Abstract
The study of the mechanisms leading to cardiac hypertrophy is essential to better understand cardiac development and regeneration. Pathological conditions such as ischemia or pressure overload can induce a release of extracellular nucleotides within the heart. We recently investigated the potential role of nucleotide P2Y receptors in cardiac development. We showed that adult P2Y4-null mice displayed microcardia resulting from defective cardiac angiogenesis. Here we show that loss of another P2Y subtype called P2Y6, a UDP receptor, was associated with a macrocardia phenotype and amplified pathological cardiac hypertrophy. Cardiomyocyte proliferation and size were increased in vivo in hearts of P2Y6-null neonates, resulting in enhanced postnatal heart growth. We then observed that loss of P2Y6 receptor enhanced pathological cardiac hypertrophy induced after isoproterenol injection. We identified an inhibitory effect of UDP on in vitro isoproterenol-induced cardiomyocyte hyperplasia and hypertrophy. The present study identifies mouse P2Y6 receptor as a regulator of cardiac development and cardiomyocyte function. P2Y6 receptor could constitute a therapeutic target to regulate cardiac hypertrophy.
Keywords: cardiac hypertrophy, cardiomyocyte, G protein-coupled receptor (GPCR), heart development, nucleotide, P2Y6 receptor, UDP, isoproterenol
Introduction
Cardiovascular pathologies are the main cause of mortality in developed countries. Heart failure is characterized by cardiac hypertrophy and fibrosis. Pathological cardiac hypertrophy can be caused inter alia by hypertension, myocardial infarct, or diabetes and is associated with cardiac cell death, cardiomyocyte hypertrophy, and increased proliferation of cardiac fibroblasts.
The role of P2Y nucleotide receptors in the heart has not been extensively studied. Cardioprotective effect of UTP has been previously described: treatment with UTP prior to myocardial infarction leads to reduced ischemic damage through P2Y2 receptor activation (1, 2). Another UTP receptor, P2Y4, plays an important role in the heart: P2Y4 knock-out (KO) mice display reduced cardiac angiogenesis and cardiac postnatal development (3), as well as lower exercise capacity and reduced exercise-induced cardiac hypertrophy (4). More recently, it has been shown that P2Y4 KO mice display lower infarct size and reduced cardiac inflammation and fibrosis in a model of ligation of the left anterior descending artery (5). The study of the role of P2Y6 UDP receptor in the heart has also been initiated. UDP can have an inotropic effect on cardiomyocytes, mediated by P2Y6 receptor (6). Nishida et al. (7) showed that inhibition of P2Y6 receptor using the specific antagonist MRS2578 decreased collagen deposition after transverse aortic constriction, without affecting cardiac hypertrophy induced in this model. Also, P2Y6 receptor seems to have a deleterious role in atherosclerosis, being enriched in the sclerotic lesions and favoring inflammation (8, 9). These various effects of nucleotides are depending on cell-specific expression of P2Y subtypes involved: mouse P2Y4 receptor is not expressed in cardiomyocytes and is mainly expressed in cardiac endothelial cells, whereas mouse P2Y2 and P2Y6 receptors were found in all tested cardiac cells (2, 3, 6, 7).
We decided here to investigate P2Y6 involvement in cardiac hypertrophy by analyzing postnatal heart development of P2Y6-null mice and by using P2Y6-null mice in a pathological cardiac hypertrophy-induced model. Cardiac hypertrophy is a hallmark of cardiac remodeling. There are two types of cardiac hypertrophy: physiological and pathological hypertrophy. Physiological hypertrophy arises during embryonic and postnatal development, pregnancy, or chronic physical exercise. This hypertrophy is reversible and characterized by normal or increased cardiac function, associated with normal sarcomeric organization and gene expression (10). Pathological hypertrophy is associated with re-expression of fetal genes considered as hypertrophy markers: Acta1 (skeletal muscle actin α1), Nppa (atrial natriuretic peptide), and Myh7 (myosin heavy chain β), and develops in response to pressure overload, myocardial infarct, or diabetes and as a consequence of genetic mutations (11), and is linked to cell death leading to fibrosis. Fibrosis decreases cardiac contractility and capillary density, which decreases oxygen availability and leads to cardiac ischemia and heart failure. This kind of hypertrophy is irreversible.
The presence of the P2Y6 receptor in mouse cardiomyocytes and its involvement in cardiac fibrosis (6, 7) led us to study its potential role in cardiac development. We analyzed the role of this UDP receptor in physiological and pathological cardiac hypertrophy using P2Y6-null mice. We used the model of intraperitoneal injection of isoproterenol (ISO),5 a non-selective agonist of β-adrenergic receptors, which is a model frequently used to induce pathological cardiac hypertrophy (12–14).
Experimental Procedures
Ethics Statement
C57Bl6/J P2Y6 knock-out (P2Y6 KO) male mice were obtained as previously described (15). Animal works were assessed according to the national and international guidelines and approved by ethic committee CEBEA (Ethic Committee of Animal Well-Being).
Cardiac Echography Experiments
Cardiac echography experiments were assessed as previously described (5). Briefly, transthoracic echography was performed on anesthetized mice (1% isoflurane), using a Vevo 2100 system equipped with a 40 MHz transducer (Visualsonics, Toronto, Canada). Cardiac parameters were measured in B mode long axis, M mode, or Doppler mode.
Immunohistochemistry Experiments
5-Day-old wild type (WT) and P2Y6 KO mice were injected with 1 mm EdU overnight to evaluate proliferation of cardiomyocytes in neonate mice. Then neonates were decapitated and hearts were harvested, rinsed, and frozen in Tissue-Tek® OCTTM (Sakura Finetek, Alphen aan den Rijn, The Netherlands). Hearts were sliced to 5 μm thickness with cryostat Leica 3050S. Then EdU was revealed with the Click-iT® EdU Imaging Kit (Invitrogen, Life Technologies) and cardiomyocytes were stained with troponin T antibody (Sigma) and nuclei with Hoechst (Thermo Scientific, Waltham, MA). Images were taken randomly with a Zeiss Axioimager Z1 and analyzed with ImageJ software. Data were obtained by counting 5 images per heart.
For histological analysis, hearts from adult mice were harvested and frozen in Tissue-Tek® OCTTM, cut at 10 μm thickness and stained with hematoxylin-eosin or Masson's trichrome for collagen staining. Cross-sectional areas and fibrosis quantification were evaluated with ImageJ software.
Flow Cytometry Analysis
Hearts were harvested from 5-day-old WT and P2Y6 KO mice and total ventricular cells were extracted. These cells were stained with Ki67 (BD Biosciences) and troponin T (antibodies-online, Atlanta, GA) antibodies. Number of double-stained cells was analyzed with BD LSRFortessa cell analyzer (BD Biosciences).
Isoproterenol Injection in Vivo
WT and P2Y6 KO mice aged between 8 and 12 weeks were intraperitoneally injected once with 50 mg/kg of ISO (Sigma) or saline solution 2 min before echographic measurements (acute injection), or with 50 mg/kg/day of ISO or saline solution, daily during 7 days. Then, mice were sacrificed, weighted, tibias were measured, and hearts were harvested, rinsed, and weighted. Ventricles were then stored in TRIzol® reagent (Life Technologies) for RNA extraction or in Tissue-Tek® OCTTM for histological examination.
RNA Sequencing Analysis
Indexed cDNA libraries were obtained using the TruSeq Stranded mRNA sample preparation kit (Illumina) following the manufacturer's recommendation. The multiplexed libraries (9.5 pm) were loaded on a high throughput HS flow cell. Sequences were produced using a TruSeq PE Cluster kit version 4 and SBS-kit version 4 (250 cycles) on a HiSeq 1500 (Illumina). 29.2 and 26.1 million paired-end reads (2 × 125 bases) corresponding to P2Y6 KO mice and control mice, respectively, were imported into the web-based Galaxy platform. Read quality reports of the sequenced libraries were obtained using the FastQC software. Reads were then trimmed to 90 bp to obtain sequences with a per base quality value >30 and remove the Illumina universal adapter sequences. Reads were mapped against the mouse reference genome (NCBI build 37.2/mm9) using TopHat2 to generate read alignmensts for each sample. The mean coverage obtained was ×45.2 and ×44.9 for the library corresponding, respectively, to P2Y6 KO and control mice. Annotations were obtained from iGenomes (Illumina). Cuffdiff were then used to calculate the level of differential gene expression. RNAseq was assessed on a mixture of RNA from three WT hearts and three P2Y6 KO hearts harvested after 7 days of saline or ISO injection. Genes with counts per million (cpm) ≥1 in KO RNA and with cpm KO/cpm WT ratio ≥2 were selected and analyzed with Enrichnet software, which sorted genes regulated in KO mice in function of KEGG pathways in its database. Regulated genes involved in interesting pathways were then analyzed with STRING software to obtain interaction networks. RNA sequencing data have been deposited and assigned GEO accession number GSE76215.
