Abstract
CD133, a widely known cancer stem cell marker, has been proved to promote tumor metastasis. However, the mechanism by which CD133 regulates metastasis remains largely unknown. Here, we report that CD133 knockdown inhibits cancer cell migration, and CD133 overexpression promotes cell migration. CD133 expression is beneficial to activate the Src-focal adhesion kinase (FAK) signaling pathway. Further studies show that CD133 could interact with Src, and the region between amino acids 845 and 857 in the CD133 C-terminal domain is indispensable for its interaction with Src. The interaction activates Src to phosphorylate its substrate FAK and to promote cell migration. Likewise, a Src binding-deficient CD133 mutant loses the abilities to increase Src and FAK phosphorylation and to promote cell migration. Inhibition of Src activity by PP2, a known Src activity inhibitor, could block the activation of FAK phosphorylation and cell migration induced by CD133. In summary, our data suggest that activation of FAK by the interaction between CD133 and Src promotes cell migration, providing clues to understand the migratory mechanism of CD133+ tumor cells.
Keywords: migration, protein phosphorylation, protein-protein interaction, protein-tyrosine kinase 2 (PTK2), focal adhesion kinase (FAK), Src, CD133
Introduction
Tumor metastasis is a process by which tumor cells detach from the primary tumor into the stroma and invade blood vessels. This is followed by a secondary tumor growth at a distant site (1). This accounts for over 90% of lethality in cancer patients (2). Increasing evidence indicates that there is a subset of tumor cells contributing to metastasis that has properties of cancer stem cells (3). CD133, a pentaspan transmembrane glycoprotein, has rapidly gained clinical value with its wide use as a cancer stem cell marker (4–13). A series of studies have shown that CD133 could directly regulate tumorigenesis, cell self-renewal, and angiogenesis (14–16). Increasing evidence has also shown that CD133 is related to tumor metastasis in a variety of solid tumors (17–20). For instance, activation of AKT/protein kinase B (PKB) signal pathway promotes invasion and migration of CD133+ cancer stem cells in brain cancer (21). G protein-coupled receptor 87 was found to promote the growth and metastasis of CD133+ cancer stem cells in hepatocellular carcinoma. CD133 expression is correlated with lymph node metastasis in pancreatic cancer (22, 23). However, the mechanisms of CD133 regulating metastasis directly remain largely unknown.
In head and neck cancer, CD133 could regulate Src kinase activity. The CD133/Src axis mediates tumor-initiating property and epithelial mesenchymal transition (24). Src is a member of the Src family of kinases, which is a non-receptor tyrosine kinase family (25, 26). Src is highly expressed or highly activated in various human cancers (27). For instance, Src is highly active in glioblastoma multiforme specimens, and it represents an effective target for CD133+ glioblastoma multiforme stem cell migration (28). Among the kinase substrates of Src, FAK4 is an important mediator of tumor progressions and metastasis through the modulation of tumor cell migration and invasion (29). FAK is a cytoplasmic protein-tyrosine kinase that is involved in extracellular matrix/integrin-mediated signaling pathways (30–32). Activation of FAK by integrin clustering leads to autophosphorylation at tyrosine (Tyr) residue 397, which is a binding site of Src family kinases (31, 33). The recruitment of Src family kinases results in the phosphorylation and activation of FAK through the kinase activity of Src (34, 35). The Src-FAK complex acts to recruit or to phosphorylate a number of signaling proteins that are involved in adhesion regulation and the invasive phenotype of cancer cells (36–38). However, the relationship between CD133 and Src-FAK signaling complex remains unclear.
In this study, we used the CD133-positive colorectal cancer cell line SW620 and CD133-negative HEK293T cell line to investigate the mechanism of CD133 regulating cell migration. In SW620 cells, CD133 knockdown reduced cell migration and phosphorylation of FAK under serum starvation condition. Ectopic expression of CD133 in HEK293T cells promoted cell migration and increased the phosphorylation level at FAK Tyr925 dramatically. Furthermore, we provide evidence that CD133 promoted migration through its interaction with Src, which activated Src to phosphorylate its substrate FAK. Inhibition of Src by PP2 could block the change of FAK phosphorylation and cell migration induced by CD133 ectopic expression or CD133 knockdown. To the best of our knowledge, this is the first study to demonstrate that CD133 regulates cell migration through its binding to Src, therefore activating the Src-FAK signaling pathway.
Results
CD133 Promotes Cell Migration and Phosphorylation of FAK
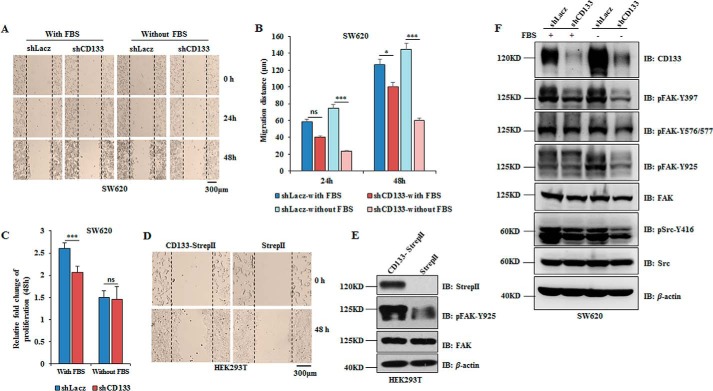
To determine the significance of CD133 in cell migration, we used lentivirus-based short hairpin RNA (shCD133) to knock down CD133 in the highly migratory cancer cell line SW620 (39, 40). Considering that serum starvation is an important condition to stimulate cancer cell migration (41), we compared the migratory ability of SW620 cells infected with Lacz short hairpin RNA (shRNA) (control) or CD133 shRNA (CD133 knockdown) lentivirus under complete medium (with fetal bovine serum (FBS)) and serum starvation (without FBS) culture conditions. CD133 knockdown obviously reduced SW620 cell migration under serum starvation condition (Fig. 1, A and B). Increasing evidence reveals that CD133 could promote tumor cell proliferation (14). To examine whether the increased migratory distance was due to cell proliferation induced by CD133, we detected the proliferative ability of SW620 cells with CD133 knockdown. We found that CD133 knockdown slightly reduced cell proliferation under complete medium but not under serum starvation culture condition (Fig. 1C). Although HEK293T cells are nontumorigenic, it has been proved that CD133 expression could strength malignancy of HEK293T cells by inducing tumor-initiating properties (42). HEK293T cells do not express CD133 endogenously. Thus, we ectopically expressed CD133 in HEK293T cells to investigate the role of CD133 in cell migration by a wound healing assay. Of note, the wound healing speed was significantly faster in cells overexpressing CD133 (Fig. 1D). Thus, CD133 has a key role in tumor cell migration.
FIGURE 1.
CD133 promotes cell migration and phosphorylation of FAK. A–C, SW620 cells infected with shLacz (control) or shCD133 (CD133 knockdown) lentivirus were cultured in complete medium (with FBS) or serum deprivation medium (without FBS), respectively. A, representative images of the wound healing assay of SW620 cells with the indicated treatments. B, relative distance of cell migration in A was calculated and is presented as the mean ± S.D. (error bars) (n = 6). Statistical analysis was performed by Student's t test (*, p < 0.05; ***, p < 0.001; ns, not significant). C, cell proliferative ability was analyzed by Cell Counting Kit 8 assay in a 96-well plate. A450 was determined, and results are presented as the mean ± S.D. (error bars) (n = 12). Statistical analysis was performed by Student's t test (***, p < 0.001; ns, not significant). D and E, HEK293T cells were infected with CD133-StrepII or control lentivirus. D, a wound healing assay was done, and the rate of migration was calculated by measuring the distance of cell migration in the same view at 0 and 48 h after scratching. E, expression of CD133, pFAK-Tyr925, and total FAK was analyzed by Western blotting analysis. β-Actin was blotted as a loading control. F, SW620 cells were infected with shLacz or shCD133 lentivirus and cultured in medium with or without FBS. pSrc-Tyr416, FAK phosphorylation (pFAK-Tyr397, pFAK-Tyr576/577, and pFAK-Tyr925), total Src, and FAK were all monitored by Western blotting analysis. IB, immunoblotting.
