Abstract
The interaction of β-actin mRNA with zipcode-binding protein 1 (ZBP1) is necessary for its localization to the lamellipod of fibroblasts and plays a crucial role in cell polarity and motility. Recently, we have shown that low ZBP1 levels correlate with tumor-cell invasion and metastasis. In order to establish a cause and effect relationship, we expressed ZBP1 in a metastatic rat mammary adenocarcinoma cell line (MTLn3) that has low endogenous ZBP1 levels and delocalized β-actin mRNA. This leads to localization of β-actin mRNA, and eventually reduces the chemotactic potential of the cells as well as their ability to move and orient towards vessels in tumors. To determine how ZBP1 leads to these two apparently contradictory aspects of cell behavior – increased cell motility but decreased chemotaxis – we examined cell motility in detail, both in cell culture and in vivo in tumors. We found that ZBP1 expression resulted in tumor cells with a stable polarized phenotype, and reduced their ability to move in response to a gradient in culture. To connect these results on cultured cells to the reduced metastatic ability of these cells, we used multiphoton imaging in vivo to examine tumor cell behavior in primary tumors. We found that ZBP1 expression actually reduced tumor cell motility and chemotaxis, presumably mediating their decreased metastatic potential by reducing their ability to respond to signals necessary for invasion.
Introduction
Localization of mRNA allows cells to spatially regulate translation and thus create functional subregions with distinct components. β-actin mRNA is specifically localized in fibroblasts (Kislauskis et al., 1993;Lawrence and Singer, 1986). Localization of β-actin protein to the leading edge where polymerization takes place is presumably dependant on this mRNA localization (Shestakova et al., 2001). This localization occurs in response to intracellular signaling (Latham, Jr et al., 1994) and is seen in a variety of cell types (Hill and Gunning, 1993; Hill et al., 1994; Hoock et al., 1991). Study of this mechanism led to the understanding that all localized mRNAs contain cis-acting elements, mostly located in the 3′UTR, that are bound by trans-acting factors to direct localization. In fibroblasts, short fragments of the β-actin 3′UTR from several species were demonstrated to be sufficient for localization when expressed in a heterologous construct (Kislauskis et al., 1994). One of these fragments, a 54-nucleotide sequence that forms a stem-loop structure, exhibited most of the localizing activity and has been termed the β-actin mRNA zipcode. Trans-acting localization factors that bound to the β-actin zipcode were identified and have been referred to as zipcode-binding proteins. A primarily cytoplasmic 68 kDa protein, which bound to the zipcode, was called zipcode-binding protein 1 (ZBP1) and contains several recognizable regions, including two RNA-recognition motifs (RRM), four hnRNP K homology (KH) domains as well as potential nuclear localization and export signals (Ross et al., 1997).
Recently, β-actin mRNA localization and ZBP1 have been implicated in metastasis. First, β-actin mRNA localization has been shown to be required for directed cell motility (Farina et al., 2003; Kislauskis et al., 1997), particularly in non-metastatic cells (Shestakova et al., 1999). Second, reduction of β-actin mRNA localization through treatment with antisense oligonucleotides targeting the zipcode, which disrupted the interaction between ZBP1 and β-actin mRNA, has been shown to convert the behavior of cells with a polarized movement phenotype to a `random walk' (Shestakova et al., 2001). Third, MTLn3 (metastatic) cells do not localize β-actin mRNA and contain significantly less ZBP1 than MTC (non-metastatic) cells derived from the same tumor, which do localize the mRNA (Wang et al., 2004). Fourth,ZBP1 was found to be highly expressed in rat mammary tumors as well as in human tumors (Noubissi et al., 2006; Yantiss et al., 2005) but its expression was shown to be suppressed specifically in the invasive subpopulation of tumor cells (Wang et al., 2004). These results suggested that the ability of tumor cells to exhibit amoeboid movement and, therefore, heightened chemotaxis ability as is characteristic of metastatic cells (Condeelis et al., 1992) depended on a random distribution of β-actin mRNA, whereas cells that are able to target β-actin mRNA retain a stable polarity that would be less responsive to a chemoattractant.
We hypothesized that ZBP1 protein induces β-actin mRNA localization (Oleynikov and Singer, 2003), which in turn suppresses chemotaxis by establishing a persistent polarity, leading to reduced responsiveness and ability to orient towards exogenous chemotactic gradients required for cellular invasiveness and hence metastatic potential. To test this hypothesis, we compared cell motility and chemotactic response, together with motility, protrusion and orientation towards vessels in tumors in cells with different levels of ZBP1 expression. To determine whether expression of ZBP1 in `random-walking' cells was sufficient to change them into `linear-walkers', we examined motility in a stable cell line expressing ZBP1 derived from random-walking metastatic cells (MTLn3). In addition, we examined the movement of cells with or without ZBP1 protein expression in the presence of chemotactic factors. Finally, the metastatic cell line expressing elevated levels of ZBP1 was tested for its ability to invade within mammary tumors to determine the role these motility changes play in vivo in an environment where metastasis occurs. Our results suggest that ZBP1 expression: (1) alters motility patterns, converting the movement of tumor cells with a random walking pattern characteristic of invasive cells into a highly polarized pattern of movement characteristic of non-invasive cells; (2) interferes with the ability of tumor cells to move in the direction of a chemotactic gradient and; (3) in living animals, ZBP1 expression leads to a similar reduction in motility towards vessels within tumors.
