Abstract
Kai-Xin-San (KXS), a Chinese herbal decoction for anti-depression, is a combination of paired-herbs, i.e. Ginseng Radix et Rhizoma (GR)-Polygalae Radix (PR) and Acori Tatarinowii Rhizoma (ATR)-Poria (PO). The make-up of the paired-herbs has been commonly revised according to syndrome differentiation and treatment variation of individual. Currently, an optimized KXS (KXS2012) was prepared by functional screening different combination of GR-PR and ATR-PO. The aim of this study was to verify the effect and underlying mechanism of KXS2012 against depression in chronic mild stress (CMS)-induced depressive rats and in primary cultures of neurons and astrocytes. In rat model, the CMS-induced depressive symptoms were markedly alleviated by the treatment with KXS2012. The CMS-suppressed neurotransmitter amounts were restored in the presence of KXS2012. And the expressions of neurotropic factors and its corresponding receptors were increased under KXS2012 administration. In cultured neurons, application of KXS2012 could promote neurogenesis by inducing the expression of synaptotagmin and dendritic spine density. Moreover, application of KXS2012 in cultured astrocytes, or in H2O2-stressed astrocytes, induced the expressions of neurotrophic factors: the increase might be associated with the modification of Erk1/2 and CREB phosphorylation. Our current results fully support the therapeutic efficacy of KXS2012 against depression in cell and animal models.
Depression, also known as major depressive disorder, is a serious mental illness characterized by constant feeling of low self-esteem and loss of interest or pleasure1. Depression is a complex disease with multiple genetic and environmental pathogenesis. The main pathological changes of depression include neuron/synapse reduction, change in neurotransmitter system and loss of neurotrophic factors, especially in cortex and hippocampus2. In addition to neurodegenerative symptoms, untreated depression may progress to suicide or metabolic disorders (diabetes and cardiovascular diseases)3,4. However, current anti-depression drugs that possess severe side effects are not effective to all the patients. Thus, developing anti-depression drug is urgently needed5.
Kai-Xin-San (KXS), a traditional Chinese medicine (TCM), was firstly recorded in Beiji Qianjin Yaofang < Thousand Formulae for Emergency > by Sun Simiao (581–685 A.D.) of Tang Dynasty in 652 A.D6,7. KXS has been used in treating mental disorders, especially depression for thousands of years in China. In animal studies, KXS was shown to relieve depression-like symptoms8,9,10, and the mechanism was proposed to be mediated by increasing the amounts of neurotransmitters and neurotrophic factors in the brain11. In cell cultures, the functions of KXS in regulating the expressions of neurotrophic factors in cultured astrocytes and neurons were reported12,13.
KXS composes of two paired-herbs, i.e. Ginseng Radix et Rhizoma (GR; root and rhizome of Panax ginseng C. A. Mey.; Araliaceae family) - Polygalae Radix (PR; root of Polygala tenuifolia Wild.; Polygalaceae family) and Acori Tatarinowii Rhizoma (ATR; rhizome of Acorus tatarinowii Schott; Acoraceae family) - Poria (PO; sclerotium of Poria cocos (Schw.) Wolf; Polyporaceae family). The make-up of these two paired-herbs in KXS has been commonly changed according to syndrome differentiation and treatment variation, which causes confusion in clinics. Thus, an optimized KXS (KXS2012, GR-PR: ATR-PO = 1:5) was prepared by functional screening the combination of GR-PR and ATR-PO14. Here, we propose it is reasonable to use KXS2012 to attenuate depression. With this in mind, we evaluate the functions of KXS2012 against depression on cell and animal studies.
Among different depressive animal models, the chronic mild stress (CMS)-induced depressive rat is usually selected for its similarity with the true state of depressive patients15,16. Thus, the CMS-induced depressive rat model promises a stable and credible approach in evaluating the function of anti-depressants, and the study on tissue samples from depressive rats indicates the underlying mechanism against depression. Moreover, neuron/synapse reduction is one of the most severe pathogenesis during depression. In depressed brain, nerve reduction supports the neurogenesis theory of depression, which posits that depression is largely caused by an impairment of the brain’s ability to produce neurons, a process known as neurogenesis. This disorder is reversible when depressed patients are treated, which presents a novel and effective therapeutic target against depression17,18. The study on neurogenesis using neuron and astrocyte cultures predicts the potential cellular mechanism against depression, which helps to represent biological functionality and integration of molecular information. In this study, we aimed to discover the functions of KXS2012 against depression as well as the potential underlying mechanism. Our results could accelerate the development of new therapy for anti-depression.