Among the metastasis-related signaling proteins, FAK is an important mediator of tumor progression and metastasis (43, 44), and phosphorylation of FAK at Tyr925 enhances cell migration and cell protrusion by activation of the p130CAS/Dock180/Rac1 signaling pathway (45). In HEK293T cells, although CD133 overexpression did not change the expression of total FAK, FAK was highly phosphorylated at Tyr925 in cells expressing exogenous CD133 (Fig. 1E). Consistent with this, CD133 knockdown inhibited the phosphorylation of FAK (pFAK-Tyr397, pFAK-Tyr576/577, and pFAK-Tyr925) and the phosphorylation of Src (pSrc-Tyr416) without changing their total protein expression levels under serum starvation condition (Fig. 1F). Altogether, CD133 promotes cell migration and increases the phosphorylation of FAK-Tyr925.
CD133 Promotes FAK Phosphorylation through Src
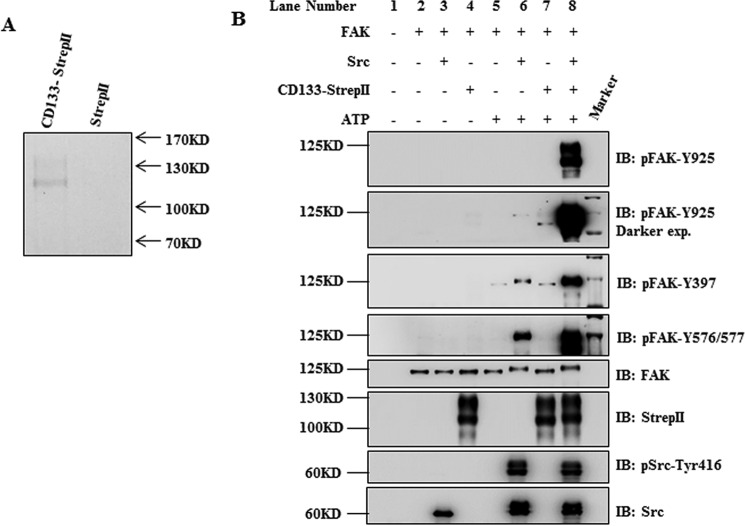
We next focused on the mechanism of CD133 promoting FAK phosphorylation. We first supposed that CD133 had protein-tyrosine kinase activity to phosphorylate FAK-Tyr925. This hypothesis was investigated by in vitro kinase assay. CD133 protein with StrepII tag was purified by Strep-Tactin affinity along with 2 m NaCl in wash buffer to eliminate nonspecific binding proteins (Fig. 2A). Although FAK-Tyr925 could be phosphorylated by its known kinase Src, CD133 could not phosphorylate FAK-Tyr925 directly (Fig. 2B, lanes 6 and 7). Thus, CD133 activates FAK-Tyr925 phosphorylation depending on another tyrosine kinase. Autophosphorylation at FAK-Tyr397 makes a binding site for the Src homology 2 (SH2) domain (31). Then the activated Src could phosphorylate FAK at tyrosine residues 576/577 and 925 (34, 35). To investigate whether phosphorylation of FAK by CD133 was mediated by Src, CD133 and Src were simultaneously added in a kinase reaction system containing FAK protein. Results showed that the FAK protein was heavily phosphorylated at Tyr925 (Fig. 2B, lane 8). In addition, the phosphorylation levels of FAK at Tyr397 and Tyr576/577 were also higher in the group containing CD133 and Src than in the group containing only Src (Fig. 2B, lanes 8 and 6). Thus, we presume that CD133 promotes FAK phosphorylation through Src.
FIGURE 2.
CD133 promotes FAK phosphorylation through Src. A, purified effect of CD133 protein was determined by Coomassie Blue staining. B, in vitro kinase assay. FAK protein was prepared by immunoprecipitation with anti-FAK antibody in HEK293T cells. CD133-StrepII protein, recombinant Src protein, and/or FAK protein was incubated in kinase assay buffer with or without ATP as indicated. The reaction was carried out for 30 min at 30 °C. The levels of CD133-StrepII, total Src, and active Src (pSrc-Tyr416), total FAK, pFAK-Tyr397, pFAK-Tyr576/577, and pFAK-Tyr925 were detected by Western blotting analysis. IB, immunoblotting; exp., exposure.
CD133 Interacts with Src
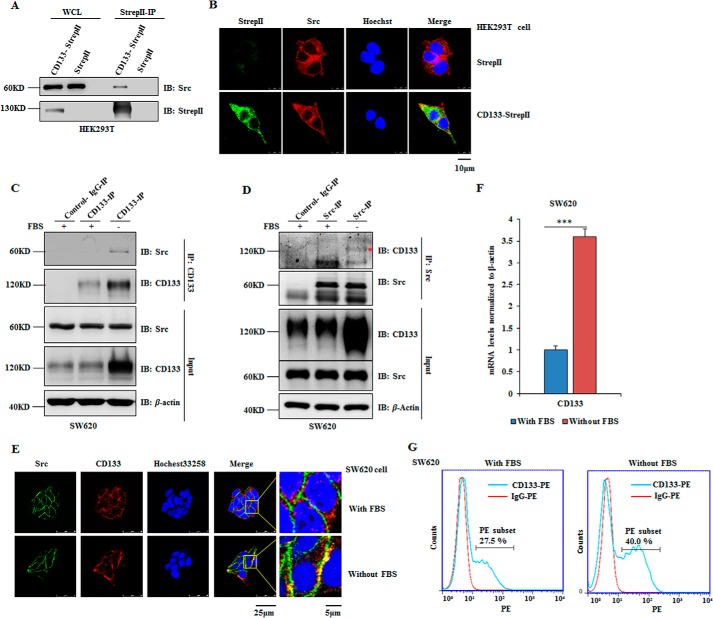
This finding motivated us to examine whether CD133 could interact with Src. CD133-StrepII was expressed in HEK293T cells, and co-immunoprecipitation (co-IP) was done with Strep-Tactin purification. Interaction between CD133 and Src was detected by Western blotting analysis (Fig. 3A). This interaction was also examined by immunofluorescence. We observed co-localization of CD133 and Src by laser confocal scanning (Fig. 3B). Next, reciprocal co-IP assays in SW620 cells showed that endogenous Src bound to endogenous CD133 under serum starvation culture condition (Fig. 3, C and D). Consistent with this, CD133 was co-localized with Src in the plasma membrane of SW620 cells under this condition (Fig. 3E).
FIGURE 3.
CD133 interacts with Src. A and B, HEK293T cells were transfected with CD133-StrepII plasmid or corresponding empty vector with StrepII tag. A, 48 h later, cells were harvested, and co-IP was done with Strep-Tactin-agarose. B, immunofluorescence assay was done to observe the co-localization of CD133 (green) and Src (red). C–G, SW620 cells were cultured in DMEM with or without FBS. C and D, co-IP analysis was performed to determine the interaction between endogenous CD133 and endogenous Src in SW620 cells. The band indicated by a red asterisk is CD133. E, an immunofluorescence assay was performed to examine the co-localization of CD133 (red) and Src (green). F, the relative mRNA level of CD133 was detected by quantitative PCR. Results are presented as the mean ± S.D. (error bars) (n = 3). Statistical analysis was performed by Student's t test (***, p < 0.001). G, flow cytometry was performed to examine the percentage of CD133-positive cells. The percentages shown in the graphs are the average percentages from the results obtained in three experiments. WCL, whole cell lysate; IB, immunoblotting.
