Abstract
Nelumbo nucifera is an evolutionary relic from the Late Cretaceous period. Sequencing the N. nucifera mitochondrial genome is important for elucidating the evolutionary characteristics of basal eudicots. Here, the N. nucifera mitochondrial genome was sequenced using single molecule real-time sequencing technology (SMRT), and the mitochondrial genome map was constructed after de novo assembly and annotation. The results showed that the 524,797-bp N. nucifera mitochondrial genome has a total of 63 genes, including 40 protein-coding genes, three rRNA genes and 20 tRNA genes. Fifteen collinear gene clusters were conserved across different plant species. Approximately 700 RNA editing sites in the protein-coding genes were identified. Positively selected genes were identified with selection pressure analysis. Nineteen chloroplast-derived fragments were identified, and seven tRNAs were derived from the chloroplast. These results suggest that the N. nucifera mitochondrial genome retains evolutionarily conserved characteristics, including ancient gene content and gene clusters, high levels of RNA editing, and low levels of chloroplast-derived fragment insertions. As the first publicly available basal eudicot mitochondrial genome, the N. nucifera mitochondrial genome facilitates further analysis of the characteristics of basal eudicots and provides clues of the evolutionary trajectory from basal angiosperms to advanced eudicots.
The size of the mitochondrial genome differs among angiosperm species, ranging from approximately 220 kb (Brassica napus)1 to 11.3 Mb (Silene conica)2, reflecting the insertion of noncoding DNA3, including DNA of plastid and nuclear origin and DNA from horizontal gene transfer (HGT)4,5. Genomic organization is also variable across angiosperm evolution, reflecting frequent intramolecular or intermolecular homologous recombination mediated through repeats6. The organization, gene content and RNA editing of the mitochondrial genome differs over a significant scale across seed plants. This variation is useful for studying the evolution of both genome structures and sequences.
Nelumbo nucifera Gaertn. (Sacred lotus) is considered an evolutionary relic, which like Ginkgo, Sequoia, Metasequoia and Liriodendron, has survived since the Late Cretaceous period7. During the Early Cretaceous period, N. nucifera was a perennial aquatic plant that flourished during the middle Albian8,9. Currently, N. nucifera has been classified in the monotypic family Nelumbonaceae, which contains a single genus Nelumbo. This genus includes two species, N. nucifera and N. lutea. As a eudicot whose lineage emerged prior to the divergence of core eudicots10, N. nucifera provides new insights into the origin of eudicots. The nuclear11,12 and chloroplast13 genomes of N. nucifera have recently been released. However, no information on the N. nucifera mitochondrial genome has been reported. Thus, it is important to sequence the N. nucifera mitochondrial genome to reveal the evolutionary characteristics of this plant and provide clues concerning the evolutionary trajectory from basal angiosperms to advanced eudicots.
Third-generation sequencing through single molecule real-time sequencing technology (SMRT)14,15 produces considerably longer (up to 30 kb) unbiased DNA sequences without PCR amplification16. This technology has previously been used in de novo assembly through the PacBio RS II platform17,18,19,20,21.
In the present study, using an optimized method for mitochondrial DNA isolation, we prepared N. nucifera mitochondrial DNA and sequenced the genome using SMRT technology. The mitochondrial genome map was constructed after de novo assembly and annotation of the sequence data. Our analyses provide insights into the evolution of gene content and order, RNA editing patterns, positively selected sites and chloroplast DNA insertions in core eudicots.
Results
N. nucifera mitochondrial DNA isolation and genome assembly
Mitochondria were purified from N. nucifera etiolated seedlings after discontinuous sucrose gradient centrifugation and DNase I digestion. Janus Green B staining showed that most isolated mitochondria were intact (Supplementary Figure S1). The 260/230 and 260/280 ratios of isolated mtDNA were 2.08 and 1.93, respectively. Semi-quantitative PCR showed that the isolated DNA was pure enough to build a library for sequencing (Supplementary Figure S2).
PacBio RSII sequencing generated 76,495 reads (341,866,338-bp in total), with a mean read quality of 0.83. After trimming off adapters and low quality regions and correcting by mapping short reads to long seeds, we have obtained 9,165 reads (42,623,117-bp in total, 4,651-bp per read on average) with an accuracy of 99%. After filtering chloroplast reads, a total of 7,151 reads (31,112,098-bp in total, 4,351-bp per read on average) were used for the assembly process, reaching a coverage depth of 59 over the N. nucifera mitochondrial genome. The assembly was verified by comparing with Sanger sequencing of PCR amplification using 18 primer pairs. ABI3730 sequencing generated a total of 20,176-bp sequences, representing 3.84% of the genome. Only one mismatch was detected at position 68,132 of the assembled mitochondrial genome (Supplementary Table S1), making the assembly accuracy of approximately 99.995%.
Genome size and content
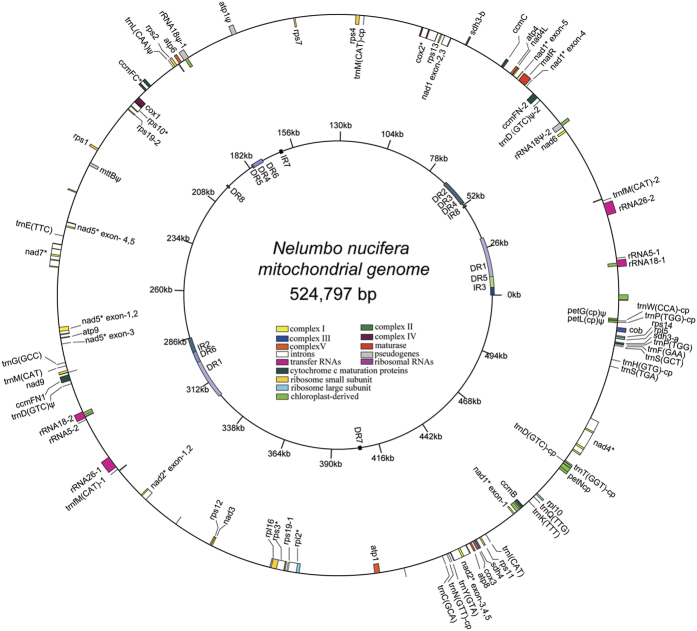
The N. nucifera mitochondrial genome is assembled into a single circular-mapping22 molecule of 524,797-bp (Table 1), with a GC content of 48.16%. To our knowledge, N. nucifera has the second highest GC content of all plant mitochondrial genomes, while the Butomus umbellatus mitochondrial genome has the highest GC content of 49.1%23 (Supplementary Table S2). Eight long repeats (>500-bp) including four direct repeats (DRs) and four inverted repeats (IRs) were identified, accounting for 9.3% (48,898-bp) of the total size. In addition to the long repeats, the N. nucifera mitochondrial genome also contained many small repeats (20- to 500-bp), comprising 3.2% (16,668-bp) of the total length. Two hundred and one simple sequence repeats (SSRs) were identified (Supplementary Table S3), accounting for 0.5% (2628-bp) of the total length.
