Supplemental Digital Content is available in the text.
Abstract
For patients with extensive burns or donor site scarring, the limited availability of autologous and the inevitable rejection of allogeneic skin drive the need for new alternatives. Existing engineered biologic and synthetic skin analogs serve as temporary coverage until sufficient autologous skin is available. Here we report successful engraftment of a self-assembled bilayered skin construct derived from autologous skin punch biopsies in a porcine model. Dermal fibroblasts were stimulated to produce an extracellular matrix and were then seeded with epidermal progenitor cells to generate an epidermis. Autologous constructs were grafted onto partial- and full-thickness wounds. By gross examination and histology, skin construct vascularization and healing were comparable to autologous skin grafts and were superior to an autologous bilayered living cellular construct fabricated with fibroblasts cast in bovine collagen. This is the first demonstration of spontaneous vascularization and permanent engraftment of a self-assembled bilayered bioengineered skin that could supplement existing methods of reconstruction.
For the treatment of patients with extensive burns, heavy scarring or large defects, xenogeneic and synthetic bioengineered alternatives to autologous skin grafts have been introduced, but none of them share the combined capacity of autologous split-thickness skin grafts (STSGs) to engraft, vascularize, provide epidermal coverage and persist long-term.1–5 Current bioengineered skin graft alternatives include cultured epithelial autografts (CEAs),6 epidermal progenitor cells, dermal regeneration templates, and composite skin substitutes.1,2,5 However, clinical utility of these is limited by rejection of allogeneic skin substitutes,7,8 absence of the epidermal component in dermal regeneration templates, variable rates of engraftment in CEA, epidermal progenitor cells, cultured skin substitute (CSS),9–12 lack of engraftment in self-assembled skin substitute (SASS),13 difficulty in handling because of lack of a stratum corneum in CEA,5,14,15 and the potentially sensitizing presence of xenogeneic cells or foreign collagen.16–18 Among composite skin substitutes, the most “anatomically correct” are bilayered living cellular construct [BLCC, first Living Skin Equivalent introduced by Bell et al.19 and found its way to commercialization as allogeneic, off-the-shelf, wound healing product: Apligraf® (Organogenesis Inc., Canton, Mass.)]20, SASS, and the CSS.5 CSS (PermaDerm, Regenicin, Little Falls, N.J.) is a bilayered construct composed of autologous fibroblasts seeded on collagen-glycosaminoglycan scaffold with an epidermal layer of differentiated autologous keratinocytes. Although CSS features engraftment (71–82%), albeit lower than STSG (>90%),5,11 there are few studies supporting its effectiveness.1,2,5 Another available composite bioengineered construct is the bilayered living cellular construct (BLCC, Apligraf, Organogenesis Inc., Canton, Mass.), which is used to promote healing of chronic venous leg and diabetic foot ulcers. The lower layer of the BLCC comprises human fibroblasts cultured in bovine collagen, and the upper layer comprises keratinocytes that have differentiated into a stratified epithelium. The BLCC is applied under allogeneic conditions and promotes secondary intention healing from the wound margins, but it does not engraft or sensitize the patient.21,22 The BLCC has not been studied under autologous conditions, and, therefore, its ability to engraft and provide immediate and lasting wound coverage is unknown. Most importantly, both the CSS and BLCC contain foreign collagen as a scaffold or gel.2,5 Random pore area, pore fraction, and structure of fabricated dermal matrix23,24 may contribute to the engraftment variability observed with the CSS or lack of engraftment of the BLCC. An ideal skin substitute, however, would be a durable bilayered construct that is morphologically and biochemically similar to native skin, replicating its texture, structure, and capacity to engraft.1,5
Dermal fibroblasts in static culture can assemble a native extracellular matrix (ECM)25; this process of dermal ECM generation is termed the self-assembly. The dermis generated by self-assembly can be seeded with keratinocytes to produce a bilayered construct (containing dermis and epidermis) structurally simillar to skin. When applied under autologous conditions, the risk of allosensitization by these constructs is eliminated.
Here, we describe the implementation and preclinical testing of a self-assembled autologous bioengineered skin, termed the autologous skin construct (ASC).25 The ASC was tested as a treatment for partial-thickness wounds (PTWs) and full-thickness wounds (FTWs) in a translational miniature swine model, which was selected because of the similarity between pig and human skin.26,27 We hypothesized that the ASC derived from autologous dermal fibroblasts and keratinocytes would engraft and persist similarly to an autologous STSG and that the engraftment and persistence of these constructs would be superior to constructs generated using a bovine collagen gel, given the similarity in tissue composition and structure between an STSG and the ASC.25 Constructs were grafted onto PTWs or FTWs of 3 animals from 2 distinct breeds (Yorkshire and Massachusetts General Hospital miniature swine) and followed for up to a year by clinical observation and histological assessment. The ASC’s behavior was compared with that of an autologous STSG, an autologous BLCC (aBLCC), and to wounds that were allowed to heal by secondary intention alone.
MATERIALS AND METHODS
Animals
All experimental procedures were carried out in accordance with the Guide for Care and Use of Laboratory Animals (Eighth edition, National Academy of Sciences Press, 2011) and were approved by the Institutional Animal Care and Use Committees of the MGH and Tufts University, Grafton, Mass. The MGH miniature swine (20687) was bred at the MGH farm and the Yorkshire swine (1, 2) were delivered by cesarean section at Cummings Tufts Veterinary School from a sow obtained from Earle M. Parsons & Sons Inc., Produce and Livestock (Hadley, Mass.). The MGH miniature swine experiments were supervised by CAH at the Transplantation Biology Research Center (TBRC), Massachusetts General Hospital, Boston, MA, and the Yorkshire swine experiments were overseen by TJB and the Organogenesis team at the Cummings School of Veterinary Medicine at Tufts University. The animals presented in this study were performed as controls for the study on the immunology of allogeneic self-assembled skin constructs, presented in a parallel manuscript.