Results and Discussion
Levels of ZBP1 in cell lines
We hypothesized that the differences in polarity, motility and metastasis seen between random walking, highly metastatic mammary-tumor-derived cells (MTLn3) and linear walking, less invasive mammary-tumor-derived cells (MTC) stem from differences in ZBP1 levels. Previous work demonstrated that levels of ZBP1 mRNA differ between these cell lines, although differences in protein levels had not been directly observed (Wang et al., 2004). Therefore, we compared ZBP1 protein levels in these cell lines as well as the cell line we generated expressing a ZBP1 fusion protein. ZBP1 levels in the stable cell line referred to as ZBPA in Fig. 1 and ZBP throughout the rest of this paper are 3.99±1.96 times that of the parental MTLn3 cell line, and are closer to that of a less metastatic cell line derived from the mammary tumor MTC, which has ZBP1 levels 5.42±2.13 times that of MTLn3 cells and 1.44±0.43 times that of the ZBP1-expressing MTLn3 stable cells (supplementary material Fig. S1).
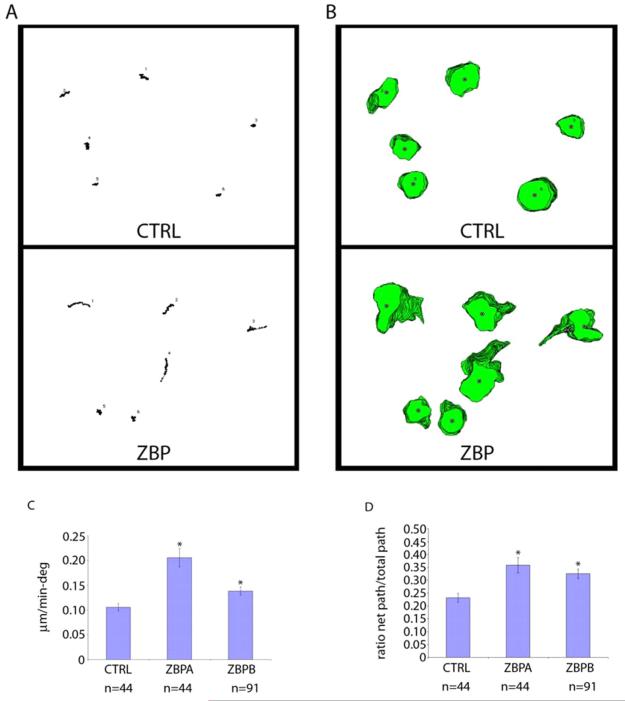
Fig. 1.
ZBP1 expression causes phenotypic conversion from random walk to directed movement, increasing persistence and directionality. Movement of GFP- and GFP-ZBP1-expressing MTLn3 cells (from two independently derived stable cell lines, indicated as ZBPA and ZBPB) was examined in 5% serum. The total time interval was 30 minutes, and time between successive frames was 1 minute. (A,B) Plots of cell (A) centroid and (B) perimeter for six GFP-expressing control MTLn3 cells (CTRL, A and B top) and six GFP-ZBP1-expressing MTLn3 cells (ZBP, A and B bottom). (C,D) Differences in (C) persistence and (D) directionality were statistically significant between the populations (*P<0.005, error bars indicate ± s.e.m.). Persistence is the speed divided by the change in direction, directionality is the net path length divided by the total path length.
ZBP1 increases the polarity of locomotion in tumor cells
Although the specific role of ZBP1 in β-actin mRNA localization and metastasis has been described, its role associating cell motility with metastasis needs to be established. To determine the effects of ZBP1 on cell motility directly, metastatic tumor cells (MTLn3) that do not express ZBP1 were transfected with a GFP-ZBP1-expressing transgene, and two stable lines were selected from independent transfections. ZBP1 function is unaffected by this fusion with GFP (Farina et al., 2003). Both transformants and the control cells, expressing GFP alone, were subjected to extensive motility analysis. Dynamic imaging analysis software (DIAS) was used to determine various parameters of cell movement and provide an accurate picture of cell position and displacement. This provided a rigorous assessment of the differences between genetically identical cell populations differing only in ZBP1 expression levels. Centroid (Fig. 1A) and perimeter (Fig. 1B) plots demonstrated that ZBP1-induced conversion of a parental `random walk' into a more linear `crawling pattern'. These experiments demonstrated that ZBP1 expression was sufficient to induce the motility phenotype consistent with a polarized cell, leading to significantly increased persistence and directionality in cell motility (Fig. 1C,D). Cell speed was also calculated from this analysis and for control cells was 0.76±0.15 μm/minute, for ZBPA was 1.05±0.27 μm/minute and for ZBPB was 0.79±0.22 μm/minute. Because cell speed was not reduced with ZBP1 expression, changes in speed cannot form the basis for changes in response to chemoattractant or in metastatic ability.
Orientation towards a chemoattractant is reduced with ZBP1 expression
We suggest that a tumor cell polarized as the result of ZBP1 expression will be less able to exhibit chemotaxis in the direction of an EGF gradient. Instead, it will continue to move in its polarized direction regardless of the orientation of the gradient. This would explain why ZBP1 could act as a metastasis suppressor (Wang et al., 2004).