Table 1. The statistics of the features of the Nelumbo nucifera mitochondrial genome.
| Category | Feature | Number | bp (%) |
|---|---|---|---|
| genome (524,797 bp) | G + C | 252,718 (48.16) | |
| Genes (total) | |||
| protein coding1 | 43 | 35651 (6.8) | |
| pseudogenes | 9 | 5541 (1.1) | |
| rRNA2 | 6 | 11407 (2.2) | |
| mt-derived tRNA | 13 | 1054 (0.2) | |
| cp-derived tRNA | 7 | 506 (0.1) | |
| ORF | 96 | 38062 (7.3) | |
| Introns | |||
| cis-spliced | 20 | 39780 (7.6) | |
| trans-spliced | 5 | n.a | |
| Repeats | |||
| Repeats(>500) | 8 | 48898 (9.3) | |
| Short Repeats(20<, <500) | 191 | 16668 (3.2) | |
| SSRs | 201 | 2628 (0.5) | |
1Each of gene ccmFN, rps19 and sdh3 has two copies.
2Each of the three ribosome RNAs (rrn26, rrn18, rrn5) has two copies.
The N. nucifera mitochondrial genome contains a total of 63 genes, including 40 protein-coding genes, three rRNA genes (rrn5, rrn18 and rrn26), 13 complete native mitochondrial tRNA genes and seven chloroplast-derived tRNA genes (Fig. 1, Table 2). Of these genes, ccmFN, rps19 and all three rRNA genes have two identical copies, while sdh3 has two different copies, sdh3-a and sdh3-b. sdh3-a is 39 bp longer than sdh3-b and has a 5-bp difference with sdh3-b at the 3′-terminal nucleotides (Supplementary Figure S3). Two different copies of a gene can result from horizontal gene transfer (HGT) events between angiosperm mitochondrial genomes5 or mtDNA recombination. However, the high sequence similarity (100% identity from 1 to 331 bases) of sdh3-a and sdh3-b suggests that these two copies result from N. nucifera mtDNA recombination rather than HGT from other species. Ninety-six unknown functional open reading frames (ORFs) were also predicted in the present study, comprising 7.3% (38,062-bp) of the total length (Table 1). The N. nucifera mitochondrial genome contained 25 Group II introns, including 20 cis-spliced and five trans-spliced introns. The total length of the 20 cis-spliced introns is 39,780-bp, accounting for 7.6% of the entire N. nucifera mitochondrial genome (Table 1),
Figure 1. The Nelumbo nucifera mitochondrial genome map.
Displayed as a circle, without implying that this is the in vivo conformation22. The genes are indicated in the outer circle, and the eight large repeats (>500-bp) with 99% identity are indicated in the inner circle. Chloroplast-derived regions are noted with a ‘-cp’ suffix. The same genes with different sequences are indicated with ‘-a,-b’. The same genes with the same sequences are indicated with ‘-1,-2′.
Table 2. List of the genes present in the mitochondrial genome of Nelumbo nucifera.
| Group of genes | Name of genes |
|---|---|
| Complex I | nad1*1, nad2*, nad3, nad4*, nad4L, nad5*, nad6, nad7*, nad9 |
| Complex II | sdh3-a, sdh3-b, sdh4 |
| Complex III | cob |
| Complex IV | cox. cox2. cox3 |
| Complex V | atp1. atp4. atp6. atp8. atp9 |
| Cytochrome c biogenesis | ccmB, ccmC, ccmFC*, ccmFN(×2) |
| Ribosome large subunit | rpl2*, rpl5, rpl10, rpl16 |
| Ribosome small subunit | rps1. rps2, rps3*. rps4. rps7. rps10*, rps11,rps12, rps13, rps14, rps19 (×2) |
| Intron maturase | matR |
| rRNA genes | rrn26(×2), rrn18(×2), rrn5(×2) |
| tRNA genes | trnC(GCA), trnE(TTC), trnG(GCC), trnK(TTT), trnM(CAT), trnfM(CAT)(×2), trnI(CAT), trnF(GAA), trnP(TGG), trnQ(TTG), trnS(GCT), trnS(TGA), trnY(GTA) |
| chloroplast-derived genes | petN(cp), trnM(CAT)-cp, trnN(GTT)-cp, trnD(GTC)-cp, trnT(GGT)-cp, trnH(GTG)-cp, trnP(TGG)-cp, trnW(CCA)-cp |
| Pseudogenes | atp1ψ, mttBψ, petG(cp)ψ, petL(cp)ψ, ycf3(cp)ψ, rRNA18-1ψ, rRNA18-2ψ, trnD(GTC)ψ, trnL(CAA)ψ |
1Genes with introns are indicated with asterisks (*).
To analyse the potential codon bias in the N. nucifera mitochondrial genome, RSCU (relative synonymous codon usage)24 was calculated using the coding sequences of 40 different mitochondrial protein-coding genes in N. nucifera. The results showed that A or U were more frequently used compared with G or C at the third position of N. nucifera mitochondrial codons (Supplementary Table S4), as observed in both chloroplast genomes and plant mitochondria genomes25,26. These results reflected a high A/T content at the third position of each codon27, that 61.27% of the N. nucifera mitochondrial codons have third position of A/T.
Conserved gene clusters
Based on the endosymbiont hypothesis that mitochondrial genomes are the remnants of a free-living prokaryote (most likely an extant α-proteobacterium), some small DNA fractions of the original genes have been identified in the mitochondrial DNA of higher order plants28. Therefore, conserved gene clusters remain present in some angiosperms despite multipartite organizations in angiosperm mitochondrial genomes29,30. A comparison of the collinearity among 38 analysed species revealed 15 collinear gene clusters in the N. nucifera mitochondrial genome relative to Liriodendron tulipifera (Supplementary Table S5): rrn18-rrn5, atp4-nad4L, atp8-cox3-sdh4, rpl2-rps19-rps3-rpl16, nad1_exon2,3-rps13, cox1-rps10, nad5_exon4,5-trnE(TTC)-nad7, nad5_exon1,2-atp9, nad3-rps12, trnN(GTT)(cp)-trnY(GTA), ccmB-trnK(TTT)-trnQ(TTG), trnS(GCT)-trnF(GAA)-trnP(TGG)-sdh3-a, cob-rps14-rpl5, trnP(TGG)(cp)- trnW(CCA)(cp) and petGψ-petLψ. Among these, six N. nucifera mitochondrial gene clusters were found conserved in other angiosperm species. Atp8-cox3-sdh4 occurred in both the ‘early-diverging’ angiosperm L. tulipifera and some eudicot species. Cox1-rps10, nad5_exon1,2-atp9, trnN(GTT)(cp)-trnY(GTA) and petGψ-petLψ were represented only in eudicots. Nad5_exon4,5-trnE(TTC)-nad7 was found only in L. tulipifera and N. nucifera. The nine remaining gene clusters were variously distributed among all of these taxa.
RNA editing of N. nucifera mitochondrial protein-coding genes
In the present study, with the exception of rpl10, all of the mitochondrial protein-coding genes of N. nucifera were successfully amplified, and a total of 33,719-bp cDNA sequences were sequenced; the amplicon for ccmFC was derived from pre-mRNA, with unspliced introns rather than mature mRNA. After aligning the DNA and cDNA sequences, 700 RNA editing sites were identified, including 612 nonsynonymous, 58 synonymous and 30 unpartitioned edits of the pseudogene mttB (Supplementary Table S6). As the same RNA editing sites were identified in both copies of sdh3, the RNA editing sites of the sdh3 genes were only counted once. Approximately 87% of the total editing sites changed the translated amino acid sequence. Three U-to-C edits were identified in ccmFN, cox1 and cox2, changing GUG (V) to GCG (A), UGC (C) to CGC (R) and UCU (S) to CCU (P), respectively. The most frequently edited genes were atp9 and ccmB, with editing frequencies of 7.111 edits/100 nt and 6.924 edits/100 nt, respectively, while the frequencies of other genes were less than five edits/100 nt. The genes with no RNA editing sites included ccmC, rps12, rps2 and sdh4. N. nucifera showed a pattern of relative editing levels across genes that was similar to other angiosperms31. For example, the ribosomal protein-coding genes had fewer edits, while the NADH dehydrogenase genes had more RNA edits.
