Abstract
Vertebral artery dissection (VAD) is an important cause of ischemic stroke and subarachnoid hemorrhage (SAH). Dissections presenting with ischemia rarely cause SAH after more than a few hours, especially without radiographic evidence of pseudoaneurysm. We successfully treated a patient for persistent vessel injury presenting with SAH 7 years after presenting with extracranial subocclusive dissection of the right vertebral artery and an associated right posterior inferior cerebellar artery stroke. This is one of only three reported cases of delayed SAH occurring more than 2 weeks after an initial ischemic presentation of a VAD, and the only one without radiographic evidence of pseudoaneurysm at standard follow-up duration.
Keywords: Dissection, Hemorrhage, Stroke, CT Angiography, Coil
Background
Intracranial artery dissections can cause ischemic stroke and subarachnoid hemorrhage (SAH).1 Dissections most commonly affect healthy young and middle-aged adults (average age 38 years) with no significant difference in prevalence between men and women.1 2 Injury to the vessel wall may stenose the lumen, dilate the vessel, or form a pseudoaneurysm.1 Subintimal dissections with preserved media and adventitia narrow the lumen and are associated with thrombus and infarction, while dissections through the media and adventitia can rupture and cause SAH.1
First-line management of asymptomatic extracranial vertebral artery dissection (VAD) comprises blood pressure management, several months of anticoagulant and antiplatelet therapy, and imaging follow-up.1 3 In contrast, intracranial extension of VAD is treated to reconstruct the lumen or deconstruct the parent artery with the primary objective of preventing intracranial hemorrhage. In dissections with persistent ischemia refractory to anticoagulation, intervention can target the source of emboli or provide reperfusion.2 Surgical intervention can also target hemorrhagic sequelae of the dissection via embolization of a pseudoaneurysm or of the parent vessel itself. Without radiographic evidence of aneurysmal dilation or intracranial extension, the risk of delayed SAH is presumed to be low.3
Case presentation
A 57-year-old man with a history of hypertension and a known right VAD causing a right posterior inferior cerebellar artery (PICA) stroke after a motor vehicle accident in 2009 presented with the worst headache of his life, nausea, and diaphoresis. During his original presentation he was evaluated with CT angiography (CTA) imaging and found to have a subocclusive dissection of the right vertebral artery (VA) at the C2 vertebral body level, with weak retrograde reconstitution of the distal right VA by the left VA and associated right PICA infarction on MRI. He was placed on warfarin for 12 months and then converted to 325 mg aspirin daily. A follow-up CTA performed 7 months after his stroke showed partially restored flow at V2 and fully reconstituted flow at V3 and V4.
Investigations
At the current presentation, CT imaging revealed SAH into the perimedullary, prepontine, and perimesencephalic cisterns (figure 1A). Further investigation with CTA and digital subtraction angiography (DSA) demonstrated occlusion of the previously dissected right VA (figure 1C–E) and retrograde filling of the right PICA from the left VA (figures 1B and 2). There was no evidence of aneurysm on DSA.
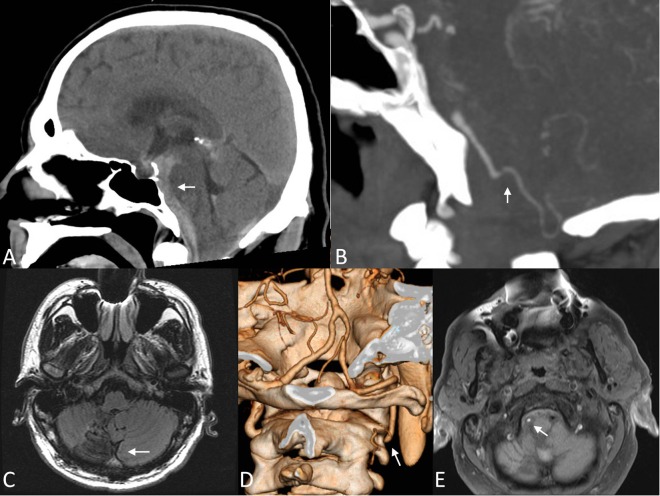
Figure 1.