To test this hypothesis, we examined the effect of ZBP1 on cell motility in a gradient. Control or ZBP1-expressing MTLn3 cells were serum-starved and imaged in a chamber that provided a stable linear gradient of EGF (Soon et al., 2005). DIAS was then used to analyze cell behavior. In this assay, ZBP1-expressing MTLn3 cells showed a reduced ability to orient and move in the direction of increasing EGF, relative to control MTLn3 cells (expressing only GFP), which efficiently locomoted towards increased concentrations (Fig. 2A,B). The ZBP1-expressing cells continued to be polarized and move irrespective of the orientation of the gradient (Fig. 2C). This confirmed that cells with an inherent polarity are less responsive to a chemoattractant (Condeelis et al., 2005). These data are consistent with a stochastic model for motility and chemotaxis, suggesting that an increase in signal decay time that may be caused by ZBP1-expression-mediated polarization, reduces orientation behavior in a gradient if the response time remains constant (Tranquillo et al., 1988). This model also suggests that factors affecting orientation in a gradient play an important role in persistence of motility in the absence of a gradient. Our results are also consistent with the observation that invasive tumor cells with low ZBP1 expression are more chemotactic and invasive in vivo (Wang et al., 2004). This suggests a motility-based mechanism by which ZBP1 expression could reduce metastasis.
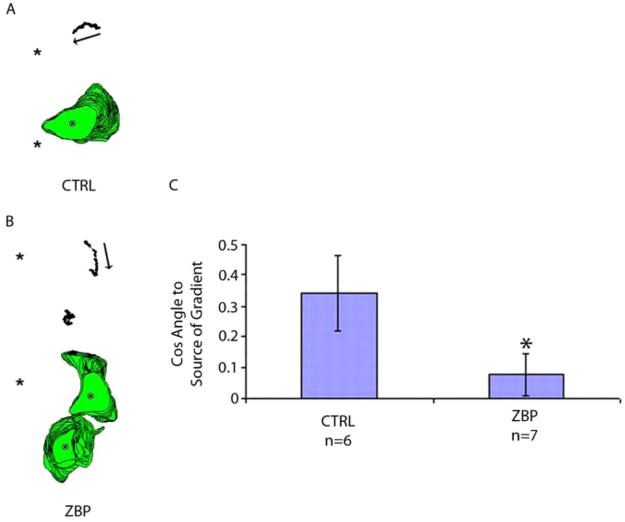
Fig. 2.
ZBP1 expression reduces movement in the direction of a chemoattractive gradient. (A,B) Perimeter and centroid plots are from (A) GFP-expressing or (B) GFP-ZBP1-expressing MTLn3 cells when exposed to a gradient of EGF after starvation. These results are representative of cell behaviors over the time frame analyzed (1 hour at 1-minute intervals; * indicates needle position, which is the source of EGF). (C) Quantification of cell movement relative to an EGF gradient. The angle between cell movement and the direction of applied chemoattractant indicates how well a cell orients to the chemoattractant, with a smaller angle suggesting greater alignment of the directions. This is reflected in our results, which take the cosine of the angle, and for which a greater alignment would give a value closer to 1 and lower degree of alignment gives a lower value, and 0 would indicate average random motion. MTLn3 cells are efficiently stimulated to move along the gradient, whereas ZBP1-expressing cells less efficiently orient and move in response to such a gradient (*P=0.035, error bars indicate ± s.e.m.).
ZBP1-expressing cells can sense and respond to a chemoattractant
Reduced orientation towards a chemoattractant could be either owing to the inability to sense the chemotactic factor or the inability to turn towards the chemoattractant because of the existence of stable cell polarity (Janetopoulos et al., 2004). In order to differentiate between these possible explanations for the inhibitory effects of ZBP1 expression on chemotaxis, we applied EGF directionally from a pipette and analyzed protrusion and retraction of the cell. When the pipette was applied in front of the leading edge of a polarized cell, both parental and ZBP1-expressing MTLn3 cells protruded towards the stimulus, and retracted from the opposite side (Fig. 3). This indicated that expressing ZBP1 did not influence the detection of the gradient. Hence, ZBP1-expressing cells can both sense and respond to a chemoattractant as long as it is aligned with the polarity of the cell. Therefore, the reduced chemotaxis of ZBP1-expressing cells probably resulted from the stable cell polarity induced by elevated ZBP1 expression.
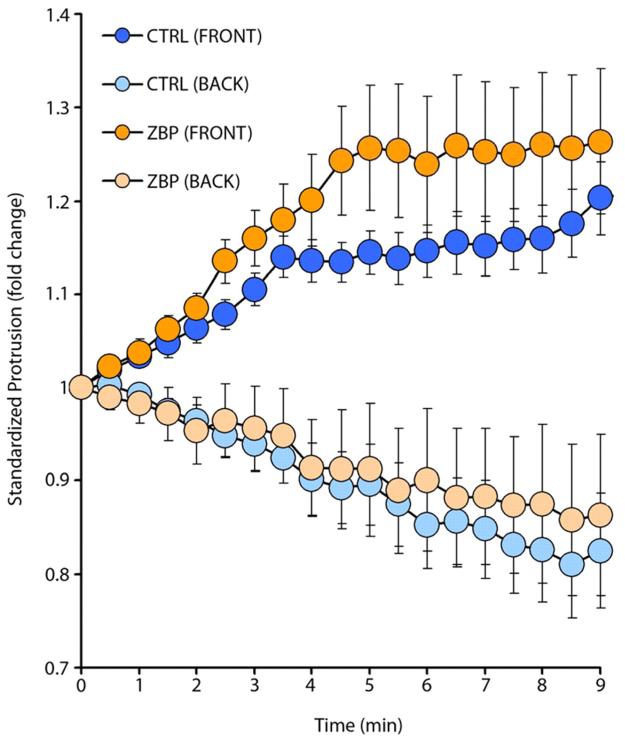
Fig. 3.