Cell Culture, ASC, and aBLCC Generation
Porcine ASCs and aBLCCs were generated at the Preclinical Research and Development Laboratory, Organogenesis Inc. Dermal fibroblast and keratinocyte cell banks were created from 6-mm skin punch biopsies of neonatal (0–3 days) MGH miniature swine (20687) and Yorkshire swine (1, 2). Punch biopsies were incubated with trypsin and collagenase to release cells (Fig. 1), which were then serially passaged in media favoring proliferation of either fibroblasts (Dulbecco’s Modified Eagle’s Medium containing 15% fetal bovine serum) or keratinocytes (minimally supplemented basal medium).28 Cultures were cryopreserved at 2 stages to generate master and working cell banks. The minimally supplemented basal medium used to expand porcine keratinocyte cultures was modified by the addition of triiodothyronine, bovine pituitary extract, and cholera toxin, as described,28 to prevent differentiation during expansion in monolayer.
Fig. 1.
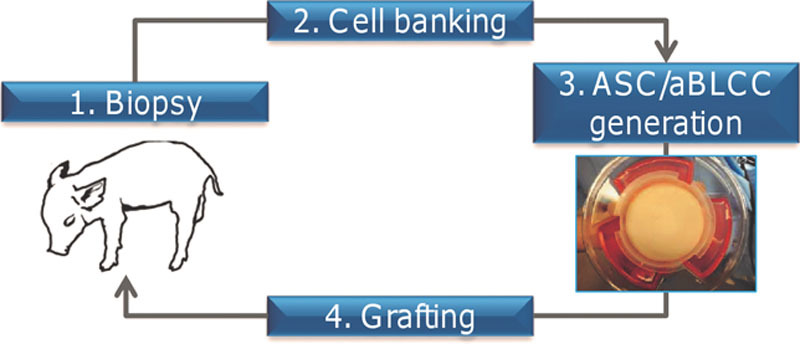
Experimental study design and ASC or aBLCC generation. Skin punch biopsies were taken from a neonatal piglet, followed by cell banking of the fibroblasts and keratinocytes, generation of the ASC and aBLCC, and grafting onto the same animal.
The lower (dermal) layer of the ASC was produced from dermal fibroblasts (30 million cells/44.2 cm2), which were thawed from cryopreserved cell banks, expanded for 1 additional passage, and seeded into a 75-mm diameter tissue culture inserts (Corning, Corning, NY) as previously described, except that cells were cultured in Dulbecco’s Modified Eagle’s Medium supplemented only with 50 µg/mL L-ascorbic acid-2-phosphate and 20% fetal bovine serum.25,29–31 The lower layer of the aBLCC was generated in 75-mm diameter tissue culture inserts as previously described.31
The upper (epidermal) layers of the ASC and aBLCC were generated from keratinocytes, which were thawed from a cryopreserved cell bank and expanded for 1 additional passage before seeding onto dermal matrices with a concentration of 10,000 cells/cm2. The tissue inserts were lifted to the air-liquid interface, with tissues receiving periodic media replenishment to promote epidermal stratification and barrier function development, which were induced to differentiate and stratify, as described previously31 (Fig. 2). Quality control was performed before the release of each ASC lot to ensure sterility, morphology, and viability.
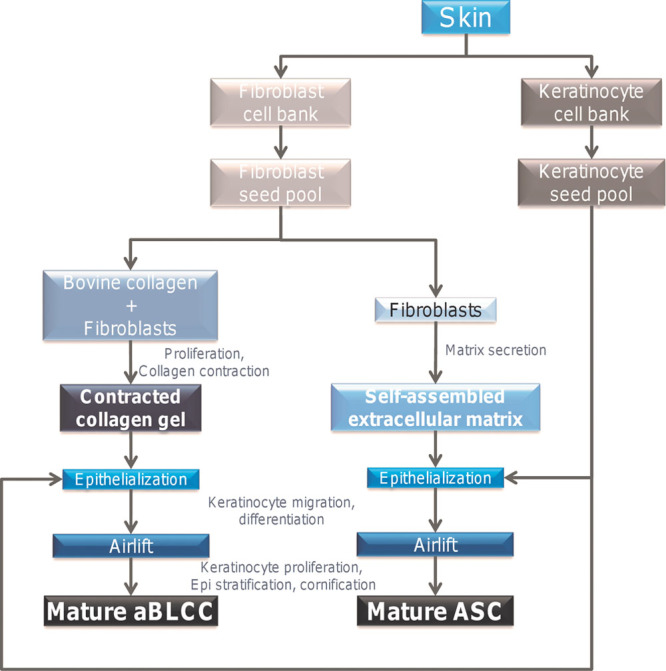
Fig. 2.
Schematic representation of tissue dissociation, cell banking, and ASC or aBLCC generation.