In addition to RNA editing site analysis, we compared the RNA editing data obtained from Amborella trichopoda, L. tulipifera, Vitis vinifera and Oryza sativa with that of N. nucifera to identify similarities in the RNA editing sites (Supplementary Table S7). The results showed that the total number of RNA editing sites of A. trichopoda, L. tulipifera and N. nucifera (818, 802 and 700, respectively) were higher compared with V. vinifera and O. sativa (403 and 489, respectively). To examine similarities in RNA editing, we summarized the number of unique and shared edits in Supplementary Figure S4 using a pairwise comparison of the RNA editing sites of these five species. Compared with A. trichopoda, the maximum number of shared edits was 444 in L. tulipifera, followed by 361 in N. nucifera, 209 in O. sativa and 176 in V. vinifera. Compared with L. tulipifera, the maximum number of shared edits was 482 in N. nucifera, followed by 261 in O. sativa and 205 in V. vinifera. Moreover, N. nucifera contained the fewest number of unique edits (Supplementary Figure S4).
Phylogenetic and selection pressure analysis
To examine the phylogenetic evolution of N. nucifera mitochondria, a maximum likelihood (ML) tree of 79 plant mitochondrial genomes, including N. nucifera, was built using the sequences of 41 mitochondrial protein-coding genes and rooted with the green algae Mesostigma viride32 (Supplementary Figure S5). The resulting tree showed that the topology was consistent with the representatives of the Angiosperm Phylogeny Group (APG) III33. In the phylogenetic tree, the algae were sisters of land plants; pteridophyte Huperzia squarrosa was a sister of the gymnosperm Cycas taitungensis and Angiospermae. Within the Angiospermae clade, Amborella trichopoda was sister of all other angiosperms. The monocots and the eudicots were monophyletic; Spirodela polyrhiza was sister of all other monocots; and N. nucifera formed an independent basal branch as a sister of all other eudicots. Each of these relationships has 100% bootstrap value support.
To explore potential horizontal gene transfer (HGT) events that occurred in N. nucifera mitochondria, Maximum Likelihood (ML) trees were reconstructed from 41 individual gene alignments using optimal substitution models (Supplementary Figure S6). The support of topological structure of each gene tree was evaluated by both bootstrap values of 1,000 bootstrap replicates and approximate likelihood ratio test (aLRT) values. The results showed that, among the 41 single gene trees, N. nucifera was located at the bottom of the angiosperm cluster as sister to the early-derived angiosperms L. tulipifera and A. trichopoda in 19 gene trees, and located as the basal branch of eudicot clusters in another 18 gene trees. In the rest four trees, N. nucifera was also embedded in the eudicot clusters: In nad4L and rps19 trees, N. nucifera was sister to V. vinifera, which formed a basal branch of eudcot cluster in the mitochondria tree (Supplementary Figure S5). In cox1 tree, N. nucifera was sister to Vaccinium macrocarpon, which was sister of all other Asterids in the mitochondria tree. And in sdh4 tree, N. nucifera was sister to Malus x domestica which located at the bottom of Rosids cluster in the mitochondria tree. Many of the 41 gene trees had topologies inconsistent with the mitochondrial tree (Supplementary Figure S5) or the phylogenetic history of some plant species. For examples, H. squarrosa was embedded into the bryophytes cluster in 15 of all 33 gene trees containing H. squarrosa. And Chara vulgaris was clustered as sister to Nitella hyalina in all 32 gene trees containing C. vulgaris, 22 of which were well supported by both the bootstrap and aLRT values. Some eudicots were found among totally unrelated plant phyla in several gene trees: In atp9 tree, B. umbellatus was clustered as sister to eudicots Silene vulgaris and Silene latifolia with bootstrap value of 85% and aLRT value of 0.89, but formed a long branch. In nad7 tree, V. macrocarpon was embedded in the monocots cluster as sister to Spirodela polyrhiza. In rps7 tree, Salvia miltiorrhiza and Boea hygrometrica were sisters to Entransia fimbriata with 100% bootstrap support and 100% aLRT support, but formed long branches. In rps11 tree, V. vinifera was embedded into alga cluster. In rpl2 tree, eudicots S. miltiorrhiza, Ajuga reptans and Daucus carota were clustered within alga branch. And in sdh3 tree, eudicot Millettia pinnata was also embedded into alga cluster. Several observations may explain the cause of these tree abnormalities. The affinity of S. miltiorrhiza and B. hygrometrica with E. fimbriata in rpl2 tree was more of a long branch attraction (LBA) of highly divergent sequences, because the alignment demonstrated that the rps7 sequence of E. fimbriata was highly divergent from that of S. miltiorrhiza and B. hygrometrica. NCBI-BLASTN searches of these rps7 genes against the Nucleotide Collection Database showed that rps7 in S. miltiorrhiza and B. hygrometrica mitochondria have shown 100% identity with rps7 of their own chloroplast genomes, while rps7 in E. fimbriata showed low similarity with other nucleotides. The abnormalities in rps11 tree and rpl2 tree could also be caused by genes of chloroplast origin, because BLASTN showed a high similarly of V. vinifera mitochondrial rps11 with its chloroplast rps11, the rpl2 of S. miltiorrhiza, A. reptans and D. carota also showed 100% similarities with their chloroplast ones. The sdh3 of M. pinnata showed extremely low similarity with records in both nucleotide collection database and non-redundant protein database of NCBI, which leads to the embedding of M. pinnata into alga cluster with a extremely long branch. The abnormalities in nad7 tree may be caused by lacking of informative sites due to the limited sequence variations, since the deletion of S. polyrhiza and Phoenix dactylifera leads to the relocation of V. macrocarpon as sister to D. carota in nad7 tree with higher branch support values. And the alignments showed that V. macrocarpon shows higher similarity with D. carota than with S. polyrhiza. The abnormalities in atp9 tree may be also caused by lacking of informative sites considering its short length (225-bp in the alignments) and the high similarity between eudicots and monocots.
Mitochondrial protein-coding genes are essential for the assembly of respiratory chain complexes and the translation of proteins. Therefore, these genes are subject to purifying selection pressures34. The improved branch-site model was used to identify the positively selected sites of 41 mitochondrial genes in N. nucifera using the alignments and gene trees of the 79 mitochondria genomes. After likelihood ratio tests (LRT), alternative models were significantly favoured in 27 genes. However, in other genes, alternative models were not preferable to the null model (Supplementary Table S8). Positively selected sites were identified in 14 genes after Bayes Empirical Bayes (BEB) analyses. Among these 14 genes, only two genes had positively selected sites that were significantly supported by posterior probability values: 96.1% and 95.6% of the 25th and 31st sites in sdh4, respectively, and 99.6% of 36th site in cox1. The low proportion of positively selected genes in N. nucifera suggested that most of the N. nucifera mitochondrial genes were conserved and remained stable during evolution. The positively selected sites in cox1 and sdh4 might imply functional variations. Thus, the structural analysis and functional verification of these two genes will be necessary in subsequent research.