ZBP1-expressing cells can sense and move towards an oriented gradient. ZBP1-expressing cells are capable of responding normally to a gradient of EGF, but only when the gradient is applied in the direction of their intrinsic polarity. A chemoattractant, EGF, was released from a needle placed in front of the leading edge of the cell while protrusion and retraction were measured from both the front and back of the cell. Standardized membrane protrusion is plotted versus time after the micropipette stimulation. Responses of ZBP1-expressing cells are not substantially different from controls expressing only GFP (15 cells were measured for each group, error bars indicate ± s.e.m.).
Protrusion and locomotion in primary tumors are reduced with ZBP1 expression
MTLn3 cells expressing either ZBP1-GFP or GFP alone were orthotopically injected into the mammary fat pads of rats and after growth, tumors were inspected by two photon microscopy. ZBP1-GFP could be seen localized at the cell peripheries and at cell-cell junctions (Fig. 4A). This localization pattern is similar to that previously seen for ZBP1 after immunohistological staining of sections from MTLn3-generated tumors (Wang et al., 2002). Orientation of cells towards vessels, cell protrusion and locomotion in primary tumors have been shown to be crucial steps in the metastatic cascade, and elevated levels of these parameters are correlated with metastasis (Wyckoff et al., 2000). To determine whether reduced metastasis of the ZBP1-expressing cells correlated with changes in any of these movement patterns, we performed intravital imaging to compare the movement of ZBP1 expressing and control cells within tumors. Whereas control cells oriented towards the nearest vessels (Fig. 4B), ZBP1-expressing cells did not (Fig. 4C). Furthermore, ZBP1-expressing cells were less protrusive in tumors than control MTLn3 cells. Finally motility was reduced in ZBP1-expressing cells. In tumors generated by control cells, motility was observed in 28% of the fields analyzed by time-lapse microscopy over the course of 20 minutes. By contrast, motility was seen in only 2% of tumors generated by ZBP1-expressing cells over the same time period. Strikingly, the ZBP1-expressing cells also showed greatly reduced orientation towards vessels when compared with the control cell line (Fig. 4D).
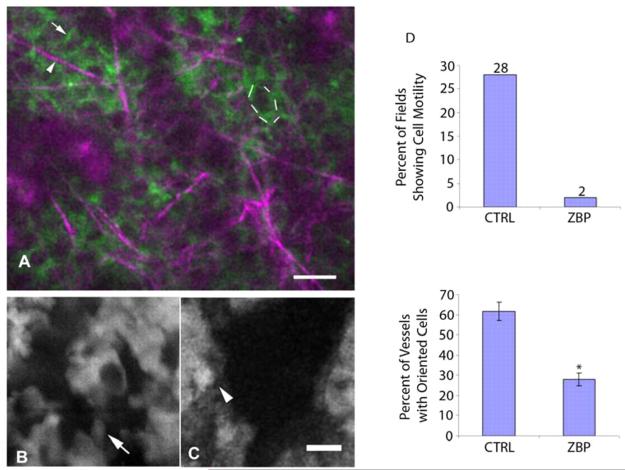
Fig. 4.
ZBP1 localizes to the periphery of cells and reduces motility and orientation towards vessels in living tumors. (A) ZBP1-GFP-expressing cells imaged in vivo using multiphoton microscopy show ZBP1 (green; one cell outlined with dotted line) localized to the periphery of cells and at cell-cell junctions (arrow). Collagen is imaged by second harmonic generated polarized light in the multiphoton microscope (purple, arrowhead). (B) MTLn3 cells transfected with CFP show elongated cell morphology (arrow) and orientation towards vessels (black spaces) in a living tumor. (C) ZBP1-expressing cells show a rounded morphology (arrowhead) along vessels. ZBP1-expressing cells do not polarize in the direction of vessels. Bars, 25 μm. (D) ZBP1 cells are less motile than cells in tumors generated by MTLn3 control cells. ZBP1 cells also show decreased orientation towards vessels than cells in tumors generated by MTLn3 control cells (*P=0.0003, error bars indicate ± s.e.m.).
Concluding remarks
Here, we show that ZBP1 expression is sufficient to convert an unpolarized cell into one with polarized morphological and movement phenotypes. Possible mechanisms by which zipcode interactions can result in these polarized phenotypes include localized expression of a variety of motility-related mRNAs (Mingle et al., 2005) (Wells et al., personal communication). ZBP1 binds such mRNAs, and presumably exerts its effects on polarity by regulating protein synthesis spatially and temporally so that motility proteins are made in the right time and place (Huttelmaier et al., 2005). In this way, the polarized phenotype can be maintained.
Previously, we have shown that MTLn3 cells induced to express ZBP1 have a reduced tendency to move through a filter in response to EGF (Boyden Chamber) and to invade a microneedle containing this chemoattractant in vivo (Wang et al., 2004). Our data suggest that the effects of ZBP1 expression on metastasis are caused by increases in polarity leading to a reduction in the ability to orient towards the chemotactic source. These results are consistent with studies in a wide variety of species showing that exogenous chemotactic signals must overcome the intrinsic polarity of the cells in order to affect motility (Devreotes and Janetopoulos, 2003).
