities, number of dissected lymph nodes (LN), postoperative complications, lymph-node ratio stage, and application of adjuvant chemotherapy. After PSM, 171 patients in TUM were matched to NCCK patients, and baseline characteristics for both cohorts were well balanced. Patients in Korea had significantly longer survival than those in Germany both before and after PSM. When the analysis was performed for each UICC stage separately, same trend was found over all UICC stages before PSM. However, significant difference in survival was observed only for UICC I after PSM.
This analysis demonstrates different survival outcomes after surgical treatment of GC on different continents in specialized centers after balancing of baseline characteristics by PSM.
Keywords: gastric cancer, outcome
1. Introduction
Gastric cancer (GC) is one of the leading causes of cancer-related deaths worldwide[1] with the highest incidence in Eastern-Asian countries (Korea,[2] Japan, China[3]) but also in Latin-America and Eastern-Europe.[4] In contrast to Korea, there are no screening programs for GC in Western countries leading to the fact that the disease is mostly diagnosed in advanced stages and commonly treated in multimodal therapy concepts.[5,6] Due to a national screening program in Korea,[7] there is an extraordinary high detection rate for early GC rendering local stomach-preserving therapies such as endoscopic submucosal dissection and sentinel-node-guided surgery possible. Despite the differences in the 2 countries, surgical resection remains the mainstay of therapy.[8–12] A former retrospective analysis in 1993 from the Surgical Department of the Technische Universitaet Muenchen (TUM/MRI) comparing outcome data to patients of the National Cancer Center in Tokyo concluded that there were no clinically meaningful differences when similar patient subgroups were compared.[8] Nonetheless there was a great heterogeneity in the 2 compared patient cohorts. These results were reproduced by several follow-up studies comparing United States and Japanese but also United States and Korean data.[9,10] The major drawbacks of these studies were that the patient cohorts revealed major disparities in patient numbers and characteristics rendering direct comparisons difficult. The purpose of this analysis was to investigate survival outcomes between 2 institutions specialized in GC treatment after equalizing disparities in patient's baseline characteristics by propensity score matching (PSM) technique.
2. Methods
The prospectively documented databases for GC were screened for patients having undergone primary surgery for GC at the National Cancer Center Korea (NCCK) and the Surgical Department of the Technische Universitaet Muenchen (TUM) from January 2002 to August 2008. Data were obtained from the medical records and transferred to the institutional databases as soon as the patients were discharged from inpatient hospital care. Eligibility criteria were: histologically proven GC, primary R0 resection. Exclusion criteria were: metastatic disease, neoadjuvant/perioperative chemotherapy, extension to the distal esophagus, gastric stump cancer, endoscopic resection for early GC, hospital mortality within 30 days, loss of follow-up within a 60 months period and residual cancer after surgery (R1/R2). All surgical procedures were performed according to the Japanese guidelines for GC treatment including standardized D2-lymphnode dissection. Korean patients received adjuvant chemotherapy according to local guidelines for UICC stages II/III. German patients received adjuvant treatment in selected cases only after multidisciplinary team review. Adjuvant chemotherapy in German patients consisted of 2 cycles cisplatin/leucovorin/5-FU, whereas in Korean patients either 12 months of S1 or 6 months of capecitabine/oxaliplatin was applied routinely for patients staged UICC II/III. All patients receiving adjuvant chemotherapy were included in this analysis. Further all patients with Siewert-type II cancers were omitted from the analysis. Siewert-type III cancers were included when involvement of the cardia was ruled out. All patients were followed by the respective outpatient departments for 60 months after oncologic surgery every 6 to 12 months. Only deceased or surviving patients with complete follow-up of at least 60 months were included in this analysis. Survival was computed from the day of surgery. The dataset consisted of patients’ age, gender, location (upper, middle, lower third), pT-, pN-, and UICC stage, grading, Lauren histotype, lymphovascular invasion (LVI), lymph-node ratio (LNR) stage, comorbidities, number of dissected lymph nodes, postoperative complications (according to the Clavien–Dindo Classification), application of adjuvant chemotherapy, type of surgery, and follow-up period with survival status. Intergroup comparisons were analyzed by χ2 testing and continuous variables are presented as mean ± standard deviation. T tests or Wilcoxon-rank sum tests were used as appropriate. Patients with missing data were excluded from further evaluation. Analysis of baseline characteristics revealed marked differences between the groups. Therefore we performed PSM in order to minimize intergroup disparities and to control for selection bias as described before.[13,14] Shortly, multivariable logistic regression was performed on center (NCCK vs TUM) using all variables with possible influence on the patients’ survival. Variables included in the multivariable logistic regression include age, gender, location, pT-, pN stages, grading, Lauren classification, LVI, comorbidity, dissected LN, postoperative complications, adjuvant CTx, and type of surgery. A propensity score was then estimated for all subjects using this logistic regression, and TUM patients were matched to NCCK patients using the nearest neighbor matching within a caliper of 0.20 times the standard deviation of the propensity score. Survival curves were estimated by the Kaplan–Meier method and statistical differences were evaluated by the log-rank test. Associations between prognostic factors and survival were estimated by the uni- and multivariable Cox proportional hazards model. The multivariable model was selected using the backward variable elimination technique with an elimination criterion of P value <0.05. Statistical analyses were performed using SAS software (Version 9, SAS Institute, Cary, NC), STATA (Version 12, Stata Corporation, College Station, TX), and R (Version 2.14.2, R-foundation, Vienna, Austria)[15] together with the Match It package (Version 2.4–18, Boston, MA).[16] Institutional Review Board (IRB) approval was obtained by the respective local boards.
