Abstract
Yeast 6-phosphofructo-1-kinase (PFK-1) has two subunits, Pfk1p and Pfk2p. Deletion of Pfk2p alters glucose-dependent V-ATPase reassembly and vacuolar acidification (Chan, C. Y., and Parra, K. J. (2014) Yeast phosphofructokinase-1 subunit Pfk2p is necessary for pH homeostasis and glucose-dependent vacuolar ATPase reassembly. J. Biol. Chem. 289, 19448–19457). This study capitalized on the mechanisms suppressing vacuolar H+-ATPase (V-ATPase) in pfk2Δ to gain new knowledge of the mechanisms underlying glucose-dependent V-ATPase regulation. Because V-ATPase is fully assembled in pfk2Δ, and glycolysis partially suppressed at steady state, we manipulated glycolysis and assessed its direct involvement on V-ATPase function. At steady state, the ratio of proton transport to ATP hydrolysis increased 24% after increasing the glucose concentration from 2% to 4% to enhance the glycolysis flow in pfk2Δ. Tighter coupling restored vacuolar pH when glucose was abundant and glycolysis operated below capacity. After readdition of glucose to glucose-deprived cells, glucose-dependent V1Vo reassembly was proportional to the glycolysis flow. Readdition of 2% glucose to pfk2Δ cells, which restored 62% of ethanol concentration, led to equivalent 60% V1Vo reassembly levels. Steady-state level of assembly (100% reassembly) was reached at 4% glucose when glycolysis reached a threshold in pfk2Δ (≥40% the wild-type flow). At 4% glucose, the level of Pfk1p co-immunoprecipitated with V-ATPase decreased 58% in pfk2Δ, suggesting that Pfk1p binding to V-ATPase may be inhibitory in the mutant. We concluded that V-ATPase activity at steady state and V-ATPase reassembly after readdition of glucose to glucose-deprived cells are controlled by the glycolysis flow. We propose a new mechanism by which glucose regulates V-ATPase catalytic activity that occurs at steady state without changing V1Vo assembly.
Keywords: glucose, glycolysis, proton pump, proton transport, vacuolar acidification, vacuolar ATPase, yeast
Introduction
Vacuolar H+-ATPase (V-ATPase)3 is a highly conserved ATP-driven proton pump distributed throughout the endomembrane system. Intracellular V-ATPase generates and maintains the acidic organelle luminal pH necessary for membrane trafficking, protein sorting and processing, and zymogen activation (1–5). V-ATPase is also present at the plasma membrane of cells specialized for active proton transport. Kidney intercalated cells (6), bone osteoclasts (7), and epididymis clear cells (6) express V-ATPases at the plasma membrane, which are necessary for systemic acid-base balance, bone resorption, and sperm maturation, respectively.
V-ATPase consists of two multisubunit domains, V1 and Vo, that are responsible for ATP hydrolysis and proton transport, respectively (8, 9). Peripheral subunits form V1, the catalytic domain attached to the cytosolic side of the membrane. V1 is bound to Vo, the integral membrane domain that forms the path for proton transport. During catalysis, ATP hydrolysis within V1 drives active transport of protons from the cytosol to the other side of the membrane via rotation of a proteolipid ring structure in Vo. Detachment of V1 from Vo is an important mechanism that inhibits V-ATPase proton transport (3, 10, 11). Disassembly and reassembly of V1Vo has been observed in yeast, insects, and mammalian cells (3, 11–13). However, the cellular mechanisms governing V-ATPase regulation by reversible disassembly are not well understood. In yeast, V-ATPase reversible disassembly is intertwined with cytosol pH changes (14, 15) the RAS/cAMP/PKA pathway (16), and glycolysis (3).
Disassembly of the yeast V1Vo complex occurs when glucose is not available. Upon glucose depletion, V1 subunit C is released into the cytoplasm, causing the rest of the V1 domain to dissociate from Vo (10, 17, 18). Disassembly completely inactivates V-ATPase pumps because V1 without Vo cannot hydrolyze ATP, and Vo without V1 cannot transport protons (2). Consequently, protons cannot not be redistributed from the cytosol into the vacuole, which alkalinizes the vacuolar lumen and acidifies the cytosol (19, 20). Disassembly is reversed by readdition of glucose to yeast cells (10, 21). After reassembly, V1Vo resumes ATP-driven proton transport, which restores vacuolar and cytosol pH homeostasis (20).
Complete glucose depletion causes about 75% of the V1Vo complexes to disassemble (10, 21); the remaining 25% of the pumps maintain the V-ATPase activity necessary to sustain basal cellular functions. Presumably yeast V1Vo disassembly preserves energy when glucose, the main energy source, is limited. This hypothesis is supported indirectly by observations that rapidly fermentable carbon sources like glucose, mannose, and fructose trigger reassembly (21), whereas less efficiently fermentable carbon sources like raffinose and galactose or non-fermentable carbon sources like glycerol and ethanol cannot trigger reassembly (10, 21). These studies indirectly linked the V1Vo assembly levels with glycolysis. Additional evidence suggesting that reassembly correlates with glycolysis comes from studies using the nonmetabolizable glucose analogue 2-deoxyglucose, which cannot substitute for glucose in triggering reassembly (21), and also from studies using a phosphoglucose isomerase (pgi1) glycolytic mutant in which fructose, but not glucose, induces V1Vo reassembly (21).
The glycolytic enzyme aldolase binds to and stabilizes V1Vo complexes (22, 23). Other glycolytic enzymes, such as 6-phosphofructo-1-kinase (PFK-1) (24, 25) and glyceraldehyde-3-phosphate dehydrogenase (23), co-immunoprecipitate with V-ATPase, although a direct regulatory role on V-ATPase assembly has not been assigned to these enzymes. PFK-1 may be necessary for human kidney V-ATPase function because V-ATPase mutations that cause distal renal tubular acidosis prevent binding of PFK-1 to V-ATPase (25).
Yeast PFK-1 is a hetero-octameric complex (α4β4) (26) that co-purifies with vacuolar membranes and co-immunoprecipitates with V-ATPase subunits (24). The genes PFK1 and PFK2 encode the subunits α (Pfk1p) and β (Pfk2p), respectively (26). Because each subunit has catalytic and regulatory functions, yeast cells can form partially active homomeric complexes upon deletion of one PFK-1 structural gene (27). The PFK-1 single deletion mutants pfk1Δ and pfk2Δ metabolize glucose (27), although at a more reduced rate than wild-type cells. Glycolysis is more severely reduced in pfk2Δ than pfk1Δ cells (27), indicating that the subunits do not contribute equally to PFK-1 complex activity. In pfk2Δ cells, the concentration of fructose 1,6-biphosphate, the PFK-1 reaction product, is 5- to 7.5-fold lower than what is found in pfk1Δ cells. Accordingly, pfk2Δ exhibits reduced downstream glycolytic intermediates, glucose consumption, and ethanol production (27–29). The fact that pfk2Δ does not have detectable glycolytic oscillations (30) indicates that glycolysis self-regulatory functions are defective in this strain.
V-ATPase function is defective in both PFK-1 deletion mutants, but it is more severely impaired in pfk2Δ (24). Glucose-dependent V1Vo reassembly is normal in pfk1Δ, but it is reduced by 40–50% in pfk2Δ. In addition, V-ATPase proton transport is partially suppressed at steady state. The cells have alkalinized vacuoles and display pH and calcium growth sensitivity, which is characteristic of yeast cells with partially defective V-ATPase activity. The mechanisms by which V-ATPase is suppressed in pfk2Δ cells are elusive. Subunit Pfk1p may regulate V-ATPase through its interaction with V1Vo in the pfk2Δ mutant. In addition, a reduction in PFK-1 function and the glycolysis flow may inhibit V-ATPase in pfk2Δ cells.
We have now investigated the interrelation between glycolysis and V-ATPase function to gain new knowledge of the mechanisms underlying glucose-dependent V-ATPase regulation. After readdition of glucose to glucose-deprived cells, V1Vo reassembly is proportional to the glycolysis flow until pfk2Δ reaches a metabolic threshold necessary to complete V1Vo reassembly. At steady state, V-ATPase also communicates with the glycolysis flow. This communication modulates proton transport to restore pH homeostasis when glucose is abundant in pfk2Δ. This is a new mechanism by which glucose regulates V-ATPase. It occurs at steady state and does not involve disassembly/reassembly.
Results
Stimulation of Glycolysis Rescues V-ATPase Function in pfk2Δ at Steady State
To test the hypothesis that V-ATPase function is adjusted in response to changes in the glycolysis flow, we manipulated glycolysis in pfk2Δ cells and measured V-ATPase functions. First, we measured PFK-1 activity in pfk2Δ cells to determine the extent of PFK-1 inhibition. As shown in Fig. 1A, pfk2Δ cells only express subunit Pfk1p. The enzymatic activity of PFK-1 was 42% lower in pfk2Δ than wild-type cells. This result is consistent with previous reports indicating that PFK-1 homomeric complexes consisting of subunit α (pfk2Δ cells) are less active than wild-type native PFK-1 hetero-octameric complexes (α4β4) (27).
FIGURE 1.
