Abstract
Introduction:
Postoperative infections are a major complication associated with tissue-expander-based breast reconstruction. The use of acellular dermal matrix (ADM) in this surgery has been identified as a potential reservoir of infection, prompting the development of sterile ADM. Although aseptic and sterile ADMs have been investigated, no study has focused on the occurrence and clinical outcome of bacterial colonization before implantation.
Methods:
Samples of aseptic AlloDerm, sterile Ready-To-Use AlloDerm, and AlloMax were taken before implantation. These samples were incubated in Tryptic soy broth overnight before being streaked on Trypticase soy agar, MacConkey agar, and 5% blood agar plates for culture and incubated for 48 hours. Culture results were cross-referenced with patient outcomes for 1 year postoperatively.
Results:
A total of 92 samples of ADM were collected from 63 patients. There were 15 cases of postoperative surgical site infection (16.3%). Only 1 sample of ADM (AlloMax) showed growth of Escherichia coli, which was likely a result of contamination. That patient did not develop any infectious sequelae. Patient outcomes showed no difference in the incidence of seroma or infection between sterile and aseptic ADMs.
Conclusions:
This study evaluates the microbiology of acellular dermal matrices before use in breast reconstruction. No difference was found in the preoperative bacterial load of either aseptic or sterile ADM. No significant difference was noted in infection or seroma formation. Given these results, we believe aseptic processing used on ADMs is equivalent to sterile processing in our patient cohort in terms of clinical infection and seroma occurrence postoperatively.
As the prevalence of breast cancer has increased so has the number of patients seeking mastectomy and reconstruction, both therapeutically and prophylactically.1 Despite the option of autologous breast reconstruction, the most common method used for breast reconstruction remains the 2-stage tissue expander and implant reconstruction.2 Traditionally, this technique involves the placement of a tissue expander under the pectorals major muscle, with the serratus anterior muscle serving as an inferolateral sling. In an attempt to expedite tissue expansion, improve cosmesis, and accommodate larger sized tissue expanders, Salzberg et al3 introduced the concept of using an acellular dermal matrix (ADM) to replace the serratus anterior muscle in 2001.
ADM gained popularity among reconstructive surgeons, who touted its benefits including the ability to perform single-stage reconstruction,4 better inframammary fold definition, and improved inferior pole projection.4–6 There have also been claims of reduced rate of capsular contracture7,8 and improved esthetic results.9–11 Recent studies have also demonstrated reduced cost of operation when using ADM to complete 1-stage reconstruction in comparison with 2-stage reconstruction with a submuscular implant.5,12
Unfortunately, numerous complications have been associated with ADM use, most notably infection and seroma. Previous work by our group associated ADM use in patients with breasts greater than 600 g with an increased risk of infection,13 a finding supported by subsequent studies.9,14–16 However, other studies have failed to demonstrate any significant difference in infectious complications between ADM and non-ADM breast reconstruction.17–19 This finding was supported by Fahrenbach et al,20 who demonstrated that aseptic ADMs are resistant to penetration by bacteria, including skin flora such as staphylococcus or streptococcus. With that being said, these studies were all completed with aseptic ADM, which requires an intraoperative rehydration and preparation process that could potentially lead to contamination.
In response to the concerns of increased rates of infection, LifeCell (Bridgewater, N.J.) created a sterile ADM regenerative tissue matrix, which does not require intraoperative rehydration and claims a sterility assurance level (SAL) of 10−6.21 Current work with sterile ADM has not shown a conclusive benefit compared to aseptic preparations. Two systematic reviews show a decreased rate of infection,21,22 whereas a third study shows no significant difference.23 The purpose of this study is to investigate the microbiology of aseptic and sterile ADMs before implantation and determine if there is a correlation with postoperative infections and seroma.
METHODS
ADM samples were obtained immediately upon opening the sterile packaging before implantation during surgical procedures. Samples measuring 1 × 1 cm were taken from AlloDerm, AlloDerm Ready-To-Use (LifeCell), and AlloMax (Bard, Warwick, R.I.) using sterile surgical instruments. This study was approved by institutional review board under exempt status, January 2012.