3. Results
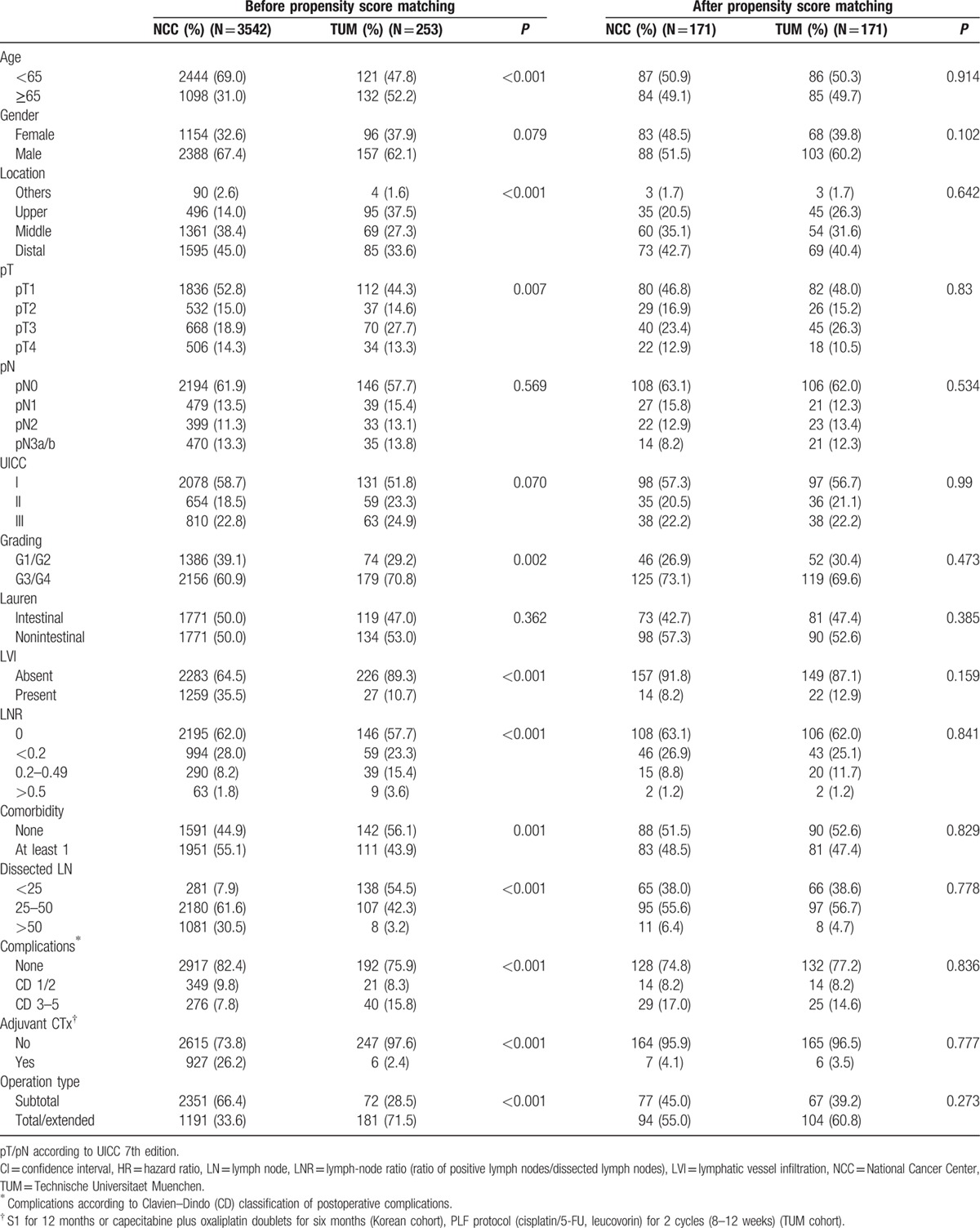
Screening of the prospectively documented databases revealed a total of 5103 surgically treated patients for GC between 2002 and 2008, 4334 patients at the NCCK and 747 patients at the TUM. Patients with neoadjuvant treatment (TUM: n = 388, NCCK: n = 91), R1 resections (TUM n = 41; NCCK: n = 93), R2 resections (TUM: n = 20, NCCK: n = 166), hospital mortality (TUM: n = 5, NCCK: n = 8), gastric stump cancer (TUM: n = 10, NCCK: n = 43), M1 (TUM: n = 16, NCCK: n = 38), and histology other than adenocarcinoma (TUM: n = 14, NCCK: n = 332) were omitted. Finally 3563 patients from NCCK and 253 patients from TUM were included in this comparative analysis. Baseline characteristics and clinicopathologic features of the NCCK and TUM patients are summarized in Table 1. There was a significant difference in age distribution with a larger amount of patients younger than 65 years at the NCCK (P < 0.001). No difference in sex distribution was noted (P = 0.079). Pathologic T-stages were more advanced in the German cohort (P = 0.007). There was no significant difference in the frequency of lymph-node (LN) metastases (P = 0.569) although there was a significantly higher amount of dissected LNs in the Korean patient cohort (44 ± 16 vs 24 ± 10, P < 0.001). Higher LNR stages were demonstrated in the German cohort (P < 0.001). German patients had less differentiated cancers than Koreans (P = 0.002). The proportion of LVI was tripled in Korean patients (P < 0.001). There were significantly less Clavien–Dindo grade 3/4 complications in the Korean cohort (P < 0.001) while comorbidity rate was significantly higher (P < 0.001). There was a significantly higher proportion of high-body tumors in the German cohort compared to more distal locations in the Korean collective (P < 0.001). Nine hundred twenty-seven patients (26.2%) from NCCK compared to only 6 TUM patients (2.4%) received adjuvant chemotherapy (P < 0.001).