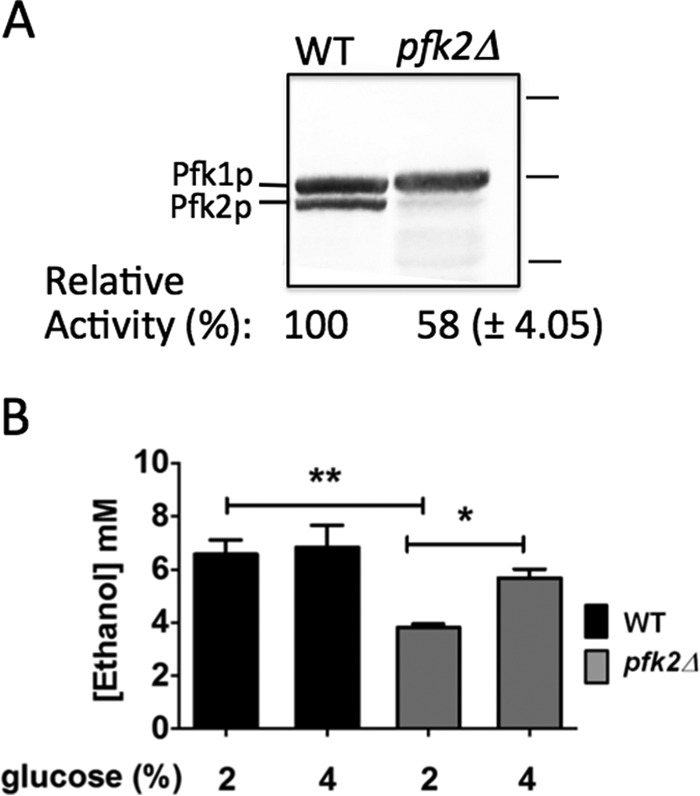
PFK-1 activity and glycolysis are defective in pfk2Δ cells. A, PFK-1 enzymatic activity is reduced in pfk2Δ cells. Wild-type and pfk2Δ cells were grown overnight to mid-log phase (optical density of 0.6–0.8 A600/ml) in YEP medium containing 2% glucose. The cells were converted to spheroplast by zymolase treatment, and protein was separated on a 10% SDS-PAGE gel. The gel was immunoblotted with primary polyclonal antibodies to PFK-1 and secondary antibodies conjugated to alkaline phosphate. Protein markers (right) are 150, 100, and 75 kDa. PFK-1 enzymatic activity was measured for an optical density of 20.0 A600 obtained from cells grown overnight to mid-log phase in medium containing 2% glucose. Data are expressed as the average ± S.D. (n = 3 separate experiments). Relative averaged values were used to express percentage activity. The wild-type PFK-1 activity in the whole cell lysate was 0.014–0.017 μmol of fructose 1,6-bisphosphate/min/optical density A600. B, ethanol concentration is lower in pfk2Δ than in wild-type cells. The cells were cultured to an optical density of 0.6–0.8 A600/ml in YEP medium containing 2% or 4% glucose, converted to spheroplasts by zymolase treatment, and resuspended in fresh medium containing 1.2 m sorbitol plus 2% or 4% glucose. After 20 min at 30 °C, the ethanol concentration was measured. Data represent three independent experiments. Error bars are standard deviation. Statistically significant differences (*, p < 0.05; **, p < 0.01) were determined by two-tailed unpaired t test.
In yeast, the final product of glycolysis, pyruvate, is fermented into ethanol, the levels of which were measured in both strains at steady state (Fig. 1B). In the presence of 2% glucose, which is the standard glucose concentration in yeast growth medium, the ethanol concentration was observed to be 6.6 ± 0.9 mm and 3.8 ± 0.2 mm in the wild-type and pfk2Δ strains, respectively. This result indicates that glycolysis was defective in pfk2Δ. The 42% ethanol concentration reduction is consistent with the 42% reduction of PFK-1 activity measured in pfk2Δ cells and with independent studies that reported reduced fructose-1,6-biphosphate, the 6-phosphofructo-1-kinase reaction product (27–29).
Previous characterizations of glycolysis (27) and V-ATPase pumps (24) in pfk2Δ cells were conducted in 2% glucose. Although 2% glucose is optimal for wild-type cells, it might not be optimal for PFK-1 mutants. We reasoned that the pfk2Δ glycolytic mutant would require larger concentrations of glucose to maintain a substantial glycolytic flow. The pfk2Δ cells were grown in medium containing 2% or 4% glucose, and the ethanol concentration was compared with wild-type cells (Fig. 1B). Although lower ethanol levels were detected in 2% glucose, the ethanol concentration increased in 4% glucose in pfk2Δ cells. It reached 83% of the ethanol concentration in the wild-type cells, indicating that glycolysis was stimulated.
Because the vacuolar pH is regulated by V-ATPase, vacuolar alkalization is a direct consequence of inhibiting V-ATPase activity. The effects of stimulating glycolysis on V-ATPase proton transport were determined by measuring the vacuolar pH in vivo. We used the pH-sensitive fluorophore BCECF, which accumulates in yeast vacuoles. The cells were loaded with BCECF-AM, and the pH was measured fluorometrically according to standard calibration curves (20, 24, 31). The vacuolar pH was measured in wild-type cells and pfk2Δ after growing the cells in 2% versus 4% glucose. vma3Δ cells were used as a control because vma3Δ completely lacks V-ATPase function (32). In wild-type cells, where the V-ATPase pumps are fully active, the vacuolar lumen was acidic (pH = 5.2–5.9) at both glucose concentrations (Fig. 2A). In 2% glucose, the pfk2Δ vacuoles were alkalinized (pH 6.6), similar to the inactive V-ATPase mutant vma3Δ (pH 6.8).
FIGURE 2.
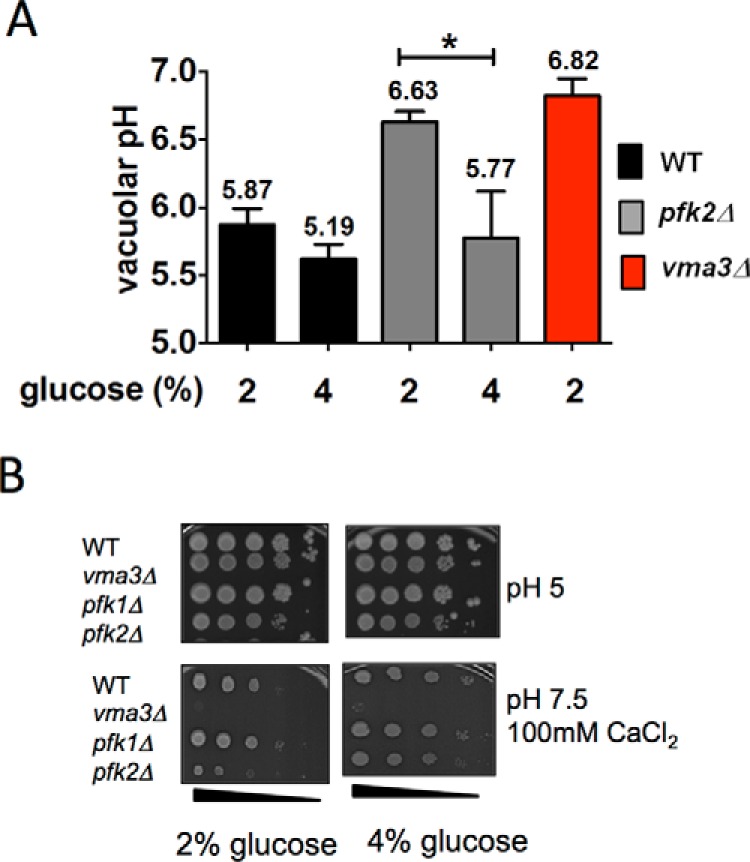
Metabolic reactivation is defective in pfk2Δ cells. A, the acidic vacuolar pH is restored in 4% glucose. Wild type, pfk2Δ, and vma3Δ cells were cultured to mid-log phase in YEP containing 2% or 4% glucose. The cells were harvested and stained with 50 μm BCECF-AM for 30 min at 30 °C. The ratio of fluorescent emission (535 nm) excited at 490 and 450 nm was measured to quantitatively assess vacuolar pH. The average fluorescence over 6 min was compared with a standard curve to generate absolute pH values. Data are presented as average pH values from three independent experiments, and error bars are standard deviation. Statistically significant differences (*, p < 0.05) were determined by two-tailed unpaired t test. B, the vma− growth phenotype is rescued in 4% glucose. Cells were cultured to mid-log phase in 2% or 4% glucose, and 10-fold serial dilutions were stamped onto YEP plates containing 2% or 4% glucose adjusted to pH 5.0 and pH 7.5 plus 100 mm CaCl2. Cell growth was monitored for 72 h at 37 °C. Shown are representative plates of triplicates.
In 4% glucose, the acidic pH of the vacuoles was rescued. The pfk2Δ vacuolar lumen pH in 4% glucose (pH 5.8) was almost identical to the wild-type vacuolar lumen pH in 2% glucose (pH 5.9). These results show that stimulation of glycolysis with 4% glucose stimulated V-ATPase proton transport in pfk2Δ cells at steady state. Thus, they indicate that V-ATPase proton transport is coupled to the flow of glycolysis at steady state.
Sensitivity to elevated pH and calcium at all temperatures is a signature of mutants that lack all V-ATPase activity (vacuolar and Golgi V-ATPase). It is known as the Vma− (vacuolar membrane ATPase) growth phenotype (33) and is only shown when over ∼70% of V-ATPase function is compromised (34–36). The vma mutants grow at pH 5.0, but growth is reduced or abolished at neutral pH in the presence of high concentrations of calcium (32). The physiological basis of Vma− growth is not fully understood because the downstream consequences of inhibiting V-ATPase are extensive (33). Vacuolar alkalization alone is not sufficient to cause the Vma− phenotype (37). However, pfk2Δ growth was fairly reduced with 2% glucose under non-permissive growth conditions at 37 °C (Fig. 2B) (24). This modest growth defect was indicative of defective vacuolar but not Golgi V-ATPase function in pfk2Δ (37).
