The state of oligo-recurrence is the most valuable prognostic factor of pulmonary oligometastases treated by stereotactic body radiotherapy.
Keywords: pulmonary oligometastases, SBRT, oligo-recurrence, sync-oligometastases
Abstract
Background
Oligometastases can be divided into sync-oligometastases and oligo-recurrence. The difference is whether the primary site is uncontrolled or controlled. The goal of this multicenter study was to evaluate treatment outcomes and factors affecting survival after stereotactic body radiotherapy for pulmonary oligometastases.
Methods
The information after stereotactic body radiotherapy from January 2004 to April 2014 was retrospectively collected. Ninety-six patients (65 males, 31 females) were enrolled. Ten cases (10%) were sync-oligometastases, 79 cases (82%) were oligo-recurrences and 7 (7%) were unclassified oligometastases with <6 months of disease-free interval. The median disease-free interval between initial therapy and stereotactic body radiotherapy was 24 months. The median calculated biological effective dose was 105.6 Gy.
Results
The median follow-up period was 32 months for survivors. The 3-year overall survival and relapse-free survival rates were 53% and 32%, respectively. No Grade 5 toxicity occurred. The median overall survival was 23.9 months for sync-oligometastases and 66.6 months for oligo-recurrence (P = 0.0029). On multivariate analysis, sync-oligometastases and multiple oligometastatic tumors were significant unfavorable factors for both overall survival and relapse-free survival.
Conclusions
In stereotactic body radiotherapy for oligometastatic lung tumors, the state of oligo-recurrence has the potential of a significant prognostic factor for survival.
Background
The concept of an oligometastatic state was first proposed by Hellman et al. (1, 2) as an intermediate state between limited primary and polymetastatic cancers in which local therapy could achieve long-term survival or cure, with no restrictions on primary lesions. In 2006, the concept of oligo-recurrence was defined by Niibe et al. (3) as the state in which patients with cancer have ≤5 metastatic or recurrent lesions with controlled primary lesions. Recently, the concept of sync-oligometastases was proposed as the state in which patients with cancer have ≤5 metastatic or recurrent lesions with active primary lesions (4). The major difference among oligometastases, oligo-recurrence and sync-oligometastases was the status of the primary lesion; oligo-recurrence has been considered to show a better prognosis than sync-oligometastases among some researchers including us. However, there was no evidence until now.
For cases with oligo-recurrent cancer in the lung, controversy exists regarding the optimal approach for these metastatic sites. Although a surgical approach is considered an alternative for a single metastasis, there are many patients with lung oligo-recurrence who are not amenable for metastasectomy. For them, less invasive techniques such as stereotactic body radiotherapy (SBRT) have been used to treat lung oligo-recurrence. In cases considered to have a favorable prognosis, radical treatment with SBRT seems to prolong the survival time. However, the role of radiotherapy and the prognostic factors for oligo-recurrence have not yet been clearly elucidated (5). This retrospective study investigated SBRT for lung oligometastases. At the same time, the difference in prognosis between oligo-recurrence and sync-oligometastases was examined.
Methods
A total of 96 patients (65 males, 31 females) who received SBRT for oligometastatic lung tumors between January 2004 and April 2014 at four high-volume institutions in Japan were enrolled in this retrospective study (Table 1). This retrospective study was approved by the ethical committees of all institutions.
Table 1.