Chloroplast DNA insertions in N. nucifera mtDNA
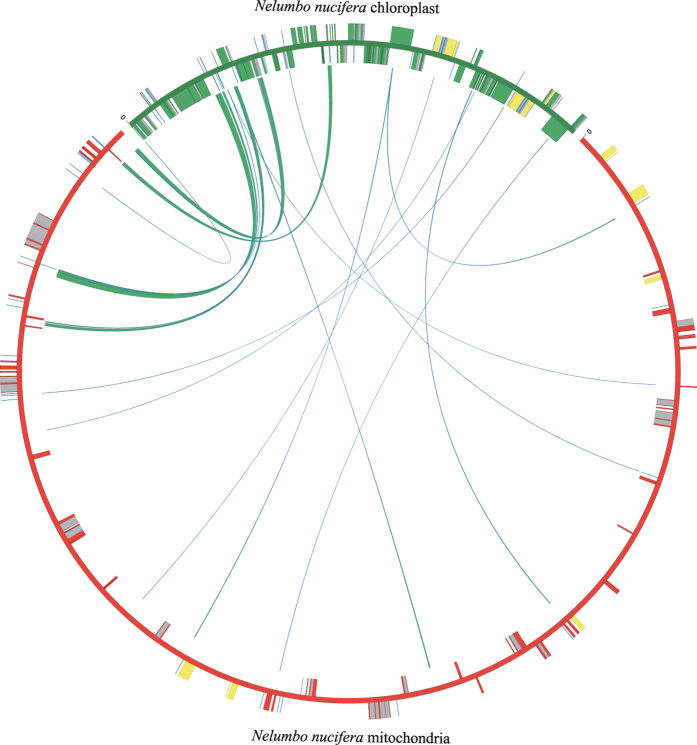
Nineteen fragments of chloroplast DNA ranging from 54 to 1,998-bp were identified in N. nucifera mtDNA (Fig. 2, Table 3). The total size was 8,256-bp, approximately 1.6% of the N. nucifera mitochondrial genome and 5.0% of the chloroplast genome. After comparing the 19 chloroplast-derived fragments with the 78 other plant mitochondrial genomes used in the present study, homologues of 15 fragments were identified in other plant mtDNA (Supplementary Table S9). None of these chloroplast-derived fragments was observed in algae, bryophytes or the lycophyte H. squarrosa. The 141-bp fragment (positions 472035-472175) and the two shortest fragments (positions 91514-91569 and 342137-342190) were unique to the N. nucifera mitochondrial genome. The third shortest fragment (positions 413440-413496) was only identified in A. trichopoda. The longest fragment (positions 469237-471234) was only identified in L. tulipifera. The 84-, 81- and 78-bp fragments containing the trnN(GTT), trnH(GTG) and trnM(CAT) genes, respectively, had homologues in more than 40 species. The 84-bp fragment was commonly identified in angiosperms, but was absent in the gymnosperm C. taitungensis, while the 81- and 78-bp fragments were commonly observed in both angiosperms and C. taitungensis.
Figure 2. Schematic representation of chloroplast DNA transferred into the Nelumbo nucifera mitochondrial genome.
The green line within the circle shows the regions of the plastid genome that has been inserted into different locations of the mitochondrial genome. The genes in plastid and mitochondria are indicated by green and red boxes, respectively. The introns are indicated by grey boxes, and rRNAs and tRNAs are indicated by yellow and blue boxes, respectively. The boxes on the inside and outside of the maps were transcribed in clockwise and counterclockwise directions, respectively.
Table 3. Chloroplast Insertions in the Nelumbo nucifera mitochondrial genome.
| No. | Length | Position | Genes Contained |
|---|---|---|---|
| 1 | 1998 | 469237–471234 | petN;psbM |
| 2 | 1387 | 523411–524797 | ycf3 |
| 3 | 1229 | 516533–517761 | petL-petG-trnW(CCA)-trnP(UGG) |
| 4 | 997 | 451200–452196 | psbC |
| 5 | 621 | 471275–471895 | trnD(GTC) |
| 6 | 321 | 230963–231283 | psbC |
| 7 | 297 | 452532–452828 | psbD;psbC |
| 8 | 211 | 181488–181698 | ndhD |
| 9 | 206 | 29058–29263 | ycf2 |
| 10 | 206 | 318795–319000 | ycf2 |
| 11 | 145 | 523258–523402 | ycf3 |
| 12 | 141 | 472035–472175 | trnT(GGT) |
| 13 | 87 | 285719–285805 | ycf2 |
| 14 | 84 | 426801–426884 | trnN(GTT) |
| 15 | 81 | 505480–505560 | trnH(GTG) |
| 16 | 78 | 123420–123497 | trnM(CAT) |
| 17 | 57 | 413440–413496 | ndhE |
| 18 | 56 | 91514–91569 | trnT(GGT) |
| 19 | 54 | 342137–342190 | trnA(TGC) |
Pseudogenes were shown in regular font. Genes with intact ORFs and genes which encode tRNAs with intact folding ability are highlighted in bold.
Discussion
We sequenced the N. nucifera mitochondrial genome using third-generation SMRT sequencing technology and constructed a genomic map. In our previous work13, a comparison between N. nucifera chloroplast assemblies obtained using Sanger, Illumina MiSeq, and PacBio RS II platforms indicated that SMRT sequencing technology showed great promise for the production of highly accurate genome sequences because it produces long reads and lacks bias. In this study, the assembly validation experiment showed a great accuracy of N. nucifera mitochondrial genome assembly.
As the first publicly available basal eudicot mitochondrial genome, the N. nucifera mitochondrial genome provides valuable insights into the pattern of the evolution from basal angiosperms to advanced eudicots, and shows ancient evolutionary features in many aspects:
Conservation of genomic content. The N. nucifera mitochondrial genome retains 40 of all the 41 protein-coding genes present in ancestral flowering plant mitochondrial genomes35. This is consistent with previously described Southern blot results36, which suggest that the Nelumbo mitochondrial genome contains the full set of protein-coding genes present in the ancestral angiosperm. To our knowledge, of all available angiosperm mitochondrial genomes, only A. trichopoda5 and L. tulipifera37 have the full set of 41 protein-coding genes. Frequent loss and transfer of mitochondrial genes, particularly of the ribosomal protein-coding genes and succinate dehydrogenase (sdh) genes, has occurred in other angiosperm mitochondrial genomes and results in variable gene content among angiosperms36. The total length of the 20 cis-spliced introns of the N. nucifera mitochondrial genome is longer than any other sequenced angiosperm mitochondrial genome37. Currently, the complete set of 20 cis-spliced introns has only been identified in the mitochondria of three angiosperm species, including N. nucifera (in the present study), the ‘early-diverging’ angiosperm L. tulipifera and the ‘early-diverging’ core eudicot Vitis vinifera. In contrast, the five trans-spliced introns in N. nucifera were conserved in all of the sequenced angiosperm mitochondrial genomes.
Conservation of gene clusters. As a sister group, the ‘early-diverging’ angiosperm L. tulipifera occurred prior to the divergence of monocots and eudicots, showing primitive features in gene content and at RNA editing sites37. Hence, the L. tulipifera mitochondrial genome is a scale to explore the evolution of N. nucifera and other angiosperms. The N. nucifera mitochondrial genome has gene clusters that can date back to the original bacterial ancestor of mitochondria (rrn18-rrn5, rpl2-rps19-rps3-rpl16), gene clusters unique to angiosperms (atp8-cox3-sdh4, nad1_exon2,3 -rps13), a gene cluster unique to eudicots (petGψ-petLψ) and a gene cluster (nad5_exon4,5-trnE(TTC)-nad7) that is only shared with L. tulipifera. This further indicates that the N. nucifera mitochondrial genome was more primitive than other advanced angiosperms in angiosperm evolution.