Table 1.
Patients’ characteristics and histopathologic data in 2 cohorts before and after propensity score matching.
Median follow-up for survivors was 79 (12–131) months. Median overall survival (OS) was not reached in the unmatched and PSM cohorts. Five-year survival rates (FYSR) in the unmatched cohort were 86.0% for Korean and 70.8% for German patients. FYSR in the PSM cohort were 85.5% for Korean and 71.7% for German patients. OS was significantly different between the 2 cohorts and over all UICC stages before propensity score matching (P < 0.001). Figure 1A displays Kaplan–Meier plots of OS for NCCK and TUM before PSM (log-rank P < 0.001). When OS was compared for each UICC stage separately, significant differences were observed for all UICC stages (Fig. 1B–D, P < 0.001). In Table 2, the results from univariable and multivariable Cox proportional hazard model are summarized. The univariable analysis on the unmatched cohorts revealed that center, pT-, pN stages, UICC stage, grading, LVI, LNR, postoperative complications and type of surgery were significantly related to OS. In multivariable analysis of the unmatched cohort center, pT-, pN stages, postoperative complications and type of surgery were independent predictors for OS (Table 2).
Figure 1.
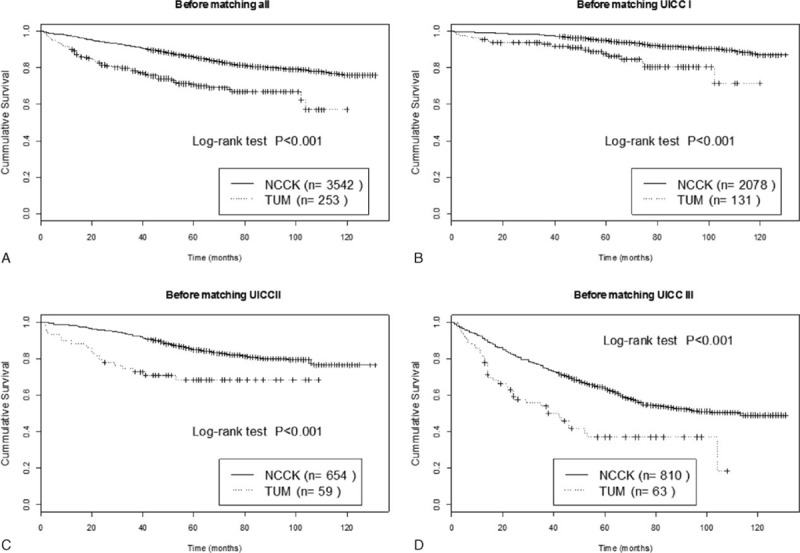
Kaplan–Meier plots of overall survival for (A) all patients and according to UICC stages before PSM in NCCK and TUM patients. (B) UICC I, (C) UICC II, (D) UICC III.
Table 2.
Results from univariable and multivariable (backward variable selection at α = 0.05) Cox regression analyses on overall survival for unmatched patients.