Split-thickness Skin and Construct Grafting
The ability of the ASC to engraft like autologous skin was tested on PTW (n = 4) and FTW (n = 2) beds. Animals were between 5 and 14 months old at the time of the surgery. On study day 0, animals were placed under general anesthesia in prone position and 4 × 4 cm PTWs or FTWs were prepared on the backs of the cell-donor animals. A dermatome was used to create PTW, until widely spaced punctate bleeding was observed (depth of approximately 1 mm into the dermis). FTW were made by scalpel until subcutaneous fat was reached. The ASC and STSG were fenestrated with a scalpel blade, applied to the wound bed dermis side down, and sutured to the wounds with prolene sutures. Standardized bolstering technique using nonadherent dressing: Adaptic (Systagenix, San Antonio, Tex.) and moist and dry gauze dressings followed by KCI V.A.C. GranuFoam (KCI, San Antonio, Tex.) were used to cover the grafts. Overall, 2 FTW and 4 PTW were treated with the ASC. Three PTW and 1 FTW were left to heal by secondary intention alone (sham) as a negative control (Supplemental Digital Content 1, http://links.lww.com/PRSGO/A199), each animal received at least 1 STSG, to allow characterization and comparison of the wound bed vascularity/quality grafting technique.
Graft Biopsy
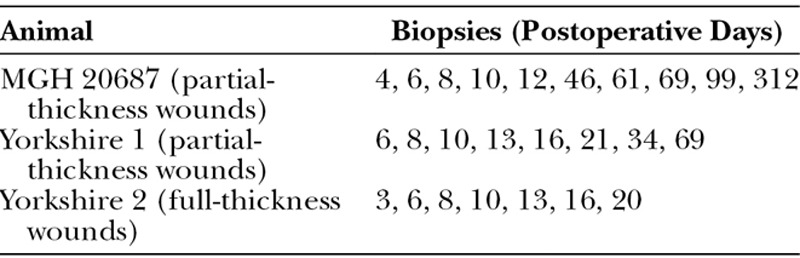
Wounds were observed clinically, photographed, and biopsied according to Table 1. Dressing changes, photographs, and 3- or 6-mm punch biopsies were performed every 2 or 3 days starting on postoperative day (POD) 3, 4 or 6 (Table 1). Engraftment was evaluated by gross examination and histology. Images were captured with Sony TX10 cyber-shot/Nikon D90; figures were prepared using PowerPoint, Adobe InDesign CS3, version 5.0 (Adobe Systems, Inc., San Jose, Calif.); for the minor processing (resizing, cropping) Adobe Photoshop CS3, version 10.0 (Adobe Systems), was used.
Table 1.
Schedule of Biopsies
Histology Preparation
Biopsies were fixed in 10% neutral buffered formalin for subsequent paraffin embedding. All paraffin embedding and tissue sectioning of research laboratory material were performed either by the MGH Department of Pathology or by the Preclinical Research and Development Laboratory at Organogenesis Inc.
Histology and Immunohistochemistry
Paraffin-embedded sections were stained with H&E for evaluation of tissue morphology or stained by immunohistochemistry for CD31 for visualization of angiogenesis with a rabbit anti-human CD31 monoclonal antibody (Clone SP164; Spring Bioscience, Pleasanton, Calif.). Sections were observed on a Zeiss Axio Imager 2 (Carl Zeiss Microscopy GmbH, Goettingen, Germany), and images were recorded at 10× magnification using AxioVision software (Carl Zeiss Microscopy) and Aperio ImageScope v10 (Leica Biosystems, Buffalo Grove, Ill.).
RESULTS
Fibroblast and keratinocyte cell banks were established from neonatal skin punch biopsies by the cellular dissociation and serial passaging described in Materials and Methods. The porcine ASC and aBLCC were generated from banked dermal fibroblasts and keratinocytes under organotypic culture conditions (Figs. 2 and 3). To generate the ASC, dermal fibroblasts were seeded at high density and induced by selective media to generate an ECM for 18 days. To generate the aBLCC, fibroblasts were resuspended in a solution of collagen, which was neutralized to initiate formation of a collagen gel. Both dermal constructs were then seeded with autologous keratinocytes, which were allowed to differentiate and cornify for 14 days. Each lot of ASCs and aBLCCs was rigorously tested for viability and morphology by histology before grafting (Fig. 3). PTWs, prepared on the backs of MGH swine 20687 and Yorkshire 1, and FTWs prepared on the back of Yorkshire 2, as described in the Materials and Methods, were treated with STSG (n = 4), ASC (n = 6), or aBLCC (n = 2) or were allowed to heal by secondary intention alone (sham) (n = 4) to test whether the ASC could engraft and persist when applied to the dermis or hypodermis, respectively. Grafts were fenestrated and after application were sutured and bolstered to prevent shearing, desiccation, and the formation of seromas and hematomas. Wounds were monitored clinically and histologically for viability, signs of rejection, inflammation, vascularization, and collagen maturation for up to a year (Figs. 4–6 and Table 1) (Supplemental Digital Content 1, http://links.lww.com/PRSGO/A199).
Fig. 3.