ADM samples were incubated in Tryptic soy broth (BD Biosciences, San Jose, Calif.) and were shaken at 225 rpm at 37°C overnight using a bacterial shaker (Benchmark Inc, Edison, N.J.). Samples were then streaked on Tryptic soy agar, MacConkey agar, and 5% blood agar plates using sterile disposable inoculation loops and incubated at 37°C overnight. Escherichia coli (E. coli) was streaked on similar plates as a positive control for bacterial growth. After 24 hours of incubation, the plates were observed for the presence of bacterial colonies. If no growth was observed, the plates were incubated for an additional 24 hours at 37°C. The absence of observable bacterial growth after 48 hours of incubation indicated the samples were free of investigated bacteria and were recorded as negative for bacterial growth.
Patient charts were also reviewed after 1 year postoperatively to assess overall outcomes. Patients were specifically evaluated for cellulitis, deep space infection, and seroma formation. Any wound cultures from patients experiencing infectious complications were documented as well. Patients were split into 2 groups based on which ADM they received. Those receiving AlloDerm were considered aseptic and those receiving AlloMax or AlloDerm RTU were considered sterile. Statistical analysis was completed using χ2 analysis.
RESULTS
Between February 2012 and May 2013, a total of 92 samples were collected from ADMs implanted in 63 random, nonconsecutive patients of homogenous demographics (Table 1). Of these, 81 samples were taken from ADMs used in immediate breast reconstruction, 3 from delayed breast reconstructions, and 8 from breast reconstruction revisions. A total of 53 samples of AlloDerm were implanted (33 patients), 24 samples of AlloDerm RTU (19 patients), and 15 samples of AlloMax (11 patients). Patient demographics are shown in Table 1.
Table 1.
Patient Demographics by Acellular Dermal Matrix Used

Cultures were positive in a single ADM sample, taken from AlloMax, which was subsequently used for an immediate breast reconstruction. The sample grew cultures on all 3 agars, and based on positive controls was a Gram-negative species, specifically E. coli. No genotyping was performed on the organism. The patient who received this ADM experienced no infectious complications and did not develop a seroma postoperatively.
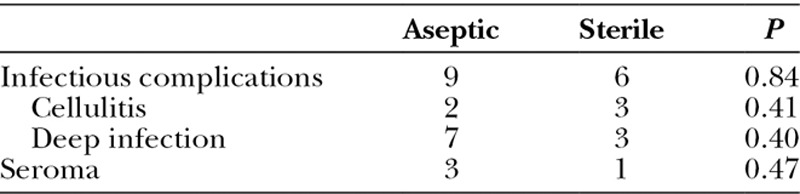
A total of 15 (23.8%) patients developed postoperative infections and 4 (6.3%) patients developed seroma. There were 9 (17.0%) infections in the aseptic cohort and 6 (15.4%) in the sterile cohort. Of the 4 samples in patients who developed seroma, 3 were from aseptic ADMs and the other 1 was from a sterile ADM. These differences were not found to be statistically significant (Table 2). No postoperative infections were noted in the patients implanted with AlloMax. There was no statistically significant difference compared with the aseptic group (P = 0.09) or to the rest of the study population (P = 0.064).
Table 2.