Retention of ancient RNA editing sites. During the evolution of advanced angiosperms, RNA editing sites showed various degrees of subsequent losses in different lineages37,38,39. Though the emergence and maintenance of RNA editing in plants has not yet been clearly explained, a hypothesis falling under the scheme of “constructive neutral evolution” holds that editing may have emerged through neutral processes, to replace functionally important cytosines that require post-transcriptional editing to produce a conserved amino acid40. According to this hypothesis, most editing sites are nonsynonymous. In N. nucifera, approximately 87% of the total editing sites changed the translated amino acid sequence. Comparing RNA editing sites of A. trichopoda, L. tulipifera, V. vinifera and O. sativa with that of N. nucifera clearly shows that the degree of editing loss in N. nucifera is much lower than that in V. vinifera and O. sativa, suggesting that the N. nucifera mitochondrial genome was more ancient.
N. nucifera was originally considered to be the closest relative to Nymphaea alba because of morphological similarities, reflecting convergent evolution with N. alba in the same aquatic environment41. However, phylogenetic analyses of angiosperms based on their nuclear genome have revealed that N. nucifera is a member of the Proteales within the basal eudicots and phylogenetic analyses using chloroplast sequences clustered N. nucifera into the basal eudicot branches as a sister to Meliosma and Platanus13. In this study, a phylogenetic tree built using the sequences of 41 mitochondrial protein-coding genes also puts N. nucifera as a sister to all other eudicots. Finally, the phylogenetic analysis in the present study, along with previous studies based on chloroplast and nuclear genome data, provided a comprehensive view of the basal location of N. nucifera in eudicots. Thus, we concluded that N. nucifera is an ‘early-diverging’ basal eudicot. In the most of the 41 gene trees, the N. nucifera sequences were clustered in the basal location in eudicots, suggesting that N. nucifera mitochondrial genes maintained low evolutionary rates. Topological incongruence was observed in different species of many single gene trees in the present study. The embedding of H. squarrosa into the bryophytes clusters confirmed Liu’s study on H. squarrosa mitochondria42 which suggests the mitochondrial genome as the most archaic form in vascular plants and shows a gene content resembling those of charophytes and most bryophytes. C. vulgaris is considered to be the most closely related alga to land plants43. Turmel’s study on C. vulgaris mitochondria43 shows that C. vulgaris mtDNA strikingly resembles Marchantia polymorpha mtDNA, supported by both genome comparisons and phylogenetic analyses. The sister-group relationship between C. vulgaris and N. hyalina in our study suggested that C. vulgaris mtDNA may have more similarities with N. hyalina mtDNA. The embedding of species into totally unrelated plant phyla identified in six gene trees was expected to be possible HGT candidates. But our further analysis showed that this topological incongruence was caused either by long branch attraction of highly divergent genes, or by lacking of informative sites due to the limited sequence variations. Of the eight highly divergent genes, six genes were found similar to their own chloroplast genes, and three of these six genes were reported as chloroplast-derived by former studies (rps11 of V. vinifera6, rpl2 of A. reptans44 and rpl2 of D. carota4). If assuming correct genome assembly and annotation, the low similarity of sdh3 of M. pinnata mitochondria compared with other nucleotide in the NCBI nucleotide collection database may show some new insights. However, in the present study, we found none of the topological incongruences showed a reliable evidence of HGT event. And among the 41 gene trees, N. nucifera was clustered with either eudicots or the early-derived angiosperms, indicating that HGT from other plant mitochondria rarely occurred in N. nucifera mitochondrial genes.
However, intracellular gene transfer (IGT) of DNA from the plastid to the mitochondrial genomes of higher plants is a common phenomenon on an evolutionary timescale. Sometimes, these IGT events may initiate a gain of functional tRNAs. Due to the slow rate of mitochondrial sequence change, some of the chloroplast-derived fragments identified in N. nucifera mitochondria may trace back to retention of an earlier HGT event. The absence of the 19 chloroplast-derived fragments in algae, bryophytes and the lycophyte H. squarrosa indicates that DNA transfer from the chloroplast genome to the mitochondrial genome in N. nucifera might have occurred after the gymnosperm/angiosperm divergence. We found that the trnN(GTT)-contained chloroplast-derived fragment was absent in the gymnosperm C. taitungensis while the trnH(GTG)- and trnM(CAT)-containing fragments were commonly observed in both angiosperms and C. taitungensis. This finding is consistent with the analysis of chloroplast-derived tRNA gene evolution37. Thus, it is likely that the chloroplast-derived genes trnH(GTG) and trnM(CAT), previously present in C. taitungensis, were obtained from an earlier transfer, while trnN(GTT) might have occurred after the separation of gymnosperms and angiosperms. The short stretches of these three fragments in N. nucifera suggested that the selection of the flanking sequences might have occurred prior to the separation of early-diverging angiosperms and basal eudicots. Plant mitochondrial tRNAs were derived from three origins: (i) ‘native’ mitochondrial tRNAs, orthologous to those in moss mitochondria and derived from a mitochondrial ancestor; (ii) chloroplast-like mitochondrial tRNAs, highly homologous to chloroplast DNA; and (iii) unknown-origin mitochondrial tRNAs, such as the bacterial-like trnC(GCA), obtained through horizontal gene transfer events45,46. Compared to all sequenced angiosperm mitochondrial genomes, the N. nucifera mitochondrial genome had a complete set of 13 intact ‘native’ mitochondrial tRNAs, which were also identified in the ‘early-diverging’ core eudicot V. vinifera, but variously presented in other angiosperms. The ‘native’ mitochondrial tRNAs retained in gymnosperms, trnL(CAA) and trnD(GTC), have become pseudogenes in N. nucifera during the evolution of angiosperms47. The absence of trnL(CAA) and trnD(GTC) was compensated with chloroplast-derived trnL(CAA) or cytosolic tRNAs3. The N. nucifera mitochondrial genome contained seven chloroplast-derived tRNA genes present in the ‘early-diverging’ core eudicot V. vinifera. Notably, the chloroplast-derived trnT(GGT) gene included in the 141-bp unique fragment in N. nucifera was also identified within a 3,571-bp chloroplast-derived fragment in V. vinifera mitochondria6 but was absent in other plant mitochondrial genomes, suggesting that the gain of the chloroplast-derived trnT(GGT) gene in N. nucifera and V. vinifera mitochondria were separate events.
Though dozens of flowering plant mitochondrial genomes are published, early-derived angiosperms or basal eudicot mitochondrial genomes are still limited. The difficulty of isolating mitochondrial DNA is a bottleneck in acquiring complete mitochondrial genome sequence data. The optimized method for the isolation of mitochondrial DNA from plants rich in secondary metabolites used in the present study would promote the sequencing of more plant mitochondria. The genomic data of other basal eudicots, such as Platanus, will provide more detailed insights into mitochondrial genome evolution in basal eudicots.
Materials and Methods
Mitochondrial genome DNA isolation
Mature seeds of N. nucifera were harvested from the Wuhan Moshan Botanical Garden (114°22′ N, 30°33′ E) of Hubei Province, China and germinated in the dark at room temperature. Discontinuous sucrose gradient centrifugation combined with DNase I digestion in vitro was used to separate mitochondrial DNA from etiolated seedlings. Three pairs of primers specific to genes in the nuclear, mitochondria and chloroplast were designed to evaluate the purity of the mtDNA through semi-quantitative PCR. A full description of the mtDNA isolation method has been provided in Supplementary Method S1.