Quantitative PCR Analysis
RNA was extracted from ventricles in TRIzol® with an RNeasy extraction kit (Qiagen, Venlo, The Netherlands), then reverse transcription was assessed using random hexamers and Superscript® II Reverse Transcriptase (Invitrogen, Life Technologies). Gene expression was then analyzed by quantitative PCR on 5 ng of cDNA and with specific primers and reactions were run on 7500 Fast Real Time PCR system (Applied Biosystems, Life Technologies). CT genes were normalized to housekeeping gene (Rpl32) CT and results were expressed on 2−ΔCT.
cAMP Assays
Hearts were harvested, boiled, and crushed in 2 ml of water. After centrifugation, the supernatants were evaporated with a concentrator centrifuge SpeedVacTM RC1010 (Thermo Scientific). Pellets were then diluted in water, cAMP was acetylated with KOH and anhydride acetic solutions, and cAMP was then measured by radioimmunoassay. cAMP was then normalized with protein content dosed with a Pierce BCA Protein Assay Kit, following the manufacturer's recommendations (Thermo Scientific).
Culture of Neonate Cardiomyocytes
1-Day-old neonates were decapitated and ventricles were harvested and rinsed in PBS. Total cells were obtained after digestion in 2 mg/ml of collagenase B, 1 mg/ml of pancreatin (Sigma), and 50 μg/ml of DNase I (Sigma). Cardiomyocytes were selected by Percoll gradient (GE Healthcare, Little Chalfont, United Kingdom) and plated in wells coated with 0.1% gelatin in medium containing half DMEM, half M199/F-12, 10% horse serum, 5% fetal bovine serum, 2% penicillin/streptomycin, and 1% l-glutamine (Gibco®, Life Technologies).
To induce hypertrophy in vitro, cells were serum deprived during 24 h and treated with 1 μm ISO and/or 100 μm UDP (Sigma) during 48 h in serum-deprived medium. After treatment, cells were fixed with 4% paraformaldehyde (Sigma) and stained with ACTA1 antibody (Proteintech group, Chicago, IL). Secondary antibody was from Jackson ImmunoResearch (Suffolk, United Kingdom).
To analyze cardiomyocyte proliferation in vitro, cells were serum deprived overnight and treated with 100 μm UDP during 48 h in serum containing medium. After treatment, cells were fixed with 4% paraformaldehyde and stained with Alexa F488-conjugated WGA (Molecular Probes, Life Technologies) and eF570-conjugated Ki67 (eBiosciences, San Diego, CA) antibodies. Immunofluorescent images (20 images per well) were taken randomly with Zeiss Axioimager Z1 and analyzed with Axiovision software.
Statistics
Results are expressed as mean ± S.E. Comparison between groups were realized using one-way analysis of variance and/or t tests (Mann-Whitney test for non-parametric data), or two-way analysis of variance test.
Results
Identification of a Physiological Cardiac Hypertrophy in P2Y6-null Mice
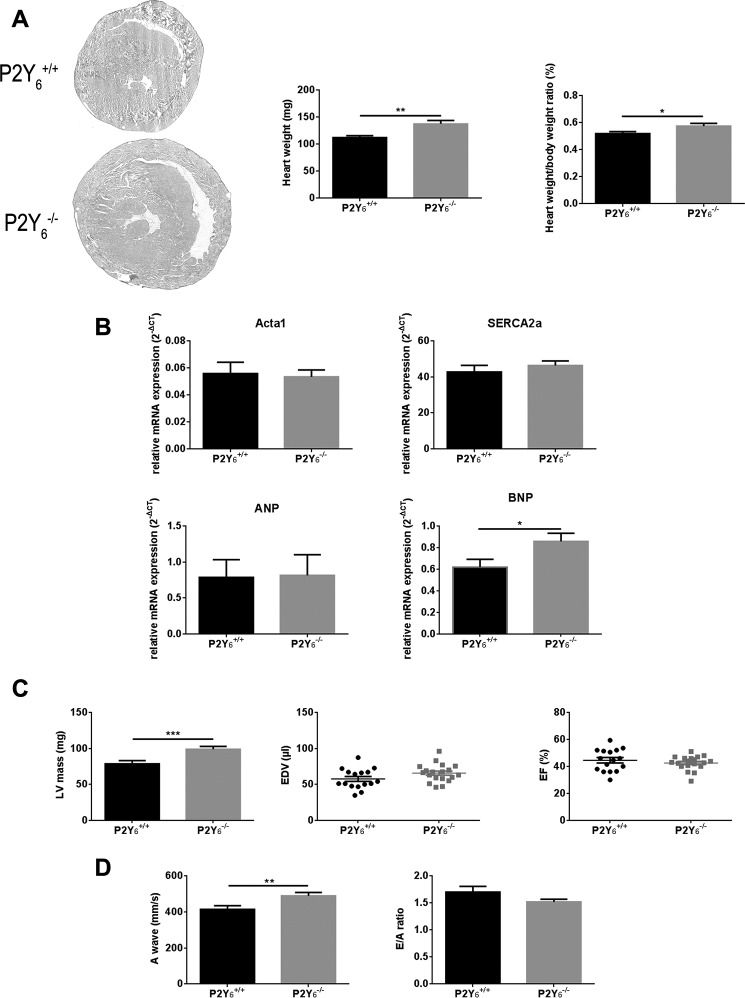
We proceeded with the observation of heart anatomy of P2Y6 KO and WT mice aged between 8 and 12 weeks. Cardiac hypertrophy was observed after hematoxylin-eosin staining of P2Y6 WT and KO hearts (Fig. 1A), but no major structural difference was observed in P2Y6 KO hearts. We observed that P2Y6 KO mice displayed a higher heart weight than that of wild type mice (137.7 ± 5.9 versus 112.3 ± 3.0 mg, respectively, mean ± S.E., n = 20 mice, **, p < 0.01, Fig. 1A, middle panel). A significant difference in heart weight was still observed after normalization to body weight (0.57 ± 0.02% versus 0.52 ± 0.01%, mean ± S.E., *, p < 0.05, Fig. 1A, right panel). Comparable expression of hypertrophy markers such as Acta1, Nppa, and Serca2a has been observed in P2Y6 WT and KO hearts (Fig. 1B). Only Nppb (gene encoding for BNP protein) showed a difference of expression between WT and P2Y6 KO mice (0.62 ± 0.07 versus 0.86 ± 0.07%, respectively, mean ± S.E., n = 11 mice, *, p < 0.05, Fig. 1B).
FIGURE 1.
Physiological cardiac hypertrophy in P2Y6 KO mice. A, left panel: staining of hearts from WT (P2Y6+/+) and P2Y6 KO (P2Y6−/−) male mice aged between 8 and 12 weeks with hematoxylin-eosin (n = 5 mice, scale bar = 1 mm). Middle and right panels, respectively, heart weight and normalization of heart weight to body weight of WT and P2Y6 KO male mice (n = 20 mice). B, quantification of expression of cardiac hypertrophy markers Acta1, Serca2a, Nppa, and Nppb by quantitative PCR in WT and P2Y6 KO hearts (n = 6 mice). C and D, echocardiographic analysis of WT and P2Y6 KO mice cardiac function. Left ventricle mass, EDV, and EF were calculated in B and M modes (panel C: nWT = 16, nKO = 19). A wave and E/A ratio were obtained by Doppler analysis for P2Y6 KO and WT mice (panel D, n = 9). Results are mean ± S.E., analyzed by t tests, ***, p < 0.001; **, p < 0.01; *, p < 0.05.
We then performed echocardiography experiments using adult WT and P2Y6 KO mice to analyze cardiac function (Fig. 1, C and D, and Table 1). These experiments revealed a higher left ventricle mass in P2Y6 KO mice than in WT mice (98.8 ± 4.0 versus 78.5 ± 4.6 mg, respectively, mean ± S.E., nWT = 16, nKO = 19 mice, ***, p < 0.001, Fig. 1C, left panel). Left ventricle hypertrophy in P2Y6 KO mice was also significant after normalization to body weight: 0.39 ± 0.02 versus 0.32 ± 0.02%, for P2Y6 KO and WT mice, respectively (*, p < 0.05, data not shown). Echocardiographic measurements of cardiac parameters in B and M modes like end-diastolic or end-systolic volumes (EDV, ESV), ejection fraction (EF) or fractional shortening (FS), the thickness of ventricular walls (LVPW), and septum (IVS) were realized for P2Y6 KO and WT mice (Fig. 1C, Table 1). No significant difference for these major cardiac parameters was observed (Fig. 1C, Table 1). Tissue Doppler measurements were also performed to investigate a potential diastolic dysfunction in P2Y6 KO mice. We observed a significant increase of the A wave value in P2Y6 KO compared with WT hearts (490.21 ± 18.40 versus 415.11 ± 20.16 mm/s, respectively, n = 9 mice; **, p < 0.01, Fig. 1D, left panel). E/A ratio was lowered in P2Y6 KO hearts but not significantly (Fig. 1D, right panel). We also observed no difference in IVRT and IVCT parameters between WT and P2Y6 KO mice (Table 1).
TABLE 1.