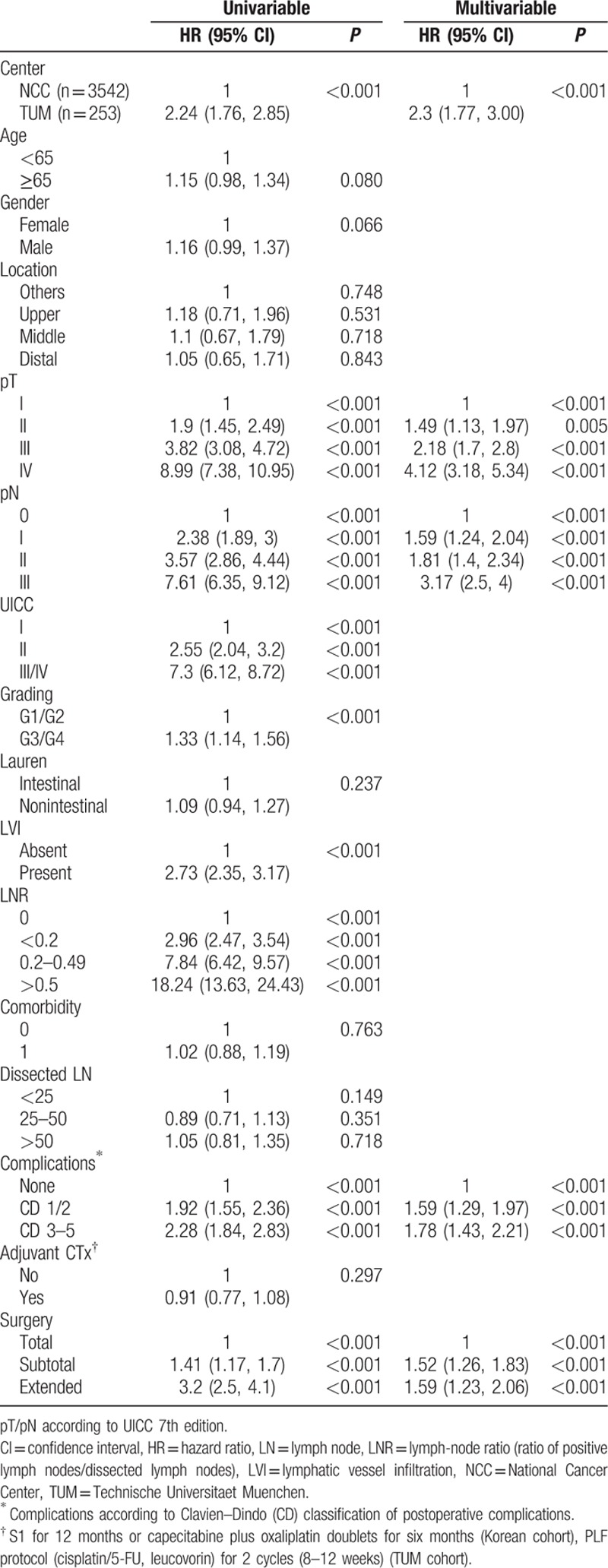
PSM matched 171 NCCK patients to 171 TUM patients. Baseline characteristics revealed balanced groups for all possibly confounding variables (age, gender, location, pT-, pN stages, UICC stage, grading, Lauren histotype, LVI, LNR, comorbidity, dissected LN, postoperative complications, adjuvant CTx and type of surgery, Table 1). Survival curves for the PSM groups (Fig. 2A) in the total matched cohort still revealed significant differences between NCCK and TUM after PSM (log-rank P = 0.003). Analyzing UICC stages separately, a statistically significant difference was only found for patients with UICC I stage (P = 0.017), but not for UICC II (P = 0.083) and UICC III (P = 0.108) (Fig. 2B–D). This is possibly due to the small number of patients in UICC II and III. Univariable regression analysis on the PSM cohort revealed that center, age, pT-, pN stages, UICC stage, LVI, LNR, postoperative complications, and type of surgery were significantly related to OS. In multivariable analysis of the PSM-cohort center, age, pN stages, and postoperative complications were predictive for OS (Table 3).
Figure 2.