Interestingly, serum starvation treatment of SW620 cells showed increased protein level of CD133 (Figs. 1F and 3, C and D). Hypoxic and nutrient-deprived condition could increase the CD133+ cell percentage in hepatocellular carcinoma cells (46). Further analyses showed that serum starvation treatment increased the mRNA of CD133 in SW620 cells (Fig. 3F). Flow cytometry analysis showed that CD133+ cell percentage increased from 27.5 to 40% after serum starvation treatment, which suggested a significant enrichment of CD133+ cells under this condition (Fig. 3G). Altogether, CD133 could interact with Src.
Region CD133(845–857) in Cytoplasmic Tail Is Indispensable for CD133 to Interact with Src
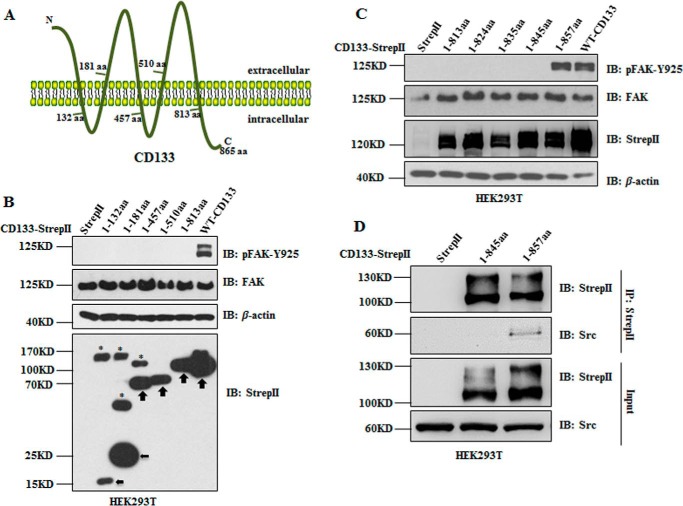
CD133 is a pentaspan transmembrane glycoprotein consisting of an N-terminal extracellular domain, five transmembrane domains with two large extracellular loops, two small intracellular loops, and a 59-amino acid cytoplasmic tail (Fig. 4A) (9). To define the region of the CD133 domain essential for activation of FAK phosphorylation, systematically truncated CD133 mutants (1–132, 1–181, 1–457, 1–510, and 1–813) with StrepII tag were expressed in HEK293T cells. These truncated mutants were constructed depending on transmembrane features of CD133 (Fig. 4A). As shown in Fig. 4B, CD133 mutant 1–813 lacking the C-terminal cytoplasmic tail could not promote phosphorylation of FAK-Tyr925. Therefore, C-terminal truncated CD133 mutants 1–813, 1–824, 1–835, 1–845, and 1–857 with StrepII tag were overexpressed in HEK293T cells, respectively. Western blotting analysis showed that truncated mutants lacking region CD133(845–857) could not promote phosphorylation of FAK-Tyr925 (Fig. 4C).
FIGURE 4.
Region CD133(845–857) is indispensable for CD133 to interact with Src and to promote pFAK-Tyr925. A, graphic representation of the proposed structural model of CD133. This protein is modeled as having an extracellular N terminus, a cytoplasmic C terminus, two small cytoplasmic loops, and two very large extracellular loops. B, wild type CD133 (WT-CD133), the five truncated mutants of CD133 (1–132, 1–181, 1–457, 1–510, and 1–813), and empty vector with StrepII tag were expressed in HEK293T cells, respectively. The levels of pFAK-Tyr925, total FAK, CD133-StrepII, and its truncated mutants were detected by Western blotting analysis. The black arrows indicate expression of CD133 mutants. The bands indicated by asterisks might be post-translationally modified CD133 mutants (1–132, 1–181, and 1–457). C, CD133 was gradually truncated at the C terminus, and five mutants with StrepII tag (1–813, 1–824, 1–835, 1–845, and 1–857) were constructed and expressed in HEK293T cells, respectively. Cell lysates were prepared to analyze pFAK-Tyr925, total FAK, CD133-StrepII, and its truncated mutants. D, truncated CD133 mutants 1–845 and 1–857 and empty vector with StrepII tag were expressed in HEK293T cells, respectively. Co-IP was done with Strep-Tactin-agarose. Whole cell lysates (Input) and IPs of StrepII-tagged CD133 mutants were detected by Western blotting with anti-StrepII antibody and anti-Src antibody. IB, immunoblotting; aa, amino acids.
Our previous finding has proved that region CD133(845–857) in the cytoplasmic tail is indispensable for CD133 activation of FAK phosphorylation (Fig. 4, B and C). Thus, we next examined whether this region of CD133 was indispensable for the interaction between CD133 and Src. Truncated mutants of CD133 (1–845 and 1–857) or control empty vector with StrepII tag were overexpressed in HEK293T cells. Co-IP with Strep-Tactin purification showed that only truncated mutant CD133(1–857) has the ability to interact with Src (Fig. 4D). Altogether, region CD133(845–857) in the cytoplasmic tail is indispensable for CD133 to interact with Src and to activate FAK phosphorylation.
CD133 Promotes Cell Migration and FAK Phosphorylation Partly Depending on Its Interaction with Src
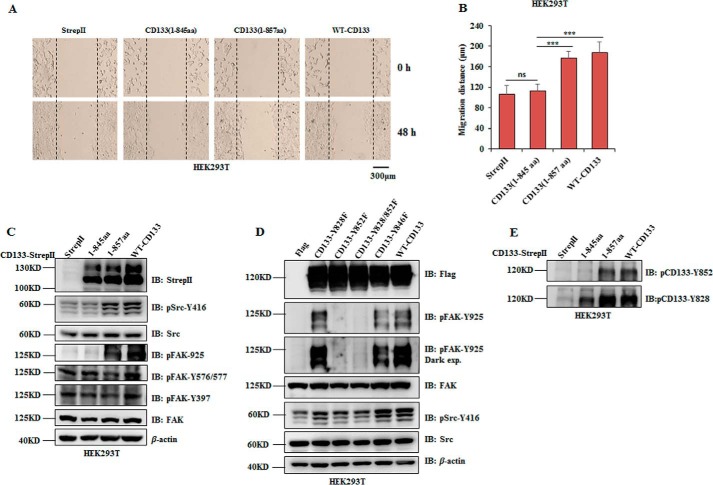
We next investigated the contribution of the interaction between CD133 and Src in CD133 promoting cell migration. A wound healing assay was performed in cells expressing wild type CD133 or truncated mutants of CD133 (1–845 and 1–857). Results showed a significant decrease of migratory distance in cells with mutant CD133(1–845) expression. Cells expressing wild type CD133 or mutant CD133(1–857) showed similar migratory distance (Fig. 5, A and B). Accordingly, wild type CD133 and mutant CD133(1–857) expression obviously increased activation of Src (pSrc-Tyr416) and phosphorylation of FAK-Tyr925 (Fig. 5C).
FIGURE 5.