Complications Comparing Aseptic and Sterile Cohorts
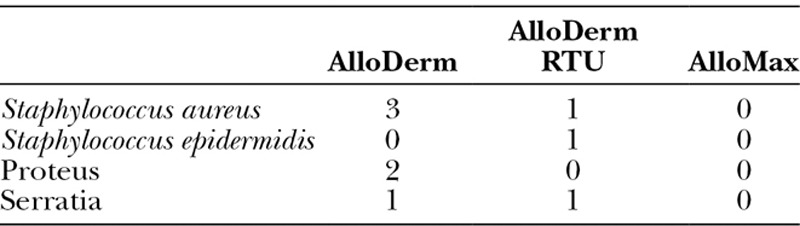
Cultures drawn after being diagnosed with a clinical infection were also documented. No patients diagnosed with cellulitis had cultures performed, although all 10 deep infection patients had cultures taken after expander removal. Of these patients, 9 yielded positive cultures (Table 3). Five of these infections were secondary to normal skin flora (Staphylococcus aureus and Staphylococcus epidermidis), whereas 4 were caused by Gram-negative organisms (Proteus mirabilis and Serratia marcescens). Overall, there was no difference between sterile and aseptic products in terms of which organism caused a deep space infection.
Table 3.
Culture Results
DISCUSSION
The use of ADM in breast reconstruction remains an important component of 1- and 2-stage alloplastic breast reconstruction. Although ADM gained widespread use in 2005,22 recent literature has advocated for a more selective utilization, specifically for patients with well-vascularized flaps, with large or ptotic breasts, or who are unable to achieve adequate inferior implant coverage with a serratus anterior muscle flap.24 More recent studies recommended selective use due to increased risk of seroma and infection.3,5,8,9,11,13–16,25–28 Given the significant expense of ADM, which is priced by the manufacturer at $25 to $30 per square centimeter, financial constraints demand assessment of surgical utilization on an individual scale, especially in the face of the associated risk profiles.3,8
Potential complications associated with ADM use include seroma formation or flap necrosis. These complications are likely caused by the use of a foreign body and a prosthesis surrounded by poorly vascularized mastectomy skin flaps, which in turn may contribute to increased infection rates.5 Failure to match the ADM dimensions to the skin flap may impair incorporation, thus creating a space for fluid buildup. The resulting seroma provides a microenvironment where bacteria may flourish and cause infection. ADM also allows for surgeons to use implants in 1-stage reconstruction or to increase the initial fill of the tissue expander. A larger implant or expander can stretch the skin flaps and further compromise blood flow, leading to flap necrosis. By compromising the flap blood supply, ADM incorporation, local immune function, and the protective nature of the skin are all impaired, potentially allowing for bacterial overgrowth and infection.
Although there is ample evidence of increased rates of infection in patients who receive ADM, these findings are far from a consensus opinion. A number of studies have found that ADM poses no significant risk of increasing the rate of clinical infection.5,17–19,29 Peled et al30 even found a statistically significant decrease in infection rate when using ADM. When investigating sterile ADM such as AlloDerm Ready-To-Use and AlloMax, early results from Weichman et al22 and Venturi et al21 indicated these ADMs decreased infection rate as well. Conversely, a study by Buseman et al23 showed no difference in infection rate, although there was an increased seroma rate associated with sterile ADM use. This conclusion was also confirmed in a meta-analysis by Macarios et al,31 comparing AlloDerm to AlloDerm RTU, which concluded that complication rates were equivocal between the 2 ADMs. These disparate opinions, likely secondary to the common use of a retrospective study design or insufficient power, further underscore the need for a proper randomized, controlled trial.
Current guidelines recommend an SAL of 10–6 for products deemed “sterile,” meaning no more than 1 organism should be present in 1,000,000 sterile products.32 In comparison, aseptic products require an SAL of 10–3. Although theoretically sterilizing a product confers a lower bacterial load, from a practical standpoint a difference in infection rates between these levels has not been proven.33 Unfortunately, the sterilization process may also affect the mechanical properties of ADMs. Mendenhall et al34 performed a study on 14 different brands of ADM, using fluorescent in situ hybridization to determine microbial growth patterns and electron microscopy to investigate the effects of the sterilization process. More bacteria per high power field were observed in the aseptically processed group, although this did not impact growth in culture (3.6 versus 1.6; P = 0.0003).34 Imaging these ADMs with electron microscopy showed a more disorganized collagen structure in the sterile ADMs, suggesting possible damage stemming from the sterilization process.34 Although the overall impact of sterilization on the mechanical properties of ADMs is uncertain, physicomechanical studies have shown decreased suture retention strength, tear resistance, and ball burst strength when comparing sterilized human ADMs to aseptically processed human ADMs.35
In our study, only 1 sample of ADM grew out any organisms in culture. The single culture likely grew out E. coli, which is an organism not typically associated with infection after breast reconstruction. This sample was likely contaminated during the preparation process or in transport from the operating room to the sample dish. This is further supported by a lack of clinical infection in the patient postoperatively. Our results show that the aseptic ADM is not colonized, especially after the preparation process, and is resistant to bacterial growth. This indicates that there may be little to no significance in increasing the SAL in these products from 10–3 to 10–6 with a sterilization procedure. With that being said, once placed inside the body, these properties may change, especially in the presence of seroma and/or flap necrosis, which have been proposed as causative mechanisms.