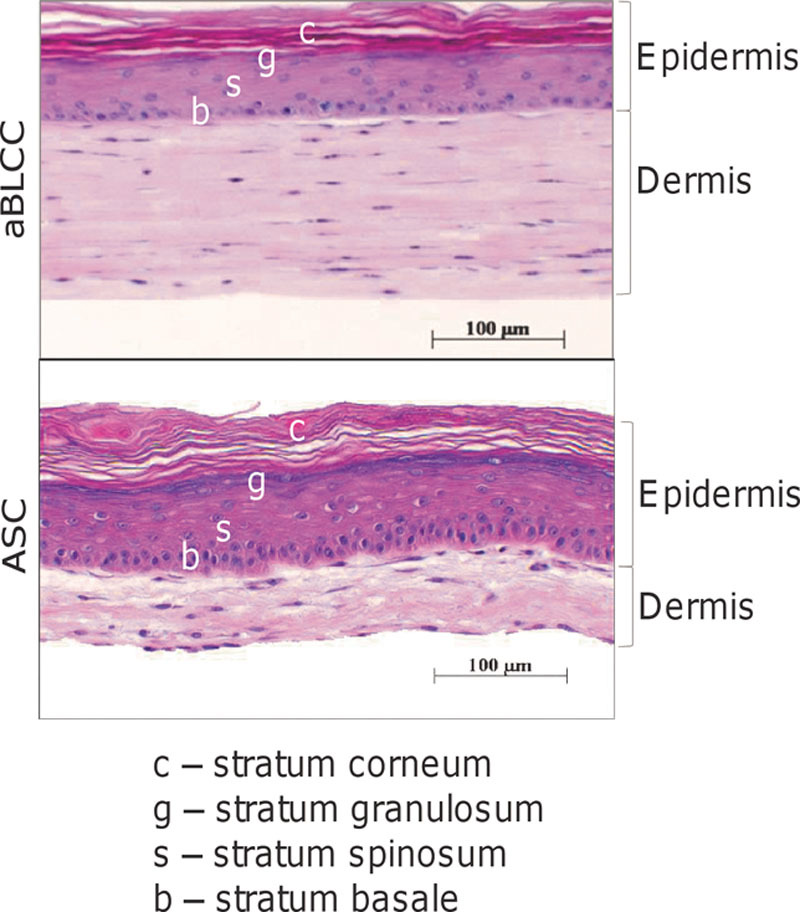
Histologically, the ASC and aBLCC contain the normal bilayered morphology of human skin, with a dermal layer containing dermal fibroblasts in either a self-assembled (generated without inclusion of foreign collagen) extracellular matrix (ASC) or a bovine type I collagen gel (aBLCC), and a stratified and differentiated epidermal layer containing a stratum basale (b), stratum spinosum (s), stratum granulosum (g), and stratum corneum (c) (H&E staining).
Fig. 4.
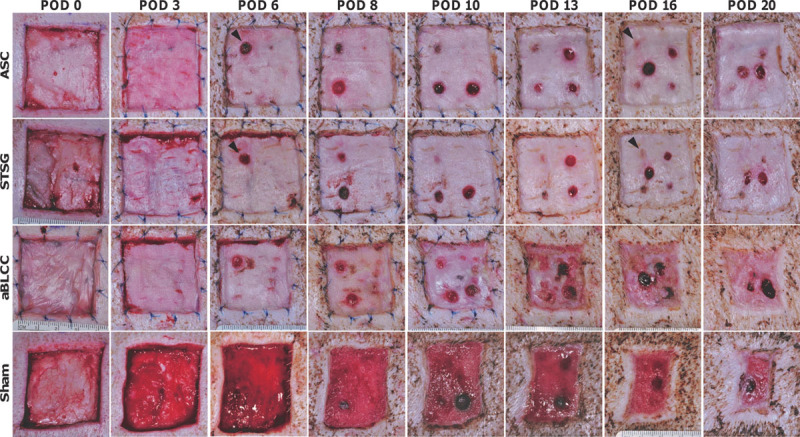
Representative time course of the FTWs treated with the ASC, STSG, aBLCC, or dressings alone (sham) acquired from Yorkshire 2. Arrowheads point to the sites of biopsies performed on STSG and ASC on POD 3, which by POD 16 have completely healed. STSG- and ASC-treated sites undergo minimal wound contraction in contrast with the aBLCC-treated and sham sites. The aBLCC sloughed partially from the wound during the POD 8 dressing change, and the remainder was lost by POD 16, after which the wound epidermalized from the margin.
Fig. 6.
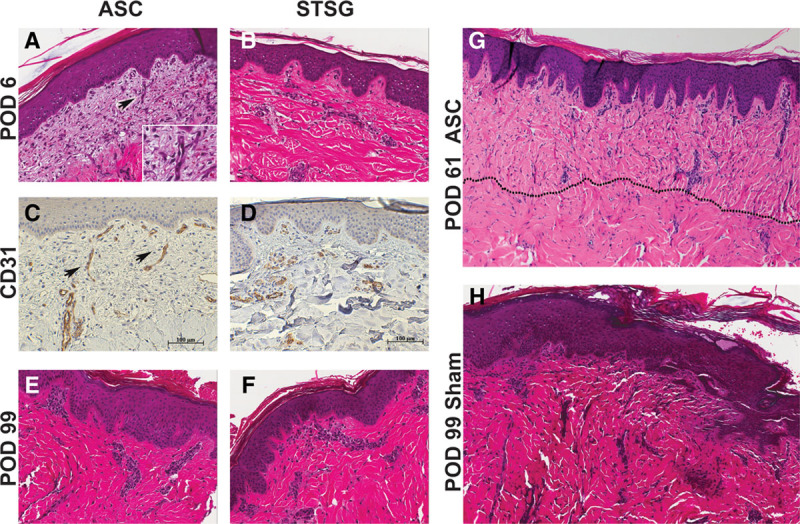
Representative histology of PTWs treated with ASC and STSG on the MGH swine (20687). A, Partial-thickness wound treated with ASC on POD 6 shows viable, engrafted construct with early angiogenesis from wound bed toward dermal-epidermal junction (H&E, 20×); arrow and inset highlight angiogenesis within the loose dermal matrix. B, Partial-thickness wound treated with STSG on POD 6 with engraftment and no inflammation. C and D, POD 6 biopsies stained with CD31 demonstrate angiogenesis in the ASC and transplanted vasculature in the STSG (20×). E and F, Late time-point biopsies showing complete engraftment and integration, no inflammation of the ASC and STSG (H&E, 20×). G, Engrafted ASC on POD 61 with a well-healed wound bed and mature collagen; vertically oriented collagen bundles and increased cellularity help define the graft-wound bed junction (dotted line). H, Histology of 1 representative sham wound (untreated with STSG or ASC) at late time-point (POD 99), entirely re-epithelialized by secondary intention with collagen disorganization and scar (gross image in Supplemental Digital Content 3, http://links.lww.com/PRSGO/A201).