CD133 promotes cell migration partly depending on its interaction with Src, and their interaction activates Src to phosphorylate FAK-Tyr925 in HEK293T cells. A–C, wild type CD133, its truncated mutants 1–845 and 1–857, or empty vector was expressed in HEK293T cells, respectively. A, representative images of the wound healing assay of the indicated cells. B, relative distance of cell migration in A was calculated and is presented as the mean ± S.D. (error bars) (n = 6). Statistical analysis was performed by Student's t test (***, p < 0.001; ns, not significant). C, expression of CD133 truncated mutants, pSrc-Tyr416, phosphorylation of FAK (pFAK-Tyr397, pFAK-Tyr576/577, and pFAK-Tyr925), total Src, and FAK were detected by Western blotting analysis. D, wild type CD133, mutants of its tyrosine residue (Y828F, Y852F, Y828F/Y852F, and Y846F), or empty vector was expressed in HEK293T cells, respectively. The levels of Src phosphorylation at Tyr416 and FAK phosphorylation at Tyr925 were detected by Western blotting analysis. E, phosphorylation of CD133-Tyr828 and CD133-Tyr852 was detected in HEK293T cells expressing WT-CD133 or CD133 mutants 1–845 and 1–857, respectively. IB, immunoblotting; exp., exposure.
Based on the report demonstrating that tyrosine phosphorylation usually affords a binding site for Src SH2 domain to activate Src (47), we hypothesized that CD133 may also afford a tyrosine phosphorylation site to activate Src. Therefore, the two known tyrosine phosphorylation site of CD133 (Tyr828 and Tyr852) and another tyrosine residue (Tyr846) located in region CD133(845–857) were mutated to phenylalanine (phospho-null mutation), and these mutants were expressed in HEK293T cells, respectively. Only CD133-Y852F reduced Src activation and almost abrogated pFAK-Tyr925 (Fig. 5D). Phosphorylation of CD133-Tyr828 and CD133-Tyr852 was detectable in HEK293T cells expressing wild type CD133 or its mutants (1–845 and 1–857) (Fig. 5E). Combined with results in Fig. 3, we conclude that CD133 promotes cell migration partly depending on its interaction with Src and therefore increases kinase activity of Src.
Inhibition of Src Activity Abrogates the Phosphorylation of FAK-Tyr925 and Cell Migratory Ability Promoted by CD133 Expression
We next determined the contribution of Src in CD133 promotion of cell migration. PP2, a Src family tyrosine kinase inhibitor (48), was used to treat SW620 cells expressing Lacz or CD133 shRNA under serum starvation condition. First, when compared with the DMSO-treated group (control group), the PP2-treated group showed reduced activation of Src (pSrc-Tyr416), which led to a decrease of pFAK-Tyr925. Second, the decrease of pSrc-Tyr416 and downstream pFAK-Tyr925 induced by CD133 knockdown were abolished by PP2 treatment (Fig. 6, A and B). HEK293T cells ectopically expressing CD133 were also treated with PP2. These cells showed a remarkable decrease of pFAK-Tyr925 (Fig. 6C).
FIGURE 6.
Inhibition of Src activity abrogates the phosphorylation of FAK-Tyr925 and cell migration induced by CD133. A and B, SW620 cells expressing shLacz or shCD133, cultured in serum starvation condition, were treated with 10 μm PP2 or DMSO for 24 h. The level of Src phosphorylation (pSrc-Tyr416), FAK phosphorylation (pFAK-Tyr397, pFAK-Tyr576/577, and pFAK-Tyr925), total Src, FAK, and CD133 were detected by Western blotting analysis. C, HEK293T cells ectopically expressing CD133 were treated with 10 μm PP2 or DMSO for 24 h. Then the levels of pFAK-Tyr925, total FAK, and CD133 were determined by Western blotting analysis. D–G, SW620 cells expressing shLacz or shCD133 were treated with 10 μm PP2 or DMSO. D and E, representative images of the wound healing assay of SW620 cells with the indicated treatments. Relative distance of cell migration was calculated and is presented as the mean ± S.D. (error bars) (n = 6). Statistical analysis was performed by Student's t test (**, p < 0.01; ***, p < 0.001; ns, not significant). F and G, representative images of the Transwell assay of SW620 cells with the indicated treatments. The number of migratory cells was calculated and is presented as the mean ± S.D. (error bars) (n = 3) Statistical analysis was performed by Student's t test (***, p < 0.001; ns, not significant). H, SW620 cells expressing shLacz or shCD133, cultured in complete medium, were treated with 10 μm PP2 or DMSO for 24 h. Cell proliferative ability was analyzed by Cell Counting Kit 8 in a 96-well plate. A450 was determine, and results are presented as the mean ± S.D. (error bars) (n = 12). Statistical analysis was performed by Student's t test (***, p < 0.001). I and J, HEK293T cells ectopically expressing CD133 were treated with 10 μm PP2 or DMSO for 24 h. I, representative image of the wound healing assay of HEK293T cells with the indicated treatments. J, relative area of cell migration in I was calculated and is presented as the mean ± S.D. (error bars) (n = 6). Statistical analysis was performed by Student's t test (***, p < 0.001). K, HEK293T cells ectopically expressing CD133 were treated with 10 μm PP1, 10 μm PP3, or DMSO for 24 h. Relative area of cell migration was calculated and is presented as the mean ± S.D. (error bars) (n = 6). Statistical analysis was performed by Student's t test (***, p < 0.001; ns, not significant). IB, immunoblotting.
Addition of PP2 had a potent inhibitory effect on CD133-induced FAK phosphorylation and markedly inhibited the cell migration induced by CD133. The effect of PP2 in inhibiting the migratory ability of SW620 cells was determined by wound healing assay and Transwell assay. PP2 treatment resulted in reduced migratory ability of both Lacz and CD133 knockdown cells. Moreover, the difference of cell migration induced by CD133 almost vanished (Fig. 6, D–G). To explore whether the migratory ability abrogated by PP2 treatment was due to a reduced proliferative ability of cells, we performed PP2 treatment and then tested the proliferative ability of SW620 cells expressing Lacz or CD133 shRNA upon complete culture condition. As shown in Fig. 1C, CD133 knockdown decreased the proliferative ability of SW620 cells under this condition. However, PP2 treatment did not change the reduced proliferative rate in CD133 knockdown cells (Fig. 6H). In HEK293T cells, PP2 treatment abrogated the increased migratory distance induced by CD133 overexpression (Fig. 6, I and J). Furthermore, another Src family tyrosine kinase inhibitor, PP1, and the negative control PP3 were used to treat HEK293T cells overexpressing CD133 (49). Similar to the effect of PP2 treatment, PP1 treatment abrogated the increased migratory distance induced by CD133 overexpression (Fig. 6K). Altogether, inhibition of Src kinase activity abrogates the phosphorylation of FAK-Tyr925 and cell migration induced by interaction between CD133 and Src.
Discussion
Cancer stem cells expressing CD133 have been reported to exhibit the capacity of aggressive tumor growth (14–16). CD133 regulates metastatic ability of cancer cells in a variety of tumors (17–20). Uncovering the mechanism by which CD133 regulates cell migration will contribute to understanding CD133+ tumor cell metastasis. Here, we used the CD133-positive colorectal cancer cell line SW620 and CD133-negative HEK293T cell line to investigate the mechanism of CD133 in regulating cell migration. First, CD133 knockdown not only decreases activation of Src and FAK but also decreases migration of SW620 cells upon serum starvation culture condition. CD133 ectopic expression increases the cell migration ability and phosphorylation level of FAK-Tyr925. Second, in vitro kinase assay proves that CD133 promotes phosphorylation of FAK through Src. Third, CD133 interacts with Src. The interaction of endogenous CD133 and Src in SW620 cells was under serum starvation condition. Region CD133(845–857) is indispensable for its interaction with Src, and tyrosine residue 852 in this region is critical to increase pSrc-Tyr416 and pFAK-Tyr925. Finally, Src inhibitor treatment abrogates the increased phosphorylation of FAK-Tyr925 and cell migration induced by CD133. Altogether, CD133 promotes cell migration at least partly through activating Src.