Patient and tumor characteristics
| Factors | Number | % |
| Age (years old) | ||
| Median | 72 | |
| Range | 25–88 | |
| Karnofsky Performance Status | ||
| 90–100% | 69 | 72% |
| 70–80% | 21 | 22% |
| 50–60% | 6 | 6% |
| Tumor diameter (cm) | ||
| Median | 1.9 | |
| Range | 0.6–4.2 | |
| Number of metastatic tumors | ||
| Single | 76 | 79% |
| Multiple | 20 | 21% |
| Disease-free interval (mo) | ||
| Median | 24 | |
| Range | 0–246 | |
| Oligometastatic state | ||
| Oligo-recurrences | 79 | 82% |
| Sync-oligometastases | 10 | 10% |
| Unclassified | 7 | 7% |
| Pathology | ||
| Adenocarcinoma | 49 | 51% |
| Squamous cell carcinoma | 19 | 20% |
| Others | 12 | 13% |
| Unknown | 16 | 17% |
| Primary site | ||
| Colorectum | 25 | 26% |
| Lung | 24 | 25% |
| Head and neck | 8 | 8% |
| Uterus | 8 | 8% |
| Others | 31 | 32% |
| Prescription point | ||
| D95 | 40 | 42% |
| Isocenter | 56 | 58% |
| BED10 (Gy10) | ||
| Median | 105.6 | |
| Range | 75.0–134.4 | |
D95: Covering 95% of the planning target volume; BED: biological effective dose.
The primary sites were the colorectum (n = 25), lung (n = 24), head and neck (n = 8), uterus (n = 8), esophagus (n = 9), stomach (n = 5), breast (n = 3), renal pelvis (n = 3), ovary (n = 2), retroperitoneum (n = 2), kidney (n = 1), pancreas (n = 1), bladder (n = 1), liver (n = 1), anal canal (n = 1), pulmonary artery (n = 1) and testis (n = 1). Ten cases were sync-oligometastases and 79 were oligo-recurrences. The median disease-free interval (DFI) between initial therapy and SBRT was 24 months (range, 0–246 months). The median calculated biological effective dose was 105.6 Gy (range, 75–134.4 Gy) using the LQ model with alpha/beta = 10 Gy. The prescribed dose was delivered to the isocenter (n = 56) or covering 95% of the planning target volume (D95, n = 40).
As to systemic chemotherapy, no concurrent chemotherapy combined with SBRT was performed. Induction chemotherapy before SBRT was given in four cases. Three cases were given carboplatin plus paclitaxel for ovary, uterine cervical and uterine body cancer, and the other was given S-1 for colon cancer. Adjuvant chemotherapy after SBRT (which was started within 8 weeks) was performed in one case. S-1 was given for colon cancer.
Statistical analysis
The definitions of oligo-recurrence, sync-oligometastases and unclassified oligometastases were that DFI ≥6, 0 and <6 months, respectively.
Overall survival (OS), the local control rate (LCR) and relapse-free survival (RFS) were calculated using Kaplan–Meier curves, and the log-rank test was used to compare the curves. Significance of univariate analyses was set at P < 0.05. The 95% confidence interval (CI) range of survival was calculated using Greenwood's formula. Multivariate analyses for OS and RFS were performed using a Cox proportional hazards model. Three factors, DFI (<24 vs. ≥24 months), oligometastatic state (sync-oligometastases vs. oligo-recurrences) and number of metastatic tumors (multiple vs. single), were included in the multivariate analysis. Significance of the multivariate analysis was set at P < 0.017 using the Bonferroni method.
Results
The median follow-up period for all patients was 21 months (range, 1–119 months) and that for survivors was 32 months (range, 1–110 months). During follow-up, local recurrence occurred in 18 lesions, 43 patients (45%) died and relapse or death occurred in 70 patients (73%). Radiation pneumonitis of Grade 3 was found in two patients, and gastrointestinal toxicity of Grade 4 was found in one patient. No Grade 5 toxicity occurred.
Univariate analysis
The 3-year OS, LCR and RFS were 53.2% (95% CI: 41.6–64.8%), 74.2% (95% CI: 62.8–85.6%) and 32.2% (95% CI: 21.6–42.8%), respectively (Table 2). The median OS, LCR and RFS times were 41.5 months (95% CI: 16.2–66.8 months), not reached and 18.0 months (95% CI: 15.6–20.4 months), respectively.
Table 2.
