Abstract
Trans-septal puncture is associated with risks of serious complications. We report a case of an obese 52-year-old man with hypertrophic cardiomyopathy who underwent preoperative coronary angiography and cardiac catheterisation complicated by left atrial perforation. We describe a direct transatrial pericardiocentesis approach to treating cardiac tamponade.
Background
Since its introduction in the late 1950s,1 trans-septal puncture technique has changed very little over time, with only small modifications incorporated by operators aiming to improve the safety of the procedure.2 This procedure has been widely used in cardiac catheterisation for diagnosis and treatment of valvular, arrhythmic and congenital disorders. Even though transoesophageal echocardiography can be useful in performing safer punctures in selected cases, trans-septal puncture is, nevertheless, performed exclusively under fluoroscopic guidance in most centres. The trans-septal puncture technique is associated with risks of serious complications such as cardiac or aortic perforation, systemic emboli and rhythm/conduction disturbances.3 Cardiac tamponade caused by atrial wall perforation can occur even in experienced centres, with or without echocardiographic guidance, and can be a fatal complication if not recognised and treated early.4 Percutaneous subxiphoid pericardiocentesis is the treatment of choice to treat cardiac tamponade. However, tamponade occurring during trans-septal puncture and cardiac catheterisation is usually due to posterior/basal trauma, and apical or parasternal punctures are not the preferred approaches. Moreover, pericardiocentesis is not always easily performed, especially in urgent situations or in obese patients.5 The aim of this report is to describe a transatrial pericardiocentesis approach for a cardiac perforation during a trans-septal diagnostic procedure.
Case presentation
A 52-year-old man presented with progressive shortness of breath (New York Heart Association grade III). He was a current smoker and was known for treated hypertension, dyslipidaemia, diabetes mellitus, obesity grade II (WHO classification) and asthma. His physical examination revealed a 2/6 mitroaortic mesosystolic murmur compatible with dynamic obstruction.
Investigations
Transthoracic echocardiography revealed normal systolic function but significant concentric left ventricular hypertrophy with diastolic dysfunction. Anteroseptal wall thickness was 16 mm with systolic anterior motion of the mitral valve chordate with a left ventricular outflow tract (LVOT) gradient of 30 mm Hg, which increased to 52 mm Hg with the Valsalva manoeuvre.
In addition to preoperative coronary angiography, a dedicated left and right heart catheterisation with formal assessment of dynamic LVOT obstruction was planned, using simultaneous LV apex (via trans-septal puncture) and LVOT (retrograde aortic) pressure measurements. Right heart catheterisation was performed with no complications and a SL1 sheath (St Jude Medical, St Paul, Minnesota, USA) was advanced to the superior cava vein to perform the trans-septal puncture, using the conventional technique.2 A pig-tail catheter was positioned in the non-coronary sinus, and both right and left anterior oblique fluoroscopic incidences were used for guidance. The system was advanced into the left atrium without complications (pressure curves confirmed its correct position). However, during the manoeuvres to cross the mitral valve with a 0.032 guidewire and a 6-French (Fr) pig-tail catheter (Cordis Corp, Miami Lakes, Florida, USA), the wire accidentally perforated the left atrium as it exited the SL1 sheath and was advanced into the pericardial space. This situation was immediately recognised and the wire maintained in the pericardium. The patient reported of chest pain and became progressively hypotensive with evident pulsus paradoxus on pressure monitoring. Clinical tamponade was clinically diagnosed and confirmed by transthoracic echocardiography.
Differential diagnosis
Conventional subxiphoid pericardiocentesis was expected to be difficult in light of the important central obesity and presence of a median hepatic lobe.