Initial cross-section imaging. (A) Midline sagittal non-contrast CT scan showing premedullary, prepontine, and premesencephalic (arrow) subarachnoid hemorrhage. (B) Sagittal contrasted CT arteriogram (CTA) showing a patent right vertebral artery distal to the right posterior inferior cerebellar artery (PICA, arrow), but not proximally. (C) Seven-year prior axial MRI T2 sequence demonstrating prior right PICA (arrow) infarction. (D) Reconstruction of CTA from 7 years prior demonstrating the dissection of the right vertebral artery at the C2 level (arrow) with reconstitution from the left vertebral artery. (E) Axial MRI T1 sequence with fat suppression demonstrating absent flow-related signal loss in the right vertebral artery (arrow) and T1 shortening consistent with dissection.
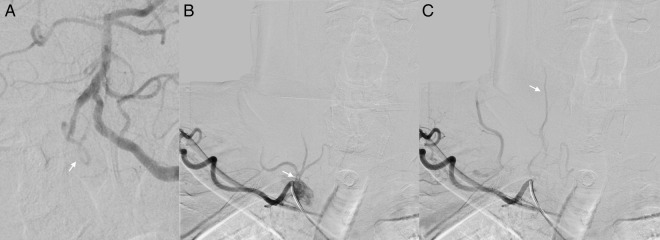
Figure 2.
Initial angiogram. (A) Anteroposterior (AP) digital subtraction angiogram (DSA) with left vertebral artery injection demonstrating retrograde opacification of the distal right vertebral artery into the right posterior inferior cerebellar artery (arrow) but not the proximal right vertebral artery. (B) AP DSA with right subclavian injection early (top) and mid (bottom) arterial phase, without demonstration of right vertebral artery patency proximally or through distal collateral anastamoses.
A repeat angiogram performed 1 week later revealed increased anterograde filling of the V3 segment of the right VA with retrograde filling of the V4 segment (figure 3A). The vessel was still occluded proximally; however, a more thorough angiogram with injection of the costocervical trunk demonstrated collateral filling from the costocervical trunk via the right ascending cervical artery (figure 3B) and thyrocervical trunk via the right deep cervical artery (figure 3C). The angiographic evidence of increased anterograde and collateral perfusion of the dysplastic vessel (arrowhead, figure 3C) raised suspicion of an acute-on-chronic dissection and elevated the risk of a rebleed, so the decision was made to embolize the right VA.
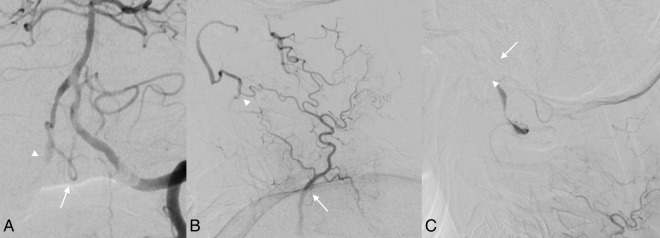
Figure 3.
Follow-up angiogram at 1 week after initial presentation. (A) Anteroposterior digital subtraction angiogram (DSA) with left vertebral artery injection demonstrating retrograde opacification of the distal right vertebral artery into the right posterior inferior cerebellar artery (PICA, arrow) and now also the proximal right vertebral artery (PICA, arrowhead). (B) DSA of the right costocervical trunk during early arterial phase: the deep cervical artery (arrow) provides collateral flow to the right vertebral artery at the C2 level via dilated anastamoses (arrowhead). (C) DSA of the right thyrocervical trunk during the late arterial phase shows the distal vertebral artery filling with a fusiform dilation (arrowhead) before filling the right PICA (arrow).
Treatment
Access to the reconstituted distal right VA was achieved in a retrograde fashion. Coils were placed proximal to the dysplastic segment and up to the origin of the right PICA. A final postoperative DSA demonstrated cessation of anterograde flow through the right VA with preserved retrograde flow into the preserved right PICA (figure 4).