To determine whether the Vma− growth is rescued after stimulation of glycolysis, pfk2Δ cells were plated on medium containing 100 mm CaCl2 buffered to pH 7.5 (Fig. 2B) that was supplemented with 2% glucose (left panels) versus 4% glucose (right panels). The Vma− growth defect was rescued in 4% glucose, indicating that the vacuolar V-ATPase function was restored. As expected, the wild-type strain grew under all the conditions, and vma3Δ, which completely lacks V-ATPase function (32), failed to grow under non-permissive conditions. The pfk1Δ mutant strain was examined for reference. The pfk1Δ strain lacks the PFK-1 subunit Pfk1p but has greater glycolysis flow than the pfk2Δ strain (27). The pfk1Δ cells closely mimicked the wild-type cells, as reported before (24). A normal pfk1Δ growth was consistent with the concept that vacuolar V-ATPase function is coupled to the glycolysis flow.
Metabolic Reactivation Is Defective in pfk2Δ Cells
Readdition of glucose to cells deprived of glucose rapidly reactivates glycolysis (38–40). We measured ethanol under V-ATPase disassembly (glucose depletion) and reassembly (glucose readdition) conditions to determine whether the glycolysis flow dictates the V-ATPase reassembly level. As described below, glucose-dependent metabolic reactivation was defective in pfk2Δ, which cannot sufficiently reassemble V1Vo after readdition of glucose.
Under disassembly conditions (10 min after glucose removal), the ethanol concentration dropped to about 2.5 ± 0.2 mm in the wild-type and pfk2Δ strains (Fig. 3A). As a control, the ethanol concentration was determined in cells treated with 2-deoxyglucose after glucose was removed. Because of its inability to activate glycolysis beyond 2-deoxyglucose-6-phosphate formation (41), the observed ethanol concentration of 2.5 mm was comparable with that of the glucose-deprived wild type. Because 2-deoxyglucose does not support ethanol production, this indicates that glycolysis had stalled upon removal of glucose.
FIGURE 3.
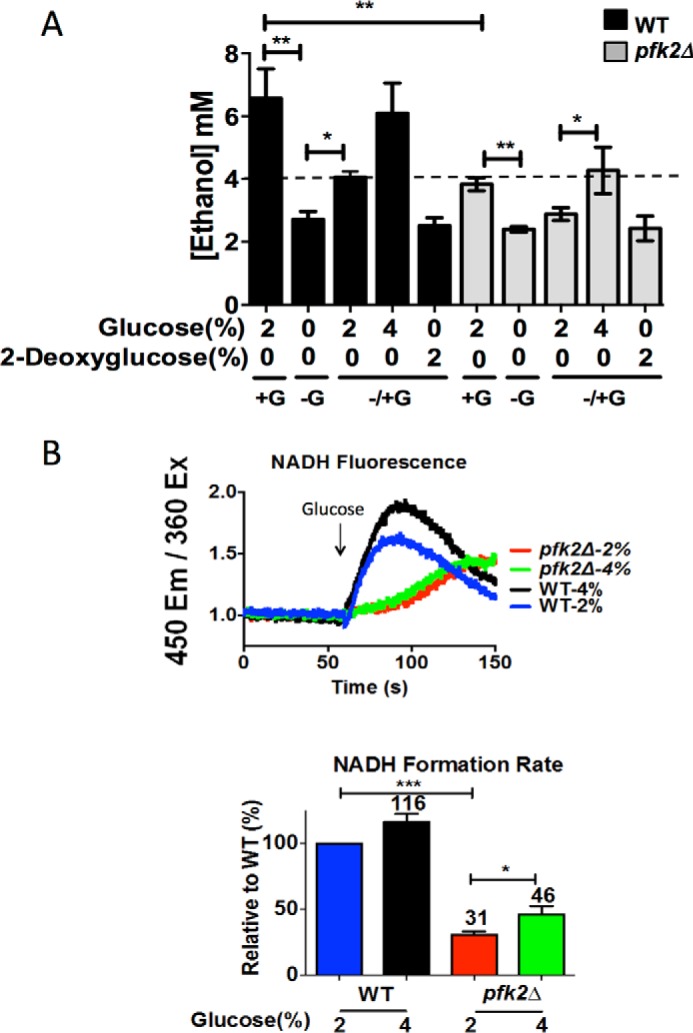
Glucose-induced metabolic reactivation is defective in pfk2Δ cells. A, glucose-dependent ethanol formation is decreased in pfk2Δ cells. The wild-type and pfk2Δ strains were cultured to an optical density of 0.6–0.8 A600/ml in YEP medium containing 2% glucose and converted to spheroplast by zymolase treatment. Spheroplasts were resuspended in fresh medium containing 1.2 m sorbitol and incubated at 30 °C as follows: in the presence of 2% glucose (+ G, 20 min), without glucose (− G, 10 min), and after readdition of 2% glucose, 4% glucose, or 2% 2-deoxyglucose to glucose-deprived cells (± G, 10 min). The ethanol concentration was measured using a colorimetric assay. Data represent three independent experiments. Error bars are standard deviation. The dashed line represents the ≥40% reassembly threshold. B, the rate of NADH formation after glucose re-addition is decreased in pfk2Δ cells. Wild-type and pfk2Δ cells were cultured overnight in medium containing 2% glucose, and then cells were resuspended in 50 mm potassium phosphate buffer (pH 6.8) for 3 h, after which NADH autofluorescence was monitored (emission (Em), 450 nm; excitation (Ex) 366 nm) for 60 s in the absence of glucose and for another 120 s after 2% or 4% glucose final concentration was added. Velocities were calculated for the initial 15 s following glucose addition and expressed relative to wild-type cells. Data represent two independent experiments. Error bars are standard deviation. Statistically significant differences (*, p < 0.05; **, p < 0.01; ***, p < 0.001) are as compared with the wild type in the presence of 2% glucose and were determined by two-tailed unpaired t test.
Under reassembly conditions (10 min after glucose readdition), the concentration of ethanol increased, indicating that glycolysis resumed. Although addition of 2% glucose increased ethanol concentrations to 4.1 ± 0.2 mm and 2.9 ± 0.2 mm in the wild-type and pfk2Δ strains, respectively, steady state levels were not reached. These results also showed that 2% glucose resumed glycolysis in the two strains, but to a lesser extent in pfk2Δ. The pfk2Δ mutant, when 2% glucose was readded, mimicked the wild-type cells deprived of glucose, suggesting that insufficient glycolytic flow upon reactivation is responsible for the V1Vo reassembly defects in pfk2Δ.
Addition of glucose after nutrient limitation is known to trigger a rapid increase of NADH/NAD+ that inhibits the glycolytic enzyme triose phosphate dehydrogenase (39) and results in a transient peak in NADH levels (42). To monitor the rate of metabolic reactivation when glucose was readded, NADH was measured by autofluorescence. Readdition of 2% glucose stimulated NADH synthesis in wild-type and pfk2Δ cells (Fig. 3B). However, the NADH synthesis rate was reduced by 70% in pfk2Δ cells. These results are consistent with the ethanol measurements, indicating that glucose-mediated metabolic reactivation was defective in pfk2Δ.
We measured metabolic reactivation after adding 4% glucose. Fig. 3, A and B, shows the level of ethanol and NADH formation that resulted from the addition of 4% glucose to pfk2Δ after a brief glucose depletion period. The concentration of ethanol reached a steady-state level of 6 mm ± 0.6 mm and 3.8 ± 0.7 mm for the wild type and pfk2Δ, respectively. The rate of NADH synthesis in wild-type and pfk2Δ cells increased by 15%. It reached up to 46% in pfk2Δ cells, which was a larger increase than that observed upon addition of 2% glucose. Thus, doubling the concentration of glucose from 2% to 4% stimulated glucose-dependent metabolic reactivation by about 50% in pfk2Δ, as indicated by ethanol levels and the NADH formation rate. Next, we exposed pfk2Δ to 2% and 4% glucose to manipulate glycolysis and directly establish the role of glycolysis in V-ATPase assembly and activity.
4% Glucose Rescues Glucose-dependent Reassembly and Vacuolar Acidification
If V1Vo reassembly is governed by the glycolysis flow, we anticipated reassembly levels to increase after stimulating glycolysis in pfk2Δ cells. Fig. 4A shows the extent of V1Vo reassembly in pfk2Δ after addition of 4% glucose. Reassembly levels were measured using biosynthetically 35S-radiolabeled cells in pulse-chase experiments as described under “Experimental Procedures.” The radiolabeled cells were chased in YEP medium containing 2% glucose for 20 min (steady-state condition, + G), YEP medium without glucose for 10 min (disassembly condition, − G), and after readdition of varied concentrations of glucose (0.1–4%) for an additional 10 min (reassembly conditions, ± G). After the chases, the V-ATPase complexes were immunoprecipitated under nondenaturing conditions with anti-V1 subunit B to immunoprecipitate V1 and V1Vo versus anti-Vo subunit a, which can only immunoprecipitate Vo when it is disassembled from V1.
FIGURE 4.