Echocardiographic data of WT and P2Y6 KO mice
Cardiac morphometric analysis was assessed by cardiac echography on WT and P2Y6 KO adult male mice in B, M, and Doppler modes. Results are mean ± S.E., nWT = 16, nKO = 19 mice, except for deceleration time, IVRT and IVCT, n = 9 mice. The abbreviations used are: IVSd, interventricular septum in diastole; IVSs, interventricular septum in systole; LVIDd, left ventricle internal diameter in diastole; LVIDs, left ventricle internal diameter in systole; LVPWd, left ventricle posterior wall in diastole; LVPWs, left ventricle posterior wall in systole; IVRT, isovolumic relaxation time; IVCT, isovolumic contraction time.
| P2Y6 WT baseline | P2Y6 KO baseline | |
|---|---|---|
| B mode long axis | ||
| EDV (μl) | 57.6 ± 3.5 | 65.7 ± 2.8 |
| ESV (μl) | 32.3 ± 2.5 | 38.1 ± 2.2 |
| EF (%) | 44.6 ± 2.0 | 42.5 ± 1.2 |
| LV mass (mg) | 78.5 ± 4.6 | 98.8 ± 4.0a |
| M mode | ||
| IVSd (mm) | 0.69 ± 0.03 | 0.70 ± 0.04 |
| IVSs (mm) | 1.01 ± 0.06 | 1.03 ± 0.06 |
| LVIDd (mm) | 3.84 ± 0.11 | 3.91 ± 0.09 |
| LVIDs (mm) | 2.77 ± 0.16 | 3.02 ± 0.11 |
| FS (%) | 28.1 ± 2.5 | 25.7 ± 1.7 |
| LVPWd (mm) | 0.72 ± 0.04 | 0.66 ± 0.03 |
| LVPWs (mm) | 1.05 ± 0.06 | 0.96 ± 0.06 |
| Heart rate (bpm) | 444.9 ± 15.4 | 421.7 ± 15.4 |
| Doppler mode | ||
| E wave (mm/s) | 684.12 ± 28.54 | 735.92 ± 25.00 |
| A wave (mm/s) | 415.11 ± 20.16 | 490.21 ± 18.40b |
| E′ wave (mm/s) | 25.07 ± 1.38 | 28.33 ± 1.45 |
| E/A ratio | 1.70 ± 0.10 | 1.52 ± 0.05 |
| E/E′ ratio | 28.15 ± 1.39 | 27.24 ± 1.60 |
| Deceleration time (ms) | 20.78 ± 2.17 | 20.60 ± 2.84 |
| IVRT (ms) | 18.31 ± 0.64 | 17.82 ± 1.06 |
| IVCT (ms) | 14.28 ± 1.71 | 14.20 ± 1.67 |
a p < 0.001.
b p < 0.01.
Loss of P2Y6 Increases Cardiomyocyte Proliferation and Size in Neonate Hearts
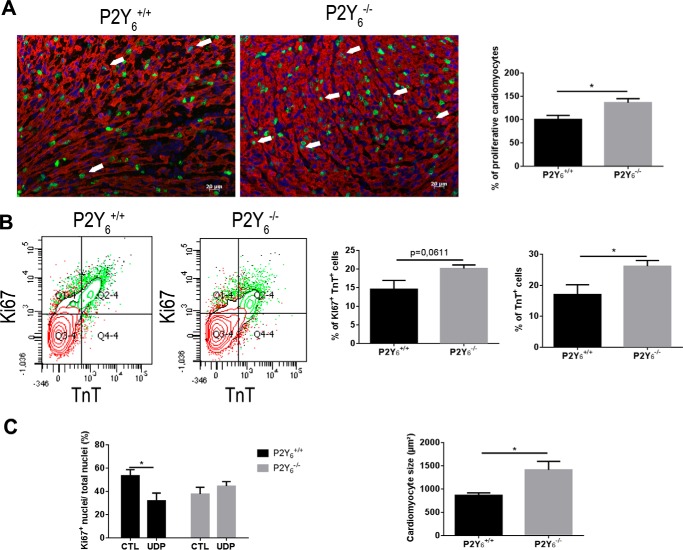
We investigated the proliferation of cardiac myocytes after EdU injection in 5-day-old WT and P2Y6 KO mice (Fig. 2). Immunofluorescence experiments showed that P2Y6 KO cardiomyocytes exhibit a proliferation increase of 36.1 ± 8.8% compared with WT cardiomyocytes (mean ± S.E., n = 10 mice, *, p < 0.05, Fig. 2A). We also assessed flow cytometry experiments using 5-day-old neonates and antibodies against troponin T (TnT) and Ki67 to stain proliferating cardiomyocytes as double positive cells (Fig. 2B). We observed an increase of proliferating cardiomyocytes of 38% in P2Y6 KO mice compared with WT mice (20.1 ± 1.0 versus 14.6 ± 2.4%, respectively, n = 5 mice, p = 0.0611, Fig. 2B). Flow cytometry experiments revealed a higher number of TnT positive cells in P2Y6 KO mice (26.1 ± 1.8 versus 17.0 ± 3.2%, mean ± S.E., n = 5 mice, *, p < 0.05, Fig. 2B, right panel), indicating that P2Y6 KO mice possess more cardiomyocytes than WT mice. Moreover, to confirm these results in vitro, we performed cultures of cardiomyocytes from WT and P2Y6 KO ventricles of 1-day-old neonates (Fig. 2C). We observed that UDP application reduced radically the proliferation of WT neonate cardiomyocytes (53.5 ± 5.2 versus 31.8 ± 6.6%, mean ± S.E. of 6 independent cultures, *, p < 0.05, Fig. 2C, left panel). This UDP effect was lost in P2Y6 KO neonate cardiomyocytes (Fig. 2C, left panel). We also observed a higher size of cardiomyocytes from P2Y6 KO mice than WT (1414 ± 181 versus 866 ± 54 μm2, respectively, mean ± S.E., n = 3, *, p < 0.05, Fig. 2C, right panel).
FIGURE 2.
Higher proliferation and size of cardiomyocytes in P2Y6 KO neonate mice. A, quantification of proliferating cardiomyocytes (arrows) was assessed after EdU (1 mm; overnight) injection in 5-day-old neonates by immunofluorescence experiments (red, troponin T; green, EdU; blue, nuclei, scale bar = 20 μm). n = 10 mice. B, flow cytometry analysis of cardiomyocyte proliferation. Anti-Ki67 and anti-troponin T antibodies were used to quantify proliferating cardiomyocytes (left panel) in 5-day-old neonates, and percentage of cardiomyocytes (TnT+ cells) in total ventricles of P2Y6 WT (P2Y6+/+) and KO (P2Y6−/−) 5-day-old neonates, quantified by flow cytometry (right panel). n = 5 mice. C, in vitro analysis of the effect of UDP on proliferation of WT and P2Y6 KO cardiomyocytes (left panel) and quantification of the size of cardiomyocytes isolated from WT and P2Y6 KO 1-day-old neonates (right panel). Data are the mean ± S.E. of 5 independent cultures, analyzed by t tests, *, p < 0.05.
P2Y6 Loss Amplifies Pathological Cardiac Hypertrophy Induced by Isoproterenol
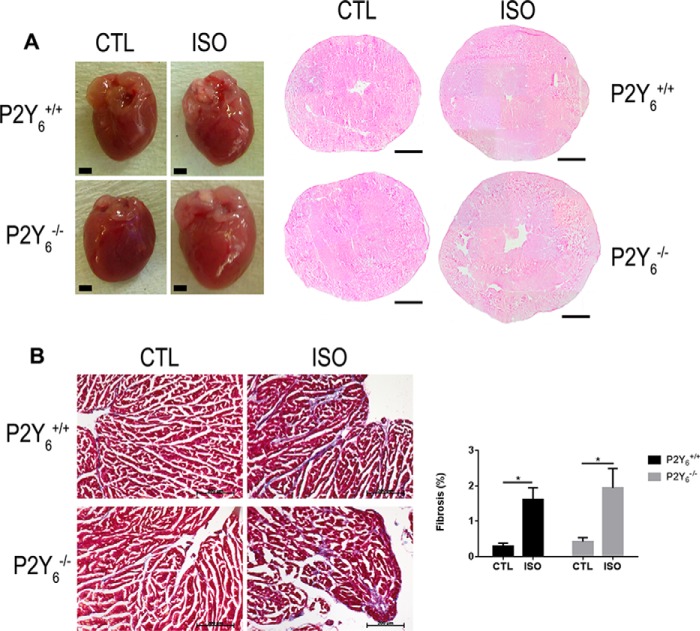
We investigated the potential role of P2Y6 receptor in pathological cardiac hypertrophy induced by isoproterenol injection. We injected 50 mg of ISO/kg/day during 7 days and analyzed cardiac hypertrophy in WT and P2Y6 KO mice. Interestingly, Fig. 3A shows that ISO-induced cardiac hypertrophy was greater in P2Y6 KO mice than in WT mice (Fig. 3A, Table 2). The following heart weight values were observed: 113.27 ± 2.07 mg versus 133.87 ± 2.18 g for untreated versus ISO-treated P2Y6 WT mice (mean ± S.E., ***, p < 0.001, n = 26) and 118.80 ± 1.95 versus 154.72 ± 3.98 g for untreated versus ISO-treated P2Y6 KO mice (mean ± S.E., ***, p < 0.001, n = 26). The percentage difference in heart weight normalized to body weight represents 31.5 ± 2.0% for P2Y6 KO mice and 22.4 ± 1.3% for WT mice. After normalization to tibia size, it represents 30.4 ± 2.4% for P2Y6 KO mice and 17.9 ± 1.6% for WT mice (n = 26 mice, **, p < 0.01, Table 2). No significant difference in body weight was observed between P2Y6 WT and KO mice in these experiments: respectively, 23.98 ± 0.41 versus 23.39 ± 0.42 g for untreated versus ISO-treated P2Y6 WT mice, and 25.30 ± 0.42 versus 24.80 ± 0.39 g for untreated versus ISO-treated P2Y6 KO mice, mean ± S.E., n = 26).