Univariate analysis for OS and RFS
| Variables | 2-year OS | SE | 3-year OS | SE | 4-year OS | SE | MST (mo) | SE | Log-rank P value | Median RFS (mo) | SE | Log-rank P value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years old) | 0.99 | 0.12 | ||||||||||
| <72 | 63.7 | 7.9 | 50.8 | 8.6 | 45.2 | 9.3 | 46 | 29 | 16.2 | 0.3 | ||
| ≥72 | 70.0 | 7.0 | 55.3 | 8.1 | 49.1 | 8.3 | 42 | 27 | 19.8 | 1 | ||
| Gender | 0.14 | 0.98 | ||||||||||
| Female | 76.7 | 8.4 | 68.2 | 9.4 | 57.0 | 10.7 | 67 | 24 | 17.3 | 2 | ||
| Male | 62.8 | 6.5 | 45.7 | 7.3 | 42.9 | 7.4 | 31 | 7.0 | 17.1 | 2 | ||
| K-PS | 0.75 | 0.16 | ||||||||||
| ≥90% | 68.8 | 6.2 | 55.7 | 6.9 | 48.0 | 7.3 | 46 | 26 | 16.2 | 0 | ||
| <90% | 62.4 | 10.0 | 46.5 | 11 | 46.5 | 11 | 33 | 3.3 | 26.1 | 8 | ||
| Disease-free interval | 0.0083 | 0.062 | ||||||||||
| <24 mo | 52.9 | 8.8 | 29.8 | 8.7 | 29.8 | 8.7 | 26 | 1.8 | 12.4 | 2 | ||
| ≥24 mo | 77.1 | 6.1 | 69.2 | 7.0 | 59.8 | 7.9 | 72 | 11 | 21.2 | 3 | ||
| Tumor diameter | 0.18 | 0.17 | ||||||||||
| <2.0 cm | 76.5 | 6.5 | 58.7 | 8.1 | 51.1 | 8.7 | 72 | 33 | 19.6 | 3 | ||
| ≥2.0 cm | 56.8 | 8.1 | 47.2 | 8.6 | 43.2 | 8.6 | 31 | 16 | 16.2 | 2 | ||
| Oligometastatic state | 0.0029 | 0.0057 | ||||||||||
| Sync-oligometastases | 50.0 | 16 | 10.0 | 9.5 | 10.0 | 9.5 | 24 | 4.1 | 4.2 | 3 | ||
| Oligo-recurrences | 68.9 | 5.7 | 61.0 | 6.3 | 54.0 | 6.8 | 67 | 19 | 18.2 | 3.0 | ||
| Number of metastatic tumors | 0.13 | 0.0013 | ||||||||||
| Multiple | 48.5 | 13 | 32.4 | 13 | 32.4 | 13 | 21 | 7.8 | 8.0 | 4.9 | ||
| Single | 71.5 | 5.6 | 58.3 | 6.4 | 51.5 | 6.8 | 67 | 20 | 19.8 | 6 | ||
| Pathological type | 0.79 | 0.96 | ||||||||||
| Adenocarcinoma | 74.6 | 7.0 | 52.5 | 8.6 | 45.7 | 8.7 | 37 | 8.6 | 18.0 | 3 | ||
| Others | 60.2 | 7.6 | 54.5 | 7.9 | 49.5 | 8.6 | 46 | 22 | 16.8 | 1 | ||
| BED10 | 0.45 | 0.29 | ||||||||||
| <100 Gy | 68.7 | 11 | 61.9 | 12 | 61.9 | 12 | 87 | 55.0 | 12.6 | 3 | ||
| ≥100 Gy | 66.7 | 6.0 | 51.0 | 6.7 | 44.3 | 6.9 | 37 | 11 | 18.0 | 3.6 |
OS = overall survival, SE = standard error, MST = median survival time, RFS = relapse-free survival.