This study is limited by the methodology in which we investigate ADM ex vivo and its limited sample size. Although the number of cultured samples is significant, the lack of power may have contributed to the inability to find a statistically significant difference in clinical outcomes. Our clinical results are also impacted by the large number of confounding variables, such as medical comorbidities, adjuvant therapies, and differences among surgeons. Further work on this topic requires a more extensive, long-term prospective study to fully validate our findings. A prospective, blinded study would be optimal, with a long-term follow-up of complications. A study of in vivo ADM samples would also help to elucidate this relationship.
CONCLUSIONS
This study evaluates the microbiology of samples of acellular dermal matrices before use in breast reconstruction. No difference was found in the preoperative bacterial load of either aseptic or sterile ADM. Further, no significant difference was noted in clinical infection or seroma formation in either group. Given these results, we feel that the aseptic processing used on ADMs was equivalent to sterile processing in our patient cohort in terms of clinical infection and seroma occurrence postoperatively.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. This study was investigator initiated and self-funded. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Pesce CE, Liederbach E, Czechura T, et al. Changing surgical trends in young patients with early stage breast cancer, 2003 to 2010: a report from the National Cancer Data Base. J Am Coll Surg. 2014;219:19–28. doi: 10.1016/j.jamcollsurg.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 2.Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131:15–23. doi: 10.1097/PRS.0b013e3182729cde. [DOI] [PubMed] [Google Scholar]
- 3.Salzberg CA, Ashikari AY, Koch RM, et al. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plast Reconstr Surg. 2011;127:514–524. doi: 10.1097/PRS.0b013e318200a961. [DOI] [PubMed] [Google Scholar]
- 4.Salzberg CA. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann Plast Surg. 2006;57:1–5. doi: 10.1097/01.sap.0000214873.13102.9f. [DOI] [PubMed] [Google Scholar]
- 5.Vardanian AJ, Clayton JL, Roostaeian J, et al. Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg. 2011;128:403e–410e. doi: 10.1097/PRS.0b013e31822b6637. [DOI] [PubMed] [Google Scholar]
- 6.Breuing KH, Colwell AS. Inferolateral AlloDerm hammock for implant coverage in breast reconstruction. Ann Plast Surg. 2007;59:250–255. doi: 10.1097/SAP.0b013e31802f8426. [DOI] [PubMed] [Google Scholar]
- 7.Colwell AS, Damjanovic B, Zahedi B, et al. Retrospective review of 331 consecutive immediate single-stage implant reconstructions with acellular dermal matrix: indications, complications, trends, and costs. Plast Reconstr Surg. 2011;128:1170–1178. doi: 10.1097/PRS.0b013e318230c2f6. [DOI] [PubMed] [Google Scholar]
- 8.Macadam SA, Lennox PA. Acellular dermal matrices: economic considerations in reconstructive and aesthetic breast surgery. Clin Plast Surg. 2012;39:187–216. doi: 10.1016/j.cps.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Antony AK, McCarthy CM, Cordeiro PG, et al. Acellular human dermis implantation in 153 immediate two-stage tissue expander breast reconstructions: determining the incidence and significant predictors of complications. Plast Reconstr Surg. 2010;125:1606–1614. doi: 10.1097/PRS.0b013e3181d4fb2a. [DOI] [PubMed] [Google Scholar]