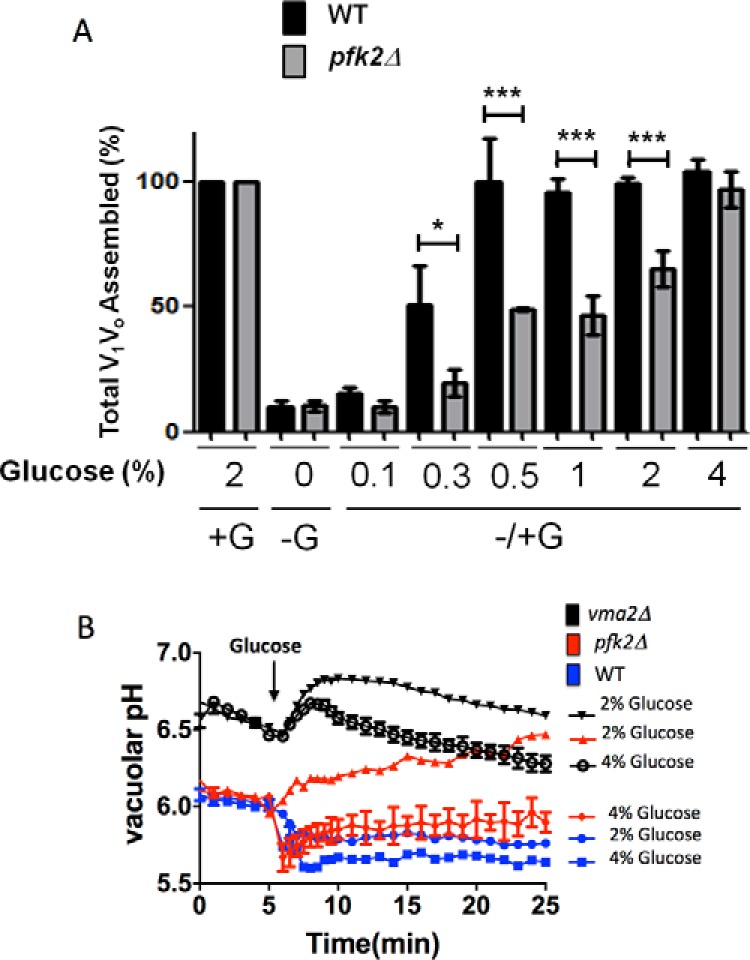
Addition of 4% glucose to glucose-deprived pfk2Δ cells rescues V-ATPase reassembly and vacuolar acidification. A, V1Vo reassembly is glucose-dose dependent. The cells were cultured in 2% glucose, converted to spheroplasts, and biosynthetically radiolabeled with Tran35S. Chases were conducted in the presence of 2% glucose (+ G) for 20 min, after glucose depletion (− G) for 10 min, and after readdition of varied concentrations of glucose (0.1–4% glucose) (± G) for an additional 10 min. The V-ATPase complexes were immunoprecipitated with monoclonal antibodies to V1 subunit B and Vo subunit a and separated by SDS-PAGE, and then the proportion of total Vo assembled in V1Vo complexes was estimated. Data were analyzed in a Fuji scanner (FLA-5100) with Multi Gauge and GraphPad Prism 5 software. Results are presented as average from three independent experiments. Error bars are standard deviation. Statistically significant differences (*, p < 0.05; ***, p < 0.001) as compared with steady state (2% glucose) were determined by two-tailed unpaired t test. Error bars show mean ± S.D. B, readdition 4% glucose restores pfk2Δ vacuolar acidification after reassembly. Cells were stained with BCECF-AM and deprived of glucose for 10 min in 1 mm HEPE-MES (pH 5.0) buffer, and glucose was readded to a final concentration of 2% or 4% (arrow). The ratio of fluorescent emission (535 nm) excited at 490 nm and 450 nm was calculated, and the vacuolar pH was measured using calibration curves. Data represent three independent experiments. Error bars are standard deviation.
About 80% of the V1Vo complexes disassembled upon glucose depletion (Fig. 4A). After glucose readdition, reassembly was proportional to the concentration of glucose added. However, pfk2Δ required greater concentrations of glucose to reach wild-type levels of reassembly. In the wild-type cells, 50% reassembly was detected after addition of 0.3% glucose; 100% reassembly was reached with 0.5% glucose. In contrast, addition of 0.3% glucose did not trigger significant reassembly in pfk2Δ cells, and 2% glucose led to only 60% reassembly. Approaching steady-state levels of assembly (100%) required 4% glucose.
Readdition of 2% glucose, which restored 62% of the steady-state ethanol concentration, triggered comparable levels of V1Vo reassembly (60%). Readdition of 4% glucose, which restored steady-state ethanol levels led to steady-state reassembly levels (100%). Collectively, these results showed that V1Vo reassembly was proportional to the glycolysis flow in pfk2Δ until the cells reached ≥40% of the steady-state wild-type ethanol concentration and NADH formation rate.
The V1Vo complexes reactivate after reassembly, which acidifies the vacuolar lumen and restores membrane gradients and secondary transport systems (20). To determine whether reassembly restored V-ATPase proton transport, we measured the vacuolar pH after readdition of 4% glucose to pfk2Δ. Vacuolar acidification was detected within 1–2 min of glucose readdition to the wild-type cells (Fig. 4B), which is within the timescale when reassembly occurs (3, 21). The V1Vo reassembly level was somewhat higher in 4% glucose (Fig. 4A), and the wild-type vacuole pH was slightly more acidic.
Glucose-induced acidification was defective in pfk2Δ in response to 2% glucose even though 60% of the V1Vo complexes reassembled in the glycolytic mutant (Fig. 4A). However, V-ATPase proton transport resumed after 4% glucose readdition. Reactivation of V-ATPase proton transport was indicated by the rapid acidification of the vacuolar lumen. The vma2Δ strain that cannot assemble V1Vo complexes (43) was used as a negative control because vma2Δ completely lacks V-ATPase activity and vacuolar buffering capacity (2). As expected, the net vacuole pH was considerably more alkaline in vma2Δ than in wild-type and pfk2Δ cells. A gradual pH drop was detected in vma2Δ at 3 min after V-ATPase reactivation (acidification) was complete in wild-type and pfk2Δ cells (Fig. 4B). This observation further indicated that glucose-mediated vacuolar acidification was V-ATPase-dependent in pfk2Δ cells.
Proton Transport Is Increased at Vacuolar Membranes
The rates of proton transport and ATP hydrolysis were measured in purified vacuolar membranes from pfk2Δ cells. V-ATPase activity was determined in the presence and absence of the specific V-ATPase inhibitor concanamycin A, and the ratio of proton pumping to ATP hydrolysis was used as a means of estimating the coupling efficiency of the enzyme when glycolysis was stimulated in pfk2Δ cells.
In wild-type cells cultured in 4% glucose, ATP hydrolysis and proton transport activities increased by 21% and 17%, respectively, compared with those grown in 2% glucose (Fig. 5A). In contrast, ATP hydrolysis was unchanged in pfk2Δ membranes from cells cultured in 2% versus 4% glucose, which was 64–67% of the wild-type ATP hydrolysis. However, proton transport significantly increased (by 30%) when pfk2Δ cells were grown in 4% glucose from that observed in 2% glucose. As a result, the proton transport/ATP hydrolysis ratio increased 24% in the pfk2Δ mutant (from 0.63 to 0.83). Western blotting analyses of vacuolar membrane fractions showed comparable levels of V1 subunit A and subunit B in 2% glucose and 4% glucose (Fig. 5B). Based on these results, we concluded that the V-ATPase pumps fine-tuned their catalytic activity in 4% glucose, which enabled pfk2Δ cells to restore vacuolar pH homeostasis.
FIGURE 5.
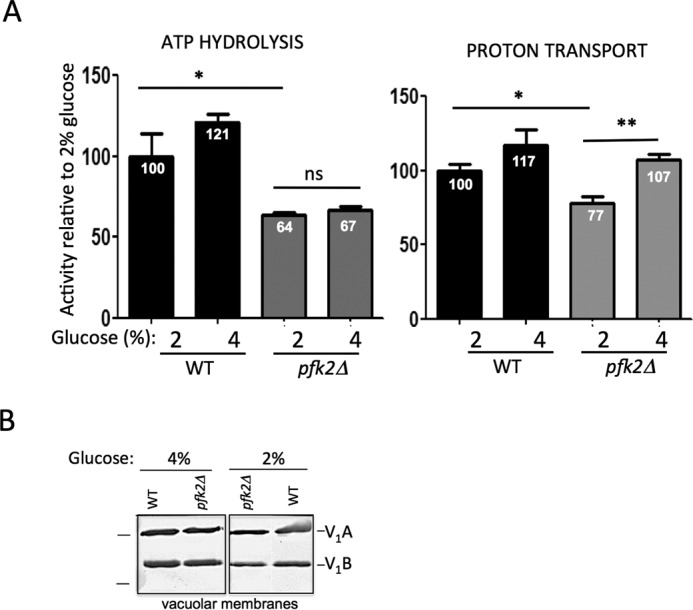
Proton transport is increased at vacuolar membranes from pfk2Δ cells cultured in 4% glucose. A, V-ATPase coupling efficiency is increased in 4% glucose. Vacuolar membrane fractions from wild-type and pfk2Δ cells were purified by density centrifugation. ATP hydrolysis (left panel) was assayed spectrophotometrically in the presence and absence of the V-ATPase inhibitor concanamycin A (100 nm) by using an enzymatic coupled assay that measures NADH oxidation at 340 nm. The average wild-type specific activity in 2% glucose for the concanamycin A-sensitive ATP hydrolysis was 3.0 μmol of ATP/min/mg of total vacuolar protein. ATP-dependent proton transport (right panel) was measured by fluorescence quenching of 1 μm 9-amino-6-chloro-2-methoxyacridin (excitation, 410 nm; emission, 490 nm) upon addition of 0.5 mm ATP/1 mm MgSO4 to 5 μg of total protein in vacuolar membrane vesicles. Initial velocities were calculated for 15 s following MgATP addition. The average wild-type slope was −1117.47 fluorescence units/15 s. Data represent six independent vacuolar preps. Statistically significant differences (*, p < 0.05; **, p < 0.01; ns, not significant) were as compared with the wild type in the presence of 2% glucose and determined by two-tailed unpaired t test. B, V-ATPase assembly is comparable in 4% glucose and 2% glucose. Vacuolar membrane vesicles were purified from pfk2Δ and wild-type cells cultured overnight in 2% glucose or 4% glucose. Membrane protein (1 μg total membrane protein/well) was separated by SDS-PAGE in 10% gels. Gels were immunoblotted with primary monoclonal antibodies to V1 subunit A and subunit B, and secondary antibodies conjugated to alkaline phosphate. Protein markers (left) are 77 and 50 kDa. This gel was modified to excise a lane containing pfk1Δ membranes.