Library construction and sequencing
A total of 20 μg of standard mtDNA was required to construct a size-selected ~20 kb SMRT-bell library. Subsequently, the SMRT-bell library was sequenced using three SMRT cells (Pacific Biosciences) with C2 chemistry on a PacBio RS II sequencing platform14. The sequencing and de novo assembly were performed at Yale University, Connecticut, America and Nextomics, Wuhan, China, respectively.
Genome assembly and annotation
The primitive reads from the Pacbio RS II were filtered and corrected by mapping short reads to long “seed” reads (6,000-bp) using SMRT Analysis 2.1 (Filtering parameters: Minimum Subread Length = 500, Minimum Polymerase Read Quality = 0.8, and Minimum Polymerase Read Length = 100. Correcting parameters: Alignment Candidates Per Chunk = 10, Total Alignment Candidates = 24, Number Of Seed Read Chunks = 6, Blasr: -noSplitSubreads -minReadLength 200 -maxScore -1000 -maxLCPLength 16 ). Then, the reads were mapped to both the Nelumbo nucifera chloroplast genome [GenBank:KM655836] and the NCBI chloroplast genome database using Blat48 with default settings to filter chloroplast reads, reads with more than 90% matched and identity of more than 90% were removed. Both Celera Assembler 7.049 and MIRA 4.0rc5 were used for the assembly process. Reads longer than 8,000-bp were extracted for assembly. In the Celera Assembler assembly process, the fastqToCA utility in Celera Assembler was used to generate wrapper LIB messages, reads were assembled into contigs using runCA (-p asm -d asm -s asm.spec) and then aligned to the contigs using the BWA-SW algorithm in BWA-0.7.3a50 with a mismatch penalty set to 5, contigs with less than 10X coverage depth were filtered. In the MIRA assembly process, the assembly was performed with parameters of “-GE:not = 20 –hirep_best -SK:mchr = 30 -HS:mnr = yes -HS:nrr = 5 PCBIOHQ_SETTINGS -CL:pec = no technology = PCBIOHQ”, redundancy was subsequently removed using self-to-self alignment performed by LastZ51. Both assembly processes output four contigs, these contigs were subsequently assembled into one contig using LastZ. At last, Quiver was used to correct the error regions and generate the best consensus sequence for the mitochondrial genome16. Default parameters were otherwise employed for processes above. To verify the assembly, 18 primer pairs were designed to target random selected intergenic regions (Supplementary Table S1).
The N. nucifera mitochondrial genome was manually annotated based on the output of NCBI-BLASTN and NCBI-BLASTX searches to a custom local database. The online tool tRNAscan-SE52 was also used to search the tRNA genes. Conserved ORFs were identified through BLASTX searches of the NCBI database. The mitochondrial genome map of N. nucifera was drawn using Genome Vx53. Large repeats (>500-bp) were identified through BLASTN searches with 99% identities. Chloroplast insertions were identified through BLASTN searches of the N. nucifera chloroplast genome with 70% identity and an e-value of 1e–5. To identify the nuclear-derived sequences, the protein-coding genes, cis-spliced introns, rDNAs, tRNAs and chloroplast-derived sequences were masked; subsequently, the masked sequences were searched in the N. nucifera nuclear genome. The RSCU (relative synonymous codon usage) of the N. nucifera mitochondrial genome was calculated from the coding sequences of 40 different protein-coding genes. MISA54 was used to screen for perfect SSRs, including mono-, di-, tri-, tetra-, penta- and hexa-nucleotide repeats. The minimum number of repeats was set at 10, 5, 4, 3, 3 and 3, respectively.
Mitochondrial genome gene collinear analysis
To evaluate the collinear relationships between genes, all protein-coding genes, tRNA genes and rRNA genes from the N. nucifera mitochondria genome were compared to 37 representative species of angiosperm mitochondria genomes available in NCBI using BLASTP/BLASTX55. The blast results were inputted into MCscan56 with default parameters to compute multiple synteny. The final gene collinearity results were generated from the MCscan output file by swapping the gene order number of each gene with their names using a Perl script.
RNA editing of N. nucifera mitochondrial protein-coding genes
Total RNA was isolated from tender leaves using the modified CTAB method57(Supplementary Figure S7, a); total cDNA was produced through RT-PCR (reverse transcription PCR) (Supplementary Figure S7,b). The genome sequence data were used to design PCR primers for all 40 protein-coding genes and one pseudogene (Supplementary Table S10). These primers were immediately flanked by genes based on the assumption that these regions comprised parts of the 5′ and 3′ untranslated regions, and the predicted PCR products were searched in the expressed sequence tags database using NCBI-BLASTN to evaluate whether the primers were in the untranslated regions. These genes were amplified from both the total DNA and total cDNA using the following conditions: 35 cycles of (40 s at 94 °C, 40 s at 48~56 °C and 1.5~2 min at 72 °C), with an initial step of 5 min at 94 °C and a final step of 10 min at 72 °C. The amplicons were detected on 1% agarose gels and purified using a Gel Extraction Kit (BioDev-Tech, Beijing, China). The purified cDNA amplicons were directly sequenced using an ABI 3730, and some of the purified products were also ligated into the pMD18-T Easy vector (TaKaRa, Dalian, China), transformed into Top10 chemically competent E. coli and subsequently sequenced. The sequenced cDNAs were compared with the genomic sequence and aligned using ClustalW58 implemented in MEGA524. The editing sites were detected according to the alignments and the chromatogram peaks of the cDNA sequences and subsequently partitioned based on a nonsynonymous or synonymous effect using a Perl script. The RNA editing data of A. trichopoda5, L. tulipifera37, O. sativa59 and V. vinifera6 were acquired from GenBank or REDIdb and parsed using Perl scripts.
Phylogenetic and selection pressure analysis
In addition to the sequenced mtDNA of N. nucifera, the protein-coding sequences from 78 published complete mitochondrial sequences of angiosperms were downloaded from the NCBI database (Supplementary Table S11). All 41 protein-coding genes were selected for phylogenetic analysis. These nucleotide sequences were separately aligned using ClustalW and manually adjusted, and the independent alignments were subsequently concatenated. The program jModelTest60 was used to identify the most appropriate substitution model and combination of gamma distribution and proportion of invariant sites, with the parameter number of rate categories set to 4. Both the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) were calculated. During the analysis, if the predicted models of AIC and BIC were not the same, AIC models were used (instead of BIC models). Phylogenetic analyses of each gene and the concatenated sequences were performed using the maximum likelihood (ML) method implemented in RAxML-HPC v8.1.261 according to the optimal substitution models under the rapid bootstrap algorithm (1000 replicates). To test support for the branch points of each gene trees, nonparametric branch support tests based on the Shimodaira-Hasegawa-like approximate likelihood ratio test (SH-like aLRT) procedure were performed using phyML62 with the tree topology search operation option of Nearest Neighbour Interchange (NNI), and the same substitution models as used in RAxML trees. R packages ape63 and ggtree64 were used to combine the branch supports of bootstrap value with SH-like aLRT value in each gene tree and present the tree structures. The alignments can be found in Supplementary Data S1, the results of jModelTest and all the trees can be found in Supplementary Data S2.