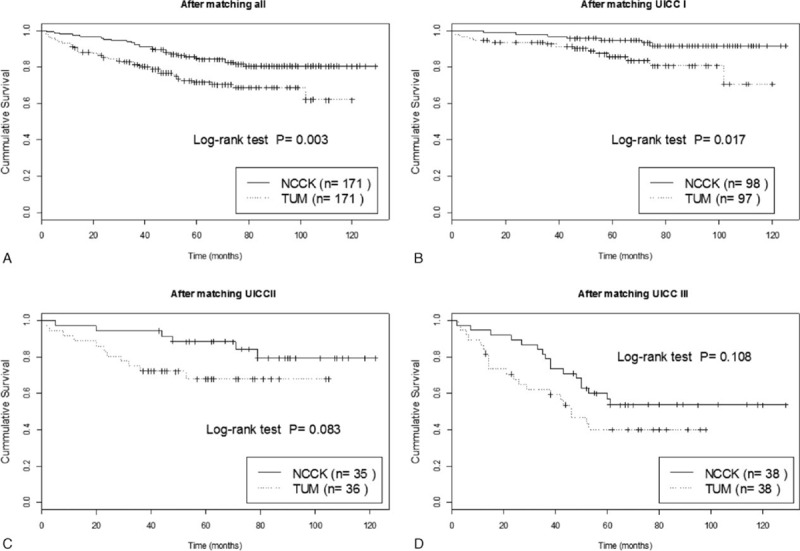
Kaplan–Meier plots of overall survival for (A) all patients and according to UICC stages after PSM in NCCK and TUM patients. (B) UICC I, (C) UICC II, (D) UICC III.
Table 3.
Results from univariable and multivariable (backward variable selection at α = 0.05) Cox regression analyses on overall survival for propensity score matched patients.
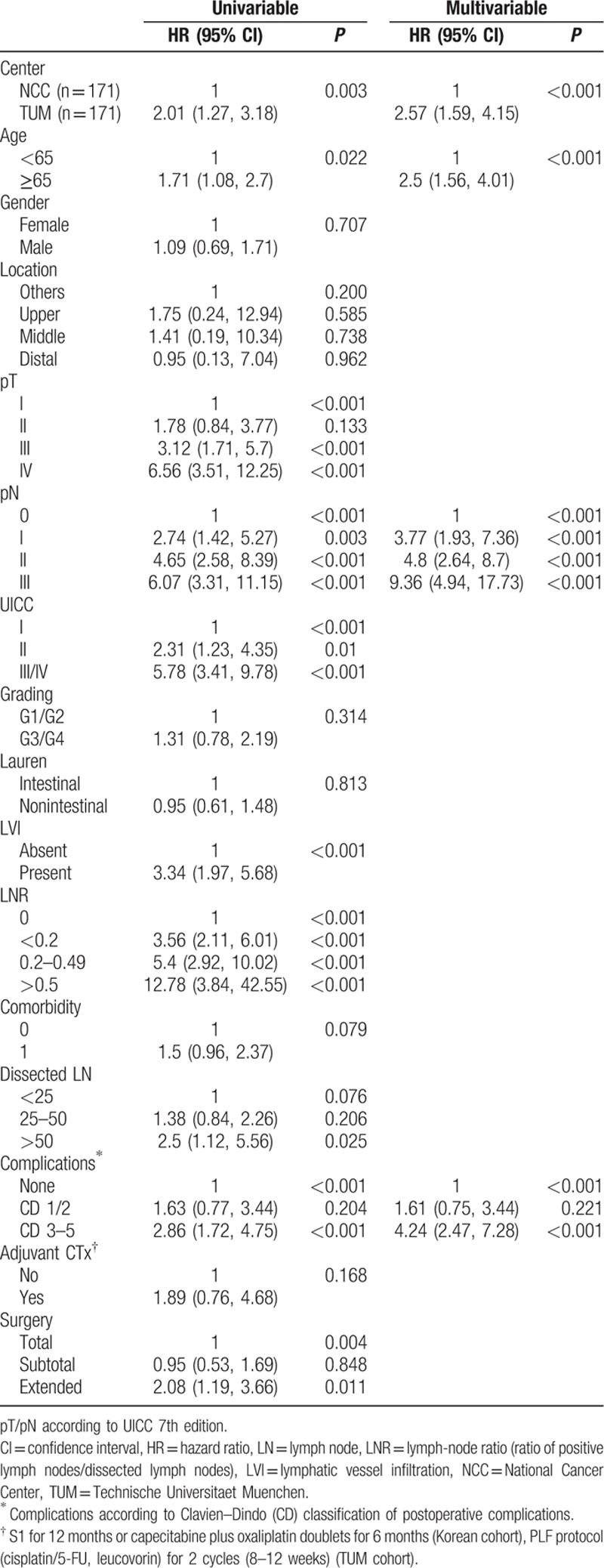
4. Discussion
Several retrospective studies on the clinical outcome of GC surgery comparing Eastern-Asian (Japanese/Korean) and Western patients (United States/European) were published over the recent years.[8–12] Almost all those analyses concluded that oncologic outcome was better in Eastern patients due to so far unknown reasons.[10–12] Some authors consider biologic properties and different ethnicity,[11,12] others speculate that surgical techniques or the application of adjuvant chemotherapy may be responsible for the improved results in Eastern-Asia.[17]
This retrospective analysis on the oncologic outcome of a high-volume Korean and a highly experienced German center after curative R0 resection for GC aimed to eradicate the differences in baseline characteristics by PSM which is an accepted method to achieve comparability in heterogeneous patient cohorts. This analysis revealed the same highly heterogeneous groups in the beginning and demonstrated persistence of the different survival outcomes after PSM despite balanced baseline characteristics. The incidence for GC is known to be 10 times higher in Korea than in Germany[1] and due to the national Korean screening program[7] GC detection rates are considerably higher,[6] explaining the huge disparity of patient numbers between the 2 centers. There was a considerably higher amount of early GCs in the unmatched Korean cohort and more advanced pT stages in the German patient group. Reasons could be the delayed diagnosis and the lack of a national screening program in Germany. German patients also revealed a higher proportion of poorly and undifferentiated cancers. Differences in the distribution of Lauren histotypes could not be detected in contrast to the most recent study by Strong et al.[10] The quality of lymph-node (LN) dissection appeared to be different. There was a significantly higher amount of dissected LNs in the Korean cohort although the German center is specialized in D2 dissection.[18] Reasons may be that either LN dissection was not performed as indicated in the surgical record or the pathologists did not continue to dissect nodes out of the specimen after identification of 15 to 25 LNs as recommended before.[19] Another conceivable issue might be that embryologic planes in surgical dissection were not respected, especially around LN station #6 and in the N2 area (LN stations 8a–12a). Unfortunately there is no detailed data on the respective LN stations in the TUM database providing a possible answer to this fact. Most of European surgeons are still reluctant to perform standardized D2 dissection although long-term results from a Dutch trial revealed significant survival benefits for those patients receiving D2 dissection.[20] Meta-analyses for Western data on this issue are not conclusive to this date[21] but experienced centers may have excellent results.