FIGURE 3.
P2Y6 loss amplifies isoproterenol-induced cardiac hypertrophy. Mice were injected with 50 mg/kg/day of ISO during 7 days to induce cardiac hypertrophy. A, hearts were harvested and stained with hematoxylin-eosin. Scale bars = 1 mm. B, slides of hearts were stained with Masson's trichrome and fibrosis was quantified after saline or ISO injection. Scale bar = 200 μm, n = 4, *, p < 0.05.
TABLE 2.
Percentage of heart increase in WT and P2Y6 KO mice after 7 days of ISO injection
After 7 days of ISO injection (50 mg/kg daily), hearts were harvested, weighted, and normalized to body weight or tibia size. Ratios are reported as percentage difference between WT ISO versus WT CTL and between P2Y6 KO ISO versus P2Y6 KO CTL (n = 26 mice).
| Percentage difference between treated and untreated WT mice | Percentage difference between treated and untreated P2Y6 KO mice | p | |
|---|---|---|---|
| Non normalized heart weight | 16.7 ± 1.8 | 30.2 ± 2.5 | <0.001 |
| Heart weight/body weight ratio | 22.4 ± 1.3 | 31.5 ± 2.0 | <0.001 |
| Heart weight/tibia size ratio | 17.9 ± 1.6 | 30.4 ± 2.4 | <0.001 |
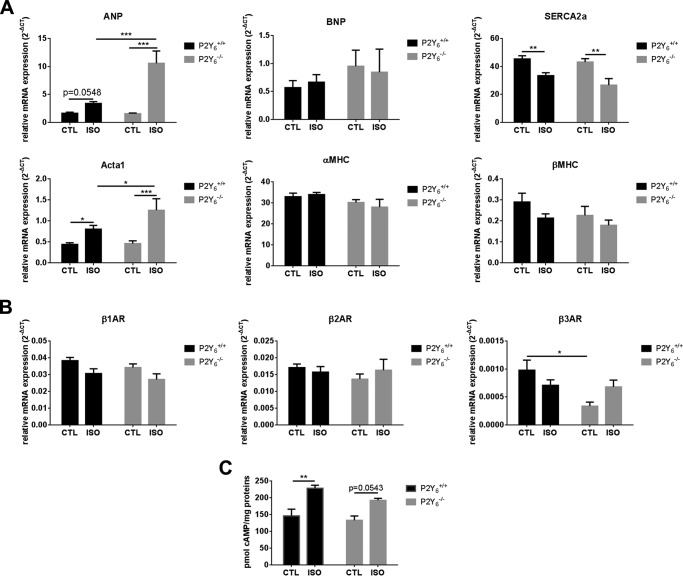
We then analyzed fibrosis deposition in P2Y6 KO mice (Fig. 3B). Only a restricted fibrosis was observed in ISO-treated hearts with no significant difference: 1.61 ± 0.32 versus 1.94 ± 0.55% in WT ISO versus KO ISO mice, respectively; mean ± S.E., n = 4 mice, Fig. 3B). Then we investigated the expression of cardiac hypertrophy markers Nppa, Nppb, Serca2a, Acta1, Myh6, and Myh7 in P2Y6 KO and WT mice. Expressions of Nppa and Acta1 were higher in P2Y6 KO than WT hearts after isoproterenol injection (Fig. 4A), which is characteristic of pathological hypertrophy, whereas the expressions of Nppb, Myh6, and Myh7 were not significantly modified between CTL and ISO-injected mice. The expression of Serca2a, which is known to be decreased in some cardiac pathologies (16–18), was lower after ISO injection, but in a comparable way in WT and P2Y6 KO mice.
FIGURE 4.
Expression of hypertrophy-linked genes and β-adrenoreceptor genes in WT and P2Y6 KO isoproterenol-treated mice. A, quantification of expression of cardiac hypertrophy markers Nppa, Nppb, Serca2a, Acta1, Myh6 (αMHC), and Myh7 (βMHC) by quantitative PCR in P2Y6 KO (P2Y6−/−) and WT (P2Y6+/+) mice injected with 50 mg/kg/day of ISO during 7 days (n = 10 mice). B, β-adrenergic receptors expression was quantified by quantitative PCR in mice injected with 50 mg/kg/day of ISO during 7 days (n = 10 mice). C, quantification of cardiac cAMP level 10 min after ISO injection (50 mg/kg) in P2Y6 KO and WT mice (n = 7 mice). Results are mean ± S.E., analyzed by two-way analysis of variance. ***, p < 0.001; **, p < 0.01; *, p < 0.05.
β-Adrenoreceptor expression was evaluated in control and ISO-treated P2Y6 KO and WT hearts (Fig. 4B). Comparable expression was observed for β1- and β2-adrenoreceptor expression (Fig. 4B) but β3-adrenoreceptor expression was decreased in the untreated P2Y6 KO heart (Fig. 4B). We also quantified the cardiac cAMP level in P2Y6 KO and WT mice after acute ISO treatment (50 mg/kg during 10 min, Fig. 4C). We observed that acute ISO induced a more significant cAMP increase in WT than in P2Y6 KO hearts (Fig. 4C).
To understand the effects of ISO on cardiac function, we assessed echocardiography experiments on acute ISO-treated mice (Table 3). We can observe that ISO injection induced modifications of cardiac contractility and heart rate, but with no difference between WT and P2Y6 KO mice (Table 3).
TABLE 3.
Echocardiographic data of WT and P2Y6 KO mice after acute ISO injection
Mice were injected intraperitoneally with ISO (50 mg/kg) or saline (NaCl 0.9%) and echography was realized 2 min after ISO injection in B and M modes. Results are mean ± S.E. before and after injection (n = 5 mice). The abbreviations used are: IVSd, interventricular septum in diastole; IVSs, interventricular septum in systole; LVIDd, left ventricle internal diameter in diastole; LVIDs, left ventricle internal diameter in systole; LVPWd, left ventricle posterior wall in diastole; LVPWs, left ventricle posterior wall in systole.
| P2Y6+/+ |
P2Y6−/− |
|||||||
|---|---|---|---|---|---|---|---|---|
| Saline |
ISO |
Saline |
ISO |
|||||
| Before injection | After injection | Before injection | After injection | Before injection | After injection | Before injection | After injection | |
| IVSd (mm) | 0.71 ± 0.01 | 0.73 ± 0.06 | 0.73 ± 0.04 | 1.00 ± 0.07a | 0.81 ± 0.09 | 0.74 ± 0.06 | 0.62 ± 0.04 | 1.02 ± 0.03a |
| IVSs (mm) | 1.01 ± 0.04 | 1.10 ± 0.13 | 1.03 ± 0.10 | 1.88 ± 0.05a | 1.09 ± 0.11 | 1.13 ± 0.09 | 1.00 ± 0.04 | 1.87 ± 0.06a |
| LVIDd (mm) | 3.75 ± 0.10 | 3.63 ± 0.06 | 3.85 ± 0.11 | 2.82 ± 0.17a | 3.77 ± 0.11 | 3.70 ± 0.10 | 3.85 ± 0.06 | 2.94 ± 0.08a |
| LVIDs (mm) | 2.79 ± 0.12 | 2.42 ± 0.16 | 2.79 ± 0.20 | 0.73 ± 0.14a | 2.75 ± 0.17 | 2.44 ± 0.11 | 2.65 ± 0.12 | 0.90 ± 0.12a |
| LVPWd (mm) | 0.71 ± 0.02 | 0.59 ± 0.03 | 0.67 ± 0.05 | 1.01 ± 0.15b | 0.63 ± 0.02 | 0.66 ± 0.04 | 0.64 ± 0.02 | 1.02 ± 0.13c |
| LVPWs (mm) | 1.04 ± 0.06 | 1.08 ± 0.08 | 1.07 ± 0.10 | 1.97 ± 0.09a | 1.02 ± 0.11 | 1.13 ± 0.09 | 1.12 ± 0.02 | 1.83 ± 0.04a |
| EDV (μl) | 73.05 ± 3.87 | 73.44 ± 4.73 | 74.10 ± 5.87 | 47.34 ± 4.74a | 77.19 ± 5.09 | 70.61 ± 4.49 | 73.25 ± 3.22 | 42.81 ± 2.92a |
| ESV (μl) | 46.91 ± 3.10 | 41.75 ± 4.63 | 43.46 ± 5.51 | 12.17 ± 1.29a | 43.70 ± 3.54 | 37.58 ± 4.40 | 44.79 ± 4.27 | 8.60 ± 1.04a |
| EF (%) | 35.92 ± 1.00 | 43.26 ± 4.18 | 42.12 ± 3.46 | 73.99 ± 2.47a | 43.21 ± 3.82 | 46.63 ± 5.60 | 39.17 ± 4.33 | 79.62 ± 2.48a |
| FS (%) | 25.70 ± 3.50 | 33.29 ± 7.84 | 28.02 ± 7.36 | 74.68 ± 8.70a | 27.15 ± 8.60 | 34.04 ± 7.82 | 31.20 ± 5.04 | 69.62 ± 8.19a |
| Heart rate (bpm) | 387.1 ± 21.1 | 411.0 ± 27.3 | 409.5 ± 20.6 | 580.1 ± 12.9a | 405.3 ± 3.0 | 392.4 ± 22.2 | 406.3 ± 16.2 | 583.2 ± 15.8a |
a p < 0.001.
b p < 0.05.
c p < 0.01.