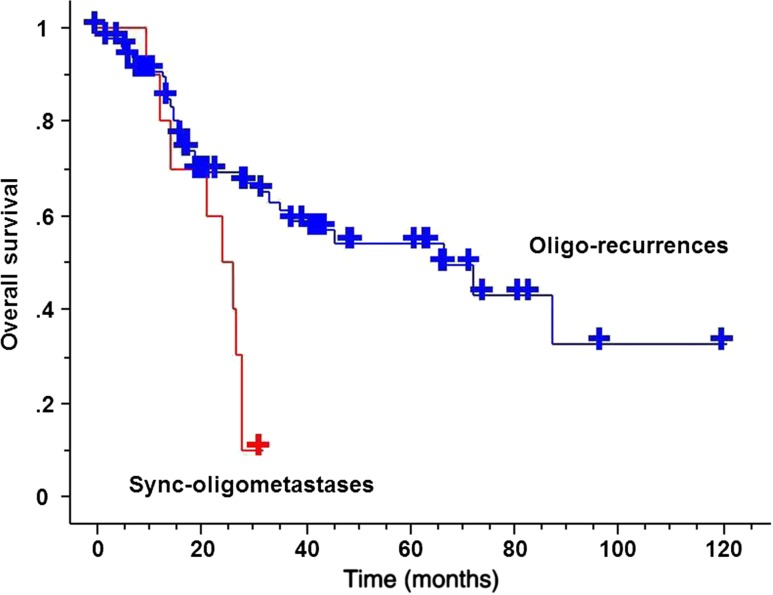
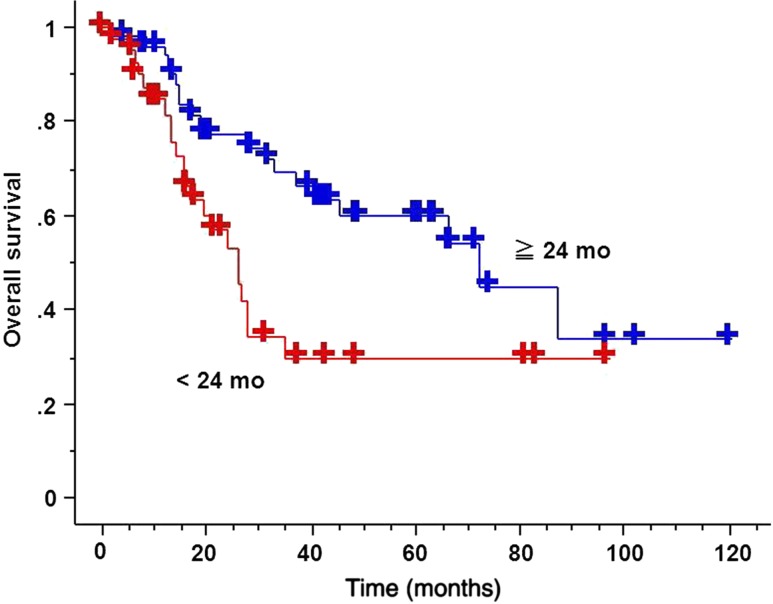
The 3-year OS for sync-oligometastases and oligo-recurrence was 10% (95% CI: 0–28.6%) and 61.0% (95% CI: 48.7–73.3%), respectively (Fig. 1). The 3-year OS for DFI ≥24 months and for DFI <24 months was 69.2% (95% CI: 55.5–82.9%) and 29.8% (95% CI: 12.7–46.9%), respectively (Fig. 2). The median survival time for sync-oligometastases and oligo-recurrence was 23.9 months (95% CI: 15.9–31.9 months) and 66.6 months (95% CI: 29.8–103.4 months), respectively (P = 0.0029). The median survival time for DFI ≥24 months and for DFI <24 months was 72.3 months (95% CI: 50.2–94.4 months) and 25.9 months (95% CI: 22.4–29.4 months), respectively (P = 0.0083).
Figure 1.
Overall survival (OS) curves comparing oligo-recurrence and sync-oligometastases. Oligo-recurrence group achieved statistically significant better OS than that of sync-oligometastases (P = 0.0029).
Figure 2.
OS curves comparing disease-free interval (DFI) not <24 months and DFI <24 months. DFI not <24 months group achieved statistically significant better OS than that of DFI <24 months (P = 0.0083).