Signaling molecules play an important role in relaying information about extracellular signals to affect cell direction (Funamoto et al., 2002; Janetopoulos et al., 2001; Yart et al., 2001). This study suggests a new role for signaling, in particular for Src, in regulating metastasis by regulating polarized protein expression. Src levels and Src kinase activity are increased in a wide variety of cancers, particularly mammary carcinomas (Jacobs and Rubsamen, 1983; Ottenhoff-Kalff et al., 1992). In addition, increases in Src activity are associated with the progression of cancer, and are higher in metastatic lesions than in primary tumors (Talamonti et al., 1993; Termuhlen et al., 1993). Src becomes rapidly activated upon activation of the EGF receptor (Osherov and Levitzki, 1994). We have shown in previous work that ZBP1 represses the translation of its bound mRNAs, particularly β-actin (Huttelmaier et al., 2005). Src phosphorylation on tyrosine 396 of ZBP1 leads to a release of bound mRNA and hence activation of β-actin translation. Therefore, local Src activation at the membrane following EGF receptor stimulation would regulate local translation of bound mRNAs that have localized near the activated Src. This way, ZBP1 might play a role in the mechanism by which increased Src activity promotes metastasis. Abnormally high Src activity may lead to widespread phosphorylation of ZBP1. This would cause bound mRNAs to be translated in inappropriate locations, resulting in the conversion of polarized cells to random walkers and sensitization to chemoattractant gradients. Reduced amounts of ZBP1 would have the same effect, to preclude polarized translation, hence resulting in an invasive phenotype. Src mislocalization from the periphery has been implicated in malignancy (Verbeek et al., 1996). Therefore, a combination of sufficient ZBP1 levels to repress translation and appropriately localized Src activity to spatially activate this translation near the leading edge would prevent inappropriate responses to growth factors leading to invasion and metastasis.
Effects in tumors
ZBP1 expression reduces the response of tumor cells to external signals both in vivo and in vitro, despite the ability of ZBP1 to enhance motility in the absence of a gradient (Shestakova et al., 2001). We propose that this is due to an inherent polarity induced by ZBP1 expression (Oleynikov and Singer, 2003). These results are also consistent with the possibility of additional mechanisms by which ZBP1 might exert these effects, particularly in tumors. Particularly, the localization of ZBP1 to cell-cell junctions suggests a role in adhesion that may further limit cell motility within a tumor and subsequent metastasis. The zipcode dependence of junctional localization in myoblasts also supports this hypothesis (Rodriguez et al., 2006). Metastatic cells in vivo become oriented and exhibit protrusive activity towards blood vessels in response to vascular chemoattractants (Wang et al., 2002; Wyckoff et al., 2000). These results extend these previous studies and show that, whether in the presence of a gradient of chemoattractant in culture or in a tumor, cells exhibit less spontaneous protrusive activity when they express ZBP1.
Some of these results appear to contradict other studies demonstrating that ZBP1-related mRNA-binding proteins are highly expressed in a variety of tumors (Hammer et al., 2005; Ross et al., 2001;Yaniv and Yisraeli, 2002), including human breast cancer (Doyle et al., 2000). It has been suggested that higher expression levels of such mRNA-binding proteins play, in fact, a causative role in tumorigenesis (Tessier et al., 2004). There are several important distinctions between these studies (Doyle et al., 2000) and those previously published by us (Wang et al., 2004). The ZBP1-expressing cells can form tumors as well as wild-type cells, and this is consistent with observations by others. However, the data presented here address the effect of ZBP1 expression specifically on the role of chemotaxis, which affects metastasis rather than tumorigenesis. In particular, we have noticed that the decrease in ZBP1 expression observed in tumors occurs only in the invasive subpopulation of tumor cells, whereas the expression of ZBP1 in the non-invasive cells remains relatively elevated in the same tumor (Wang et al., 2004). Since the invasive tumor cells are a minor fraction of the tumor mass their contribution to the ZBP1 expression status of the whole tumor would not be detected by gross studies of expression levels in tumors. Since we have focused particularly on the stage in which tumor cells migrate and prepare for intravasation – a prerequisite for metastasis – we can determine effects of ZBP1 that may be particularly crucial for outcome prediction and treatment of cancer patients with regard to metastasis.
Materials and Methods
Cell culture and cell lines
MTLn3 cells (rat mammary adenocarcinoma cell line) stably expressing EGFP-FLAG-ZBP1 were generated as previously described (Wang et al., 2004). MTLn3 cells stably expressing ECFP were generated as previously described (Sahai et al., 2005). Cells were grown in α-modified Eagle's medium containing 5% fetal bovine serum, and the antibiotics penicillin and streptomycin as previously described (Bailly et al., 1998; Segall et al., 1996).
Analysis of expression levels
Cell lysates were diluted with SDS sample buffer, boiled for 5 minutes and separated on SDS-PAGE. Proteins were transferred to Hybond ECL membranes (Amersham) by wet blotting. Primary rabbit polyclonal antibody raised against full-length His-tagged recombinant ZBP1 was used at 1:4500 and primary mouse monoclonal antibody against α-tubulin (Rockland) was used at 1:500. Secondary anti-mouse Alexa Fluor 680 (Invitrogen Molecular Probes) was used at 1:10,000 and anti-rabbit IRDye 800 (Rockland) was used at 1:5000. Signal was visualized using the Odyssey Infrared Imaging System (Li-Cor) and analyzed using IPLab software (BD Biosciences).
Motility in serum
To determine motility characteristics, cells were imaged in their growth medium on 35-mm glass-bottomed MatTek dishes prepared as previously described on a microscope with a computer-controlled CCD camera as described previously (Lorenz et al., 2004). Multiple fields were collected concurrently using a macro that cycles stage positions, and images were collected using IPlab software (BD Biosciences) and analyzed using a combination of NIH image (developed by the National Institute of Health available at http://rsb.info.nih.gov/nih-image/) to trace cell perimeters and DIAS software (Solltech) to analyze persistence (speed divided by the direction change), and directionality (net path length divided by total path length).