UDP Inhibits in Vitro Isoproterenol-induced Cardiomyocyte Hypertrophy
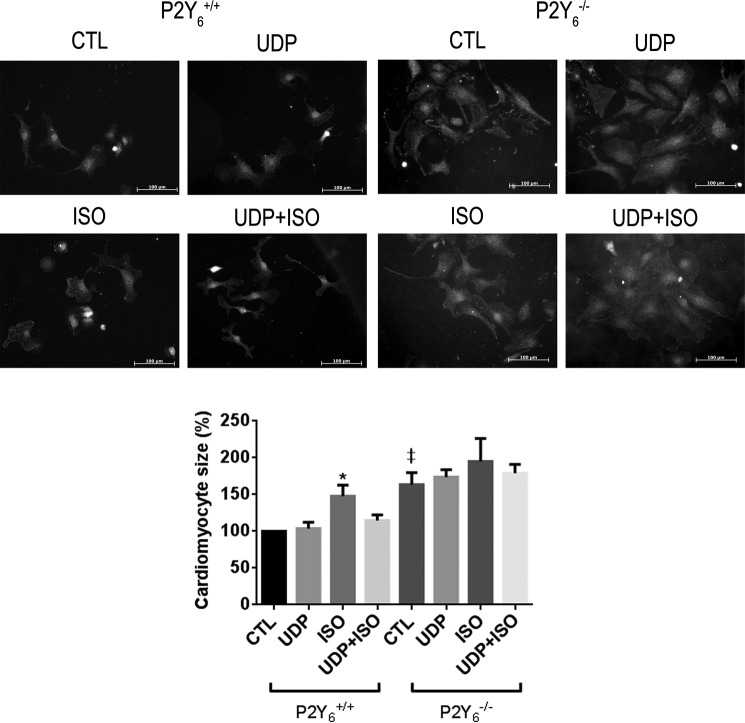
To investigate if the positive effect of P2Y6 receptor loss on ISO-induced cardiac hypertrophy was linked to a negative effect of the ligand UDP on cardiomyocyte hypertrophy, we isolated and treated 1-day-old neonate P2Y6 WT and KO cardiomyocytes with 1 μm ISO and/or 100 μm UDP. After 2 days of cardiomyocyte stimulation, we observed that UDP alone had no effect on cardiomyocyte size, whereas ISO was able to induce hypertrophy of cardiomyocytes (47.8 ± 20.5%, mean ± S.E. of 4 independent experiments; *, p < 0.05, Fig. 5). Interestingly, UDP was able to significantly inhibit the positive effect of isoproterenol on cardiomyocyte size (Fig. 5). As previously observed in Fig. 2C, the increase in basal cardiomyocyte size in P2Y6 KO cultures was confirmed (Fig. 5). ISO and UDP effects on P2Y6 KO myocyte size were not significant (Fig. 5).
FIGURE 5.
Inhibitory effect of UDP on isoproterenol-induced hypertrophy of cardiomyocytes. Quantification of cardiomyocyte size after ISO stimulation (1 μm) during 48 h in the presence or absence of 100 μm UDP in vitro. Cardiomyocyte size was estimated after immunostaining with ACTA1 and quantified with ImageJ software. Images are representative images of 4 independent experiments. Scale bars = 100 μm, data are the mean ± S.E. of 4 independent cultures. *, p < 0.05 for ISO-treated WT (P2Y6+/+ ISO) mice versus ISO + UDP-treated WT (P2Y6+/+ ISO+UDP), and ‡, p < 0.05 for P2Y6−/− CTL versus P2Y6+/+ CTL.
Identification of Genes Regulated in Control and Isoproterenol-treated P2Y6 KO Hearts
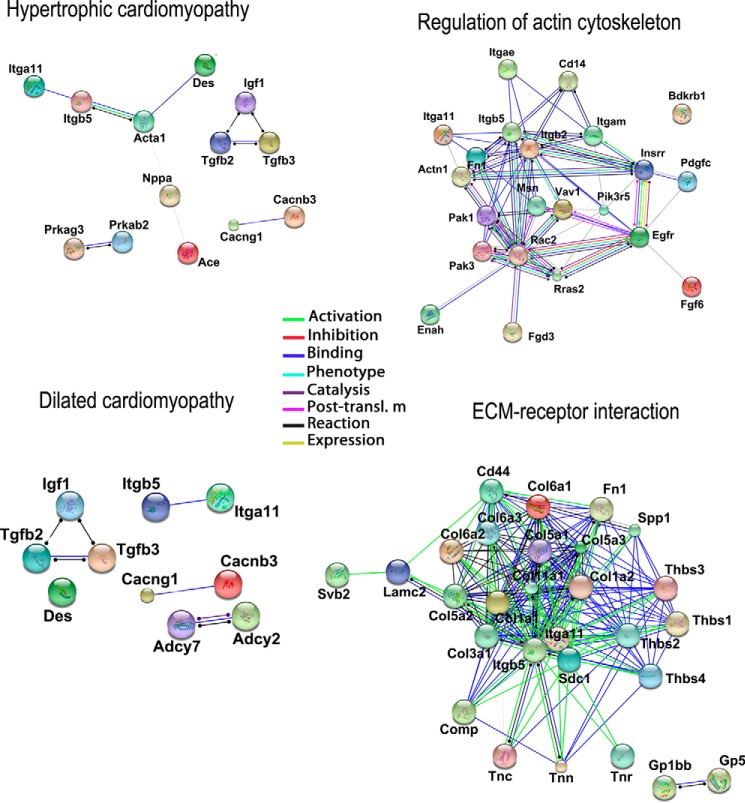
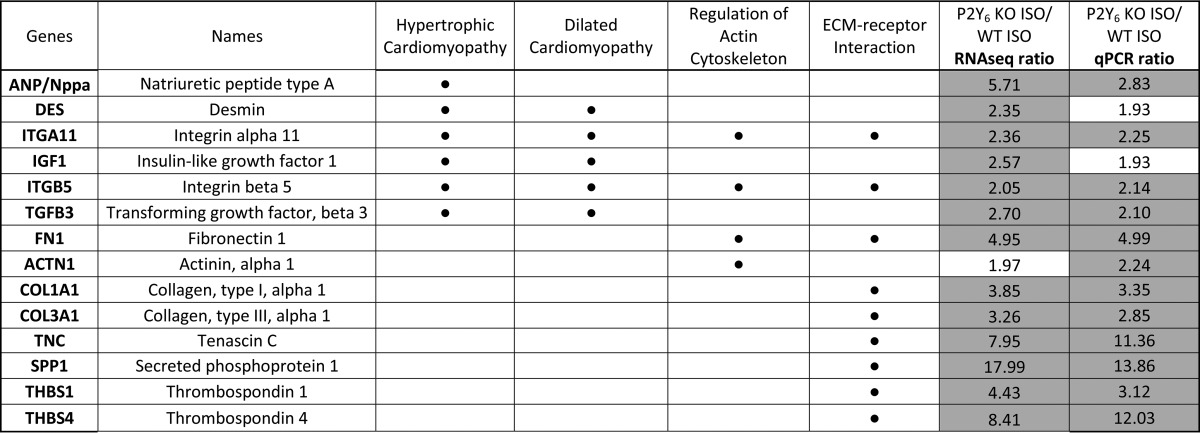
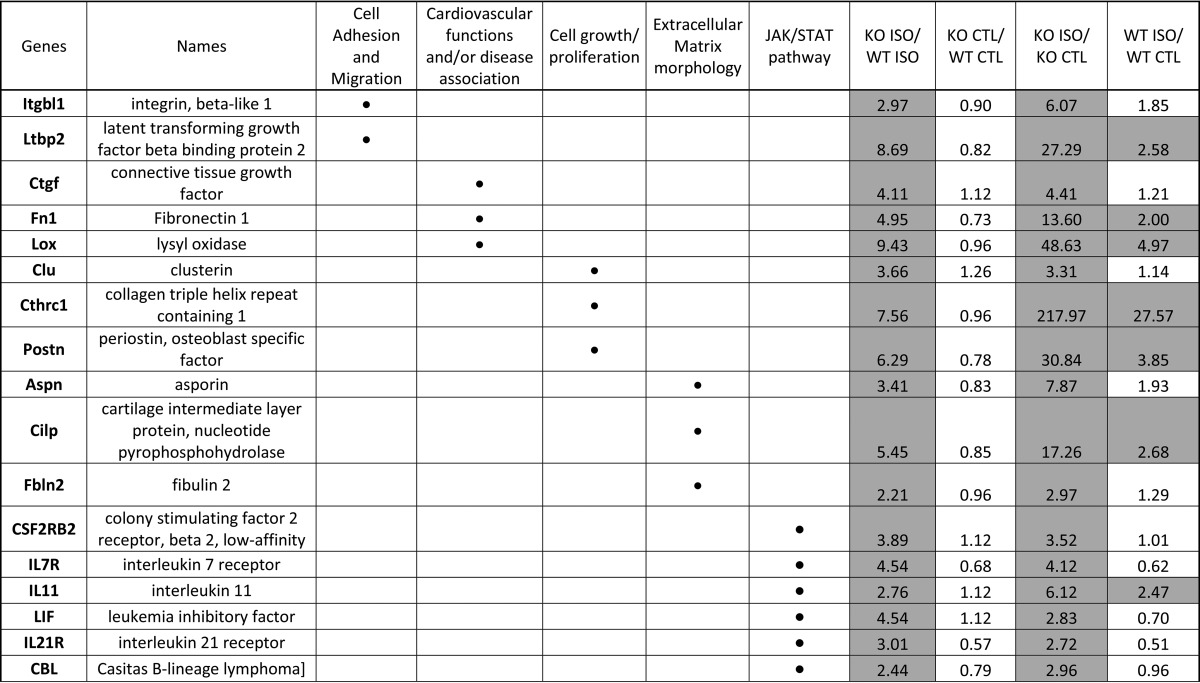
RNAseq experiments were assessed on hearts isolated from WT and P2Y6 KO mice 7 days after saline or ISO injection (supplemental Tables S1 and S2). Comparison of gene expression between P2Y6 KO and WT basal mice showed that only about 120 genes were differentially regulated. After analysis with Enrichnet (KEGG pathway) and STRING softwares, supplemental Table S1 revealed genes involved in MAPK, peroxisome proliferator-activated receptor, and Toll-like receptor signaling pathways, metabolic pathways, and cell cycle. Then, we compared the regulation of genes between ISO-injected P2Y6 KO versus ISO-injected WT mice (supplemental Table S2 and Fig. 6). Among the 1130 regulated genes in ISO-treated P2Y6 KO compared with WT mice, analysis with Enrichnet (KEGG pathway) and STRING softwares revealed genes involved in processes like hypertrophic or dilated cardiomyopathies (Acta1, Des, Itga11, and Itgb5), regulation of actin cytoskeleton (Vav1, Rac2, Pak 1 and 3), or extracellular matrix-receptor interactions (Fig. 6, supplemental Table S2). We also identified P2Y6 target genes in RNAseq experiments that are involved in signaling pathways such as the JAK/STAT or MAPK pathways (supplemental Table S2). Additionally, quantitative PCR experiments have been performed to confirm up-regulation of some target genes involved inter alia in hypertrophic and dilated cardiomyopathy (Table 4). The shading in Tables 4 and 5 indicate the gene regulations corresponding to a ratio superior or equal to 2 (≥2). Table 5 is focused on genes significantly regulated by ISO in P2Y6 KO hearts. We identified inter alia genes with an important KO ISO/WT ISO ratio involved in cardiovascular function (Lox, Ctgf, and Fn1) or JAK/STAT pathway (Lif, Il7r, Il21r, and Il11).
FIGURE 6.
Schematic representation of regulated pathways in isoproterenol-treated P2Y6 KO hearts. RNA sequencing experiments were performed on hearts from P2Y6 WT and KO mice after 7 days of ISO injection (50 mg/kg daily). String views show interactions between the regulated targets and their action mode.
TABLE 4.
Confirmation of gene regulations in ISO-treated P2Y6 KO vs ISO-treated WT mice by RNAseq and quantitative PCR
Mice were injected daily with ISO (50 mg/kg daily) during 7 days. Then, hearts were harvested and RNA was extracted to perform RNAseq experiments using a RNA pool from 3 hearts for each condition. Some genes up-regulated in ISO-injected P2Y6 KO mice compared to ISO-injected WT mice (P2Y6 KO ISO/WT ISO) were confirmed by quantitative qPCR analysis using 10 mice per condition. Regulated genes were analyzed with Enrichnet (KEGG pathway) and STRING softwares and have been classified in different pathways as represented by a black dot (•).
TABLE 5.
Genes more strongly upregulated after ISO injection in P2Y6 KO than in WT mice