[22] Surgical strategies were different between the cohorts. Almost two-thirds of the patients received subtotal gastrectomies in the Korean group whereas most of the patients in the German group received total or even extended gastrectomies. This may be related to the fact that in the German cohort the tumors were predominantly located in the upper part of the stomach and extension to the distal esophagus was considered to be necessary from an oncological point of view, although in the final pathology report no involvement of the GE junction was reported. Another explanation could be surgical philosophy. Whereas subtotal gastrectomies for cancers located in the middle third of the stomach are considered oncologically safe in Korea, TUM surgeons are reluctant to perform subtotal resections for these patients. However, these factors were equalized by the PSM algorithm for further analysis and the surgical procedure differences and different frequencies of tumor location were balanced in the PSM cohort.
OS rates were excellent for both centers. OS in the Korean cohort was comparable to Japanese standards and OS for the German patients was above European standard compared to previously published data on patients having undergone primary resection for GC.[23,24] This may be reflected by a centralization effect. Traditionally the Munich department is experienced in GC treatment and cares for nationwide patients similar to the NCCK. Though, survival was significantly better at NCCK over all UICC stages in the unmatched cohorts. The largest and probably clinically most important differences were found for UICC stages II/III. This may be related to the fact that in the German cohort only patients without any chemotherapeutic treatment were included, whereas patients having undergone adjuvant chemotherapy in the NCCK group were not omitted. Adjuvant chemotherapy significantly improved survival in a Korean randomized controlled clinical.[25] Patients undergoing neoadjuvant chemotherapy were omitted from this analysis because preoperative chemotherapy was not a standard of care in the period (2002–2008) analyzed here. Further on, chemotherapy regimens were not standardized at that time which may have influenced the results considerably. Besides that, preoperative chemotherapy was applied mostly for patients undergoing treatment for locally irresectable, metastatic or clinically noncurative cancers in the TUM cohort.
Overall complication rate was significantly higher in Germans compared to Koreans in the unmatched cohort. Several groups reported that survival of postoperative complications leads to worsened long-term outcomes after oncologic surgery.[26–28] Postoperative complications were survival predictors for both patient cohorts in this analysis not only in the unmatched but also in the PSM analysis. There were significantly more Clavien–Dindo Class III–V complications in the unmatched TUM group, which may have caused a higher mortality rate in the follow-up period of the unmatched patients. However, postoperative complications were matched by the PSM algorithm and therefore should not translate into a possible confounding factor in the matched cohorts.