Response to chemoattractant
Cells were starved and EGF (25 nM) was introduced through a micropipette as previously described (Mouneimne et al., 2004). In the Soon Chamber Assay, cells were plated on coverslips on glass bottom dishes, and stimulated by a gradient of EGF produced by release from the micropipette at the side of a dam (Soon et al., 2005). Cells were imaged on an inverted Olympus IX-70 microscope (Olympus) every minute for 1 hour for this assay, whereas in the micropipette assay cells were stimulated and imaged every minute for 10 minutes. Analysis of motility in the Soon Chamber Assay was also performed using DIAS, by tracing cell perimeter, calculating centroid position at each time point, and determining the cosine of the angle between a line connecting the centroid movement between timepoints and a line connecting the centroid to the tip of the micropipette at the side of the dam.
Micropipette-stimulation assay
After starvation and micropipette stimulation with EGF as described above, membrane protrusion was monitored by time-lapse microscopy. Quantification designated the side facing the EGF stimulation as front side and the side facing away as the back side. Front and back protrusions were measured along a line passing through the centroid and the tip of the micropipette. Measurements recorded at 30-second intervals, after introduction of the micropipette, were calculated using ImageJ (http://rsb.info.nih.gov/ij/), and were all standardized over the values of the same cell at 0 seconds (immediately before stimulation). Standardized measurements were averaged and plotted versus the time after stimulation.
Intravital imaging
MTLn3-ZBP1 or MTLn3-GFP cells were injected into the mammary fat pads of female Fisher 344 rats or SKID mice to derive tumors. After 3-4 weeks of growth rats were placed under isoflurane anesthesia and the tumor was exposed using a simple skin flap surgery. The animal was then placed onto an inverted Olympus IX-70 multiphoton microscope (Olympus), using a 20× objective and time-lapse images were acquired. Approximately three fields of each tumor were imaged for 20-30 minutes each. These procedures have been previously described in detail (Wyckoff et al., 2000).
Image quantification
Time lapse movies from tumors were reconstructed with Image J and directly evaluated for cell extension, retraction and locomotion as described (Wyckoff et al., 2000).
Supplementary Material
ZBP1-expressing MTLn3 cells show similar ZBP1 levels to endogenous MTC cells. (A) ZBP1 levels in EGFP-ZBP1-expressing MTLn3 and control MTLn3 cells were compared by western blot using an antibody against ZBP1, normalized to a tubulin loading control. In addition, the expression level in each of these lines was compared with a less metastatic mammary tumor cell line (MTC) previously shown to have higher ZBP1 mRNA levels. (B) Ratios of normalized ZBP1 levels for EGFP-ZBP1-expressing MTLn3 cells, control MTLn3 cells and MTC cells were calculated from three independent western blots (error bars indicate ± s.e.m.)
Acknowledgments
This work was supported by NIH grant AR41480 to R.H.S. and NIH grant CA100324 to J.C. We thank Shailesh Shenoy for his help with the figures, and Amber Wells and Stefan Huttelmaier for their helpful suggestions.
References
- Bailly M, Yan L, Whitesides GM, Condeelis JS, Segall JE. Regulation of protrusion shape and adhesion to the substratum during chemotactic responses of mammalian carcinoma cells. Exp. Cell Res. 1998;241:285–299. doi: 10.1006/excr.1998.4031. [DOI] [PubMed] [Google Scholar]
- Condeelis J, Jones J, Segall JE. Chemotaxis of metastatic tumor cells: clues to mechanisms from the Dictyostelium paradigm. Cancer Metastasis Rev. 1992;11:55–68. doi: 10.1007/BF00047603. [DOI] [PubMed] [Google Scholar]
- Condeelis J, Singer RH, Segall JE. The great escape: when cancer cells hijack the genes for chemotaxis and motility. Annu. Rev. Cell Dev. Biol. 2005;21:695–718. doi: 10.1146/annurev.cellbio.21.122303.120306. [DOI] [PubMed] [Google Scholar]
- Devreotes P, Janetopoulos C. Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J. Biol. Chem. 2003;278:20445–20448. doi: 10.1074/jbc.R300010200. [DOI] [PubMed] [Google Scholar]
- Doyle GA, Bourdeau-Heller JM, Coulthard S, Meisner LF, Ross J. Amplification in human breast cancer of a gene encoding a c-myc mRNA-binding protein. Cancer Res. 2000;60:2756–2759. [PubMed] [Google Scholar]
- Farina KL, Huttelmaier S, Musunuru K, Darnell R, Singer RH. Two ZBP1 KH domains facilitate beta-actin mRNA localization, granule formation, and cytoskeletal attachment. J. Cell Biol. 2003;160:77–87. doi: 10.1083/jcb.200206003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funamoto S, Meili R, Lee S, Parry L, Firtel RA. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–623. doi: 10.1016/s0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Hammer NA, Hansen TO, Byskov AG, Rajpert-De Meyts E, Grondahl ML, Bredkjaer HE, Wewer UM, Christiansen J, Nielsen FC. Expression of IGF-II mRNA-binding proteins (IMPs) in gonads and testicular cancer. Reproduction. 2005;130:203–212. doi: 10.1530/rep.1.00664. [DOI] [PubMed] [Google Scholar]