- 10.Forsberg CG, Kelly DA, Wood BC, et al. Aesthetic outcomes of acellular dermal matrix in tissue expander/implant-based breast reconstruction. Ann Plast Surg. 2014;72:S116–S120. doi: 10.1097/SAP.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 11.Ho G, Nguyen TJ, Shahabi A, et al. A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction. Ann Plast Surg. 2012;68:346–356. doi: 10.1097/SAP.0b013e31823f3cd9. [DOI] [PubMed] [Google Scholar]
- 12.Jansen LA, Macadam SA. The use of AlloDerm in postmastectomy alloplastic breast reconstruction: part II. A cost analysis. Plast Reconstr Surg. 2011;127:2245–2254. doi: 10.1097/PRS.0b013e3182131c6b. [DOI] [PubMed] [Google Scholar]
- 13.Lanier ST, Wang ED, Chen JJ, et al. The effect of acellular dermal matrix use on complication rates in tissue expander/implant breast reconstruction. Ann Plast Surg. 2010;64:674–678. doi: 10.1097/SAP.0b013e3181dba892. [DOI] [PubMed] [Google Scholar]
- 14.Chun YS, Verma K, Rosen H, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg. 2010;125:429–436. doi: 10.1097/PRS.0b013e3181c82d90. [DOI] [PubMed] [Google Scholar]
- 15.Kim JY, Davila AA, Persing S, et al. A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg. 2012;129:28–41. doi: 10.1097/PRS.0b013e3182361fd6. [DOI] [PubMed] [Google Scholar]
- 16.Liu AS, Kao HK, Reish RG, et al. Postoperative complications in prosthesis-based breast reconstruction using acellular dermal matrix. Plast Reconstr Surg. 2011;127:1755–1762. doi: 10.1097/PRS.0b013e31820cf233. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim AM, Shuster M, Koolen PG, et al. Analysis of the National Surgical Quality Improvement Program database in 19,100 patients undergoing implant-based breast reconstruction: complication rates with acellular dermal matrix. Plast Reconstr Surg. 2013;132:1057–1066. doi: 10.1097/PRS.0b013e3182a3beec. [DOI] [PubMed] [Google Scholar]
- 18.Sbitany H, Serletti JM. Acellular dermis-assisted prosthetic breast reconstruction: a systematic and critical review of efficacy and associated morbidity. Plast Reconstr Surg. 2011;128:1162–1169. doi: 10.1097/PRS.0b013e318230c29e. [DOI] [PubMed] [Google Scholar]
- 19.Nahabedian MY. AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation. Plast Reconstr Surg. 2009;124:1743–1753. doi: 10.1097/PRS.0b013e3181bf8087. [DOI] [PubMed] [Google Scholar]
- 20.Fahrenbach EN, Qi C, Ibrahim O, et al. Resistance of acellular dermal matrix materials to microbial penetration. JAMA Dermatol. 2013;149:571–575. doi: 10.1001/jamadermatol.2013.1741. [DOI] [PubMed] [Google Scholar]
- 21.Venturi ML, Mesbahi AN, Boehmler JH, 4th, et al. Evaluating sterile human acellular dermal matrix in immediate expander-based breast reconstruction: a multicenter, prospective, cohort study. Plast Reconstr Surg. 2013;131:9e–18e. doi: 10.1097/PRS.0b013e3182729d4f. [DOI] [PubMed] [Google Scholar]
- 22.Weichman KE, Wilson SC, Saadeh PB, et al. Sterile “ready-to-use” AlloDerm decreases postoperative infectious complications in patients undergoing immediate implant-based breast reconstruction with acellular dermal matrix. Plast Reconstr Surg. 2013;132:725–736. doi: 10.1097/PRS.0b013e31829fe35b. [DOI] [PubMed] [Google Scholar]