Results
Standardization of herbal extracts
GR-PR and ATR-PO extracts were prepared according to ancient preparation of Chinese herbal mixture. The extraction efficiency was about 25.79 ± 3.25% for GR-PR extract and 10.32 ± 2.78% for ATR-PO extract (Mean ± SD, n = 3). Two approaches were chosen to control the quality of paired-herb extracts: (i) chemical fingerprinting; and (ii) assessment of chemical markers. Known chemicals were identified in chemical fingerprints (Fig. S1A), which accorded to our published reports7,14. Eight chemical markers were selected: e.g. GR-derived ginsenosides (Rb1, Rd, Re, Rg1), PO-derived pachymic acid, PR-derived 3,6′-disinapoyl sucrose (330 nm) and ATR-derived α-asarone (258 nm) and β-asarone (258 nm) (Fig. S1). In GR-PR extract, the amount in mg/g of dried herbal extract was about 4.287 ± 0.065 for ginsenosides Rb1, 0.397 ± 0.046 for ginsenosides Rd, 3.648 ± 0.079 for ginsenosides Re, 3.304 ± 0.084 for Rg1 and 9.678 ± 0.067 for 3,6′-disinapoyl sucrose. In ATR-PO extract, the amount in mg/g of dried herbal extract was about 6.292 ± 0.017 for α-asarone, 0.736 ± 0.014 for β-asarone and 0.021 ± 0.003 for pachymic acid (Mean ± SD, n = 3). The established chemical parameters served as the control for repeatability of the below animal and biochemical analyses. Thus, KXS2012 was referring to a mixture of GR-PR extract plus ATR-PO extract at 1: 5 weight ratio.
KXS2012 reverses the depression-like symptoms in CMS-induced depressive rats
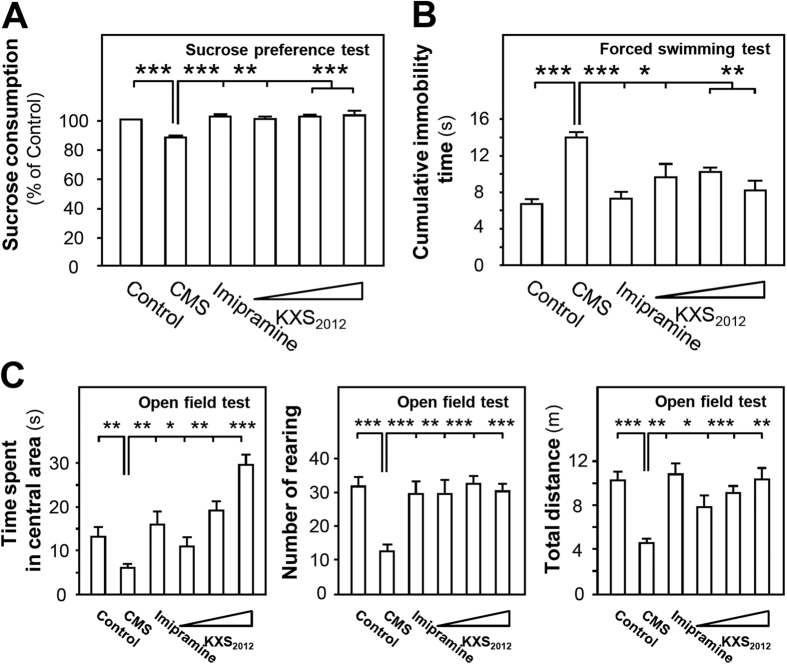
Three animal behavior tests including sucrose preference, forced swimming and open field tests were employed to evaluate the function of KXS2012 against depression in rat. After the treatment of herbal extracts for 6 weeks, KXS2012 at three doses (low dose: 60.9 mg/kg/day, medium dose: 182.7`mg/kg/day and high dose: 548.1 mg/kg/day) alleviated sucrose preference (~20%) of CMS-induced depressive rats (Fig. 1A). In forced swimming test, the CMS-induced depressive rats doubled cumulative immobility time, while KXS2012 at all doses restored the cumulative immobility time (Fig. 1B). In open field test, the CMS-induced depressive rats showed a decrease of over 50% movements. However, the treatment of KXS2012 at all doses reversed the time spent in central area of CMS-induced depressive rats: this treatment also affected the horizontal and vertical movements of KXS2012-treated rats (Fig. 1C). Imipramine (20 mg/kg/day) was set as a positive control, and non-stressed rats were set as another control.
Figure 1. KXS2012 alleviates the depression-like symptoms in CMS-induced depressive rats.
The CMS-induced depressive rats were randomly divided into six groups: control, CMS model, imipramine (20 mg/kg/day), KXS2012 low dose (60.9 mg/kg/day), KXS2012 medium dose (182.7 mg/kg/day) and KXS2012 high dose (548.1 mg/kg/day). After drug treatment, sucrose preference (A), forced swimming (B) and open field tests (C) were carried out, as described in the method session. Values are expressed in percentage of un-stressed control, second (s), number or miter (m), Mean ± SEM, n = 8, *p < 0.05, **p < 0.01 and ***p < 0.001 compared to the control.