Pfk1p Subunit Binding to V-ATPase Decreases in 4% Glucose
The individual PFK-1 subunits expressed in pfk1Δ (subunit Pfk2p) and pfk2Δ (subunit Pfk1p) cells co-immunoprecipitate with V-ATPase and co-purify with vacuolar membrane fractions (24). Thus, deletion of one PFK-1 subunit does not prevent interaction of the other subunit with V-ATPase.
We asked whether binding of the subunit Pfk1p to V-ATPase was affected in 4% glucose in the pfk2Δ mutant. The level of Pfk1p subunit co-immunoprecipitated with the anti-V1 subunit A monoclonal antibodies that recognize V1 and V1Vo complexes (21) was analyzed. About 60% fewer Pfk1p co-immunoprecipitated with V1 subunits in pfk2Δ cells in 4% glucose (Fig. 6). This decrease in Pfk1p-V-ATPase binding was not caused by change in expression of Pfk1p and/or V-ATPase subunits because these protein expression levels were comparable in whole cell lysates from 2% and 4% glucose (Fig. 6, Input). Rather, Pfk1p-V-ATPase binding was decreased in 4% glucose in pfk2Δ, which was suggestive of lower Pfk1p-V-ATPase binding affinity in this mutant.
FIGURE 6.
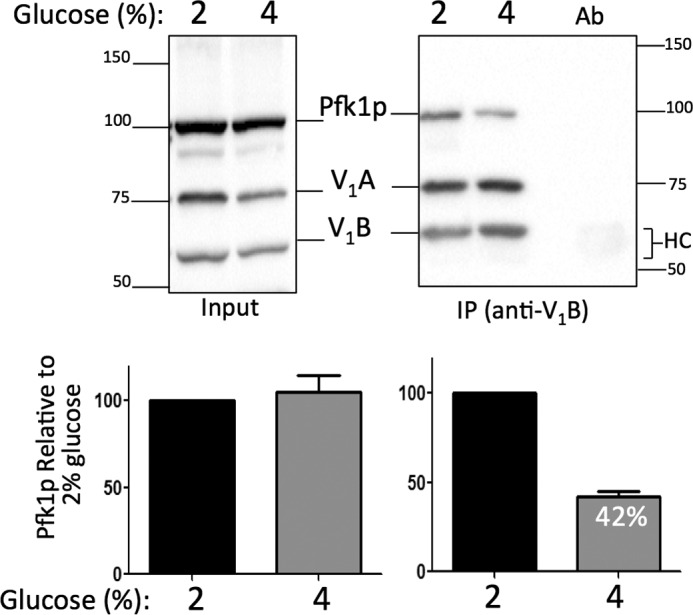
Pfk1p subunit binding to V-ATPase decreases in 4% glucose. Overnight mid-log phase cultures (optical density of 0.8–1.0 A600/ml) were lysed, and V-ATPase complexes were immunoprecipitated with anti-A monoclonal antibody. Immunoprecipitated protein (IP) and total lysate protein (Input) were loaded on 10% SDS-PAGE gels. Pfk1p and V-ATPase (V1 subunits A and B) were detected by immunoblots using, respectively, anti-PFK-1 polyclonal antibodies and anti-B and anti-A monoclonal antibodies and horseradish peroxidase secondary antibodies. Ab, antibody alone; HC, antibody heavy chain. Protein markers are 150, 100, 75, and 50 kDa. A representative gel is shown (top panel). Gels from two independent experiments were scanned using a Bio-Rad ChemiDoc XRS+, and data were analyzed using Multi Gauge V3.0 and GraphPad Prism 5 software. Data were expressed as –fold increase Pfk1p:V1 subunit ratio ± S.D. relative to the wild type (bottom panel).
Discussion
This study capitalized on the cellular mechanisms suppressing V-ATPase function in pfk2Δ to gain new knowledge of the mechanisms underlying glucose-dependent V-ATPase regulation. We took advantage of the fact that V-ATPase is fully assembled in pfk2Δ cells at steady state and glycolysis partially suppressed to manipulate the glycolysis flow and assess its direct involvement on V-ATPase function.
At steady state, the ratio of proton transport to ATP hydrolysis increases in response to high glucose levels in pfk2Δ. Enhanced V-ATPase proton transport restores vacuolar pH homeostasis. It likely allows cells to preserve energy when glycolysis is suboptimal and glucose abundant (4% glucose). One importance of these findings is that they revealed V-ATPase elasticity of coupling as a new mechanism of how glucose regulates V-ATPase pumps without changing the V1Vo assembly state. This study also showed that, under V1Vo reassembly conditions, the level of glucose-induced reassembly directly corresponds to the glycolysis flow in pfk2Δ cells. V1Vo reassembly is complete after the rate of glycolysis reaches a threshold (40–46% of the wild-type rate in 2% glucose) when metabolism resumes after glucose is readded to glucose-deprived cells.
The Glycolysis Flow Communicates with V-ATPase and Regulates Its Activity at Steady State
At steady state, in 2% glucose, the ethanol concentration is significantly reduced (by 42%) in pfk2Δ cells. This level of glycolytic reduction mimics the level of PFK-1 activity reduction (42%) (Fig. 1A), as expected, because PFK-1 catalyzes the second limiting step reaction of the pathway.
V-ATPase proton transport is suppressed in 2% glucose in pfk2Δ. Consequently, the vacuolar lumen is alkalinized (Fig. 2A). This alteration in V-ATPase function is not due to V1Vo disassembly or catalytic defects because pfk2Δ cells cultured in 2% glucose display wild-type levels of V1Vo complexes at the membrane and express catalytically competent wild-type V-ATPase pumps (24). Rather, vacuolar V-ATPase function is inhibited in vivo in pfk2Δ cells cultured in 2% glucose. This study indicates that V-ATPase activity is suppressed in pfk2Δ because glycolysis is suppressed. Regulation of V-ATPase activity at steady state by the glycolysis flow has not been reported before.
V-ATPase activity is remarkably sensitive to changes in the glycolysis flow. Addition of 4% glucose restores 83% of wild-type ethanol concentration at steady state (Fig. 1B). This significant stimulation of glycolysis also restores the vacuolar acidic pH (Fig. 2A). This result is indicative of a causal correlation between the glycolysis flow and V-ATPase activity. It is evidence that glucose regulates V-ATPase activity at steady state. Accordingly, the PFK-1 subunit deletion mutant pfk1Δ, which has milder glycolysis defects than pfk2Δ (27), has milder V-ATPase alterations (24). Thus, V-ATPase proton transport is adjusted in response to the glycolytic flow at steady state.
V-ATPase Adjusts Catalytic Activity in Response to Glucose When Glycolysis Is Suboptimal
V-ATPase catalytic coupling is tighter in pfk2Δ cells in 4% glucose (Fig. 5A) when glycolysis operates below capacity at steady state. The proton transport/ATPase ratio increases by 24%, indicating that V-ATPase is more efficient (transports more protons per ATP hydrolyzed), probably to help cells to preserve energy. Importantly, these findings indicate that ATP hydrolysis and proton transport by V-ATPase pumps may not always be optimally coupled in vivo.
Using pfk2Δ cells, this study showed that glucose can control V-ATPase activity without changing the V-ATPase assembly state. Until now, glucose-dependent regulation of yeast V-ATPase has been via V1Vo disassembly and reassembly in response to glucose depletion and readdition, respectively (3). Although the ability of V-ATPase to change coupling of ATP hydrolysis and proton pumping has been described before (44), the involvement of glucose and glycolysis has not been reported. Increased efficiency of coupling was observed in some genetic mutants (45) and when V-ATPase pumps were exposed to low ATP concentrations in vitro (46). In yeast, the N-end domain of Vo subunit a (47, 48) and a non-homologous region of the V1 subunit A (45) have been implicated in this type of regulation.
Changes in the proton transport to ATPase activity ratio may be linked to “loose” conformations required for disassembly and reassembly because, during catalysis, relative rotation of V1 and Vo subunits requires stable V1Vo assembly. Accordingly, catalytic slip does not occur in the molecular motors that do not reversibly disassemble, such as the F1Fo ATP synthase. One possibility is that, in 4% glucose, V1Vo intermediary structures that slip during catalysis are stabilized in pfk2Δ to restore the membrane pH gradients necessary for an array of other transporters when glucose concentration is high (20).
Complete V1Vo Reassembly after Glucose Readdition Requires the Glycolysis Flow to Reach a Threshold
Glucose depletion, which inhibits glycolysis, leads to V1 dissociation from Vo, and its readdition, which resumes glycolysis, causes V1 to reassociate with Vo (3). Thus, yeast V-ATPase assembly is intimately linked to the glycolysis flow.
We reasoned that if a metabolic input drives V1Vo reassembly, then it would reach a certain threshold within the first minutes after glucose readdition to glucose-deprived cells. Below this threshold, reassembly is defective. We found the threshold to be 40–46% of the wild-type glycolytic capacity. We have chosen to monitor the glycolytic intermediate NADH to establish the metabolic threshold for reassembly after glucose readdition. The NADH formation rate corresponds to the rate of glycolysis. In addition, NADH autofluorescence is measureable in intact cells in real time. The wild-type NADH formation rate in 2% glucose is used as a reference for these studies because 2% is the standard glucose concentration in yeast growth medium.