Treatment
A decision was made to advance a 6-Fr pig-tail catheter directly over the 0.032″ trans-septal wire already in the pericardium, allowing safer and more direct access to the pericardium. Manual aspiration was performed repeatedly to avoid a recurrent pericardial tamponade, extracting a total of 200 cc of blood. A bolus of 3000 U of unfractionated heparin was administered through the pig-tail in order to avoid thrombus formation in the left atrium on the indwelling catheter while waiting for the operating room. Coronary angiography was performed from the femoral artery and revealed no significant coronary artery stenosis (figure 1). Repeat transthoracic echocardiography confirmed the complete drainage and absence of recurrent pericardial effusion (figure 2), and the patient was referred for surgical septal myectomy and left atrial repair. The patient was operated the same day in stable condition without further complications. Median sternotomy and aortic cannulation for extracorporeal circulation were performed in the usual manner. The exact location of the perforation was facilitated by the presence of the pig-tail catheter left in place and there was no blood in the pericardial sac at the time of surgery. The pig-tail catheter was withdrawn, the atrial perforation repaired using a 4/0 polypropylene pledge suture and the haemorrhage was brought under control. No further bleeding occurred. Septal myectomy was realised by standard technique.6
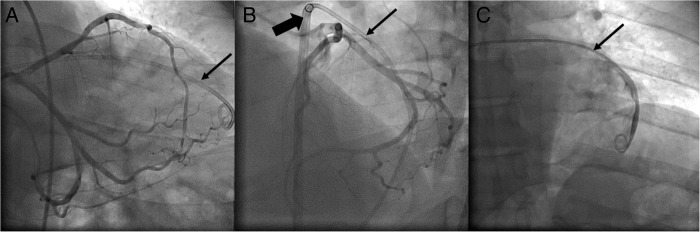
Figure 1.
(A) LAO caudal view of coronary angiogram performed while pig-tail catheter (thin arrow) remained in the pericardial space. (B) LAO cranial view of coronary angiogram performed while pig-tail catheter (thin arrow) remained in the pericardial space through SL1 sheath (thick arrow). (C) Contrast injection in the pig-tail catheter (thin arrow) to verify its position in the pericardial space. LAO, left anterior oblique.
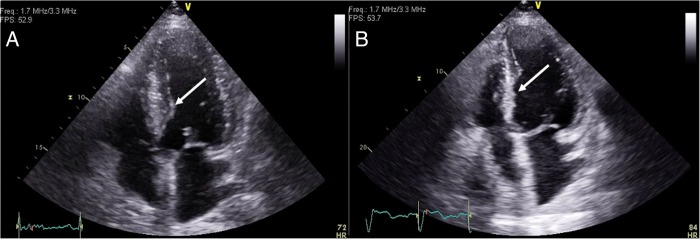
Figure 2.
Per-operative transoesophageal echocardiography showing no pericardial bleeding before pig-tail removal (A: four-chamber view; B: three-chamber view).
Outcome and follow-up
Postoperative echocardiography (figure 3) showed a baseline LVOT gradient of 16 mm Hg, which did not increase with the Valsalva manoeuvre, and no defect on the left atrium. The patient was discharged 6 days later after an uneventful postoperative course.
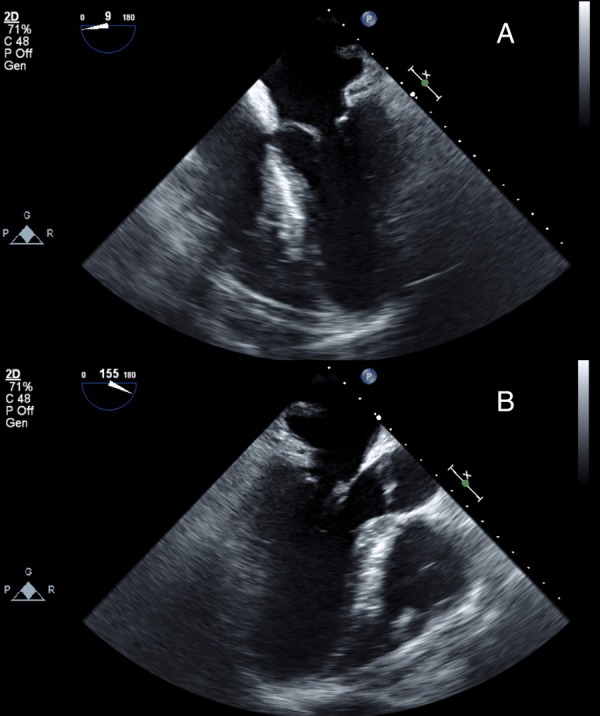
Figure 3.