How does the interaction between CD133 and Src promote cell migration? Activated Src could form complexes with a series of cytoplasmic proteins such as FAK (50–52). FAK is critical for focal adhesion complex formation and actin cytoskeleton dynamics (53). In tumor tissues, FAK is involved in metastasis and progression of hepatocellular carcinoma and thyroid lesions (54–56). Our findings proved the relationship between CD133 expression and pFAK-Tyr925. Higher migratory capacity and phosphorylation level of FAK-Tyr925 were detected in HEK293T cells expressing exogenous CD133. CD133 knockdown in SW620 cells significantly reduced Src-FAK signaling pathway activation and cell migration. Thus, CD133 promotes FAK phosphorylation in vivo. Furthermore, CD133 protein showed an obvious synergy effect in promoting Src to phosphorylate FAK, which was proved by in vitro kinase assay. Thus, CD133 promotes FAK phosphorylation in vivo and in vitro. Inhibition of Src activity by PP2 could block the activation of FAK phosphorylation and cell migration induced by CD133 overexpression. Moreover, the region of CD133 interacting with Src is in accordance with the region of CD133 promoting FAK activation and cell migration. Thus, we presume that interaction between CD133 and Src might promote cell migration through activation of FAK.
We also examined the molecular mechanism of the interaction between CD133 and Src. We found that the 13-amino acid region (between amino acids 845 and 857) in the CD133 cytoplasmic tail is critical for its interaction with Src. Tyrosine phosphorylation usually affords a binding site for Src SH2 domain to activate Src (47), so we explored whether the two known tyrosine phosphorylation sites of CD133 (Tyr828 and Tyr852) (57) or CD133-Tyr846 located in region CD133(845–857) could affect Src-FAK activation. Results showed that only mutation of CD133-Tyr852 reduced Src activation and abrogated pFAK-Tyr925. Thus, phosphorylation of CD133-Tyr852 might afford a binding site for Src to increase its kinase activity. However, the contribution of CD133-Tyr852 phosphorylation in the interaction between CD133 and Src should be further examined.
An interesting finding was that the interaction with CD133 depends on serum starvation culture condition. Serum starvation increased the CD133+ cell percentage of SW620 cells and promoted CD133 expression at transcription and translation levels. The effect of serum starvation on CD133 modification or expression should be further explored in the future. Furthermore, we also found that CD133 regulated Src and FAK activation and cell migration upon serum starvation condition in SW620 cells. As we know, nutritional deficiency, such as serum deficiency or hypoxia, would stimulate cancer cell metastasis (41, 58). These phenomena indicate that CD133+ cancer cells are more tolerant of nutritional deficiency condition and that nutritional deficiency condition leads to a changes in intracellular signaling pathways, such as CD133-Src-FAK signaling, upon serum starvation condition that result in increased migratory ability of CD133+ cancer cells.
In summary, CD133 interacts with Src affording a binding site for Src to increase its kinase activity. Activation of Src promotes phosphorylation of FAK. Then the activated Src and FAK maybe form complex and interact with downstream substrates to promote actin remodeling and cellular migration as previous report (53, 59, 60). Our results demonstrate a new mechanism of promoting cell migration by interaction between CD133 and Src. This finding provides clues to understand the highly migratory ability of CD133+ tumor cells.
Experimental Procedures
Reagents and Antibodies
Restriction enzymes were obtained from New England Biolabs (Beverly, MA). PCR reagents and Pfu DNA polymerase were obtained from Takara. Transfection reagents Lipofectamine 2000 and Dulbecco's modified Eagle's medium (DMEM) were obtained from Invitrogen. FBS was obtained from Biological Industries. Protease inhibitor mixture was obtained from Roche Applied Science. Strep-Tactin Superflow agarose, desthiobiotin, and StrepII tag antibody were obtained from Novagen. Src recombinant protein and PP2 were obtained from Millipore. PP1 and PP3 were obtained from Calbiochem Novabiochem Biosciences. Mouse anti-FAK antibody was obtained from BD Biosciences. Rabbit anti-pFAK-Tyr397 antibody, anti-pFAK-Tyr576/577 antibody, anti-pFAK-Tyr925 antibody, anti-Src antibody, anti-pSrc-Tyr416 antibody, and anti-FLAG antibody were obtained from Cell Signaling Technology (Danvers, MA). Mouse anti-β-actin antibody and Hoechst 33258 were obtained from Sigma. Mouse anti-CD133 (W6B3C1) antibody, CD133-PE antibody, and IgG-PE antibody were obtained from Miltenyi Biotec. Rabbit anti-pCD133-Tyr828 antibody and anti-pCD133-Tyr852 antibody were obtained from Abgent. HRP-conjugated secondary antibodies goat anti-mouse IgG antibody and goat anti-rabbit IgG antibody were obtained from Santa Cruz Biotechnology. Streptavidin-Alexa Flour 488 and donkey anti-rabbit Alexa Flour 594 were obtained from Invitrogen.
Cell Culture
HEK293T cells and colorectal cancer cell line SW620 were maintained in DMEM with 10% FBS, 100 units/ml penicillin, and 50 mg/ml streptomycin at 37 °C in a humidified 5% CO2 incubator. For serum starvation treatment of SW620 cell, cells were washed for four times with PBS and cultured in DMEM without FBS for 36–48 h.
Plasmids, Transfection, Lentivirus Production, and Infection
The cDNAs of CD133 and its truncated mutants were amplified by PCR and cloned into the pRRLSIN.cPPT.PGK vector to create full-length CD133 and CD133 deletion mutants with StrepII tag. The three tyrosine residues (Tyr828, Tyr846, and Tyr852) of CD133 were respectively mutated to phenylalanine by overlap extension PCR and then cloned into pRRLSIN.cPPT.PGK vector. All the mutations were verified by DNA sequencing. For ectopic expression of CD133, transient transfections of HEK293T cells were carried out using Lipofectamine 2000. Cells were harvested at 48–72 h after transfection.
Lentivirus production and infection were performed as described previously (15). The pLL3.7-shLacz, which contains an shRNA insert that targets β-galactosidase, was used for experimental control. The pLL3.7-shCD133, which contains an shRNA insert that targets CD133, was used to knock down the expression of CD133 mRNA. The shRNA sequences were as follows: shLacz, 5′-GTGACCAGCGAATACCTGT-3′; shCD133, 5′-GCTCAGAACTTCATCACAA-3′.
Immunoprecipitation and CD133 Protein Purification
The HEK293T cells transfected with CD133 or CD133 truncated mutants were lysed at 4 °C for 2 h using lysis buffer (150 mm NaCl, 100 mm Tris (pH 8.0), 0.5% Triton X-100, 1 mm EDTA, protease inhibitor mixture, 1 mm β-glycerophosphate, 1 mm Na3VO4, 1 mm NaF), and then the insoluble materials were removed by centrifugation at 12,000 × g for 10 min. The supernatants of cell lysates were incubated with Strep-Tactin-agarose at 4 °C for 12–16 h. After incubation, the agarose was washed three times in lysis buffer to eliminate nonspecific binding proteins. 2.5 mm desthiobiotin was used to elute StrepII-tagged CD133 and its truncated mutants. The elution was concentrated to a volume of 20 μl using an ultrafiltration tube (Millipore).
To purify CD133 protein, the lysis and incubation processes of HEK293T cells transfected with CD133-StrepII were the same as those described above. After incubation, agarose was washed three times with 2 m NaCl in lysis buffer to eliminate nonspecific binding proteins on agarose and proteins interacting with CD133. Then 2.5 mm desthiobiotin was used to elute CD133-StrepII protein. The elution of CD133 protein was concentrated to a small volume, and desthiobiotin was removed with PBS using an ultrafiltration tube. The purified effect of CD133 protein was determined by Coomassie Blue staining.