When 2% glucose is readded, the NADH formation rate is only 31% of the wild-type rate. Under these conditions, the pfk2Δ cells cannot sufficiently reassemble V1Vo. Addition of 4% glucose, which stimulates the rate of glycolysis to 46% (the proposed NADH threshold), prompts wild-type levels of V1Vo reassembly in pfk2Δ (Fig. 4A). Likewise, V1Vo reassembly is complete when the cells produce ≥40% the wild-type ethanol levels (Fig. 3A). With reassembly, V1Vo proton transport resumes, and the vacuoles are acidified after 1–2 min of 4% glucose readdition to pfk2Δ.
The finding that V1Vo remains silenced in pfk2Δ after readdition of 2% glucose even though 60% of the V-ATPase complexes are reassembled is intriguing. It can be explained if inhibitory MgADP is trapped in the catalytic sites (49) because glycolysis is significantly suppressed. Alternatively, a regulatory subunit such as V1 subunit H, which silences V1 after disassembly (49) and activates V1Vo complexes at the membrane (50), could have retained an inhibitory conformation preventing proton transport in 2% glucose (51, 52).
Although glycolytic ATP formation is an attractive possibility to signal reassembly and/or reactivation in 4% glucose, the levels of ATP during glucose deprivation and readdition do not appear to correlate to the V-ATPase assembly state (21). Wild-type yeast cells recover steady-state ATP concentrations in the absence of glucose (21).
How Does PFK-1 Regulate V-ATPase Activity?
The finding that V-ATPase function is fully rescued in 4% glucose in pfk2Δ cells that lack the PFK2 gene argues against direct regulation of V-ATPase through its physical interaction with the PFK-1 subunit Pfk2p. However, the PFK-1 subunit Pfk1p may play an inhibitory role in pfk2Δ cells. The level of Pfk1p that co-immunoprecipitates with V-ATPase subunits is significantly reduced in pfk2Δ cells in 4% glucose (Fig. 6). This decrease can be explained if Pfk1p is recruited to support greater glycolytic demands in 4% glucose, after which V-ATPase proton transport is enhanced. Whether binding of subunit Pfk1p to V-ATPase is inhibitory is an interesting possibility that will be addressed in future studies.
This study showed that at least one mechanism by which PFK-1 modulates V-ATPase is via the glycolysis flow. The RAS/cAMP/PKA signaling pathway, which has been linked to glucose-dependent V1Vo disassembly and reassembly, is also intertwined with PFK-1. Activation of the RAS/cAMP/PKA pathway enhances the glycolysis flow because PKA stimulates formation of fructose-2,6-bisphosphate, the most potent activator of PFK-1. Thus, RAS/cAMP/PKA could control V-ATPase assembly via glycolysis (16).
We cannot eliminate the possibility that glucose-dependent pH changes could also regulate V-ATPase. Cytosol alkalization is glucose concentration-dependent and correlates to the level of V1Vo assembly (14, 53). A larger ΔpH between the cytosolic and the luminal sides of the vacuolar membrane may stimulate V1Vo activity. Similar to the activating effect ΔpH has on the Fo subunit a of the evolutionary related F-ATP synthase (54), binding of protons to the membrane-bound Vo subunit a of the V-ATPase could prime V1Vo proton transport. In Arabidopsis, the V-ATPase coupling ratios are sensitive to the cytosol and vacuolar pH (55). In the lemon fruit, variable coupling and pH-dependent slip regulate the V-ATPase pump (56).
Clearly, the scope of glucose-dependent V-ATPase regulation is more complex than initially anticipated. It extends beyond V1Vo disassembly and/or reassembly and is intimately linked to the metabolic state of a cell, specifically the glycolysis flow. The finding that V-ATPase changes catalytic efficiency when the cellular demands for membrane transport increase (4% glucose) and glycolytic ATP production operates below capacity can have implications in human health, particularly for distal renal tubular acidosis (25), viral infections (57), and the metabolic switch in cancers (58, 59), where glucose-dependent regulation of V-ATPase is conserved.
Experimental Procedures
Materials and Strains
The Tran[35S]-label was from MP Biomedicals (Santa Ana, CA). The antibodies anti-Myc, anti-phosphoglycerate kinase 1 (22C5D8), and anti-V-ATPase (10D7 and 13D11) were from Invitrogen. Alkaline phosphatase-conjugated secondary antibodies were from Promega (Madison, WI) and horseradish peroxidase secondary antibodies from Invitrogen. Zymolase 100T was purchased from Seikagaku (Tokyo, Japan), concanamycin A from Wako Biochemicals (Richmond, VA), and Ficoll and carbonyl cyanide m-chlorophenylhydrazone from United Stated Biologicals (Swampscott, MA). The ATP assay kit (K354-)100 was purchased from Biovision (Milpitas, CA) and the ethanol assay kit (ab65343) from Abcam (Cambridge, MA). All other reagents were from Sigma. The Saccharomyces cerevisiae strains referred to throughout are BY4742 wild-type (MATα; his3Δ1; leu2 Δ; lys2Δ; ura3Δ), BY4742 pfk1 (pfk1Δ:KanMX6), BY4742 pfk2Δ (pfk2Δ:KanMX6), and BY4742 vma3Δ (vm3Δ:KanMX6). The mutant strains pfk2Δ and pfk1Δ were verified by PCR using primers to amplify the PFK2 and PFK1 genes with 5′-XhoI and 3′-XmaI cutting sites.
Growth Phenotype
Cells were grown overnight to 0.6–1.0 A600/ml in YEPD medium buffered to pH 5.0 with 50 mm succinic acid/50 mm sodium phosphate. Cultures were washed twice with sterile double-distilled H2O and cells with an optical density of 2.5 A600 were resuspended into 1 ml of sterile double-distilled H2O. 10-fold serial dilutions were stamped onto YEP containing 2% glucose and 4% glucose. Plates were buffered to pH 5.0 with 50 mm succinic acid/50 mm sodium phosphate (pH 7.5) with 50 mm MES/50 mm MOPS or pH 7.5 with 100 mm calcium chloride added. The plates were incubated for 72 h at 30 °C and 37 °C.
Vacuolar pH
Vacuolar pH was measured using the ratiometric fluorescent dye BCECF-AM (31, 60). Cells were grown to an optical density of 0.6–1.0 A600/ml and stained with 50 μm BCECF-AM for 30 min at 30 °C. Calibration curves were generated as described before (20) in calibration buffers (pH 5.5, 5.75, 6.0, 6.25, 6.5, and 7) consisting of 50 mm MES, 50 mm HEPES, 50 mm KCl, 50 mm NaCl, 200 mm ammonium acetate, 10 mm sodium azide, 10 mm 2-deoxyglucose, and 50 μm carbonyl cyanide m-chlorophenylhydrazone. For steady-state analyses of the vacuolar pH, the BCECF-stained cells were transferred to 1 mm HEPE/MES (pH 5.0) buffer with 2% or 4% glucose (final concentration), and BCECF fluorescence (ratio of 490/450 nm, 525 nm excitation) was measured for 10 min at 30 °C in a FluoroMax 4 spectrofluorometer (Horiba Jobin Yvon Inc.). Data collected between 3 and 8 min were averaged. For V-ATPase disassembly/reassembly conditions, BCECF-stained cells were deprived of glucose on ice for 10 min, and the vacuolar pH was monitored 20 min after 2% or 4% glucose readdition (final concentration).
Immunoprecipitations
Pulse-chase experiments were conducted at the indicated glucose concentrations and times following protocols described before (21). The V-ATPase complexes were immunoprecipitated from whole cell lysates with the monoclonal antibodies 13D11 (anti-V1 subunit B) and 10D7 (anti-Vo subunit a, Vph1p isoform) and the protein separated by SDS-PAGE (13% acrylamide gels). The gels were dried, scanned in a Fuji scanner (FLA-5100), and analyzed using Multi Gauge and GraphPad Prism 5 software as described previously (24). The proportion of Vo assembled into V1Vo, determined by comparing the amount of Vo subunit a immunoprecipitated with 13D11 with the total amount of Vo subunit a immunoprecipitated with both antibodies. For non-radiolabeled immunoprecipitations, the 13D11 antibody was used to immunoprecipitate V-ATPase from whole cell lysates (61). Protein was separated by SDS-PAGE in 10% gels and analyzed by Western blotting using the monoclonal antibodies 8B1 (anti-V1 subunit A) and 13D77 (anti-V1 subunit B) and yeast PFK-1 polyclonal antibodies. The nitrocellulose membranes were blotted with horseradish peroxidase secondary antibody and scanned using a Bio-Rad ChemiDoc XRS+, and then the intensity of protein bands was quantified using Multi Gauge and GraphPad Prism 5 software.
NADH Autofluorescence
NADH was monitored as described by Poulsen et al. (42) with the following modifications. The cells were grown overnight to an optical density of 0.6–1.0 A600/ml in YEPD, harvested, and cells with an optical density of 100 A600 were resuspended in 50 mm potassium phosphate buffer (pH 6.8) up to a density of 10% by weight. The cells were starved of glucose by incubation in the same phosphate buffer for 3 h on a rotary shaker at 30 °C and placed on ice for 10 min. The NADH fluorescence intensity (excitation at 366 nm, emission at 450 nm) was monitored without glucose for 60 s and then continuously recorded for an additional 90 s with readdition of 2% glucose or 4% glucose at 30 °C in a FluoroMax 4 spectrofluorometer (Horiba Jobin Yvon Inc.).