(A) Transthoracic preprocedural echocardiography showing hypertrophic obstructive cardiomyopathy (16 mm septal wall thickness, thin arrow). (B) Transthoracic echocardiography after septal myectomy (thin arrow).
Discussion
The main observation of this report is that a cardiac perforation complicated by tamponade after a trans-septal puncture can be safely and effectively managed with direct transatrial drainage through the trans-septal puncture if a guidewire is left in place or can be positioned in the pericardium safely.
In this case, cardiac surgery was already indicated before the trans-septal complication occurred and, considering the potential difficulties to perform a subxiphoid pericardiocentesis in this specific patient, we intentionally leveraged the guidewire position (in the pericardial space) to perform direct transatrial pericardiocentesis by advancing a pig-tail catheter into the pericardial space. This procedure allowed us to conclude the coronary angiography and refer the patient to surgery in a non-emergent setting, with stable haemodynamic parameters and without the risk of tamponade. As a limitation, one could argue that left heart catheterisation was not mandatory in the first place. Indeed, alternative methods such as dual arterial puncture or dual-lumen catheter can be used to assess dynamic outflow obstruction.7 Two arterial punctures can be performed, placing one catheter in the left ventricle and one in the ascending aorta just above the aortic valve. The major limitation of this technique is obviously the need for two arterial punctures, which may increase discomfort to the patient and cause vascular complications. Alternatively, a single arterial puncture can be used when a dual-lumen catheter is used. One can manipulate the dual lumen pig-tail catheter within the left ventricle to induce premature ventricular contractions and observe for the Brockenbrough-Braunwald-Morrow sign (postextra systolic reduction of the pulse pressure due to reduced stroke volume caused by increased dynamic obstruction, which is due to postextrasystolic potentiation). Nevertheless, left heart catheterisation through trans-septal puncture is often performed for presurgery evaluation in our institution, and has low complications rates. Moreover, the purpose of this case-report is to show the management of this potential complication.
The trans-septal puncture technique has been widely used, and the number of procedures has increased with the development of structural heart disease interventions (mitral valve edge-to-edge repair, left atrial appendage occlusion, etc) or atrial fibrillation ablation.8 9 Nevertheless, because of its invasiveness, the trans-septal technique is associated with risks of serious complications even in most experienced centres. Fisher et al10 reported two cases of atrial perforation with a long sheath being introduced into the pericardial space during left-side anomalous pathway ablations. In those two cases, the atrial perforations were not initially noticed and mapping/ablation were attempted before cardiac tamponade became evident. In both cases, protamine sulfate was administered to reverse anticoagulation, the 8.5-Fr sheath was advanced over the ablating catheter into the pericardial space and blood could be aspirated. Afterwards, the sheath was exchanged via a 0.032″ guidewire for a 5-Fr multipurpose catheter and further aspirations could be performed. The 5-Fr was withdrawn leaving the guidewire alone in the pericardial space until bleeding stopped, when it was finally removed. The authors were the first to describe that downsizing the sheath transfixing the atrium to a 5-Fr catheter and maintaining a guidewire in the pericardial space was sufficient to stop pericardial bleeding and avoid a subxiphoid percutaneous pericardiocentesis. The patients had an unremarkable evolution after this transcardiac pericardiocentesis and underwent successful accessory pathway ablation the next day. More recently, Scanavacca et al11 reported two cases of left atrium perforation safely managed with no cardiac tamponade and with conclusion of planned procedures. The two patients were referred for atrial fibrillation ablation. In both cases, maintaining the long sheaths in the pericardial space during the procedure, therefore occluding the atrial perforation, prevented pericardial bleeding. This decision allowed the continuation of atrial fibrillation ablation and the initiation of safe systemic anticoagulation. After finishing the atrial fibrillation ablation, anticoagulation was reverted with protamine sulfate, and a subxiphoid pericardial puncture was performed to prepare for transatrial pericardial sheath withdrawal. Surprisingly, no blood was aspirated while removing the transatrial sheath, neither from the pig-tail catheter introduced by the subxiphoid access, nor from the catheter introduced through the long sheath in the pericardial space.