SW620 cells were lysed in a modified radioimmunoprecipitation assay buffer (150 mm NaCl, 50 mm Tris-HCl (pH 7.4), 2 mm EDTA, 1% Triton X-100, protease inhibitor mixture, 1 mm β-glycerophosphate, 1 mm Na3VO4, 1 mm NaF). Lysates were centrifuged at 12,000 × g for 10 min. The supernatants of cell lysates were cleared by incubation with 25 μl of Protein G-agarose (Roche Applied Science) for 1.5 h at 4 °C. The precleared supernatant was subjected to IP using the indicated antibodies at 4 °C overnight. Antibodies used in IP included mouse monoclonal anti-CD133 (W6B3C1 clone) and rabbit polyclonal anti-Src. The collected protein complexes were washed three times with IP buffer and analyzed by Western blotting.
Western Blotting Analysis
Cells were lysed with 0.5% sodium dodecyl sulfate (SDS) containing 5% mercaptoethanol and 1% protease inhibitor mixture. Western blotting analysis was performed as described previously (15). The dilution ratios of primary antibodies were as follows: CD133 (W6B3C1), 1:500; pCD133-Tyr828, 1:500; pCD133-Tyr852, 1:500; FAK, 1:1,000; pFAK-Tyr397, 1:1,000; pFAK-Tyr576/577, 1:1,000; pFAK-Tyr925, 1:1,000; StrepII, 1:4,000; Src, 1:1,000; pSrc-Tyr416, 1:1,000; FLAG, 1:1,000; and β-actin, 1:5,000.
Immunofluorescence
Cells were grown on coverglasses coated with polylysine, fixed with 4% paraformaldehyde for 40 min at room temperature, washed three times with PBS, and blocked with a PBS-based solution containing 5% normal serum and 0.3% Triton X-100. For HEK293T cells, cells were incubated overnight at 4 °C with rabbit anti-Src (1:100). For SW620 cells, cells were incubated overnight at 4 °C with rabbit anti-Src (1:100) and mouse monoclonal anti-CD133 (1:40). After being washed three times with PBS for 0.5 h, HEK293T cells were incubated with streptavidin-Alexa Flour 488 (1:400) and donkey anti-rabbit Alexa Fluor 594 IgG (1:800), and SW620 cells were incubated with donkey anti-rabbit Alexa Fluor 488 IgG (1:800) and donkey anti-mouse Alexa Fluor 594 IgG (1:800) for double immunofluorescence staining. Nuclei were counterstained with Hoechst 33258 (10 μg/ml). Immunofluorescence images were collected on a Leica TCS SP5 confocal microscope and analyzed using LAS AF software.
In Vitro Kinase Assay
HEK293T cells were lysed in radioimmunoprecipitation assay buffer. Lysates were centrifuged and cleared by incubation with 25 μl of Protein G-agarose for 1.5 h at 4 °C. The precleared supernatant was subjected to IP using the FAK antibody (Cell Signaling Technology; 1:100) and 20 μl of protein G-Agarose at 4 °C overnight. The FAK protein collected on protein G-Agarose was washed two times with radioimmunoprecipitation assay buffer and another two times with the buffer used for the phosphorylation reaction (20 mm Hepes·NaOH (pH 7.5), 5 mm MgCl2, 1 mm DTT, 0.2 mm Na3VO4). Then the FAK protein on the protein G-agarose was incubated with 0.5 μg of CD133 protein, 0.25 μg of recombinant Src protein (Millipore), or both in the phosphorylation reaction buffer described above with 0.1 mm ATP for 1 h at 30 °C. The kinase assay was stopped by adding 1% SDS and 10% mercaptoethanol. Proteins were separated by SDS-PAGE and analyzed by Western blotting with antibodies against FAK, StrepII, pFAK-Tyr397, pFAK-Tyr576/577, pFAK-Tyr925, Src, or pSrc-Tyr416.
Real Time PCR
Total RNA was extracted using TRIzol solution (Invitrogen), and reverse transcription PCR was performed using an Advantage RT-for-PCR kit (Takara) according to the manufacturers' instructions. Real time PCR was performed on triplicate samples in a reaction mixture containing SYBR Green PCR Master Mix (Invitrogen).
Wound Healing Assay
In wound healing assays, cells were digested, and cell suspensions were added to a 6-well plate. The cell monolayers were wounded with a tip when they were 70–80% confluent. The scratched cells were then rinsed three times with PBS to remove non-adherent cells, and fresh culture medium without FBS was added. The rate of migration was calculated by measuring the distance of cell migration in the same view at 0, 24, and 48 h after scratching.
Transwell Assay
Transwell assays were conducted using Transwell chambers (8 μm; Corning Costar Co., Cambridge, MA) according to the manufacturer's instructions. Briefly, cells were trypsinized and resuspended in serum-free medium to a final concentration of 2 × 105/ml, and the cell suspension (200 μl) was then pipetted into the top chamber. Medium (600 μl) with 10% FBS was added to the lower chamber as a chemoattractant. After 24-h incubation, the cells on the upper side of the membrane were mechanically removed with cotton swabs, and cells that migrated to the lower surface were fixed with 5% glutaraldehyde for 10 min and stained with 1% crystal violet in 2% ethanol for 20 min. The cells were counted in five fields for triplicate chambers.
Detection of Proliferation by Cell Counting Kit 8
Cell proliferation was detected with Cell Counting Kit 8 (Dojindo) according to the manufacturer's instructions. Briefly, at the end of tests, a mixture of 10 μl of the reagent and 90 μl of medium was placed in each well of a 96-well plate, the plate was incubated for 2 h, and the absorbance at 450 nm was measured using the Gen5 system (BioTek).
Flow Cytometry
1 × 106 cells were collected and washed with 1% BSA in cold PBS two times by centrifugation at 300 × g for 10 min at 4 °C. Anti-CD133-PE antibody and mouse IgG-PE isotype control antibody (1:50) were incubated with cells for 15 min on ice in the dark. Samples were analyzed using a FACS apparatus (MoFlo XDP, Beckman Coulter).
Statistical Analysis
Statistical analysis was performed using Microsoft Excel. Comparisons of categorical data were carried out by unpaired two-tailed Student's t test. Data are presented as the mean ± S.D. p values <0.05 were considered statistically significant.
Author Contributions
C. L. conducted most of the experiments, analyzed the results, and wrote most of the paper. J. J., Y. W., and Z. A. designed the research. Y. L. and Y. X. constructed part of the plasmids. B. C., F. Y., and T. Y. contributed valuable discussions during the study.
Acknowledgments
We thank Prof. Yalin Huang (Fudan University) for technical assistance on confocal analysis, Prof. Maurizio Pesce for providing plasmid of pRRLSIN.cPPT.hPGK-GFP.WPRE, and Dr. Bernd Giebel and Dr. Denis Corbeil for providing CD133 plasmid.
This work was supported by Program for National Natural Scientific Foundation of China Grants 81272435, 81472724, and 31370807 and National Basic Research Program of China Grant 2013CB910503. The authors declare that they have no financial and personal relationships with other people or organizations that could inappropriately influence (bias) the work in this study.
- FAK
- focal adhesion kinase
- IP
- immunoprecipitation
- DMSO
- dimethyl sulfoxide
- SH2
- Src homology 2
- pFAK
- phosphorylated FAK
- pSrc
- phosphorylated Src
- pCD133
- phosphorylated CD133
- PE
- phycoerythrin.
