Abstract
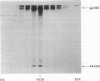
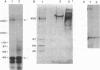
Using antibodies isolated from glomeruli of nephritic rats we have previously identified a 330-kDa cell surface glycoprotein (gp330) as a major pathogenic antigen of Heymann nephritis (HN), an experimental model of human membranous glomerulonephritis. Recently, we have isolated a cDNA clone, C14, encoding a polypeptide that contains a pathogenic epitope of HN responsible for the initiation of the disease. Subsequently, another protein, alpha 2-macroglobulin receptor-associated protein (alpha 2-MRAP), which is a subunit of the receptor for human alpha 2-macroglobulin/low density lipoprotein receptor-related protein (LRP), was shown to possess a high degree of sequence homology to the C14 protein (C14p). In this report, we have investigated the relationship between gp330, C14p, and alpha 2-MRAP. Immunoprecipitation studies demonstrate that gp330 forms a heterodimeric association with a 44-kDa polypeptide that is stable to detergent extraction and long-term centrifugation. Further, immunoblotting analysis on the purified complex indicates that the 44-kDa associated protein shares immunological identity to C14p and alpha 2-MRAP. In addition, antibodies eluted from glomeruli of HN rats and antibodies to a C14 fusion protein immunoprecipitated gp330 and the 44-kDa protein, demonstrating that the epitopes responsible for the initial events of HN are accessible within the complex. Based on these data, three models are proposed to explain how pathogenic epitopes in the gp330-44-kDa, HN antigenic complex may be presented at the cell surface and initiate the onset of HN.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres D. A., Rhodes J. D., Meisel R. L., Dixon J. E. Characterization of the carboxyl-terminal sequences responsible for protein retention in the endoplasmic reticulum. J Biol Chem. 1991 Aug 5;266(22):14277–14282. [PubMed] [Google Scholar]
- Copeland C. S., Doms R. W., Bolzau E. M., Webster R. G., Helenius A. Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J Cell Biol. 1986 Oct;103(4):1179–1191. doi: 10.1083/jcb.103.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couser W. G. Mechanisms of glomerular injury in immune-complex disease. Kidney Int. 1985 Sep;28(3):569–583. doi: 10.1038/ki.1985.167. [DOI] [PubMed] [Google Scholar]
- Couser W. G., Steinmuller D. R., Stilmant M. M., Salant D. J., Lowenstein L. M. Experimental glomerulonephritis in the isolated perfused rat kidney. J Clin Invest. 1978 Dec;62(6):1275–1287. doi: 10.1172/JCI109248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgington T. S., Glassock R. J., Dixon F. J. Autologous immune complex nephritis induced with renal tubular antigen. I. Identification and isolation of the pathogenetic antigen. J Exp Med. 1968 Mar 1;127(3):555–572. doi: 10.1084/jem.127.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Ozawa M., Huang R. P., Muramatsu T. A heparin binding protein whose expression increases during differentiation of embryonal carcinoma cells to parietal endoderm cells: cDNA cloning and sequence analysis. J Biochem. 1990 Aug;108(2):297–302. doi: 10.1093/oxfordjournals.jbchem.a123197. [DOI] [PubMed] [Google Scholar]
- Glenn K. C., Carney D. H., Fenton J. W., 2nd, Cunningham D. D. Thrombin active site regions required for fibroblast receptor binding and initiation of cell division. J Biol Chem. 1980 Jul 25;255(14):6609–6616. [PubMed] [Google Scholar]
- Gutmann E. J., Niles J. L., McCluskey R. T., Brown D. Colchicine-induced redistribution of an apical membrane glycoprotein (gp330) in proximal tubules. Am J Physiol. 1989 Aug;257(2 Pt 1):C397–C407. doi: 10.1152/ajpcell.1989.257.2.C397. [DOI] [PubMed] [Google Scholar]
- HEYMANN W., HACKEL D. B., HARWOOD S., WILSON S. G., HUNTER J. L. Production of nephrotic syndrome in rats by Freund's adjuvants and rat kidney suspensions. Proc Soc Exp Biol Med. 1959 Apr;100(4):660–664. doi: 10.3181/00379727-100-24736. [DOI] [PubMed] [Google Scholar]