- 23.Buseman J, Wong L, Kemper P, et al. Comparison of sterile versus nonsterile acellular dermal matrices for breast reconstruction. Ann Plast Surg. 2013;70:497–499. doi: 10.1097/SAP.0b013e31827f52c8. [DOI] [PubMed] [Google Scholar]
- 24.Jordan SW, Khavanin N, Fine NA, et al. An algorithmic approach for selective acellular dermal matrix use in immediate two-stage breast reconstruction: indications and outcomes. Plast Reconstr Surg. 2014;134:178–188. doi: 10.1097/PRS.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 25.Clemens MW, Kronowitz SJ. Acellular dermal matrix in irradiated tissue expander/implant-based breast reconstruction: evidence-based review. Plast Reconstr Surg. 2012;130(5 suppl 2):27S–34S. doi: 10.1097/PRS.0b013e318265f690. [DOI] [PubMed] [Google Scholar]
- 26.de Blacam C, Momoh AO, Colakoglu S, et al. Cost analysis of implant-based breast reconstruction with acellular dermal matrix. Ann Plast Surg. 2012;69:516–520. doi: 10.1097/SAP.0b013e318217fb21. [DOI] [PubMed] [Google Scholar]
- 27.Jordan SW, Khavanin N, Fine NA, et al. An algorithmic approach for selective acellular dermal matrix use in immediate two-stage breast reconstruction: indications and outcomes. Plast Reconstr Surg. 2014;134:178–188. doi: 10.1097/PRS.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 28.Krishnan NM, Chatterjee A, Van Vliet MM, et al. A comparison of acellular dermal matrix to autologous dermal flaps in single-stage, implant-based immediate breast reconstruction: a cost-effectiveness analysis. Plast Reconstr Surg. 2013;131:953–961. doi: 10.1097/PRS.0b013e3182865a24. [DOI] [PubMed] [Google Scholar]
- 29.Reish RG, Damjanovic B, Austen WG, Jr, et al. Infection following implant-based reconstruction in 1952 consecutive breast reconstructions: salvage rates and predictors of success. Plast Reconstr Surg. 2013;131:1223–1230. doi: 10.1097/PRS.0b013e31828bd377. [DOI] [PubMed] [Google Scholar]
- 30.Peled AW, Foster RD, Garwood ER, et al. The effects of acellular dermal matrix in expander-implant breast reconstruction after total skin-sparing mastectomy: results of a prospective practice improvement study. Plast Reconstr Surg. 2012;129:901–908. doi: 10.1097/PRS.0b013e31824ec447. [DOI] [PubMed] [Google Scholar]
- 31.Macarios D, Griffin L, Chatterjee A, et al. A meta-analysis assessing postsurgical outcomes between aseptic and sterile AlloDerm regenerative tissue matrix. Plast Reconstr Surg Glob Open. 2015;3:e409. doi: 10.1097/GOX.0000000000000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Woedtke T, Kramer A. The limits of sterility assurance. GMS Krankenhhyg Interdiszip. 2008;3:Doc19. [PMC free article] [PubMed] [Google Scholar]
- 33.Winckels HW, Dorpema W. Risk assessment as a basis for the definition of sterility. Med Dev Technol. 1994;5:38–43. [Google Scholar]
- 34.Mendenhall SD, Daugherty TC, Cosenza NM, et al. A microbiologic comparison of acellular dermal matrices as an aseptic reconstructive material and a scaffold for stem cell in-growth. Plast Reconstr Surg. 2015;135(5 suppl 1):101. [Google Scholar]
- 35.Deeken CR, Eliason BJ, Pichert MD, et al. Differentiation of biologic scaffold materials through physicomechanical, thermal, and enzymatic degradation techniques. Ann Surg. 2012;255:595–604. doi: 10.1097/SLA.0b013e3182445341. [DOI] [PubMed] [Google Scholar]