ASC and STSG Behave Comparably in PTWs
The autologous ASCs and STSGs applied to PTWs were adherent at early time-points (POD 3–4) and were pinkish-red, contoured well to the wound bed, and had well-integrated edges. By POD 6, the ASC and STSG were well-integrated into the wound bed and contained a healthy and stratified epidermis (Figs. 4–6) (Supplemental Digital Content 1, http://links.lww.com/PRSGO/A199 and Supplemental Digital Content 2, http://links.lww.com/PRSGO/A200). All STSGs and ASCs, at later time-points, appeared clinically healthy, with normal turgor; no hematomas, seromas, or infection were observed. Desquamation of the STSGs and ASCs was noted early during the course of healing. ASCs and STSGs were comparable in long-term follow-up (Fig. 4) (Supplemental Digital Content 1, http://links.lww.com/PRSGO/A199). Histology showed absence to minimal inflammation, comprising mostly polymorphonuclear cells of the innate immune system starting on POD 6 (Figs. 5, 6); inflammation was similar to ASC- and STSG-treated sites. Dermal capillaries extending up to the dermal-epidermal junction were observed in the ASC starting on POD 6, highlighted by CD31 staining of the endothelial cells (Figs. 5, 6). Complete engraftment of ASCs and STSGs was observed for up to 1 year (Supplemental Digital Content 1, http://links.lww.com/PRSGO/A199).
Fig. 5.
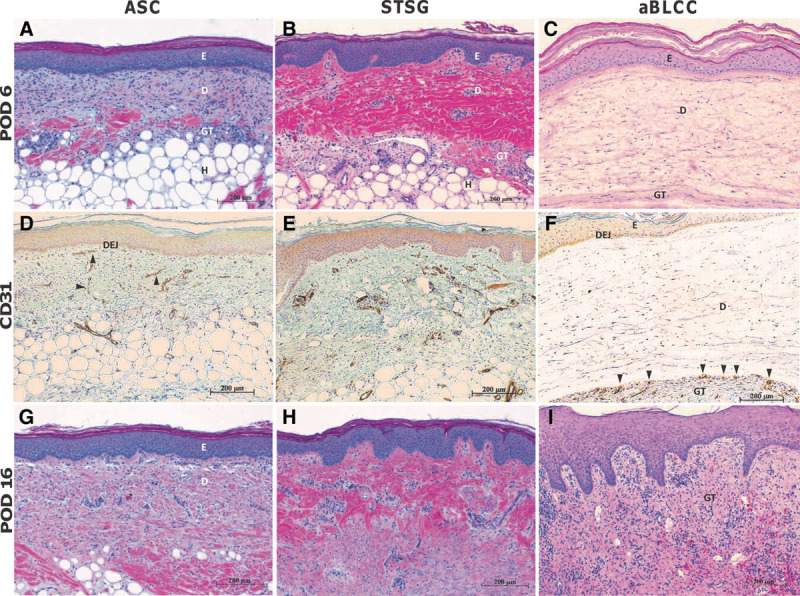
Representative histology of FTWs treated with ASC, STSG, and aBLCC. A, ASC-treated wound on POD 6 showing a viable, engrafted construct. B, STSG-treated wound on POD 6 demonstrates similar viability and engraftment. C, aBLCC on POD 6 showing hydrated dermal layer. D, CD31 staining of ASC-treated wound on POD 6 highlighting early angiogenesis (arrowheads) from wound bed toward dermal-epidermal junction (DEJ) of the construct. E, CD31 staining of STSG on POD 6. F, CD31 staining of aBLCC-treated site on POD 6 showing vasculature limited to granulation tissue (GT) immediately adjacent to the bottom of the construct but not penetrating. G, ASC-treated wound on POD 16 with interval deposition of collagen bundles throughout the fibroblast layer of the construct, most notably at the graft bed, indicating successful integration and maturation. H, STSG-treated wound on POD 16 showing continued healing. I. aBLCC-treated site on POD 16. Wound site comprises granulation tissue and a neo-epidermis derived from the wound margin. Inflammation is absent on POD 16 in both STSG- and ASC-treated sites. All sections H&E- or anti-CD31-stained and captured at 10× magnification. ASC, STSG, and aBLCC epidermal (E) and dermal (D) layers and wound bed granulation tissue (GT) and hypodermis (H), are indicated within the images.
The ASC and STSG Behave Differently Than the aBLCC in FTWs
The ASCs and STSGs applied to FTWs under autologous conditions behaved similarly to those applied to PTWs (see above). In contrast, the aBLCCs applied to FTWs were generally adherent on POD 3 but had a waxy surface and a whitish-pink appearance; however, they did not contour as well to the wound bed as the ASCs and STSGs and were peeling slightly at their peripheral edges. On POD 6, the edges of the aBLCCs continued to peel. By POD 8, both aBLCCs sloughed off partially. The sloughing continued and was complete on POD 16 after which the aBLCC-treated wounds healed by secondary intention. After each biopsy, the ASC- and STSG-treated sites healed by granulation from the wound bed, followed by epidermalization. As these biopsies were taken from the wound bed and as FTWs did not contain appendages and could only therefore heal from their peripheral edges, it indicated that epidermalization of the biopsy sites was facilitated by graft keratinocytes, indicating that both the ASC and the STSG were viable and physiologically active throughout the study time course (Fig. 4) (Supplemental Digital Content 1, http://links.lww.com/PRSGO/A199). In contrast to the ASC- and STSG-treated FTWs, healing in the aBLCC-treated wound sites by epidermalization from the graft did not occur. This occurred despite the fact that the epidermis of the aBLCC appeared by histology to be viable normally stratified (Fig. 3).