Mice were injected daily with ISO (50 mg/kg daily) during 7 days. Then, hearts were harvested and RNA was extracted to perform RNAseq experiments using a RNA pool from 3 hearts for each condition. Ratios were reported for ISO-injected P2Y6 KO mice compared to ISO-injected WT mice (KO ISO/WT ISO), P2Y6 KO compared to WT saline-injected mice (KO control (CTL)/WT CTL), ISO-injected compared to saline-injected P2Y6 KO mice (KO ISO/KO CTL), ISO-injected compared to saline-injected WT mice (WT ISO/WT CTL). Regulated genes were analyzed with Enrichnet (KEGG pathway) and STRING softwares and have been classified in different pathways as represented by a black dot (•).
Discussion
During the first weeks after birth, cardiomyocyte proliferation accompanied by massive growth of capillaries and coronary vessels leads to an important development of the heart (19, 20). Cardiomyocyte hypertrophy plays a major role in further growth of the heart with a determinant role of α1A/C and α1B receptors, indicating the role of sympathetic innervation (21). The observation of increased heart weight in P2Y6 KO mice was very intriguing. The expression of P2Y6 in cardiomyocytes was previously demonstrated (22). We observed increased cardiomyocyte size in 1-day-old P2Y6 KO neonates compared with WT neonates. Additionally in vivo proliferation of cardiomyocytes was significantly increased in the hearts of 5-day-old P2Y6-null mice compared with wild type mice. Increased heart weight at adult age in P2Y6-null mice appears as a direct consequence of increased proliferation and size of neonate cardiomyocytes. P2Y6 receptor appeared thus as a negative regulator of both early hypertrophy and hyperplasia of cardiac myocytes.
We have observed that Nppb expression is increased in P2Y6 KO adult mice, compared with WT. BNP is a circulating hormone playing an important role in vascular tone regulation and compensation in heart failure (23). Dysregulation of heart development was reported for mice deficient for many target genes and mainly related to loss of their expression in cardiomyocytes and cardiomyocyte dysfunction. Neuropilin-1 or creatine kinase knock-out mice display hearts with an enhanced size due to cardiomyocyte hypertrophy (24, 25). The cardiac phenotype of P2Y6-null mice could also be compared with that of p27KIP1 knock-out mice (26) and transgenic mice expressing c-myc constitutively in cardiomyocytes (27), which is characterized by increased heart weight with enhanced proliferation of cardiomyocytes. Until now, the study of UDP effects in the heart was very restricted and P2Y6 receptor only reported to be involved in overload-induced cardiac fibrosis (7). The use of P2Y6 antagonist MRS2578 had no effect on transverse aortic constriction-induced cardiac hypertrophy (7). Even if our model of ISO injection in P2Y6-null mice also implies adrenoreceptor activation, it differs from transverse aortic constriction-induced pathological cardiac hypertrophy involving endogenous catecholamines with the use of a P2Y6 antagonist having possible secondary effects.
We demonstrated previously that mice deficient for another P2Y receptor, the P2Y4 receptor, display smaller hearts, a microcardia phenotype related to a cardiac angiogenic defect without any change in cardiac function (3) but a reduced exercise capacity (4). P2Y6 KO and WT mice display comparable values for major cardiac parameters in B and M modes. Interestingly Doppler measurements revealed a significant increase of A wave value in P2Y6 KO hearts. E/A ratio was lowered in P2Y6 KO hearts but not significantly. Tissue Doppler imaging suggests a potential diastolic dysfunction related to cardiac hypertrophy in P2Y6 KO mice.
Cardiac and urine levels of norepinephrine and dopamine were decreased in P2Y4-null mice (4), whereas isoproterenol action on cardiac hypertrophy was enhanced in P2Y6-null mice. The potentiation or inhibition of adrenergic responses could be a determinant in the action of extracellular nucleotides on cardiac development and hypertrophy.
The present study supports P2Y6 involvement in both physiological and pathological conditions. Besides the macrocardia phenotype of P2Y6-null mice, we observed a more pronounced cardiac-induced hypertrophy in these mice. Effectively, isoproterenol injection induced a more robust increase in heart weight and expression of hypertrophy markers such as Nppa and Acta1 in P2Y6-null mice than in wild type mice. These in vivo data were correlated with inhibitory effects of UDP in vitro on hyperplasia and isoproterenol-induced hypertrophy of cardiomyocytes. Echocardiography experiments showed comparable cardiac parameters after acute ISO treatment of P2Y6 KO and WT mice.
Interestingly, β3-adrenoceptors expression was lower in P2Y6 KO compared with WT basal hearts, whereas P2Y6 KO and WT mice displayed comparable β3-adrenoreceptor cardiac expression 7 days after ISO injection. Overexpression of β3-adrenoceptors was reported to inhibit hypertrophic response to ISO in vitro and in vivo through endothelial nitric-oxide synthase stimulation (28). Third generation β-adrenoreceptor blockers such as Nebivolol have further properties including stimulation of NOS and/or β3-adrenoceptors (29).