The median RFS time for sync-oligometastases and oligo-recurrence was 4.2 months (95% CI: 0–9.7 months) and 18.2 months (95% CI: 12.3–24.1 months), respectively (P = 0.0057). The median RFS time for multiple metastases and single metastasis was 8.0 months (95% CI: 0–17.6 months) and 19.8 months (95% CI: 8.8–30.8 months), respectively (P = 0.0013).
Multivariate analysis
On multivariate analysis, sync-oligometastases (P = 0.0029 and hazard ratio (HR) = 2.56) and multiple oligometastatic tumors (P = 0.0024 and HR = 2.36) were significant unfavorable factors for OS. Similarly, sync-oligometastases (P = 0.0021 and HR = 3.23) and multiple oligometastatic tumors (P = 0.0009 and HR = 2.87) were significant unfavorable factors for RFS (Table 3).
Table 3.
Multivariate analysis (Cox regression) for OS, RFS and LCR
| Factors | OS | RFS | LCR | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Disease-free interval | 0.23 | 0.42 | 0.18 | |||
| <24 mo | 1 | 1 | 1 | |||
| ≥24 mo | 0.65 (0.32–1.31) | 0.80 (0.46–1.38) | 0.81 (0.30–2.16) | |||
| Oligometastatic state | 0.029 | 0.0021 | ||||
| Sync-oligometastases | 1 | 1 | 2.42 (0.30–19.79) | 0.41 | ||
| Oligo-recurrences | 0.39 (0.16–0.91) | 0.31 (0.15–0.65) | 1 | |||
| Number of metastatic tumors | 0.024 | 0.0009 | ||||
| Multiple | 2.36 (1.12–4.97) | 2.87 (1.54–5.35) | 1.51 (0.43–5.25) | 0.52 | ||
| Single | 1 | 1 | 1 | |||
LCR = local control rate, HR = hazard ratio, CI = confidence interval.
Discussion
The Japanese guidelines recommend local therapy with curative intent for lung oligometastases from colorectal cancer (6). In the NCCN guidelines version 1.2015 for non–small-cell lung cancer, it is stated that aggressive local therapy may be appropriate for selected patients with limited-site involvement, such as brain oligometastatic disease. SBRT for lung oligometastases without other active disease is also covered by insurance in Japan.
In this study, 3-year OS and the LCR were 53% and 74%, respectively, for the whole patient group. The present results are comparable to other studies of lung oligometastases (7–9). Norihisa et al. (10) also previously reported the results of SBRT for 43 metastatic lung cancers. In their series, the survival rates and LCR at 2 years were reported to be 84.3% and 90%, respectively. Ricardi et al. (11) also reported a study of SBRT for oligometastatic lung tumors. In 61 patients treated with SBRT, local control was achieved in 89% and 2-year survival was 66.5%. Onishi et al. (12) reported an excellent case report about sequential oligo-recurrence.
Several studies (10, 13) have shown that local control after SBRT for lung metastases from colorectal cancer is worse than that from other origins. However, in this study, lung metastases from colorectal cancer were not significantly worse, but the statistical power might be low since there were only 96 patients and only 25 colorectal cancers.
Takahashi et al. (14) reported that a shorter DFI of less than 31.9 months was the prognostic factor in a study of SBRT for pulmonary oligo-recurrence. In another study, a DFI ≥36 months was associated with better prognosis than that of <36 months (15). The 3-year OS was 69 vs. 30% for a DFI of ≥24 vs. <24 months, respectively. A longer DFI was also found to be associated with a good prognosis in this study.
In SBRT for lung metastases, limited toxicity rates have been reported by several authors. In the present series, no patient had serious late toxicities.