The program Codeml in the PAML package65 was used to identify the positively selected genes in the N. nucifera mitochondrial genome. The phylogenetic trees of 41 protein-coding genes were used as tree structure files in Codeml, and N. nucifera was indicated as foreground in all gene trees. Test 2 of Model A in the branch-site model was selected, using the following parameters: in the null hypothesis, model = 2, NSsites = 2, fix_omega = 1, omega = 1; in the alternative hypothesis, model = 2, NSsites = 2, fix_omega = 0, omega = 1.5; and other parameters were left by default. Likelihood ratio tests (LRT) were performed, and the P value was calculated using the chi2 program in PAML packages.
Chloroplast insertions in N. nucifera mtDNA
After sequence annotation, the protein-coding genes, tRNA genes and rRNA genes of the N. nucifera mitochondrial genome were masked to eliminate BLAST hits of the conserved household genes in chloroplast and mitochondrial genomes. The masked genome sequence was BLASTed against the chloroplast genome of N. nucifera. The chloroplast-derived fragments were subsequently BLASTed against a local database containing all Viridiplantae mitochondrial genomes available in NCBI to identify homologues with an identity greater than 80% and full-length or nearly full-length (more than 90%) alignment. The maps of N. nucifera mitochondrial and chloroplast genomes and the chloroplast fragment transferences were drawn using Circos v.0.6766.
Availability of supporting data
The precise mitochondrial genome of N. nucifera has been submitted to GenBank [GenBank: KR610474]. The raw sequence data have been deposited in the Short Read Archieve (SRA) database of NCBI [SRA: SRP066826], note that only data from SMRT cell 3 (named “mt_s3.fastq”) was used for the assembly in this study. Other data sets supporting the results of the present study are included within the article and supplementary information.
Additional Information
How to cite this article: Gui, S. et al. The mitochondrial genome map of Nelumbo nucifera reveals ancient evolutionary features. Sci. Rep. 6, 30158; doi: 10.1038/srep30158 (2016).
Supplementary Material
Acknowledgments
The authors thank Professor Palmer JD (Department of Biology, Indiana University) for direction on mtDNA isolation, and Jiajian Zhou (BSgenomics Co., Ltd., ShenZhen) for bioinformatic help. This research is financially supported by National Natural Science Foundation of China (31271310).
Footnotes
Author Contributions Designed the general idea and experiments of the study: Y.D., Z.W. Isolated the N. nucifera mtDNA and carried out most of the experiments, and drafted the manuscript: S.G., Z.W. Performed annotation of mtDNA and some of the experiments: H.Z., Y.Z., Z.Z. and D.L. Modified the final manuscript: S.G., Z.W. and Y.D.
References
- Handa H. The complete nucleotide sequence and RNA editing content of the mitochondrial genome of rapeseed (Brassica napus L.): comparative analysis of the mitochondrial genomes of rapeseed and Arabidopsis thaliana. Nucleic Acids Res 31, 5907–5916 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan D. B. et al. Rapid evolution of enormous, multichromosomal genomes in flowering plant mitochondria with exceptionally high mutation rates. PLoS Biol 10, e1001241 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T. & Newton K. J. Angiosperm mitochondrial genomes and mutations. Mitochondrion 8, 5–14 (2008). [DOI] [PubMed] [Google Scholar]
- Iorizzo M. et al. De novo assembly of the carrot mitochondrial genome using next generation sequencing of whole genomic DNA provides first evidence of DNA transfer into an angiosperm plastid genome. BMC Plant Biol 12, doi: 10.1186/1471-2229-12-61 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D. W. et al. Horizontal transfer of entire genomes via mitochondrial fusion in the angiosperm Amborella. Science 342, 1468–1473 (2013). [DOI] [PubMed] [Google Scholar]
- Goremykin V. V., Salamini F., Velasco R. & Viola R. Mitochondrial DNA of Vitis vinifera and the issue of rampant horizontal gene transfer. Mol Biol Evol 26, 99–110 (2009). [DOI] [PubMed] [Google Scholar]
- Hsu J. Late Cretaceous and Cenozoic vegetation in China, emphasizing their connections with North America. Ann Mo Bot Gard 70, 490–508 (1983). [Google Scholar]
- Upchurch G. R. Jr, Crane P. R. & Drinnan A. N. The megaflora from the Quantico locality (Upper Albian), lower cretaceous Potomac group of Virginia. Mem Virginia Mus Nat Hist 4, 1–57 (1994). [Google Scholar]
- Crane P. R. & Herendeen P. S. Cretaceous floras containing angiosperm flowers and fruits from eastern North America. Rev Palaeobot Palynol 90, 319–337 (1996). [Google Scholar]
- Moore M. J., Soltis P. S., Bell C. D., Burleigh J. G. & Soltis D. E. Phylogenetic analysis of 83 plastid genes further resolves the early diversification of eudicots. Proc Natl Acad Sci USA 107, 4623–4628 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming R. et al. Genome of the long-living sacred lotus (Nelumbo nucifera Gaertn). Genome Biol 14(5), 1–11 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. et al. The sacred lotus genome provides insights into the evolution of flowering plants. Plant J 76, 557–567 (2013). [DOI] [PubMed] [Google Scholar]
- Wu Z. et al. A precise chloroplast genome of Nelumbo nucifera (Nelumbonaceae) evaluated with Sanger, Illumina MiSeq, and PacBio RS II sequencing platforms: insight into the plastid evolution of basal eudicots. BMC Plant Biol 14, doi: 10.1186/s12870-014-0289-0 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail M. A. et al. A tale of three next generation sequencing platforms: comparison of Ion Torrent, Pacific Biosciences and Illumina MiSeq sequencers. BMC Genomics 13, doi: 10.1186/1471-2164-13-341 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. J., Carneiro M. O. & Schatz M. C. The advantages of SMRT sequencing. Genome Biol 14, doi: 10.1186/gb-2013-14-7-405 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin C. S. et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods 10, 563–569 (2013). [DOI] [PubMed] [Google Scholar]
- Pugh T. J. et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature 488, 106–110 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarini M. et al. An evaluation of the PacBio RS platform for sequencing and de novo assembly of a chloroplast genome. BMC Genomics 14, doi: 10.1186/1471-2164-14-670 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichot E. B. & Norman R. S. Microbial phylogenetic profiling with the Pacific Biosciences sequencing platform. Microbiome 1, doi: 10.1186/2049-2618-1-10 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. et al. High-throughput platform for real-time monitoring of biological processes by multicolor single-molecule fluorescence. Proc Natl Acad Sci USA 111, 664–669 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K. et al. First complete genome sequence of Pseudomonas aeruginosa (Schroeter 1872) Migula 1900 (DSM 50071T), determined using PacBio single-molecule real-time technology. Genome Announc 3, e00932–00915 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendich A. J. Structural analysis of mitochondrial DNA molecules from fungi and plants using moving pictures and pulsed-field gel electrophoresis. J Mol Biol 255, 564–588 (1996). [DOI] [PubMed] [Google Scholar]