- Herz J., Goldstein J. L., Strickland D. K., Ho Y. K., Brown M. S. 39-kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. J Biol Chem. 1991 Nov 5;266(31):21232–21238. [PubMed] [Google Scholar]
- Herz J., Kowal R. C., Goldstein J. L., Brown M. S. Proteolytic processing of the 600 kd low density lipoprotein receptor-related protein (LRP) occurs in a trans-Golgi compartment. EMBO J. 1990 Jun;9(6):1769–1776. doi: 10.1002/j.1460-2075.1990.tb08301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtley S. M., Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Kanalas J. J., Makker S. P. Identification of the rat Heymann nephritis autoantigen (GP330) as a receptor site for plasminogen. J Biol Chem. 1991 Jun 15;266(17):10825–10829. [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J Exp Med. 1983 Feb 1;157(2):667–686. doi: 10.1084/jem.157.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5557–5561. doi: 10.1073/pnas.79.18.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Miettinen A., Farquhar M. G. Initial events in the formation of immune deposits in passive Heymann nephritis. gp330-anti-gp330 immune complexes form in epithelial coated pits and rapidly become attached to the glomerular basement membrane. J Exp Med. 1987 Jul 1;166(1):109–128. doi: 10.1084/jem.166.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Noronha-Blob L., Sacktor B., Farquhar M. G. Microdomains of distinctive glycoprotein composition in the kidney proximal tubule brush border. J Cell Biol. 1984 Apr;98(4):1505–1513. doi: 10.1083/jcb.98.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus W., Seyer J. M., Beachey E. H. Vimentin-cross-reactive epitope of type 12 streptococcal M protein. Infect Immun. 1989 Aug;57(8):2457–2461. doi: 10.1128/iai.57.8.2457-2461.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maunsbach A. B. Cellular mechanisms of tubular protein transport. Int Rev Physiol. 1976;11:145–167. [PubMed] [Google Scholar]
- Miettinen A., Dekan G., Farquhar M. G. Monoclonal antibodies against membrane proteins of the rat glomerulus. Immunochemical specificity and immunofluorescence distribution of the antigens. Am J Pathol. 1990 Oct;137(4):929–944. [PMC free article] [PubMed] [Google Scholar]
- Moestrup S. K., Gliemann J. Purification of the rat hepatic alpha 2-macroglobulin receptor as an approximately 440-kDa single chain protein. J Biol Chem. 1989 Sep 15;264(26):15574–15577. [PubMed] [Google Scholar]
- Pietromonaco S., Kerjaschki D., Binder S., Ullrich R., Farquhar M. G. Molecular cloning of a cDNA encoding a major pathogenic domain of the Heymann nephritis antigen gp330. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1811–1815. doi: 10.1073/pnas.87.5.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychowdhury R., Niles J. L., McCluskey R. T., Smith J. A. Autoimmune target in Heymann nephritis is a glycoprotein with homology to the LDL receptor. Science. 1989 Jun 9;244(4909):1163–1165. doi: 10.1126/science.2786251. [DOI] [PubMed] [Google Scholar]
- Rodman J. S., Kerjaschki D., Merisko E., Farquhar M. G. Presence of an extensive clathrin coat on the apical plasmalemma of the rat kidney proximal tubule cell. J Cell Biol. 1984 May;98(5):1630–1636. doi: 10.1083/jcb.98.5.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland D. K., Ashcom J. D., Williams S., Battey F., Behre E., McTigue K., Battey J. F., Argraves W. S. Primary structure of alpha 2-macroglobulin receptor-associated protein. Human homologue of a Heymann nephritis antigen. J Biol Chem. 1991 Jul 15;266(20):13364–13369. [PubMed] [Google Scholar]
- Van Damme B. J., Fleuren G. J., Bakker W. W., Vernier R. L., Hoedemaeker P. J. Experimental glomerulonephritis in the rat induced by antibodies directed against tubular antigens. V. Fixed glomerular antigens in the pathogenesis of heterologous immune complex glomerulonephritis. Lab Invest. 1978 Apr;38(4):502–510. [PubMed] [Google Scholar]
- van Deurs B., Christensen E. I. Endocytosis in kidney proximal tubule cells and cultured fibroblasts: a review of the structural aspects of membrane recycling between the plasma membrane and endocytic vacuoles. Eur J Cell Biol. 1984 Jan;33(1):163–173. [PubMed] [Google Scholar]