On day 6, the epidermis of the aBLCC exhibited a healthy stratified morphology (Fig. 5). Imbibition of wound fluid within the construct was manifested as a thickening of the dermal component and a separation of swollen collagen fibers in the plane parallel to that of the wound bed (Fig. 5). The construct was not adherent to the wound bed, unlike the ASC and STSG, which were well-integrated on POD 6 (Fig. 5). Clearly demarcated layers of inflammatory cells accumulated at the surface of the granulation tissue, immediately adjacent to the aBLCC (Fig. 5). This inflammatory cell layer was composed mostly of macrophages, lymphocytes, and endothelial and connective tissue cells. In contrast to the ASC and STSG, there was no cellular infiltration or vascularization within the aBLCC. However, numerous blood vessels were observed in the granulation tissue immediately beneath the aBLCC as evidenced by CD31 immunohistochemical staining of endothelial cells (Fig. 5). The bovine collagen layer of the aBLCC appeared to present a barrier to infiltration of vessels and inflammatory cells. In comparison, infiltration of both cells and vasculature into the ASC appeared to be uninhibited.
By POD 10, wound exudation had stopped. A significant inflammatory infiltrate, comprising neutrophils, lymphocytes, and monocytes/macrophages, was observed, which coincided with complete necrosis of the cells within the aBLCC. Because the aBLCC was applied under autologous conditions and contained the same cells as the ASC, it is assumed that the necrosis was the result of lack of both serum exudation and vasculature within the construct. By POD 16, the aBLCC was no longer present on the wound (Fig. 5).
The STSG and ASC Minimize FTW Contraction
Both full-thickness and all partial-thickness sites treated with the ASC and STSG maintained their original size, without apparent contraction; in contrast, the full-thickness sham site and the aBLCC sites were highly contracted and moderately contracted, respectively (Fig. 4) (Supplemental Digital Content 1, http://links.lww.com/PRSGO/A199). Furthermore, the ASC- and STSG-treated sites contained very little granulation tissue, whereas the aBLCC and sham sites granulated well (Fig. 5). Further maturation and remodeling of the fibroblast layer of the ASC, as indicated by thickening and reorganization of collagen fibers, was seen on POD 16 and continued until study termination (Figs. 5, 6). Late time-point biopsies showed complete engraftment and integration, with no inflammation of the ASC or STSG (Fig. 6). At late time-points, vertically oriented collagen bundles and vessels and the absence of a well-developed superficial vascular plexus were the only features that distinguished the ASC from the STSG sites (Fig. 6).
DISCUSSION
The ASC Is a Viable Alternative to the STSG for Delayed Coverage of PTWs and FTWs
Our results demonstrate that an autologous composite skin substitute with a self-assembled dermis is an efficacious alternative to STSGs for FTWs or PTWs. In contrast to the CSS, which requires multiple grafting procedures because of a lower engraftment rate, ASC revascularization was successful even in a less vascularized wound such as the FTWs lacking the perforator vessels of the dermis. The similar short- and long-term appearance of the wounds treated with the ASC and STSG in this translational model further suggests that they may have comparable cosmetic outcomes in the clinical setting. Minimal contraction of the wounds treated with ASCs was comparable with the STSGs, despite the significant difference in the thickness (ASC approximately 200 µm and STSG approximately 350 µm). It is believed that dermal thickness is inversely proportional to the degree of the resulted contraction. Regardless of small thickness of ASC, the contraction is comparable with STSG and has an advantage if compared with the sham and aBLCC sites (Fig. 4).
Manipulation and application of the ASC were similar to those of the STSG because of the presence of a robust stratum corneum (Fig. 3), which gave the construct strength and durability. In addition, integration into the wound bed and revascularization were comparable in speed to inosculation of the STSG. Notably, the ASC does not require preformed vessels for successful engraftment, contrary to what Tremblay et al.32 showed, but undergoes angiogenesis early after implantation, in contrast to the STSG, which contains vessels that undergo inosculation. The ability to quickly and efficiently induce a new vascular supply is thought to be a major factor affecting graft survival.32 All existing bilayered skin constructs contain foreign collagen scaffolds and, with the exception of CSS, they do not possess the essential property of a matrix—the capacity to engraft.2,5,10,11,13,33 The construct, proposed by Boa et al.,13 also fails to engraft, despite the fact that it is autologous and is generated through self-assembly.
The ASC has important clinical limitations. As it is derived exclusively from dermal fibroblasts and keratinocytes, the ASC does not contain appendages, such as, hair follicles and eccrine glands, which will affect cosmesis. ASCs also lack pigmentation, which may potentially be addressed by introducing melanocytes within the construct.1,2,34 Further research will address these issues, and other unanswered questions, including the need for cell banking in construct generation and the influence of the age of the donor. Nevertheless, we believe that the self-assembly approach is a major advance toward a true substitute for skin with excellent engraftment and long-term viability. Our translational model demonstrated successful engraftment without rejection and with a cosmetic outcome comparable with STSG in PTWs and FTWs as judged by clinical observation. It is important to mention the clinical testing of the SASS for chronic wounds, which is a different construct, however, using a similar approach: the self-assembly. SASS requires 6–7 construct applications for successful venous ulcer healing.13 The ASC contains only 1 sheet of ECM, in contrast to SASS, which combines multiple sheets, and the media formulation is different, which may have affected the intrinsic ECM properties, such as, porosity, ligand density, and cross-linking factors, that critically affect bioavailability in case of matrices.23,24 Also, we applied constructs to acute wounds, in contrast with the lower extremity ulcer, studied by Boa et al.,13 which may have had a poor vascular supply.