Signaling pathways involved in the development of cardiac hypertrophy are diverse and complex. Generally, physiological cardiac hypertrophy is mediated by the IGF-1/PI3K/AKT pathway, effects mediated by a receptor-tyrosine kinase (7, 11). The causes of pathological cardiac hypertrophy are also complex and multiple: mechanical stress or extracellular stimuli like catecholamines, endothelin-1, and angiotensin-II that activate G-protein coupled receptors. ISO-induced cardiac hypertrophy has been shown to be mediated first by Gs proteins then in a PKA-dependent manner by Gi proteins, leading to Src and Ras activation (12). Development of myocardial hypertrophy starts with neurohumoral factors, such as angiotensin II and norepinephrine, which activate receptors coupled to members of the Gq, G12, and Gi families (30–32) and hypertrophic gene expression in cardiomyocytes through the Ca2+-dependent pathway (33). Most of P2Y receptors including P2Y6 receptor are coupled to Gαq. The major role of the Gq family in the pathogenesis of hypertrophy was demonstrated by the use of transgenic overexpression or conditional knock-out mice (34, 35).
Our data support that the P2Y6 receptor acts as a negative regulator of hyperplasia during postnatal heart development as well as an inhibitor of isoproterenol-induced hypertrophy in adult mice. Interestingly RNAseq analysis revealed different sets of genes regulated by loss of P2Y6 in basal and ISO-treated hearts (supplemental Table S1 and S2), suggesting differential involvement of this receptor in physiological and pathological cardiac hypertrophy. RNAseq analysis of the hearts of isoproterenol-treated mice showed inter alia the up-regulation in P2Y6-null mice of genes directly involved in hypertrophic and dilated cardiomyopathies (Nppa, Acta1, Igf-1, Tgf-β2, Tgf-β3, and integrins Itga11 and Itgb5), regulation of actin cytoskeleton (Vav1, Rac2, and integrins Itgb2 and Itgam) and also up-regulation of genes implicated in the JAK/STAT pathway (Il-10ra, Il-7r, Il-9r, Socs1, and Socs3), which is involved in the development of pathological hypertrophy. We confirmed the regulation of many genes in ISO-treated control mice that were previously described as ISO target genes in the heart (36). Interestingly the ISO-mediated regulation of some of these genes such as lectin-like oxidized LDL receptor-1 (Lox-1) and periostin was strongly amplified in the P2Y6-null mice (Table 5). Lox-1 abrogation is known to reduce cardiac hypertrophy by reducing oxidative stress (37), whereas periostin is described as a factor in cardiac remodeling after clinical unloading of the failing heart (38).
UTP release was observed in human heart during myocardial infarction (6) and mechanical stretch releases nucleotides from cardiomyocytes through pannexin hemichannels (7). Uracil nucleotides can protect cardiomyocytes from hypoxic stress (39) as well as reduce infarct size and improve heart function after myocardial infarct (2), reflecting the multiple effects of UTP on P2Y2 receptors expressed on cardiomyocytes and endothelial P2Y2 and P2Y4 receptors. We recently demonstrated P2Y4 involvement in cardiac fibrosis and protection against cardiac ischemia (5). It appears thus that P2Y2, P2Y4, and P2Y6 receptors could play various roles at different levels in cardiac function, development, and remodeling.
The present study constitutes an important advance in the field of extracellular nucleotide receptors and cardiac development. UDP appears as an inhibitor of physiological and pathological cardiac hypertrophy acting through P2Y6 receptor activation. P2Y6 agonists could reduce cardiac hypertrophy and associated damages and constitute a therapeutic target as a regulator of cardiac remodeling.
Author Contributions
S. C., L. D. P., E. P. D., H. E., M. H., M. V., and A. L. performed experiments; S. C. and D. C. designed the research and wrote the paper; S. C., M. H., M. V., A. L., C. B., J. L. B., J. M. B., and D. C. analyzed results and helped edit the paper.
Supplementary Material
Acknowledgments
We thank Sophie De Man, Anne Lefort, Claude Massart, and Bernard Robaye for help and technical assistance in respectively, echocardiographic and RNA sequencing experiments (RNA sequencing platform headed by Frédérick Libert, IRIBHM, ULB).
This work was supported in part by the Fonds de la Recherche Scientifique Médicale of Belgium, Action de Recherche Concertée of the Communauté Française de Belgique, an Interuniversity Attraction Pole grant from the Politique Scientifique Fédérale (IAP-P7/40), the Prime Minister's Office, Federal Service for Science, Technology and Culture, by Fonds Lokumo, managed by the Fondation Roi Baudouin, grants from the Fonds de la Recherche Scientifique Médicale (F.R.S.M.), the Fonds d'Encouragement à la Recherche (F.E.R.), the Fonds Emile DEFAY, the Walloon Region (Programme d'Excellence CIBLES), and LifeSciHealth program of the European Community Grant LSHB-2003-503337. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Tables S1 and S2.
- ISO
- isoproterenol
- EdU
- 5-ethynyl-2′-deoxyuridine
- EDV
- end-diastolic volume
- ESV
- end-systolic volume
- EF
- ejection fraction
- LV
- left ventricle
- FS
- fractional shortening
- LVPW
- left ventricle posterior wall
- IVS
- interventricular septum
- TnT
- troponin T.
References
- 1. Cohen R., Shainberg A., Hochhauser E., Cheporko Y., Tobar A., Birk E., Pinhas L., Leipziger J., Don J., and Porat E. (2011) UTP reduces infarct size and improves mice heart function after myocardial infarct via P2Y2 receptor. Biochem. Pharmacol. 82, 1126–1133 [DOI] [PubMed] [Google Scholar]
- 2. Yitzhaki S., Shainberg A., Cheporko Y., Vidne B. A., Sagie A., Jacobson K. A., and Hochhauser E. (2006) Uridine-5′-triphosphate (UTP) reduces infarct size and improves rat heart function after myocardial infarct. Biochem. Pharmacol. 72, 949–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Horckmans M., Robaye B., Léon-Gómez E., Lantz N., Unger P., Dol-Gleizes F., Clouet S., Cammarata D., Schaeffer P., Savi P., Gachet C., Balligand J. L., Dessy C., Boeynaems J. M., and Communi D. (2012) P2Y4 nucleotide receptor: a novel actor in post-natal cardiac development. Angiogenesis 15, 349–360 [DOI] [PubMed] [Google Scholar]
- 4. Horckmans M., Léon-Gómez E., Robaye B., Balligand J. L., Boeynaems J. M., Dessy C., and Communi D. (2012) Gene deletion of P2Y4 receptor lowers exercise capacity and reduces myocardial hypertrophy with swimming exercise. Am. J. Physiol. Heart Circ. Physiol. 303, H835–843 [DOI] [PubMed] [Google Scholar]
- 5. Horckmans M., Esfahani H., Beauloye C., Clouet S., di Pietrantonio L., Robaye B., Balligand J. L., Boeynaems J. M., Dessy C., and Communi D. (2015) Loss of mouse P2Y4 nucleotide receptor protects against myocardial infarction through endothelin-1 downregulation. J. Immunol. 194, 1874–1881 [DOI] [PubMed] [Google Scholar]
- 6. Wihlborg A. K., Balogh J., Wang L., Borna C., Dou Y., Joshi B. V., Lazarowski E., Jacobson K. A., Arner A., and Erlinge D. (2006) Positive inotropic effects by uridine triphosphate (UTP) and uridine diphosphate (UDP) via P2Y2 and P2Y6 receptors on cardiomyocytes and release of UTP in man during myocardial infarction. Circ. Res. 98, 970–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nishida M., Sato Y., Uemura A., Narita Y., Tozaki-Saitoh H., Nakaya M., Ide T., Suzuki K., Inoue K., Nagao T., and Kurose H. (2008) P2Y6 receptor-Ga12/13 signalling in cardiomyocytes triggers pressure overload-induced cardiac fibrosis. EMBO J. 27, 3104–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garcia R. A., Yan M., Search D., Zhang R., Carson N. L., Ryan C. S., Smith-Monroy C., Zheng J., Chen J., Kong Y., Tang H., Hellings S. E., Wardwell-Swanson J., Dinchuk J. E., Psaltis G. C., et al. (2014) P2Y6 receptor potentiates pro-inflammatory responses in macrophages and exhibits differential roles in atherosclerotic lesion development. PLoS One 9, e111385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stachon P., Peikert A., Michel N. A., Hergeth S., Marchini T., Wolf D., Dufner B., Hoppe N., Ayata C. K., Grimm M., Cicko S., Schulte L., Reinöhl J., von zur Muhlen C., Bode C., Idzko M., and Zirlik A. (2014) P2Y6 deficiency limits vascular inflammation and atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 34, 2237–2245 [DOI] [PubMed] [Google Scholar]