- Hill MA, Gunning P. Beta and gamma actin mRNAs are differentially located within myoblasts. J. Cell Biol. 1993;122:825–832. doi: 10.1083/jcb.122.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MA, Schedlich L, Gunning P. Serum-induced signal transduction determines the peripheral location of beta-actin mRNA within the cell. J. Cell Biol. 1994;126:1221–1229. doi: 10.1083/jcb.126.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoock TC, Newcomb PM, Herman IM. Beta actin and its mRNA are localized at the plasma membrane and the regions of moving cytoplasm during the cellular response to injury. J. Cell Biol. 1991;112:653–664. doi: 10.1083/jcb.112.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttelmaier S, Zenklusen D, Lederer M, Dictenberg J, Lorenz M, Meng X, Bassell GJ, Condeelis J, Singer RH. Spatial regulation of beta-actin translation by Src-dependent phosphorylation of ZBP1. Nature. 2005;438:512–515. doi: 10.1038/nature04115. [DOI] [PubMed] [Google Scholar]
- Jacobs C, Rubsamen H. Expression of pp60c-src protein kinase in adult and fetal human tissue: high activities in some sarcomas and mammary carcinomas. Cancer Res. 1983;43:1696–1702. [PubMed] [Google Scholar]
- Janetopoulos C, Jin T, Devreotes P. Receptor-mediated activation of heterotrimeric G-proteins in living cells. Science. 2001;291:2408–2411. doi: 10.1126/science.1055835. [DOI] [PubMed] [Google Scholar]
- Janetopoulos C, Ma L, Devreotes PN, Iglesias PA. Chemoattractant-induced phosphatidylinositol 3,4,5-trisphosphate accumulation is spatially amplified and adapts, independent of the actin cytoskeleton. Proc. Natl. Acad. Sci. USA. 2004;101:8951–8956. doi: 10.1073/pnas.0402152101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kislauskis EH, Li Z, Singer RH, Taneja KL. Isoform-specific 3′-untranslated sequences sort alpha-cardiac and beta-cytoplasmic actin messenger RNAs to different cytoplasmic compartments. J. Cell Biol. 1993;123:165–172. doi: 10.1083/jcb.123.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kislauskis EH, Zhu X, Singer RH. Sequences responsible for intracellular localization of beta-actin messenger RNA also affect cell phenotype. J. Cell Biol. 1994;127:441–451. doi: 10.1083/jcb.127.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kislauskis EH, Zhu X, Singer RH. beta-Actin messenger RNA localization and protein synthesis augment cell motility. J. Cell Biol. 1997;136:1263–1270. doi: 10.1083/jcb.136.6.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham VM, Jr, Kislauskis EH, Singer RH, Ross AF. Beta-actin mRNA localization is regulated by signal transduction mechanisms. J. Cell Biol. 1994;126:1211–1219. doi: 10.1083/jcb.126.5.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JB, Singer RH. Intracellular localization of messenger RNAs for cytoskeletal proteins. Cell. 1986;45:407–415. doi: 10.1016/0092-8674(86)90326-0. [DOI] [PubMed] [Google Scholar]
- Lorenz M, DesMarais V, Macaluso F, Singer RH, Condeelis J. Measurement of barbed ends, actin polymerization, and motility in live carcinoma cells after growth factor stimulation. Cell Motil. Cytoskeleton. 2004;57:207–217. doi: 10.1002/cm.10171. [DOI] [PubMed] [Google Scholar]
- Mingle LA, Okuhama NN, Shi J, Singer RH, Condeelis J, Liu G. Localization of all seven messenger RNAs for the actin-polymerization nucleator Arp2/3 complex in the protrusions of fibroblasts. J. Cell Sci. 2005;118:2425–2433. doi: 10.1242/jcs.02371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouneimne G, Soon L, DesMarais V, Sidani M, Song X, Yip SC, Ghosh M, Eddy R, Backer JM, Condeelis J. Phospholipase C and cofilin are required for carcinoma cell directionality in response to EGF stimulation. J. Cell Biol. 2004;166:697–708. doi: 10.1083/jcb.200405156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noubissi FK, Elcheva I, Bhatia N, Shakoori A, Ougolkov A, Liu J, Minamoto T, Ross J, Fuchs SY, Spiegelman VS. CRD-BP mediates stabilization of betaTrCP1 and c-myc mRNA in response to beta-catenin signalling. Nature. 2006;441:898–901. doi: 10.1038/nature04839. [DOI] [PubMed] [Google Scholar]
- Oleynikov Y, Singer RH. Real-time visualization of ZBP1 association with beta-actin mRNA during transcription and localization. Curr. Biol. 2003;13:199–207. doi: 10.1016/s0960-9822(03)00044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osherov N, Levitzki A. Epidermal-growth-factor-dependent activation of the src-family kinases. Eur. J. Biochem. 1994;225:1047–1053. doi: 10.1111/j.1432-1033.1994.1047b.x. [DOI] [PubMed] [Google Scholar]
- Ottenhoff-Kalff AE, Rijksen G, van Beurden EA, Hennipman A, Michels AA, Staal GE. Characterization of protein tyrosine kinases from human breast cancer: involvement of the c-src oncogene product. Cancer Res. 1992;52:4773–4778. [PubMed] [Google Scholar]
- Rodriguez AJ, Shenoy SM, Singer RH, Condeelis J. Visualization of mRNA translation in living cells. J. Cell Biol. 2006;175:67–76. doi: 10.1083/jcb.200512137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AF, Oleynikov Y, Kislauskis EH, Taneja KL, Singer RH. Characterization of a beta-actin mRNA zipcode-binding protein. Mol. Cell. Biol. 1997;17:2158–2165. doi: 10.1128/mcb.17.4.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J, Lemm I, Berberet B. Overexpression of an mRNA-binding protein in human colorectal cancer. Oncogene. 2001;20:6544–6550. doi: 10.1038/sj.onc.1204838. [DOI] [PubMed] [Google Scholar]