The successful engraftment of all animals in our study with a single ASC application is encouraging and could be a step forward toward clinical trials. No infection was noticed within our experiments; however, it may be attributed to a low number of animals and rigorous aseptic care. It is known that infection is capable of inhibiting engraftment of an STSG.35
Despite the current timeframe for generation of the construct (approximately 6 weeks), the clinical outcome closely resembles the gold standard of burn repair: the autologous skin graft. Further study is underway to streamline the manufacturing process. However, clinical studies and further development are needed to test the feasibility and to decrease ASC production time. Nevertheless, this report represents the first demonstration of spontaneous vascularization and permanent engraftment of a self-assembled bioengineered skin construct, and this technology could supplement existing methods used in reconstructive surgery.
ACKNOWLEDGMENTS
We would like to acknowledge Drs. Vimukthi Patiraja and Raimon Duran-Struuck for performing initial biopsies, Ashley Gusha and Edward Harrington for excellent technical help, Obed Oposada and Kim Flink for animal care, J. Scott Arn for facilitating the MGH farm, Nicole Brousaides for pathology preparation, Dr. Robert Colvin for pathology review, and Dr. Curtis Cetrulo for the critical review of the manuscript, advice, and plastic surgical expertise. We would like to acknowledge our funding sources, the training grant NIH T32 AI007529-15, which funded MC and EAF, animal facility grant CO6RR020135-01, and Organogenesis Inc.
Supplementary Material
Footnotes
Disclosure: Supported and performed in collaboration with Organogenesis Inc. Dr. Thomas J. Bollenbach, Erika Medeiros, Dr. Jizeng Qiao, Dr. Cecile F. Rousseau, Dr. Waldemar Racki, Dr. Shumin Dong and Agatha Zawadzka were employees of Organogenesis Inc. The Article Processing Charge was paid for by the authors.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Biedermann T, Boettcher-Haberzeth S, Reichmann E. Tissue engineering of skin for wound coverage. Eur J Pediatr Surg. 2013;23:375–382. doi: 10.1055/s-0033-1352529. [DOI] [PubMed] [Google Scholar]
- 2.Kamel RA, Ong JF, Eriksson E, et al. Tissue engineering of skin. J Am Coll Surg. 2013;217:533–555. doi: 10.1016/j.jamcollsurg.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 3.Pomahac B, Svensjo T, Yao F, et al. Tissue engineering of skin. Crit Rev Oral Biol Med. 1998;9:333–344. doi: 10.1177/10454411980090030601. [DOI] [PubMed] [Google Scholar]
- 4.Shevchenko RV, James SL, James SE. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J R Soc Interface. 2010;7:229–258. doi: 10.1098/rsif.2009.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(Pt 1):493–508. doi: 10.1097/01.ASW.0000288217.83128.f3. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor NE, Mulliken JB, Banks-Schlegel S, et al. Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet. 1981;1:75–78. [PubMed] [Google Scholar]
- 7.Myers S, Navsaria H, Sanders R, et al. Transplantation of keratinocytes in the treatment of wounds. Am J Surg. 1995;170:75–83. doi: 10.1016/s0002-9610(99)80258-x. [DOI] [PubMed] [Google Scholar]
- 8.Nyame TT, Chiang HA, Orgill DP. Clinical applications of skin substitutes. Surg Clin North Am. 2014;94:839–850. doi: 10.1016/j.suc.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Boyce ST, Glatter R, Kitzmiller WJ. Case studies: treatment of chronic wounds with cultured skin substitutes. Ostomy Wound Manage. 1995;41:26–28, 30, 32. [PubMed] [Google Scholar]
- 10.Boyce ST, Goretsky MJ, Greenhalgh DG, et al. Comparative assessment of cultured skin substitutes and native skin autograft for treatment of full-thickness burns. Ann Surg. 1995;222:743–752. doi: 10.1097/00000658-199512000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyce ST, Kagan RJ, Greenhalgh DG, et al. Cultured skin substitutes reduce requirements for harvesting of skin autograft for closure of excised, full-thickness burns. J Trauma. 2006;60:821–829. doi: 10.1097/01.ta.0000196802.91829.cc. [DOI] [PubMed] [Google Scholar]
- 12.Boyce ST, Kagan RJ, Yakuboff KP, et al. Cultured skin substitutes reduce donor skin harvesting for closure of excised, full-thickness burns. Ann Surg. 2002;235:269–279. doi: 10.1097/00000658-200202000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boa O, Cloutier CB, Genest H, et al. Prospective study on the treatment of lower-extremity chronic venous and mixed ulcers using tissue-engineered skin substitute made by the self-assembly approach. Adv Skin Wound Care. 2013;26:400–409. doi: 10.1097/01.ASW.0000433102.48268.2a. [DOI] [PubMed] [Google Scholar]