- Cuenca A., Petersen G. & Seberg O. The complete sequence of the mitochondrial genome of Butomus umbellatus–a member of an early branching lineage of Monocotyledons. PLoS One 8, e61552 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K. et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28, 2731–2739 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raubeson L. A. et al. Comparative chloroplast genomics: analyses including new sequences from the angiosperms Nuphar advena and Ranunculus macranthus. BMC Genomics 8, doi: 10.1186/1471-2164-8-174 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan D. B. & Taylor D. R. Testing for selection on synonymous sites in plant mitochondrial DNA: the role of codon bias and RNA editing. J Mol Biol 70, 479–491 (2010). [DOI] [PubMed] [Google Scholar]
- Morton B. R. & Levin J. A. The atypical codon usage of the plant psbA gene may be the remnant of an ancestral bias. Proc Natl Acad Sci USA 94, 11434–11438 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. W. Evolution of organellar genomes. Curr Opin Genet Dev 9, 678–687 (1999). [DOI] [PubMed] [Google Scholar]
- Nakazono M. et al. The rps3-rpl16-nad3-rps12 gene cluster in rice mitochondrial DNA is transcribed from alternative promoters. Curr Genet 27, 184–189 (1995). [DOI] [PubMed] [Google Scholar]
- Sugiyama Y. et al. The complete nucleotide sequence and multipartite organization of the tobacco mitochondrial genome: comparative analysis of mitochondrial genomes in higher plants. Mol Genet Genomics 272, 603–615 (2005). [DOI] [PubMed] [Google Scholar]
- Alverson A. J. et al. Insights into the evolution of mitochondrial genome size from complete sequences of Citrullus lanatus and Cucurbita pepo (Cucurbitaceae). Mol Biol Evol 27, 1436–1448 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turmel M., Otis C. & Lemieux C. The complete mitochondrial DNA sequence of Mesostigma viride identifies this green alga as the earliest green plant divergence and predicts a highly compact mitochondrial genome in the ancestor of all green plants. Mol Biol Evol 19, 24–38 (2002). [DOI] [PubMed] [Google Scholar]
- Bremer B. et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc 161, 105–121 (2009). [Google Scholar]
- Lynn D. J. et al. Bioinformatic discovery and initial characterisation of nine novel antimicrobial peptide genes in the chicken. Immunogenetics 56, 170–177 (2004). [DOI] [PubMed] [Google Scholar]
- Mower J. P., Sloan D. B. & Alverson A. J. Plant mitochondrial genome diversity: the genomics revolution. In Plant Genome Diversity. Vol. 1 (eds. Wendel J. F. et al. ) 123–144 (Springer-Verlag Wien, 2012). [Google Scholar]
- Adams K. L., Qiu Y. L., Stoutemyer M. & Palmer J. D. Punctuated evolution of mitochondrial gene content: high and variable rates of mitochondrial gene loss and transfer to the nucleus during angiosperm evolution. Proc Natl Acad Sci USA 99, 9905–9912 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A. O., Rice D. W., Young G. J., Alverson A. J. & Palmer J. D. The “fossilized” mitochondrial genome of Liriodendron tulipifera: ancestral gene content and order, ancestral editing sites, and extraordinarily low mutation rate. BMC Biol 11, doi: 10.1186/1741-7007-11-29 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields D. C. & Wolfe K. H. Accelerated evolution of sites undergoing mRNA editing in plant mitochondria and chloroplasts. Mol Biol Evol. 14, 344–349 (1997). [DOI] [PubMed] [Google Scholar]
- Mower J. P. Modeling sites of RNA editing as a fifth nucleotide state reveals progressive loss of edited sites from angiosperm mitochondria. Mol Biol Evol 25, 52–61 (2008). [DOI] [PubMed] [Google Scholar]
- Stoltzfus A. On the possibility of constructive neutral evolution. J Mol Biol 49, 169–181 (1999). [DOI] [PubMed] [Google Scholar]
- Kreunen S. S. & Osborn J. M. Pollen and anther development in Nelumbo (Nelumbonaceae). Am J Bot 86, 1662–1676 (1999). [PubMed] [Google Scholar]
- Liu Y. et al. The mitochondrial genome of the lycophyte Huperzia squarrosa: the most archaic form in vascular plants. PLoS One 7, e35168 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turmel M., Otis C. & Lemieux C. The mitochondrial genome of Chara vulgaris: insights into the mitochondrial DNA architecture of the last common ancestor of green algae and land plants. Plant Cell 15, 1888–1903 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu A., Guo W., Jain K. & Mower J. P. Unprecedented heterogeneity in the synonymous substitution rate within a plant genome. Mol Biol Evol 31, 1228–1236 (2014). [DOI] [PubMed] [Google Scholar]
- Kitazaki K. et al. A horizontally transferred tRNACys gene in the sugar beet mitochondrial genome: evidence that the gene is present in diverse angiosperms and its transcript is aminoacylated. Plant J 68, 262–272 (2011). [DOI] [PubMed] [Google Scholar]
- Knie N., Polsakiewicz M. & Knoop V. Horizontal gene transfer of chlamydial-like tRNA genes into early vascular plant mitochondria. Mol Biol Evol 32, 629–634 (2015). [DOI] [PubMed] [Google Scholar]
- Chaw S. M., Shih A. C. C., Wang D., Wu Y. W. & Liu S. M. The mitochondrial genome of the gymnosperm Cycas taitungensis contains a novel family of short interspersed elements, Bpu sequences, and abundant RNA editing sites. Mol Biol Evol 25, 603–615 (2008). [DOI] [PubMed] [Google Scholar]
- Kent W. J. BLAT—the BLAST-like alignment tool. Genome Res 12, 656–664 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. R. et al. Aggressive assembly of pyrosequencing reads with mates. Bioinformatics 24, 2818–2824 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. & Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. S. Improved pairwise alignment of genomic DNA. PhD thesis, Pennsylvania State Univ. (2007). [Google Scholar]
- Schattner P., Brooks A. N. & Lowe T. M. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res 33, W686–W689 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant G. C. & Wolfe K. H. GenomeVx: simple web-based creation of editable circular chromosome maps. Bioinformatics 24, 861–862 (2008). [DOI] [PubMed] [Google Scholar]
- Thiel T., Michalek W., Varshney R. & Graner A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.). Theor Appl Genet 106, 411–422 (2003). [DOI] [PubMed] [Google Scholar]
- McGinnis S. & Madden T. L. BLAST: at the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Res 32, W20–W25 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H. et al. Unraveling ancient hexaploidy through multiply-aligned angiosperm gene maps. Genome Res 18, 1944–1954 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambino G., Perrone I. & Gribaudo I. A rapid and effective method for RNA extraction from different tissues of grapevine and other woody plants. Phytochem Anal 19, 520–525 (2008). [DOI] [PubMed] [Google Scholar]
- Larkin M. A. et al. Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948 (2007). [DOI] [PubMed] [Google Scholar]
- Notsu Y. et al. The complete sequence of the rice (Oryza sativa L.) mitochondrial genome: frequent DNA sequence acquisition and loss during the evolution of flowering plants. Mol Genet Genomics 268, 434–445 (2002). [DOI] [PubMed] [Google Scholar]
- Posada D. jModelTest: phylogenetic model averaging. Mol Biol Evol 25, 1253–1256 (2008). [DOI] [PubMed] [Google Scholar]
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S. & Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52, 696–704 (2003). [DOI] [PubMed] [Google Scholar]
- Paradis E., Claude J. & Strimmer K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 (2004). [DOI] [PubMed] [Google Scholar]
- Yu G., Smith D., Zhu H., Guan Y. & Lam T. T. Y. ggtree: a phylogenetic tree viewer for different types of tree annotations, Available at: https://github.com/Bioconductor-mirror/ggtree/tree/ release-3.2 (Accessed: 12 April 2016) (2015). [Google Scholar]
- Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol 24, 1586–1591 (2007). [DOI] [PubMed] [Google Scholar]
- Krzywinski M. et al. Circos: an information aesthetic for comparative genomics. Genome Res 19, 1639–1645 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.