Several limitations of this study warrant mention. First, it was a retrospective review from multiple institutions with a limited number of patients and limited follow-up. There was a wide range of doses prescribed and a variety of fractionation schema at each institution. SBRT for lung oligometastatic disease without controlling the primary tumor is not covered by insurance in Japan. In other words, SBRT for synchronous pulmonary metastases is not justified in general clinical setting. Furthermore, in this study, only in special situations, such as if lung metastases were controlled by SBRT, was the primary tumor surgically removed and SBRT was performed. Briefly, SBRT for lung metastases was performed for only patients with schedule of surgery for the primary tumor in some institutions. Nevertheless, the clinical outcome of SBRT for sync-oligometastases was dismal. In these situations, systemic therapy is more important than local treatment.
Conclusions
Patients with single oligo-recurrent tumor in the lung could be good candidates for SBRT. Clinicians might consider SBRT in cases of single oligo-recurrence because this powerful local therapy could benefit such patients.
Funding
This study was supported partly by a grant-in-aid from JSPS KAKENHI Grant Number 21791209 and 25461926, and a grant-in-aid from JASTRO (Japanese Society for Radiation Oncology) Grant Name JASTRO Study 2015–2016.
Conflict of interest statement
None declared.
References
- 1.Weichselbaum RR, Hellman S.. Oligometastases revisited. Nat Rev Clin Oncol 2011;8:378–82. [DOI] [PubMed] [Google Scholar]
- 2.Hellman S, Weichselbaum RR.. Oligometastases. J Clin Oncol 1995;13:8–10. [DOI] [PubMed] [Google Scholar]
- 3.Niibe Y, Hayakawa K.. Oligometastases and oligorecurrence: the new era of cancer therapy. Jpn J Clin Oncol 2010;40:107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niibe Y, Chang JY.. Novel insights of oligometastases and oligo-recurrence and review of the literature. Pulm Med 2012;2012:261096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jabbour SK, Daroui P, Moore D, et al. A novel paradigm in the treatment of oligometastatic non-small cell lung cancer. J Thorac Dis 2011;3:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe T, Itabashi M, Shimada Y, et al. Japanese society for cancer of the colon and rectum. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol 2012;17:1–29. [DOI] [PubMed] [Google Scholar]
- 7.Okunieff P, Petersen AL, Philip A, et al. Stereotactic body radiation therapy (SBRT) for lung metastases. Acta Oncol (Madr) 2006;45:808–17. [DOI] [PubMed] [Google Scholar]
- 8.Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol 2009;27:1579–84. [DOI] [PubMed] [Google Scholar]
- 9.Oh D, Ahn YC, Seo JM, et al. Potentially curative stereotactic body radiation therapy (SBRT) for single or oligometastasis to the lung. Acta Oncol (Madr) 2012;51:596–602. [DOI] [PubMed] [Google Scholar]
- 10.Norihisa Y, Nagata Y, Takayama K, et al. Stereotactic body radiotherapy for oligometastatic lung tumors. Int J Radiat Oncol Biol Phys 2008;72:398–403. [DOI] [PubMed] [Google Scholar]
- 11.Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for lung metastases. Lung Cancer 2012;75:77–81. [DOI] [PubMed] [Google Scholar]
- 12.Onishi H, Ozaki M, Kuriyama K, et al. Stereotactic body radiotherapy for metachronous multisite oligoreccurrence: a long-surviving case with sequential oligorecurrence in four different organs treated using locally radical radiotherapy and a review of the literature. Pulm Med 2012;2012:713073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeda A, Kunieda E, Ohashi T, et al. Stereotactic body radiotherapy (SBRT) for oligometastatic lung tumors from colorectal cancer and other primary cancers in comparison with primary lung cancer. Radiother Oncol 2011;101:255–59. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi W, Yamashita H, Niibe Y, Shiraishi K, Hayakawa K, Nakagawa K.. Stereotactic body radiotherapy for metastatic lung cancer as oligo-recurrence: an analysis of 42 cases. Pulm Med 2012;2012:454107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue T, Katoh N, Onimaru R, Shirato H.. Clinical outcomes of stereotactic body radiotherapy for patients with lung tumors in the state of oligo-recurrence. Pulm Med 2012;2012:369820. [DOI] [PMC free article] [PubMed] [Google Scholar]