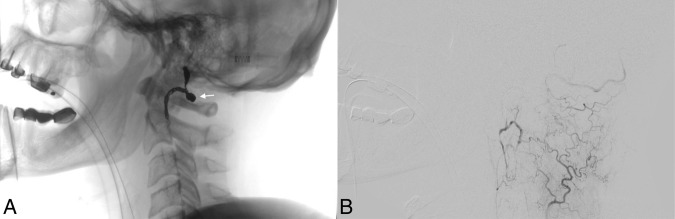
Figure 4.
Angiogram after coil embolization of the right vertebral artery. (A) Lateral fluoroscopy demonstrating the coil mass (arrow) within the C1 and C2 levels of the right vertebral artery. (B) Lateral digital subtraction angiogram with right thyrocervical trunk injection demonstrating no further opacification of the right vertebral artery dilation.
Outcome and follow-up
The patient recovered well and was neurologically intact at 2-month follow-up.
Discussion
VADs presenting with ischemia rarely cause simultaneous or subsequent SAH, especially more than a few hours after the initial dissection.2 The pathogenesis of this transition has not yet been elucidated due to the limited reports of the phenomenon.1 Fewer than a dozen cases of delayed SAH of a VAD after an ischemic presentation, and only two delays of longer than 2 weeks, have been reported.4–10 Naito et al described a patient who presented with brainstem infarction, was treated with antiplatelet therapy, and developed SAH 51 months later. Imaging revealed an occluded V4 segment flanked by two dilated aneurysmal sacs, one of which was coiled.5 Sagoh et al7 treated a patient for SAH 11 years after a left cerebellar infarction and a ‘pearl and string’ sign on angiography with persistent dilation of the pseudoaneurysm over the course of the delay. VADs typically resolve with restored perfusion or complete occlusion; only rarely do they sustain persistent pseudoaneurysmal dilation.3 7 Reported cases of delayed VAD SAH showed evidence of aneurysmal dilation, including the two with the longest delays described by Naito et al and Sagoh et al.
The patient in this case had no evidence of intracranial extension of the dissection or aneurysmal dilation during initial imaging or at most recent follow-up (7 months after dissection). During initial CTA and angiogram after delayed SAH there was new occlusion of the right VA proximal to the right PICA. Although of unclear duration, the occluded VA was not considered a vulnerable substrate for new SAH. Since incomplete visualization of the V4 segment with VA injections is not sufficient to evaluate all intracranial sources of bleeding, follow-up angiography fully evaluated the cervical arterial supply. This revealed that, despite the occlusion of the right VA, the diseased segment persisted and extended intracranially to the origin of the right PICA via anterograde supply from cervical soft tissue collaterals. The recanalization of the right VA via thyrocervical and costocervical collaterals represented a recurrent risk of hemorrhage due to persistent flow through the dysplastic right VA, hence it was critical to embolize the dissected vessel to prevent future episodes of rebleed or infarction.
This case shows that hemorrhagic transition of a previously dissected VA can occur several years after the initial dissection, even with reconstituted vessel lumen on early follow-up (7 months in this case). It is exceedingly rare for a VAD to cause SAH with no radiographic evidence of aneurysmal dilation, and it is even more rare for this to occur 7 years after an initial ischemic presentation. In this case, the dissection progressed to the intracranial segment despite a stable appearance after the typical medical management and clinical and radiographic follow-up. Furthermore, this case represents persistence of a diseased segment via recruitment of occult cervical collateral supply despite occlusion of the proximal parent vessel. The only other reported cases of delayed hemorrhage more than 2 weeks after an ischemic VAD showed radiographic evidence of aneurysmal dilation during the routine course of management.5 7
There may be a persistent risk of SAH despite improving vessel anatomy on imaging. Even if no radiographic evidence of persistent vessel irregularity or pseudoaneurysm exists on initial presentation of a VAD, a transition to SAH can occur as many as 7 years later and patients should be monitored accordingly, such as with fat-suppressed T1 sequence MRI.
Learning points.
Delayed acute on chronic vertebral artery dissection (VAD), although rare, is one of the causes of subarachnoid hemorrhage (SAH) and should be considered when evaluating a patient with SAH, especially in patients with a history of intracranial dissection.