Several previously published studies found that differences in patients’ characteristics created difficulties in direct comparisons of Eastern and Western GC patients.[8–12] Therefore we aimed to find a way eliminating those baseline differences. Simple matching is rightfully prone to criticism of selection bias. In order to create homogenous groups we used PSM for patients after primary R0 resection without preoperative chemotherapy regardless of adjuvant chemotherapy status. PSM is a statistical method applied to reduce possible selection bias in observational/nonrandomized studies, which was initially proposed by Rosenbaum and Rubin in 1983,[13] ruling out confounders in nonrandomized studies. There are even hints that PSM may be a suitable tool for evaluation of treatment outcomes when prospective randomized controlled trials are not feasible or possible.[29,30] Postsurgical variables were also considered for matching in this analysis because the intervention of interest in this study was to compare cohorts that were as homogenous as possible. The matched groups revealed balanced baseline characteristics for the relevant pathologic and epidemiologic factors. Despite the PSM, oncologic outcomes of the respective cohorts were still different regarding stage-dependent survival rates. However, this effect was statistically significant only for UICC stage I but not for UICC stages II/III which may be related to the low patient numbers in the PSM cohorts. It is important to realize that the treatment center itself was predictive for OS in the univariable and multivariable analysis after PSM and the differences certainly would have reached statistical significance had the numbers bin higher. The exact reasons for this remain elusive, because not only preoperative factors (age, gender, tumor location) and surgical procedures (type of gastrectomy, number of dissected lymph nodes) but also postsurgical variables such as the application of adjuvant chemotherapy, postoperative complication rates, and LNR stages were also included in the matching algorithm. LNR staging proposed by Kong[31] appears to be more appropriate in comparative studies between Eastern and Western patients in order to overcome the known drawbacks of stage migration effects due to more extensive LN dissections in Eastern patients.[31]
There are several limitations for this analysis; although PSM is an accepted tool to overcome selection bias, the data were analyzed retrospectively. Another limitation is that there is a huge disparity of the patient numbers between the centers which is related to the tenfold higher GC-incidence rate in Korea. Therefore no clear conclusions can be drawn on those many patients not having been included in the PSM cohort. Moreover, PSM has some limitations which are the inability to account for unmeasured factors like surgical quality, biologic and genetic differences and the need for the statistical analysis to account for the paired nature of the matched samples, possibly explaining the remaining differences in outcome. Further, patients undergoing neoadjuvant treatments were not included here due to the reasons described above, which may have led to a certain kind of selection bias.
Conclusively this report reveals that previously published differences in oncologic outcome between Eastern-Asian and European patients undergoing surgical treatment for GC may not be exclusively related to different clinical baseline characteristics, but also to biological and ethnical differences. Further, this analysis reveals that GC survival after surgical treatment in a specialized Western center can reach excellent results in Western terms, but does not reach Eastern Asian prognosis in early and advanced GC stages. However, biologic and genetic differences as much as the effect of adjuvant chemotherapy cannot be completely ruled out by this analysis, as only a small amount of German patients was compared to Koreans. Not only surgical principles of Eastern GC surgeons but also oncologic principles including standardization of postoperative chemotherapy should be adopted by Western clinicians in order to obtain improved oncologic results for their respective patients regardless of biological differences.
Footnotes
Abbreviations: CD = postoperative complications according to Clavien and Dindo, EGD = esophagogastroduodenoscopy, ESD = endoscopic submucosal dissection, FYSR = five-year survival rate, GC = gastric cancer, GE = gastroesophageal, LN = lymph node, LNR = lymph-node ratio, ratio of positive lymph nodes/dissected lymph nodes, LVI = lymphatic vessel infiltration, NCCK = National Cancer Center Korea, OS = overall survival, PS = propensity score, PSM = propensity score matching, propensity score matched, RFS = recurrence-free survival, TUM = Technische Universitaet Muenchen.
YWK and JJ contributed equally to this article as first author.
Funding: This work was supported by the National Cancer Center grant NCC-1410130-1. We thank Hyun Jung Park (Research Nurse) and Soo Hee Kim (Research Nurse) for the extraordinary support in data collection and preparation of data. This work was also supported by the German Research Foundation (DFG) and the Technical University of Munich (TUM) in the framework of the Open Access Publishing Program.