- 14.Ehrenreich M, Ruszczak Z. Update on tissue-engineered biological dressings. Tissue Eng. 2006;12:2407–2424. doi: 10.1089/ten.2006.12.2407. [DOI] [PubMed] [Google Scholar]
- 15.Williamson JS, Snelling CF, Clugston P, et al. Cultured epithelial autograft: five years of clinical experience with twenty-eight patients. J Trauma. 1995;39:309–319. doi: 10.1097/00005373-199508000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Badylak SF. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol. 2004;12:367–377. doi: 10.1016/j.trim.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Charriere G, Bejot M, Schnitzler L, et al. Reactions to a bovine collagen implant. Clinical and immunologic study in 705 patients. J Am Acad Dermatol. 1989;21:1203–1208. doi: 10.1016/s0190-9622(89)70330-3. [DOI] [PubMed] [Google Scholar]
- 18.Lynn AK, Yannas IV, Bonfield W. Antigenicity and immunogenicity of collagen. J Biomed Mater Res B Appl Biomater. 2004;71:343–354. doi: 10.1002/jbm.b.30096. [DOI] [PubMed] [Google Scholar]
- 19.Bell E, Ehrlich HP, Buttle DJ, et al. Living tissue formed in vitro and accepted as skin-equivalent tissue of full thickness. Science. 1981;211:1052–1054. doi: 10.1126/science.7008197. [DOI] [PubMed] [Google Scholar]
- 20.Eaglstein WH, Falanga V. Tissue engineering and the development of Apligraf, a human skin equivalent. Clin Ther. 1997;19:894–905. doi: 10.1016/s0149-2918(97)80043-4. [DOI] [PubMed] [Google Scholar]
- 21.Eaglstein WH, Alvarez OM, Auletta M, et al. Acute excisional wounds treated with a tissue-engineered skin (Apligraf). Dermatol Surg. 1999;25:195–201. doi: 10.1046/j.1524-4725.1999.08186.x. [DOI] [PubMed] [Google Scholar]
- 22.Falanga V, Margolis D, Alvarez O, et al. Rapid healing of venous ulcers and lack of clinical rejection with an allogeneic cultured human skin equivalent. Human Skin Equivalent Investigators Group. Arch Dermatol. 1998;134:293–300. doi: 10.1001/archderm.134.3.293. [DOI] [PubMed] [Google Scholar]
- 23.Lloyd C, Besse J, Boyce S. Controlled-rate freezing to regulate the structure of collagen-glycosaminoglycan scaffolds in engineered skin substitutes. J Biomed Mater Res B Appl Biomater. 2015;103:832–840. doi: 10.1002/jbm.b.33253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yannas IV. Emerging rules for inducing organ regeneration. Biomaterials. 2013;34:321–330. doi: 10.1016/j.biomaterials.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Pouyani T, Ronfard V, Scott PG, et al. De novo synthesis of human dermis in vitro in the absence of a three-dimensional scaffold. In Vitro Cell Dev Biol Anim. 2009;45:430–441. doi: 10.1007/s11626-009-9213-6. [DOI] [PubMed] [Google Scholar]
- 26.Debeer S, Le Luduec JB, Kaiserlian D, et al. Comparative histology and immunohistochemistry of porcine versus human skin. Eur J Dermatol. 2013;23:456–466. doi: 10.1684/ejd.2013.2060. [DOI] [PubMed] [Google Scholar]
- 27.Jacobi U, Kaiser M, Toll R, et al. Porcine ear skin: an in vitro model for human skin. Skin Res Technol. 2007;13:19–24. doi: 10.1111/j.1600-0846.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 28.Johnson EW, Meunier SF, Roy CJ, et al. Serial cultivation of normal human keratinocytes: a defined system for studying the regulation of growth and differentiation. In Vitro Cell Dev Biol. 1992;28A:429–435. doi: 10.1007/BF02634047. [DOI] [PubMed] [Google Scholar]
- 29.Cvetkovska B, Islam N, Goulet F, et al. Identification of functional markers in a self-assembled skin substitute in vitro. In Vitro Cell Dev Biol Anim. 2008;44:444–450. doi: 10.1007/s11626-008-9140-y. [DOI] [PubMed] [Google Scholar]
- 30.Michel M, L’Heureux N, Pouliot R, et al. Characterization of a new tissue-engineered human skin equivalent with hair. In Vitro Cell Dev Biol Anim. 1999;35:318–326. doi: 10.1007/s11626-999-0081-x. [DOI] [PubMed] [Google Scholar]
- 31.Wilkins LM, Watson SR, Prosky SJ, et al. Development of a bilayered living skin construct for clinical applications. Biotechnol.Bioeng. 1994;43:747–756. doi: 10.1002/bit.260430809. [DOI] [PubMed] [Google Scholar]
- 32.Tremblay PL, Hudon V, Berthod F, et al. Inosculation of tissue-engineered capillaries with the host’s vasculature in a reconstructed skin transplanted on mice. Am J Transplant. 2005;5:1002–1010. doi: 10.1111/j.1600-6143.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 33.Griffiths M, Ojeh N, Livingstone R, et al. Survival of Apligraf in acute human wounds. Tissue Eng. 2004;10:1180–1195. doi: 10.1089/ten.2004.10.1180. [DOI] [PubMed] [Google Scholar]
- 34.Regnier M, Duval C, Schmidt R. Potential cosmetic applications of reconstructed epidermis. Int J Cosmet Sci. 1999;21:51–58. doi: 10.1046/j.1467-2494.1999.183571.x. [DOI] [PubMed] [Google Scholar]
- 35.Teh BT. Why do skin grafts fail? Plast Reconstr Surg. 1979;63:323–332. doi: 10.1097/00006534-197903000-00005. [DOI] [PubMed] [Google Scholar]