Patient's perspective.
Translated from French.
“Hopefully, everything was quickly managed. I did not even feel that a complication happened.”
Learning points.
Accidental atrial perforation during procedures involving trans-septal punctures can potentially be managed by positioning a drainage catheter into the pericardial space or by downsizing the pre-existing sheath to a smaller catheter.
When pericardiocentesis is necessary, transatrial puncture is an alternative approach to be considered in selected cases where conventional subxiphoid access is unsuccessful or difficult, especially when a guidewire is already in the pericardium.
When a drainage catheter is placed into the pericardial space through transatrial puncture, careful and monitored systemic anticoagulation should be kept in order to avoid thrombus formation in the left-sided circulation.
Footnotes
Twitter: Follow Quentin de Hemptinne at @qdehempt
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Ross J, Braunwald E, Morrow AG. Transseptal left atrial puncture; new technique for the measurement of left atrial pressure in man. Am J Cardiol 1959;3:653–5. 10.1016/0002-9149(59)90347-9 [DOI] [PubMed] [Google Scholar]
- 2.De Ponti R, Zardini M, Storti C et al. Trans-septal catheterization for radiofrequency catheter ablation of cardiac arrhythmias. Results and safety of a simplified method. Eur Heart J 1998;19:943–50. 10.1053/euhj.1998.0979 [DOI] [PubMed] [Google Scholar]
- 3.Liu TJ, Lai HC, Lee WL et al. Immediate and late outcomes of patients undergoing transseptal left-sided heart catheterization for symptomatic valvular and arrhythmic diseases. Am Heart J 2006;151:235–41. 10.1016/j.ahj.2005.02.034 [DOI] [PubMed] [Google Scholar]
- 4.Bunch TJ, Asirvatham SJ, Friedman PA et al. Outcomes after cardiac perforation during radiofrequency ablation of the atrium. J Cardiovasc Electrophysiol 2005;16:1172–9. 10.1111/j.1540-8167.2005.50135.x [DOI] [PubMed] [Google Scholar]
- 5.Katritsis GD, Siontis GC, Giazitzoglou E et al. Complications of transseptal catheterization for different cardiac procedures. Int J Cardiol 2013;168:5352–4. 10.1016/j.ijcard.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 6.Morrow AG, Lambrew CT, Braunwald E. Idiopathic hypertrophic subaortic stenosis: II. Operative treatment and the results of pre- and postoperative hemodynamic evaluations. Circulation 1964;29(Suppl 4):120–51. 10.1161/01.CIR.29.5S4.IV-120 [DOI] [PubMed] [Google Scholar]
- 7.Fusman B, Faxon D, Feldman T. Hemodynamic rounds: transvalvular pressure gradient measurement. Catheter Cardiovasc Interv 2001;53:553–61. 10.1002/ccd.1222 [DOI] [PubMed] [Google Scholar]
- 8.De Ponti R, Cappato R, Curnis A et al. Trans-septal catheterization in the electrophysiology laboratory: data from a multicenter survey spanning 12 years. J Am Coll Cardiol 2006;47:1037–42. 10.1016/j.jacc.2005.10.046 [DOI] [PubMed] [Google Scholar]
- 9.Babaliaros VC, Green JT, Lerakis S et al. Emerging applications for transseptal left heart catheterization. Old techniques for new procedures. J Am Coll Cardiol 2008;51:2116–22. 10.1016/j.jacc.2008.01.061 [DOI] [PubMed] [Google Scholar]
- 10.Fisher JD, Kim SG, Ferrick KJ et al. Internal transcardiac pericardiocentesis for acute tamponade. Am J Cardiol 2000;86:1388–9. 10.1016/S0002-9149(00)01252-2 [DOI] [PubMed] [Google Scholar]
- 11.Scanavacca M, Pisani CF, Lara S et al. Management of posterior atrial wall perforation during transseptal approach for left atrium ablation. Heart Rhythm 2009;6:1222–5. 10.1016/j.hrthm.2009.03.030 [DOI] [PubMed] [Google Scholar]