- 10. Catalucci D., Latronico M. V., Ellingsen O., and Condorelli G. (2008) Physiological myocardial hypertrophy: how and why? Front. Biosci. 13, 312–324 [DOI] [PubMed] [Google Scholar]
- 11. Bernardo B. C., Weeks K. L., Pretorius L., and McMullen J. R. (2010) Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol. Ther. 128, 191–227 [DOI] [PubMed] [Google Scholar]
- 12. Zou Y., Komuro I., Yamazaki T., Kudoh S., Uozumi H., Kadowaki T., and Yazaki Y. (1999) Both Gs and Gi proteins are critically involved in isoproterenol-induced cardiomyocyte hypertrophy. J. Biol. Chem. 274, 9760–9770 [DOI] [PubMed] [Google Scholar]
- 13. Boluyt M., Long X., Eschenhagen T., Mende U., Schmitz W., Crow M. T., and Lakatta E. G. (1995) Isoproterenol infusion induces alterations in expression of hypertrophy-associated genes in rat heart. Am. J. Physiol. 269, H638–647 [DOI] [PubMed] [Google Scholar]
- 14. Murad N., and Tucci P. J. (2000) Isoproterenol-induced hypertrophy may result in distinct left ventricular changes. Clin. Exp. Pharmacol. Physiol. 27, 352–357 [DOI] [PubMed] [Google Scholar]
- 15. Bar I., Guns P. J., Metallo J., Cammarata D., Wilkin F., Boeynams J. M., Bult H., and Robaye B. (2008) Knockout mice reveal a role for P2Y6 receptor in macrophages, endothelial cells, and vascular smooth muscle cells. Mol. Pharmacol. 74, 777–784 [DOI] [PubMed] [Google Scholar]
- 16. Mitsuyama S., Takeshita D., Obata K., Zhang G. X., and Takaki M. (2013) Left ventricular mechanical and energetic changes in long-term isoproterenol-induced hypertrophied hearts of SERCA2a transgenic rats. J. Mol. Cell Cardiol. 59, 95–106 [DOI] [PubMed] [Google Scholar]
- 17. Prasad V., Lorenz J. N., Lasko V. M., Nieman M. L., Huang W., Wang Y., Wieczorek D. W., and Shull G. E. (2015) SERCA2 haploinsufficiency in a mouse model of Darier disease causes a selective predisposition to heart failure. Biomed. Res. Int. 2015, 251598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Erkens R., Kramer C. M., Lückstädt W., Panknin C., Krause L., Weidenbach M., Dirzka J., Krenz T., Mergia E., Suvorava T., Kelm M., and Cortese-Krott M. M. (2015) Left ventricular diastolic dysfunction in Nrf2 knock out mice is associated with cardiac hypertrophy, decreased expression of SERCA2a, and preserved endothelial function. Free Radic. Biol. Med. 89, 906–917 [DOI] [PubMed] [Google Scholar]
- 19. Bellomo D., Headrick J. P., Silins G. U., Paterson C. A., Thomas P. S., Gartside M., Mould A., Cahill M. M., Tonks I. D., Grimmond S. M., Townson S., Wells C., Little M., Cummings M. C., Hayward N. K., and Kay G. F. (2000) Mice lacking the vascular endothelial growth factor-B gene (Vegfb) have smaller hearts, dysfunctional coronary vasculature, and impaired recovery from cardiac ischemia. Circ. Res. 86, e29–35 [DOI] [PubMed] [Google Scholar]
- 20. Hudlicka O., and Brown M. D. (1996) Postnatal growth of the heart and its blood vessels. J. Vasc. Res. 33, 266–287 [DOI] [PubMed] [Google Scholar]
- 21. O'Connell T. D., Ishizaka S., Nakamura A., Swigart P. M., Rodrigo M. C., Simpson G. L., Cotecchia S., Rokosh D. G., Grossman W., Foster E., and Simpson P. C. (2003) The α(1A/C)- and α(1B)-adrenergic receptors are required for physiological cardiac hypertrophy in the double-knockout mouse. J. Clin. Invest. 111, 1783–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheung K. K., Marques-da-Silva C., Vairo L., dos Santos D. S., Goldenberg R., Coutinho-Silva R., and Burnstock G. (2015) Pharmacological and molecular characterization of functional P2 receptors in rat embryonic cardiomyocytes. Purinergic Signal. 11, 127–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nishikimi T., Maeda N., and Matsuoka H. (2006) The role of natriuretic peptides in cardioprotection. Cardiovasc. Res. 69, 318–328 [DOI] [PubMed] [Google Scholar]
- 24. Ellmers L. J., Knowles J. W., Kim H. S., Smithies O., Maeda N., and Cameron V. A. (2002) Ventricular expression of natriuretic peptides in Nrp1−/− mice with cardiac hypertrophy and fibrosis. Am. J. Physiol. Heart Circ. Physiol. 283, H707–H714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nahrendorf M., Spindler M., Hu K., Bauer L., Ritter O., Nordbeck P., Quaschning T., Hiller K. H., Wallis J., Ertl G., Bauer W. R., and Neubauer S. (2005) Creatine kinase knockout mice show left ventricular hypertrophy and dilatation, but unaltered remodeling post-myocardial infarction. Cardiovasc. Res. 65, 419–427 [DOI] [PubMed] [Google Scholar]
- 26. Poolman R. A., Li J. M., Durand B., and Brooks G. (1999) Altered expression of cell cycle proteins and prolonged duration of cardiac myocyte hyperplasia in p27KIP1 knockout mice. Circ. Res. 85, 117–127 [DOI] [PubMed] [Google Scholar]
- 27. Jackson T., Allard M. F., Sreenan C. M., Doss L. K., Bishop S. P., and Swain J. L. (1990) The c-myc proto-oncogene regulates cardiac development in transgenic mice. Mol. Cell. Biol. 10, 3709–3716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Belge C., Hammond J., Dubois-Deruy E., Manoury B., Hamelet J., Beauloye C., Markl A., Pouleur A.-C., Bertrand L., Esfahani H., Jnaoui K, Konrad R. G., Nikolaev V. O., Vanderper A., Herijers P., et al. (2014) Enhanced expression of β3-adrenoceptors in cardiac myocytes attenuates neurohormone-induced hypertrophic remodeling through nitric oxide synthase. Circulation 129, 451–462 [DOI] [PubMed] [Google Scholar]
- 29. Ozakca I., Arioglu-Inan E., Esfahani H., Altan V. M., Balligand J. L., Kayki-Mutlu G., and Ozcelikay A. T. (2013) Nebivolol prevents desensitization of β-adrenoceptor signaling and induction of cardiac hypertrophy in response to isoprenaline beyond β1-adreneceptor blockage. Am. J. Physiol. Heart Circ. Physiol. 304, H1267–H1276 [DOI] [PubMed] [Google Scholar]
- 30. Arai K., Maruyama Y., Nishida M., Tanabe S., Takagahara S., Kozasa T., Mori Y., Nagao T., and Kurose H. (2003) Differential requirement of Gα12, Gα13, Gαq, and Gβg for endothelin-1-induced c-Jun NH2-terminal kinase and extracellular signal-regulated kinase activation. Mol. Pharmacol. 63, 478–488 [DOI] [PubMed] [Google Scholar]
- 31. Gohla A., Schultz G., and Offermanns S. (2000) Role for G12/G13 in agonist-induced vascular smooth muscle cell contraction. Circ. Res. 87, 221–227 [DOI] [PubMed] [Google Scholar]
- 32. Sadoshima J., and Izumo S. (1997) The cellular and molecular response of cardiac myocytes to mechanical stress. Annu. Rev. Physiol. 59, 551–571 [DOI] [PubMed] [Google Scholar]
- 33. Onohara N., Nishida M., Inoue R., Kobayashi H., Sumimoto H., Sato Y., Mori Y., Nagao T., and Kurose H. (2006) TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 25, 5305–5316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Adams J. W., Sakata Y., Davis M. G., Sah V. P., Wang Y., Liggett S. B., Chien K. R., Brown J. H., and Dorn G. W. 2nd (1998) Enhanced Gαq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc. Natl. Acad. Sci. U.S.A. 95, 10140–10145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wettschureck N., Rütten H., Zywietz A., Gehring D., Wilkie T. M., Chen J., Chien K. R., and Offermanns S. (2001) Absence of pressure overload induced myocardial hypertrophy after conditional inactivation of Gαq/Gα11 in cardiomyocytes. Nat. Med. 7, 1236–1240 [DOI] [PubMed] [Google Scholar]
- 36. Galindo C. L., Skinner M. A., Errami M., Olson L. D., Watson D. A., Li J., McCormick J. F., McIver L. J., Kumar N. M., Pham T. Q., and Garner H. R. (2009) Transcriptional profile of isoproterenol-induced cardiomyopathy and comparison to exercise-induced cardiac hypertrophy and human cardiac failure. BMC Physiol. 9, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lu J., Wang X., Wang W., Muniyappa H., Hu C., Mitra S., Long B., Das K., and Mehta J. L. (2012) LOX-1 abrogation reduces cardiac hypertrophy and collagen accumulation following chronic ischemia in the mouse. Gene. Ther. 19, 522–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stansfield W. E., Andersen N. M., Tang R.-H., and Selzman C. H. (2009) Periostin is a novel factor in cardiac remodeling after experimental and clinical unloading of the failing heart. Ann. Thorac. Surg. 88, 1916–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yitzhaki S., Shneyvays V., Jacobson K. A., and Shainberg A. (2005) Involvement of uracil nucleotides in protection of cardiomyocytes from hypoxic stress. Biochem. Pharmacol. 69, 1215–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.