If preliminary non-invasive imaging does not reveal an obvious cause of the SAH, the patient should undergo a complete cerebral angiogram with attention to the extracranial vessels that can anastomose with the vertebral artery and cause SAH.
Vessel caliber can evolve following dissection and repeat cerebral angiography can demonstrate early or delayed recanalization of dissections and pseudoaneurysms.
Despite routine follow-up of dissections showing recanalization of the vessel lumen, the long-term outcome may involve variations of occlusion and collateral vessel reconstitution with persistence of a dissected segment.
The majority of cases of VAD respond well to medical management and do not require surgical treatment. Patients with repeated focal neurological symptoms despite optimal medical management should be evaluated carefully for more aggressive surgical treatment of ischemic or hemorrhagic complications.
Footnotes
Contributors: MAS analyzed the data, drafted the paper, and revised the paper. APS treated the patient in the study, collected the data, analyzed the data, compiled the figures, helped draft the paper, and revised the paper. PK treated the patient in the study, collected the data, analyzed the data, and revised the paper. NJP treated the patient in the study and revised the paper. MAA-S designed the study, oversaw the study, treated the patient in the study, and revised the paper; he is the guarantor.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The technical methodology has been disclosed in its entirety within the case report. The representative case images are not relevant for data sharing.
References
- 1.Hart RG, Easton JD. Dissections. Stroke 1985;16:925–7. 10.1161/01.STR.16.6.925 [DOI] [PubMed] [Google Scholar]
- 2.Anxionnat R, de Melo Neto JF, Bracard S et al. Treatment of hemorrhagic intracranial dissections. Neurosurgery 2003;53:289–300; discussion 300–1 10.1227/01.NEU.0000073417.01297.93 [DOI] [PubMed] [Google Scholar]
- 3.Kitanaka C, Tanaka J, Kuwahara M et al. Nonsurgical treatment of unruptured intracranial vertebral artery dissection with serial follow-up angiography. J Neurosurg 1994;80:667–74. 10.3171/jns.1994.80.4.0667 [DOI] [PubMed] [Google Scholar]
- 4.Chou CL, Lin YJ, Po HL et al. Subarachnoid hemorrhage—a rare complication after intravenous thrombolysis in an ischemic stroke patient. Am J Emerg Med 2010;28:984.e1–3. 10.1016/j.ajem.2009.12.024 [DOI] [PubMed] [Google Scholar]
- 5.Naito I, Iwai T, Sasaki T. Management of intracranial vertebral artery dissections initially presenting without subarachnoid hemorrhage. Neurosurgery 2002;51:930–7; discussion 937–8. [DOI] [PubMed] [Google Scholar]
- 6.Ogane K, Fujita S, Ohkuma H et al. [A case of delayed subrachnoid hemorrhage from vertebrobasilar artery dissecting aneurysm]. No To Shinkei 2000;52: 827–31. [PubMed] [Google Scholar]
- 7.Sagoh M, Hirose Y, Murakami H et al. Late hemorrhage from persistent pseudoaneurysm in vertebral artery dissection presenting with ischemia: case report. Surg Neurol 1999;52:480–3; discussion 483–4 10.1016/S0090-3019(99)00093-2 [DOI] [PubMed] [Google Scholar]
- 8.Shinoda S, Murata H, Waga S et al. Bilateral spontaneous dissection of the posteroinferior cerebellar arteries: case report. Neurosurgery 1998;43: 357–9. 10.1097/00006123-199808000-00111 [DOI] [PubMed] [Google Scholar]
- 9.Tsutsumi M, Kawano T, Kawaguchi T et al. Dissecting aneurysm of the vertebral artery causing subarachnoid hemorrhage after non-hemorrhagic infarction—case report. Neurol Med Chir (Tokyo) 2000;40:628–31. 10.2176/nmc.40.628 [DOI] [PubMed] [Google Scholar]
- 10.Yakushiji Y, Haraguchi Y, Soejima S et al. A hyperdense artery sign and middle cerebral artery dissection. Intern Med 2006;45:1319–22. 10.2169/internalmedicine.45.1888 [DOI] [PubMed] [Google Scholar]