This manuscript fully consists of original material, which has not been published or submitted to another journal. All authors declare that they participated in the analysis and that they have seen and approved the final version. Data acquisition and analysis was performed according to local ethics standards and GCP guidelines.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol 2013; 107:230–236. [DOI] [PubMed] [Google Scholar]
- 2.Shin A, Kim J, Park S. Gastric cancer epidemiology in Korea. J Gastric Cancer 2011; 11:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin Y, Ueda J, Kikuchi S, et al. Comparative epidemiology of gastric cancer between Japan and China. World J Gastroenterol 2011; 17:4421–4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel R, Naishadham D, Jemal A. Cancer statistics for Hispanics/Latinos, 2012. CA Cancer J Clin 2012; 62:283–298. [DOI] [PubMed] [Google Scholar]
- 5.Reim D, Gertler R, Novotny A, et al. Adenocarcinomas of the esophagogastric junction are more likely to respond to preoperative chemotherapy than distal gastric cancer. Ann Surg Oncol 2012; 19:2108–2118. [DOI] [PubMed] [Google Scholar]
- 6.Moehler M, Al-Batran SE, Andus T, et al. AWMF. [German S3-guideline “Diagnosis and treatment of esophagogastric cancer”]. Z Gastroenterol 2011; 49:461–531. [DOI] [PubMed] [Google Scholar]
- 7.Suh M, Choi KS, Lee YY, et al. Trends in cancer screening rates among Korean men and women: results from the Korean National Cancer Screening Survey, 2004–2012. Cancer Res Treat 2013; 45:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bollschweiler E, Boettcher K, Hoelscher AH, et al. Is the prognosis for Japanese and German patients with gastric cancer really different? Cancer 1993; 71:2918–2925. [DOI] [PubMed] [Google Scholar]
- 9.Noguchi Y, Yoshikawa T, Tsuburaya A, et al. Is gastric carcinoma different between Japan and the United States? Cancer 2000; 89:2237–2246. [PubMed] [Google Scholar]
- 10.Strong VE, Song KY, Park CH, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg 2010; 640–646. [DOI] [PubMed] [Google Scholar]
- 11.Theuer CP, Kurosaki T, Ziogas A, et al. Asian patients with gastric carcinoma in the United States exhibit unique clinical features and superior overall and cancer specific survival rates. Cancer 2000; 89:1883–1892. [DOI] [PubMed] [Google Scholar]
- 12.Theuer CP. Asian gastric cancer patients at a southern California comprehensive cancer center are diagnosed with less advanced disease and have superior stage-stratified survival. Am Surg 2000; 66:821–826. [PubMed] [Google Scholar]
- 13.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983; 70:41–55. [Google Scholar]
- 14.Ho D, Imai K, King G, et al. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal 2007; 15:199–236. [Google Scholar]
- 15.R Development Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2008. ISBN 3-900051-07-0, Available at: URL http://www.R-project.org Accessed January 20, 2016. [Google Scholar]
- 16.Ho D, Imai K, King G, et al. Matchit: nonparametric preprocessing for parametric causal inference. J Stat Softw 2007; 42:1–28. [Google Scholar]
- 17.Griffin SM. Gastric cancer in the East: same disease, different patient. Br J Surg 2005; 92:1055–1056. [DOI] [PubMed] [Google Scholar]
- 18.Roder JD, Böttcher K, Siewert JR, et al. Prognostic factors in gastric carcinoma. Results of the German Gastric Carcinoma Study 1992. Cancer 1993; 72:2089–2097. [DOI] [PubMed] [Google Scholar]
- 19.Schlemper RJ, Itabashi M, Kato Y, et al. Differences in diagnostic criteria for gastric carcinoma between Japanese and western pathologists. Lancet 1997; 349:1725–1729. [DOI] [PubMed] [Google Scholar]
- 20.Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomized nationwide Dutch D1D2 trial. Lancet Oncol 2010; 11:439–449. [DOI] [PubMed] [Google Scholar]
- 21.Memon MA, Subramanya MS, Khan S, et al. Meta-analysis of D1 versus D2 gastrectomy for gastric adenocarcinoma. Ann Surg 2011; 253:900–911. [DOI] [PubMed] [Google Scholar]
- 22.Hanna GB, Boshier PR, Knaggs A, et al. Improving outcomes after gastroesophageal cancer resection: can Japanese results be reproduced in Western centers? Arch Surg 2012; 147:738–745. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355:11–20. [DOI] [PubMed] [Google Scholar]
- 24.Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol 2010; 28:5210–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012; 379:315–321. [DOI] [PubMed] [Google Scholar]
- 26.Toner A, Hamilton M. The long-term effects of postoperative complications. Curr Opin Crit Care 2013; 19:364–368. [DOI] [PubMed] [Google Scholar]
- 27.Kodera Y, Ito S, Yamamura Y, et al. Obesity and outcome of distal gastrectomy with D2 lymphadenectomy for carcinoma. Hepatogastroenterology 2004; 51:1225–1228. [PubMed] [Google Scholar]
- 28.Moriwaki Y, Kunisaki C, Kobayashi S, et al. Does body mass index (BMI) influence morbidity and long-term survival in gastric cancer patients after gastrectomy? Hepatogastroenterology 2003; 50:284–288. [PubMed] [Google Scholar]
- 29.Kuss O, Legler T, Börgermann J. Treatments effects from randomized trials and propensity score analyses were similar in similar populations in an example from cardiac surgery. J Clin Epidemiol 2011; 64:1076–1084. [DOI] [PubMed] [Google Scholar]
- 30.Lonjon G, Boutron I, Trinquart L, et al. Comparison of treatment effect estimates from prospective nonrandomized studies with propensity score analysis and randomized controlled trials of surgical procedures. Ann Surg 2014; 259:18–25. [DOI] [PubMed] [Google Scholar]
- 31.Kong SH, Lee HJ, Ahn HS, et al. Stage migration effect on survival in gastric cancer surgery with extended lymphadenectomy: the reappraisal of positive lymph node ratio as a proper N-staging. Ann Surg 2012; 255:50–58. [DOI] [PubMed] [Google Scholar]