References
- 1. Chambers A. F., Groom A. C., and MacDonald I. C. (2002) Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2, 563–572 [DOI] [PubMed] [Google Scholar]
- 2. Weigelt B., Peterse J. L., and van 't Veer L. J. (2005) Breast cancer metastasis: markers and models. Nat. Rev. Cancer 5, 591–602 [DOI] [PubMed] [Google Scholar]
- 3. Li F., Tiede B., Massagué J., and Kang Y. (2007) Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res. 17, 3–14 [DOI] [PubMed] [Google Scholar]
- 4. O'Brien C. A., Pollett A., Gallinger S., and Dick J. E. (2007) A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445, 106–110 [DOI] [PubMed] [Google Scholar]
- 5. Yin S., Li J., Hu C., Chen X., Yao M., Yan M., Jiang G., Ge C., Xie H., Wan D., Yang S., Zheng S., and Gu J. (2007) CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int. J. Cancer 120, 1444–1450 [DOI] [PubMed] [Google Scholar]
- 6. Collins A. T., Berry P. A., Hyde C., Stower M. J., and Maitland N. J. (2005) Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 65, 10946–10951 [DOI] [PubMed] [Google Scholar]
- 7. Monzani E., Facchetti F., Galmozzi E., Corsini E., Benetti A., Cavazzin C., Gritti A., Piccinini A., Porro D., Santinami M., Invernici G., Parati E., Alessandri G., and La Porta C. A. (2007) Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur. J. Cancer 43, 935–946 [DOI] [PubMed] [Google Scholar]
- 8. Eramo A., Lotti F., Sette G., Pilozzi E., Biffoni M., Di Virgilio A., Conticello C., Ruco L., Peschle C., and De Maria R. (2008) Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 15, 504–514 [DOI] [PubMed] [Google Scholar]
- 9. Miraglia S., Godfrey W., Yin A. H., Atkins K., Warnke R., Holden J. T., Bray R. A., Waller E. K., and Buck D. W. (1997) A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood 90, 5013–5021 [PubMed] [Google Scholar]
- 10. Yin A. H., Miraglia S., Zanjani E. D., Almeida-Porada G., Ogawa M., Leary A. G., Olweus J., Kearney J., and Buck D. W. (1997) AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 90, 5002–5012 [PubMed] [Google Scholar]
- 11. Singh S. K., Clarke I. D., Terasaki M., Bonn V. E., Hawkins C., Squire J., and Dirks P. B. (2003) Identification of a cancer stem cell in human brain tumors. Cancer Res. 63, 5821–5828 [PubMed] [Google Scholar]
- 12. Olempska M., Eisenach P. A., Ammerpohl O., Ungefroren H., Fandrich F., and Kalthoff H. (2007) Detection of tumor stem cell markers in pancreatic carcinoma cell lines. Hepatobiliary Pancreat. Dis. Int. 6, 92–97 [PubMed] [Google Scholar]
- 13. Wu Y., and Wu P. Y. (2009) CD133 as a marker for cancer stem cells: progresses and concerns. Stem Cells Dev. 18, 1127–1134 [DOI] [PubMed] [Google Scholar]
- 14. Mak A. B., Nixon A. M., Kittanakom S., Stewart J. M., Chen G. I., Curak J., Gingras A.-C., Mazitschek R., Neel B. G., Stagljar I., and Moffat J. (2012) Regulation of CD133 by HDAC6 promotes β-catenin signaling to suppress cancer cell differentiation. Cell Rep. 2, 951–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wei Y., Jiang Y., Zou F., Liu Y., Wang S., Xu N., Xu W., Cui C., Xing Y., Liu Y., Cao B., Liu C., Wu G., Ao H., Zhang X., and Jiang J. (2013) Activation of PI3K/Akt pathway by CD133-p85 interaction promotes tumorigenic capacity of glioma stem cells. Proc. Natl. Acad. Sci. U.S.A. 110, 6829–6834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adini A., Adini I., Ghosh K., Benny O., Pravda E., Hu R., Luyindula D., and D'Amato R. J. (2013) The stem cell marker prominin-1/CD133 interacts with vascular endothelial growth factor and potentiates its action. Angiogenesis 16, 405–416 [DOI] [PubMed] [Google Scholar]
- 17. Zhang S. S., Han Z. P., Jing Y. Y., Tao S. F., Li T. J., Wang H., Wang Y., Li R., Yang Y., Zhao X., Xu X. D., Yu E. D., Rui Y. C., Liu H. J., Zhang L., and Wei L. X. (2012) CD133+CXCR4+ colon cancer cells exhibit metastatic potential and predict poor prognosis of patients. BMC Med. 10, 85–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen K. L., Pan F., Jiang H., Chen J. F., Pei L., Xie F. W., and Liang H. J. (2011) Highly enriched CD133+CD44+ stem-like cells with CD133+CD44high metastatic subset in HCT116 colon cancer cells. Clin. Exp. Metastasis 28, 751–763 [DOI] [PubMed] [Google Scholar]
- 19. Hou Y., Zou Q., Ge R., Shen F., and Wang Y. (2012) The critical role of CD133+CD44+/high tumor cells in hematogenous metastasis of liver cancers. Cell Res. 22, 259–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kohga K., Tatsumi T., Takehara T., Tsunematsu H., Shimizu S., Yamamoto M., Sasakawa A., Miyagi T., and Hayashi N. (2010) Expression of CD133 confers malignant potential by regulating metalloproteinases in human hepatocellular carcinoma. J. Hepatol. 52, 872–879 [DOI] [PubMed] [Google Scholar]
- 21. Eyler C. E., Foo W. C., LaFiura K. M., McLendon R. E., Hjelmeland A. B., and Rich J. N. (2008) Brain cancer stem cells display preferential sensitivity to Akt inhibition. Stem Cells 26, 3027–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yan M., Li H., Zhu M., Zhao F., Zhang L., Chen T., Jiang G., Xie H., Cui Y., Yao M., and Li J. (2013) G protein-coupled receptor 87 (GPR87) promotes the growth and metastasis of CD133+ cancer stem-like cells in hepatocellular carcinoma. PLoS One 8, e61056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maeda S., Shinchi H., Kurahara H., Mataki Y., Maemura K., Sato M., Natsugoe S., Aikou T., and Takao S. (2008) CD133 expression is correlated with lymph node metastasis and vascular endothelial growth factor-C expression in pancreatic cancer. Br. J. Cancer 98, 1389–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen Y. S., Wu M. J., Huang C. Y., Lin S. C., Chuang T. H., Yu C. C., and Lo J. F. (2011) CD133/Src axis mediates tumor initiating property and epithelial-mesenchymal transition of head and neck cancer. PLoS One 6, e28053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martin G. S. (2001) The hunting of the Src. Nat. Rev. Mol. Cell Biol. 2, 467–475 [DOI] [PubMed] [Google Scholar]
- 26. Irby R. B., and Yeatman T. J. (2000) Role of Src expression and activation in human cancer. Oncogene 19, 5636–5642 [DOI] [PubMed] [Google Scholar]
- 27. Yeatman T. J. (2004) A renaissance for SRC. Nat. Rev. Cancer 4, 470–480 [DOI] [PubMed] [Google Scholar]
- 28. Han X., Zhang W., Yang X., Wheeler C. G., Langford C. P., Wu L., Filippova N., Friedman G. K., Ding Q., Fathallah-Shaykh H. M., Gillespie G. Y., and Nabors L. B. (2014) The role of Src family kinases in growth and migration of glioma stem cells. Int. J. Oncol. 45, 302–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McLean G. W., Carragher N. O., Avizienyte E., Evans J., Brunton V. G., and Frame M. C. (2005) The role of focal-adhesion kinase in cancer—a new therapeutic opportunity. Nat. Rev. Cancer 5, 505–515 [DOI] [PubMed] [Google Scholar]