- Sahai E, Wyckoff J, Philippar U, Segall JE, Gertler F, Condeelis J. Simultaneous imaging of GFP, CFP and collagen in tumors in vivo using multiphoton microscopy. BMC Biotechnol. 2005;5:14. doi: 10.1186/1472-6750-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall JE, Tyerech S, Boselli L, Masseling S, Helft J, Chan A, Jones J, Condeelis J. EGF stimulates lamellipod extension in metastatic mammary adenocarcinoma cells by an actin-dependent mechanism. Clin. Exp. Metastasis. 1996;14:61–72. doi: 10.1007/BF00157687. [DOI] [PubMed] [Google Scholar]
- Shestakova EA, Wyckoff J, Jones J, Singer RH, Condeelis J. Correlation of beta-actin messenger RNA localization with metastatic potential in rat adenocarcinoma cell lines. Cancer Res. 1999;59:1202–1205. [PubMed] [Google Scholar]
- Shestakova EA, Singer RH, Condeelis J. The physiological significance of beta-actin mRNA localization in determining cell polarity and directional motility. Proc. Natl. Acad. Sci. USA. 2001;98:7045–7050. doi: 10.1073/pnas.121146098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soon L, Mouneimne G, Segall J, Wyckoff J, Condeelis J. Description and characterization of a chamber for viewing and quantifying cancer cell chemotaxis. Cell Motil. Cytoskeleton. 2005;62:27–34. doi: 10.1002/cm.20082. [DOI] [PubMed] [Google Scholar]
- Talamonti MS, Roh MS, Curley SA, Gallick GE. Increase in activity and level of pp60c-src in progressive stages of human colorectal cancer. J. Clin. Invest. 1993;91:53–60. doi: 10.1172/JCI116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termuhlen PM, Curley SA, Talamonti MS, Saboorian MH, Gallick GE. Site-specific differences in pp60c-src activity in human colorectal metastases. J. Surg. Res. 1993;54:293–298. doi: 10.1006/jsre.1993.1046. [DOI] [PubMed] [Google Scholar]
- Tessier CR, Doyle GA, Clark BA, Pitot HC, Ross J. Mammary tumor induction in transgenic mice expressing an RNA-binding protein. Cancer Res. 2004;64:209–214. doi: 10.1158/0008-5472.can-03-2927. [DOI] [PubMed] [Google Scholar]
- Tranquillo RT, Lauffenburger DA, Zigmond SH. A stochastic model for leukocyte random motility and chemotaxis based on receptor binding fluctuations. J. Cell Biol. 1988;106:303–309. doi: 10.1083/jcb.106.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek BS, Vroom TM, Adriaansen-Slot SS, Ottenhoff-Kalff AE, Geertzema JG, Hennipman A, Rijksen G. c-Src protein expression is increased in human breast cancer. An immunohistochemical and biochemical analysis. J. Pathol. 1996;180:383–388. doi: 10.1002/(SICI)1096-9896(199612)180:4<383::AID-PATH686>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Wang W, Wyckoff JB, Frohlich VC, Oleynikov Y, Huttelmaier S, Zavadil J, Cermak L, Bottinger EP, Singer RH, White JG, et al. Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer Res. 2002;62:6278–6288. [PubMed] [Google Scholar]
- Wang W, Goswami S, Lapidus K, Wells AL, Wyckoff JB, Sahai E, Singer RH, Segall JE, Condeelis JS. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res. 2004;64:8585–8594. doi: 10.1158/0008-5472.CAN-04-1136. [DOI] [PubMed] [Google Scholar]
- Wyckoff JB, Jones JG, Condeelis JS, Segall JE. A critical step in metastasis: in vivo analysis of intravasation at the primary tumor. Cancer Res. 2000;60:2504–2511. [PubMed] [Google Scholar]
- Yaniv K, Yisraeli JK. The involvement of a conserved family of RNA binding proteins in embryonic development and carcinogenesis. Gene. 2002;287:49–54. doi: 10.1016/s0378-1119(01)00866-6. [DOI] [PubMed] [Google Scholar]
- Yantiss RK, Woda BA, Fanger GR, Kalos M, Whalen GF, Tada H, Andersen DK, Rock KL, Dresser K. KOC (K homology domain containing protein overexpressed in cancer): a novel molecular marker that distinguishes between benign and malignant lesions of the pancreas. Am. J. Surg. Pathol. 2005;29:188–195. doi: 10.1097/01.pas.0000149688.98333.54. [DOI] [PubMed] [Google Scholar]
- Yart A, Laffargue M, Mayeux P, Chretien S, Peres C, Tonks N, Roche S, Payrastre B, Chap H, Raynal P. A critical role for phosphoinositide 3-kinase upstream of Gab1 and SHP2 in the activation of ras and mitogen-activated protein kinases by epidermal growth factor. J. Biol. Chem. 2001;276:8856–8864. doi: 10.1074/jbc.M006966200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ZBP1-expressing MTLn3 cells show similar ZBP1 levels to endogenous MTC cells. (A) ZBP1 levels in EGFP-ZBP1-expressing MTLn3 and control MTLn3 cells were compared by western blot using an antibody against ZBP1, normalized to a tubulin loading control. In addition, the expression level in each of these lines was compared with a less metastatic mammary tumor cell line (MTC) previously shown to have higher ZBP1 mRNA levels. (B) Ratios of normalized ZBP1 levels for EGFP-ZBP1-expressing MTLn3 cells, control MTLn3 cells and MTC cells were calculated from three independent western blots (error bars indicate ± s.e.m.)