Ethanol Concentration
For steady-state analyses, the cells were cultured overnight to an optical density of 0.6–1.0 A600/ml in YEP (pH 5.0) medium (wild-type and pfk2Δ) containing 2% or 4% final glucose concentration. Cells with a total of 2.0 optical density A600/ml per condition were harvested and then converted to spheroplasts by zymolase treatment (35). The spheroplasts were incubated at 30 °C for 10 min in YEP adjusted to pH 5.0 with 50 mm succinic acid/50 mm sodium phosphate containing 2% or 4% glucose plus 1.2 m sorbitol. The ethanol concentration was measured using an ethanol assay kit (ab65343, Abcam) according to the instructions of the manufacturer. For glucose-depletion and readdition analyses, the cells were grown in medium containing 2% glucose overnight and converted to spheroplasts as described above, and then the spheroplasts were incubated in YEP medium with 2% or 4% glucose for 20 min, in YEP for 10 min, and in YEP for 10 min, followed by addition of 2% glucose, 4% glucose, or 2% 2-deoxyglucose for 10 min.
PFK-1 Enzymatic Activity
Wild-type and pfk2Δ cells were cultured overnight to an optical density of 0.6–1.0 A600/ml in YEPD (pH 5.0) medium. Cells were converted to spheroplasts and lysed in 15 mm MES-Tris (pH 6.9) containing 5% glycerol at a final concentration of 1.0 optical density A600/μl. PFK-1 activity was measured spectrophotometrically at 37 °C using the coupled enzyme assay of Lotscher et al. (62). Whole cell lysates (20.0 optical density A600) was added to the enzymatic assay mixture (25 mm Tris (pH 6.9), 2 mm ATP, 5 mm MgCl2, 2 mm phosphoenol pyruvate, 30 units/ml pyruvate kinase, 30 units/ml l-lactic dehydrogenase, and 0.5 mm NADH), and the reaction was started by addition of 5 mm fructose-6-phospate. NADH oxidation was monitored spectrophotometrically at 340 nm for 5 min. One unit of PFK-1 activity is defined as 1 μmol fructose 1,6-bisphosphate formed/min in an optical density of 20 A600.
Other Methods
Vacuolar membranes fractions were purified by Ficoll density gradient centrifugation as described before (35, 48, 61). Protein concentration was measured by the Bradford assay (63). ATP hydrolysis was measured by monitoring NADH oxidation spectrophotometrically (62) using 5 μg of total vacuolar membrane protein in the presence and absence of 100 nm concanamycin-A. Proton transport was measured by monitoring 9-amino-6-chloro-2-methoxyacridin quenching after addition of MgATP (48).
Author Contributions
K. J. P. conceived and coordinated the study, analyzed the results, and wrote the paper. C. Y. C. designed, performed, and analyzed the experiments. D. D. performed the PFK1 activity measurements.
Acknowledgments
We thank Dr. Jürgen Heinisch (Osnabrück) for generously providing the PFK-1 polyclonal antibodies. We thank Dr. Wayne Frasch for helpful discussions.
This work was supported by National Institutes of Health Grant R01GM086495 (to K. J. P.) and American Heart Association Grant 14PRE19020015 (to C. Y. C.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- V-ATPase
- vacuolar proton-translocating ATPase
- PFK-1
- 6-phosphofructo-1-kinase
- BCECF-AM
- 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein acetoxymethyl ester
- YEP
- yeast extract, peptone medium
- YEPD
- yeast extract, peptone, 2% dextrose medium.
References
- 1. Forgac M. (2007) Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat. Rev. Mol. Cell Biol. 8, 917–929 [DOI] [PubMed] [Google Scholar]
- 2. Kane P. M. (2006) The where, when, and how of organelle acidification by the yeast vacuolar H+-ATPase. Microbiol. Mol. Biol. Rev. 70, 177–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parra K. J., Chan C. Y., and Chen J. (2014) Saccharomyces cerevisiae vacuolar H+-ATPase regulation by disassembly and reassembly: one structure and multiple signals. Eukaryot. Cell 13, 706–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marshansky V., Rubinstein J. L., and Grüber G. (2014) Eukaryotic V-ATPase: novel structural findings and functional insights. Biochim. Biophys. Acta 1837, 857–879 [DOI] [PubMed] [Google Scholar]
- 5. Cotter K., Stransky L., McGuire C., and Forgac M. (2015) Recent insights into the structure, regulation, and function of the V-ATPases. Trends Biochem. Sci. 40, 611–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Breton S., and Brown D. (2013) Regulation of luminal acidification by the V-ATPase. Physiology 28, 318–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hinton A., Bond S., and Forgac M. (2009) V-ATPase functions in normal and disease processes. Pflugers Arch. 457, 589–598 [DOI] [PubMed] [Google Scholar]
- 8. Muench S. P., Trinick J., and Harrison M. A. (2011) Structural divergence of the rotary ATPases. Q. Rev. Biophys. 44, 311–356 [DOI] [PubMed] [Google Scholar]
- 9. Zhao J., Benlekbir S., and Rubinstein J. L. (2015) Electron cryomicroscopy observation of rotational states in a eukaryotic V-ATPase. Nature 521, 241–245 [DOI] [PubMed] [Google Scholar]
- 10. Kane P. M. (1995) Disassembly and reassembly of the yeast vacuolar H+-ATPase in vivo. J. Biol. Chem. 270, 17025–17032 [PubMed] [Google Scholar]
- 11. Sumner J. P., Dow J. A., Earley F. G., Klein U., Jäger D., and Wieczorek H. (1995) Regulation of plasma membrane V-ATPase activity by dissociation of peripheral subunits. J. Biol. Chem. 270, 5649–5653 [DOI] [PubMed] [Google Scholar]
- 12. Liberman R., Bond S., Shainheit M. G., Stadecker M. J., and Forgac M. (2014) Regulated assembly of vacuolar ATPase is increased during cluster disruption-induced maturation of dendritic cells through a phosphatidylinositol 3-kinase/mTOR-dependent pathway. J. Biol. Chem. 289, 1355–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Trombetta E. S., Ebersold M., Garrett W., Pypaert M., and Mellman I. (2003) Activation of lysosomal function during dendritic cell maturation. Science 299, 1400–1403 [DOI] [PubMed] [Google Scholar]
- 14. Dechant R., Binda M., Lee S. S., Pelet S., Winderickx J., and Peter M. (2010) Cytosolic pH is a second messenger for glucose and regulates the PKA pathway through V-ATPase. EMBO J. 29, 2515–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dechant R., Saad S., Ibáñez A. J., and Peter M. (2014) Cytosolic pH regulates cell growth through distinct GTPases, Arf1 and Gtr1, to promote Ras/PKA and TORC1 activity. Mol. Cell 55, 409–421 [DOI] [PubMed] [Google Scholar]
- 16. Bond S., and Forgac M. (2008) The Ras/cAMP/protein kinase A pathway regulates glucose-dependent assembly of the vacuolar (H+)-ATPase in yeast. J. Biol. Chem. 283, 36513–36521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oot R. A., Huang L. S., Berry E. A., and Wilkens S. (2012) Crystal structure of the yeast vacuolar ATPase heterotrimeric EGC(head) peripheral stalk complex. Structure 20, 1881–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tabke K., Albertmelcher A., Vitavska O., Huss M., Schmitz H. P., and Wieczorek H. (2014) Reversible disassembly of the yeast V-ATPase revisited under in vivo conditions. Biochem. J. 462, 185–197 [DOI] [PubMed] [Google Scholar]
- 19. Kane P. M. (2016) Proton transport and pH control in fungi. Adv. Exp. Med. Biol. 892, 33–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martínez-Muñoz G. A., and Kane P. (2008) Vacuolar and plasma membrane proton pumps collaborate to achieve cytosolic pH homeostasis in yeast. J. Biol. Chem. 283, 20309–20319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parra K. J., and Kane P. M. (1998) Reversible association between the V1 and V0 domains of yeast vacuolar H+-ATPase is an unconventional glucose-induced effect. Mol. Cell. Biol. 18, 7064–7074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu M., Ammar D., Ives H., Albrecht F., and Gluck S. L. (2007) Physical interaction between aldolase and vacuolar H+-ATPase is essential for the assembly and activity of the proton pump. J. Biol. Chem. 282, 24495–24503 [DOI] [PubMed] [Google Scholar]
- 23. Lu M., Sautin Y. Y., Holliday L. S., and Gluck S. L. (2004) The glycolytic enzyme aldolase mediates assembly, expression, and activity of vacuolar H+-ATPase. J. Biol. Chem. 279, 8732–8739 [DOI] [PubMed] [Google Scholar]
- 24. Chan C. Y., and Parra K. J. (2014) Yeast phosphofructokinase-1 subunit Pfk2p is necessary for pH homeostasis and glucose-dependent vacuolar ATPase reassembly. J. Biol. Chem. 289, 19448–19457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Su Y., Blake-Palmer K. G., Sorrell S., Javid B., Bowers K., Zhou A., Chang S. H., Qamar S., and Karet F. E. (2008) Human H+ATPase a4 subunit mutations causing renal tubular acidosis reveal a role for interaction with phosphofructokinase-1. Am. J. Physiol. Renal Physiol. 295, F950–F958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Banaszak K., Mechin I., Obmolova G., Oldham M., Chang S. H., Ruiz T., Radermacher M., Kopperschläger G., and Rypniewski W. (2011) The crystal structures of eukaryotic phosphofructokinases from baker's yeast and rabbit skeletal muscle. J. Mol. Biol. 407, 284–297 [DOI] [PubMed] [Google Scholar]