- 30. Sulzmaier F. J., Jean C., and Schlaepfer D. D. (2014) FAK in cancer: mechanistic findings and clinical applications. Nat. Rev. Cancer 14, 598–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schaller M. D., Hildebrand J. D., Shannon J. D., Fox J. W., Vines R. R., and Parsons J. T. (1994) Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol. Cell. Biol. 14, 1680–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schaller M. D., Borgman C. A., Cobb B. S., Vines R. R., Reynolds A. B., and Parsons J. T. (1992) pp125FAK, a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc. Natl. Acad. Sci. U.S.A. 89, 5192–5196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cobb B. S., Schaller M. D., Leu T. H., and Parsons J. T. (1994) Stable association of pp60src and pp59fyn with the focal adhesion-associated protein tyrosine kinase, pp125FAK. Mol. Cell. Biol. 14, 147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Calalb M. B., Polte T. R., and Hanks S. K. (1995) Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol. Cell. Biol. 15, 954–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Calalb M. B., Zhang X., Polte T. R., and Hanks S. K. (1996) Focal adhesion kinase tyrosine-861 is a major site of phosphorylation by Src. Biochem. Biophys. Res. Commun. 228, 662–668 [DOI] [PubMed] [Google Scholar]
- 36. Tai Y. L., Chu P. Y., Lai I. R., Wang M. Y., Tseng H. Y., Guan J. L., Liou J. Y., and Shen T. L. (2015) An EGFR/Src-dependent beta4 integrin/FAK complex contributes to malignancy of breast cancer. Sci. Rep. 5, 16408–16421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thiyagarajan V., Tsai M. J., and Weng C. F. (2015) Antroquinonol targets FAK-signaling pathway suppressed cell migration, invasion, and tumor growth of C6 glioma. PLoS One 10, e0141285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim H. I., Lee H. S., Kim T. H., Lee J. S., Lee S. T., and Lee S. J. (2015) Growth-stimulatory activity of TIMP-2 is mediated through c-Src activation followed by activation of FAK, PI3-kinase/AKT, and ERK1/2 independent of MMP inhibition in lung adenocarcinoma cells. Oncotarget 6, 42905–42922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leibovitz A., Stinson J. C., McCombs W. B. 3rd, McCoy C. E., Mazur K. C., and Mabry N. D. (1976) Classification of human colorectal adenocarcinoma cell lines. Cancer Res. 36, 4562–4569 [PubMed] [Google Scholar]
- 40. Futschik M., Jeffs A., Pattison S., Kasabov N., Sullivan M., Merrie A., and Reeve A. (2002) Gene expression profiling of metastatic and nonmetastatic colorectal cancer cell lines. Genome Lett. 1, 26–34 [Google Scholar]
- 41. Ye Q., Kantonen S., and Gomez-Cambronero J. (2013) Serum deprivation confers the MDA-MB-231 breast cancer line with an EGFR/JAK3/PLD2 system that maximizes cancer cell invasion. J. Mol. Biol. 425, 755–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Canis M., Lechner A., Mack B., Zengel P., Laubender R. P., Koehler U., Heissmeyer V., and Gires O. (2013) CD133 induces tumour-initiating properties in HEK293 cells. Tumour Biol. 34, 437–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Abbi S., Ueda H., Zheng C., Cooper L. A., Zhao J., Christopher R., and Guan J. L. (2002) Regulation of focal adhesion kinase by a novel protein inhibitor FIP200. Mol. Biol. Cell 13, 3178–3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ilić D., Furuta Y., Kanazawa S., Takeda N., Sobue K., Nakatsuji N., Nomura S., Fujimoto J., Okada M., and Yamamoto T. (1995) Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377, 539–544 [DOI] [PubMed] [Google Scholar]
- 45. Deramaudt T. B., Dujardin D., Hamadi A., Noulet F., Kolli K., De Mey J., Takeda K., and Rondé P. (2011) FAK phosphorylation at Tyr-925 regulates cross-talk between focal adhesion turnover and cell protrusion. Mol. Biol. Cell 22, 964–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Song Y. J., Zhang S. S., Guo X. L., Sun K., Han Z. P., Li R., Zhao Q. D., Deng W. J., Xie X. Q., Zhang J. W., Wu M. C., and Wei L. X. (2013) Autophagy contributes to the survival of CD133+ liver cancer stem cells in the hypoxic and nutrient-deprived tumor microenvironment. Cancer Lett. 339, 70–81 [DOI] [PubMed] [Google Scholar]
- 47. Pawson T., and Schlessingert J. (1993) SH2 and SH3 domains. Curr. Biol. 3, 434–442 [DOI] [PubMed] [Google Scholar]
- 48. Hanke J. H., Gardner J. P., Dow R. L., Changelian P. S., Brissette W. H., Weringer E. J., Pollok B. A., and Connelly P. A. (1996) Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 271, 695–701 [DOI] [PubMed] [Google Scholar]
- 49. Liu Y., Bishop A., Witucki L., Kraybill B., Shimizu E., Tsien J., Ubersax J., Blethrow J., Morgan D. O., and Shokat K. M. (1999) Structural basis for selective inhibition of Src family kinases by PP1. Chem. Biol. 6, 671–678 [DOI] [PubMed] [Google Scholar]
- 50. Bjorge J. D., Jakymiw A., and Fujita D. J. (2000) Selected glimpses into the activation and function of Src kinase. Oncogene 19, 5620–5635 [DOI] [PubMed] [Google Scholar]
- 51. Aleshin A., and Finn R. S. (2010) SRC: a century of science brought to the clinic. Neoplasia 12, 599–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mitra S. K., and Schlaepfer D. D. (2006) Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 18, 516–523 [DOI] [PubMed] [Google Scholar]
- 53. Westhoff M. A., Serrels B., Fincham V. J., Frame M. C., and Carragher N. O. (2004) SRC-mediated phosphorylation of focal adhesion kinase couples actin and adhesion dynamics to survival signaling. Mol. Cell. Biol. 24, 8113–8133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Choi C. W., Kim Y. H., Sohn J. H., Lee H., and Kim W.-S. (2015) Focal adhesion kinase and Src expression in premalignant and malignant skin lesions. Exp. Dermatol. 24, 361–364 [DOI] [PubMed] [Google Scholar]
- 55. Shang N., Arteaga M., Zaidi A., Stauffer J., Cotler S. J., Zeleznik-Le N. J., Zhang J., and Qiu W. (2015) FAK is required for c-Met/β-catenin-driven hepatocarcinogenesis. Hepatology 61, 214–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen J. S., Huang X. H., Wang Q., Chen X. L., Fu X. H., Tan H. X., Zhang L. J., Li W., and Bi J. (2010) FAK is involved in invasion and metastasis of hepatocellular carcinoma. Clin. Exp. Metastasis 27, 71–82 [DOI] [PubMed] [Google Scholar]
- 57. Boivin D., Labbé D., Fontaine N., Lamy S., Beaulieu E., Gingras D., and Béliveau R. (2009) The stem cell marker CD133 (prominin-1) is phosphorylated on cytoplasmic tyrosine-828 and tyrosine-852 by Src and Fyn tyrosine kinases. Biochemistry 48, 3998–4007 [DOI] [PubMed] [Google Scholar]
- 58. Nagelkerke A., Bussink J., Mujcic H., Wouters B. G., Lehmann S., Sweep F. C., and Span P. N. (2013) Hypoxia stimulates migration of breast cancer cells via the PERK/ATF4/LAMP3-arm of the unfolded protein response. Breast Cancer Res. 15, R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Playford M. P., and Schaller M. D. (2004) The interplay between Src and integrins in normal and tumor biology. Oncogene 23, 7928–7946 [DOI] [PubMed] [Google Scholar]
- 60. Guarino M. (2010) Src signaling in cancer invasion. J. Cell. Physiol. 223, 14–26 [DOI] [PubMed] [Google Scholar]