- 27. Heinisch J. (1986) Construction and physiological characterization of mutants disrupted in the phosphofructokinase genes of Saccharomyces cerevisiae. Curr. Genet. 11, 227–234 [DOI] [PubMed] [Google Scholar]
- 28. Arvanitidis A., and Heinisch J. J. (1994) Studies on the function of yeast phosphofructokinase subunits by in vitro mutagenesis. J. Biol. Chem. 269, 8911–8918 [PubMed] [Google Scholar]
- 29. Heinisch J. (1986) Isolation and characterization of the two structural genes coding for phosphofructokinase in yeast. Mol. Gen. Genet. 202, 75–82 [DOI] [PubMed] [Google Scholar]
- 30. Schrøder T. D., Özalp V. C., Lunding A., Jernshøj K. D., and Olsen L. F. (2013) An experimental study of the regulation of glycolytic oscillations in yeast. FEBS J. 280, 6033–6044 [DOI] [PubMed] [Google Scholar]
- 31. Brett C. L., Tukaye D. N., Mukherjee S., and Rao R. (2005) The yeast endosomal Na+K+/H+ exchanger Nhx1 regulates cellular pH to control vesicle trafficking. Mol. Biol. Cell 16, 1396–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ohya Y., Umemoto N., Tanida I., Ohta A., Iida H., and Anraku Y. (1991) Calcium-sensitive cls mutants of Saccharomyces cerevisiae showing a Pet- phenotype are ascribable to defects of vacuolar membrane H+-ATPase activity. J. Biol. Chem. 266, 13971–13977 [PubMed] [Google Scholar]
- 33. Kane P. M. (2007) The long physiological reach of the yeast vacuolar H+-ATPase. J. Bioenerg. Biomembr. 39, 415–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu J., and Kane P. M. (1996) Mutational analysis of the catalytic subunit of the yeast vacuolar proton-translocating ATPase. Biochemistry 35, 10938–10948 [DOI] [PubMed] [Google Scholar]
- 35. Owegi M. A., Carenbauer A. L., Wick N. M., Brown J. F., Terhune K. L., Bilbo S. A., Weaver R. S., Shircliff R., Newcomb N., and Parra-Belky K. J. (2005) Mutational analysis of the stator subunit E of the yeast V-ATPase. J. Biol. Chem. 280, 18393–18402 [DOI] [PubMed] [Google Scholar]
- 36. Curtis K. K., Francis S. A., Oluwatosin Y., and Kane P. M. (2002) Mutational analysis of the subunit C (Vma5p) of the yeast vacuolar H+-ATPase. J. Biol. Chem. 277, 8979–8988 [DOI] [PubMed] [Google Scholar]
- 37. Smardon A. M., and Kane P. M. (2014) Loss of vacuolar H+-ATPase activity in organelles signals ubiquitination and endocytosis of the yeast plasma membrane proton pump Pma1p. J. Biol. Chem. 289, 32316–32326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smets B., Ghillebert R., De Snijder P., Binda M., Swinnen E., De Virgilio C., and Winderickx J. (2010) Life in the midst of scarcity: adaptations to nutrient availability in Saccharomyces cerevisiae. Curr. Genet. 56, 1–32 [DOI] [PubMed] [Google Scholar]
- 39. Kresnowati M. T., van Winden W. A., Almering M. J., ten Pierick A., Ras C., Knijnenburg T. A., Daran-Lapujade P., Pronk J. T., Heijnen J. J., and Daran J. M. (2006) When transcriptome meets metabolome: fast cellular responses of yeast to sudden relief of glucose limitation. Mol. Syst. Biol. 2, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lenzen S. (2014) A fresh view of glycolysis and glucokinase regulation: history and current status. J. Biol. Chem. 289, 12189–12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ibáñez A. J., Fagerer S. R., Schmidt A. M., Urban P. L., Jefimovs K., Geiger P., Dechant R., Heinemann M., and Zenobi R. (2013) Mass spectrometry-based metabolomics of single yeast cells. Proc. Natl. Acad. Sci. U.S.A. 110, 8790–8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Poulsen A. K., Andersen A. Z., Brasen J. C., Scharff-Poulsen A. M., and Olsen L. F. (2008) Probing glycolytic and membrane potential oscillations in Saccharomyces cerevisiae. Biochemistry 47, 7477–7484 [DOI] [PubMed] [Google Scholar]
- 43. Doherty R. D., and Kane P. M. (1993) Partial assembly of the yeast vacuolar H+-ATPase in mutants lacking one subunit of the enzyme. J. Biol. Chem. 268, 16845–16851 [PubMed] [Google Scholar]
- 44. Nelson N. (2003) A journey from mammals to yeast with vacuolar H+-ATPase (V-ATPase). J. Bioenerg. Biomembr. 35, 281–289 [DOI] [PubMed] [Google Scholar]
- 45. Shao E., Nishi T., Kawasaki-Nishi S., and Forgac M. (2003) Mutational analysis of the non-homologous region of subunit A of the yeast V-ATPase. J. Biol. Chem. 278, 12985–12991 [DOI] [PubMed] [Google Scholar]
- 46. Arai H., Pink S., and Forgac M. (1989) Interaction of anions and ATP with the coated vesicle proton pump. Biochemistry 28, 3075–3082 [DOI] [PubMed] [Google Scholar]
- 47. Kawasaki-Nishi S., Bowers K., Nishi T., Forgac M., and Stevens T. H. (2001) The amino-terminal domain of the vacuolar proton-translocating ATPase a subunit controls targeting and in vivo dissociation, and the carboxyl-terminal domain affects coupling of proton transport and ATP hydrolysis. J. Biol. Chem. 276, 47411–47420 [DOI] [PubMed] [Google Scholar]
- 48. Chan C. Y., Prudom C., Raines S. M., Charkhzarrin S., Melman S. D., De Haro L. P., Allen C., Lee S. A., Sklar L. A., and Parra K. J. (2012) Inhibitors of V-ATPase proton transport reveal uncoupling functions of tether linking cytosolic and membrane domains of V0 subunit a (Vph1p). J. Biol. Chem. 287, 10236–10250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Parra K. J., Keenan K. L., and Kane P. M. (2000) The H subunit (Vma13p) of the yeast V-ATPase inhibits the ATPase activity of cytosolic V1 complexes. J. Biol. Chem. 275, 21761–21767 [DOI] [PubMed] [Google Scholar]
- 50. Ho M. N., Hirata R., Umemoto N., Ohya Y., Takatsuki A., Stevens T. H., and Anraku Y. (1993) VMA13 encodes a 54-kDa vacuolar H(+)-ATPase subunit required for activity but not assembly of the enzyme complex in Saccharomyces cerevisiae. J. Biol. Chem. 268, 18286–18292 [PubMed] [Google Scholar]
- 51. Diab H., Ohira M., Liu M., Cobb E., and Kane P. M. (2009) Subunit interactions and requirements for inhibition of the yeast V1-ATPase. J. Biol. Chem. 284, 13316–13325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jefferies K. C., and Forgac M. (2008) Subunit H of the vacuolar (H+) ATPase inhibits ATP hydrolysis by the free V1 domain by interaction with the rotary subunit F. J. Biol. Chem. 283, 4512–4519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Parra K. J., and Kane P. M. (1996) Wild-type and mutant vacuolar membranes support pH-dependent reassembly of the yeast vacuolar H+-ATPase in vitro. J. Biol. Chem. 271, 19592–19598 [DOI] [PubMed] [Google Scholar]
- 54. Moore K. J., and Fillingame R. H. (2008) Structural interactions between transmembrane helices 4 and 5 of subunit a and the subunit c ring of Escherichia coli ATP synthase. J. Biol. Chem. 283, 31726–31735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rienmüller F., Dreyer I., Schönknecht G., Schulz A., Schumacher K., Nagy R., Martinoia E., Marten I., and Hedrich R. (2012) Luminal and cytosolic pH feedback on proton pump activity and ATP affinity of V-type ATPase from Arabidopsis. J. Biol. Chem. 287, 8986–8993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Müller M. L., Jensen M., and Taiz L. (1999) The vacuolar H+-ATPase of lemon fruits is regulated by variable H+/ATP coupling and slip. J. Biol. Chem. 274, 10706–10716 [DOI] [PubMed] [Google Scholar]
- 57. Kohio H. P., and Adamson A. L. (2013) Glycolytic control of vacuolar-type ATPase activity: a mechanism to regulate influenza viral infection. Virology 444, 301–309 [DOI] [PubMed] [Google Scholar]
- 58. Fogarty F. M., O'Keeffe J., Zhadanov A., Papkovsky D., Ayllon V., and O'Connor R. (2014) HRG-1 enhances cancer cell invasive potential and couples glucose metabolism to cytosolic/extracellular pH gradient regulation by the vacuolar-H ATPase. Oncogene 33, 4653–4663 [DOI] [PubMed] [Google Scholar]
- 59. Sennoune S. R., and Martinez-Zaguilan R. (2012) Vacuolar H(+)-ATPase signaling pathway in cancer. Curr. Protein Pept. Sci. 13, 152–163 [DOI] [PubMed] [Google Scholar]
- 60. Ali R., Brett C. L., Mukherjee S., and Rao R. (2004) Inhibition of sodium/proton exchange by a Rab-GTPase-activating protein regulates endosomal traffic in yeast. J. Biol. Chem. 279, 4498–4506 [DOI] [PubMed] [Google Scholar]
- 61. Ediger B., Melman S. D., Pappas D. L. Jr., Finch M., Applen J., and Parra K. J. (2009) The tether connecting cytosolic (N terminus) and membrane (C terminus) domains of yeast V-ATPase subunit a (Vph1) is required for assembly of V0 subunit d. J. Biol. Chem. 284, 19522–19532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lötscher H. R., deJong C., and Capaldi R. A. (1984) Modification of the F0 portion of the H+-translocating adenosinetriphosphatase complex of Escherichia coli by the water-soluble carbodiimide 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide and effect on the proton channeling function. Biochemistry 23, 4128–4134 [DOI] [PubMed] [Google Scholar]
- 63. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]