KXS2012 restores the levels of neurotransmitters in CMS-induced depressive rat brain
According to previous study, a systematic method was used to determine the amounts of neurotransmitters in rat brain by HPLC-fluorescence detector (FLD)19,20. The detected neurotransmitters included: norepinephrine, dopamine and serotonin. In addition, 5-hydroxyindoleacetic acid (5-HIAA), a major metabolite of serotonin (5-HT), was also determined. The ratio of 5-HIAA/5-HT represents the metabolic state of serotonin. Thus, the ratio of 5-HIAA/5-HT was calculated to interpret the metabolism of serotonin. The HPLC conditions for neurotransmitter detection were determined. The analytic parameters are listed in Table S1. The chromatograms of neurotransmitters are shown in Fig. S2.
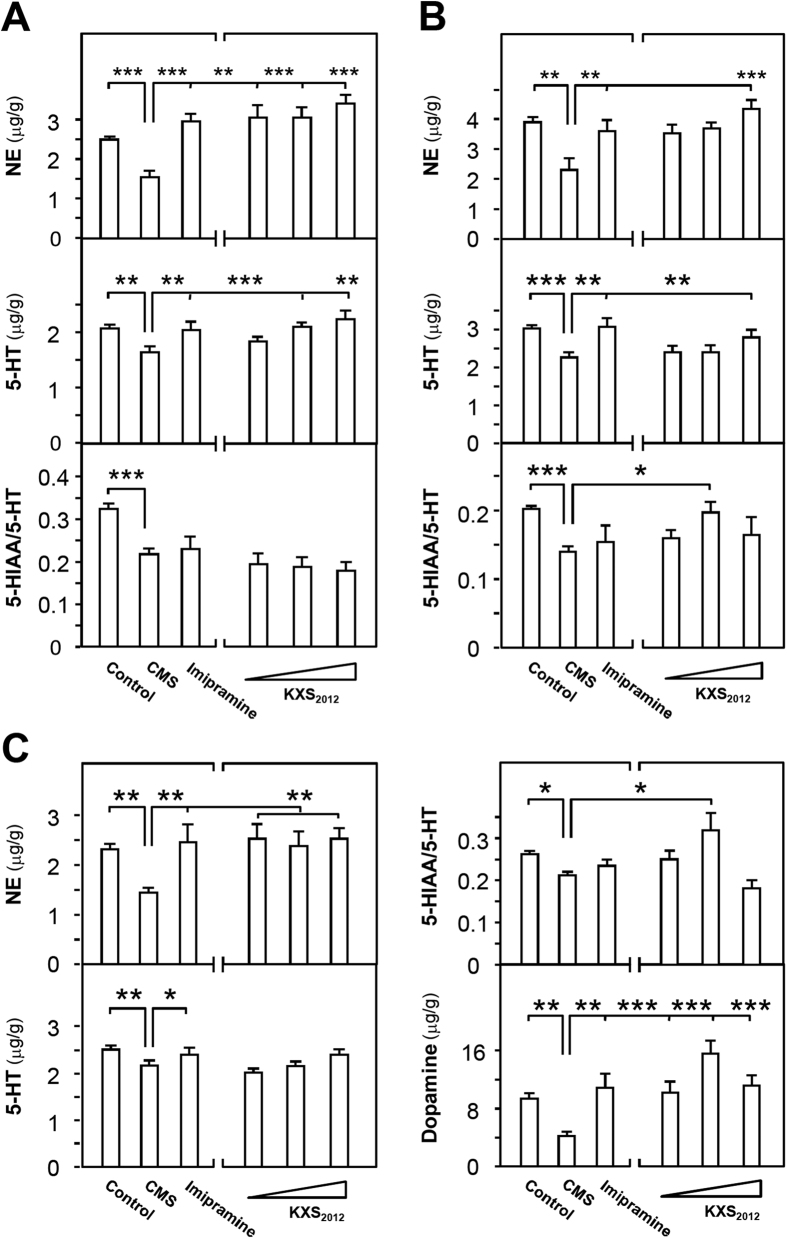
Three brain regions were selected here, i.e. hippocampus, cerebral cortex and striatum. In CMS-induced depressive rat hippocampus, the amounts of serotonin and norepinephrine, as well as the ratios of 5-HIAA/5-HT, were decreased to ~80%, ~60% and ~65%, respectively (Fig. 2A). The treatment of KXS2012 at all doses restored the levels of serotonin and norepinephrine. However, the KXS2012 treatment failed to reverse the ratio of 5-HIAA/5-HT caused by CMS (Fig. 2A). In depressive rat cerebral cortex, the reduction in amounts of serotonin (~25%), norepinephrine (~40%) and ratio of 5-HIAA/5-HT (~25%) were observed. KXS2012 at all doses restored the levels of serotonin and norepinephrine in cortex. In parallel, KXS2012 at medium dose (182.7 mg/kg/day) increased the ratio of 5-HIAA/5-HT to normal state (Fig. 2B). In depressive rat striatum, the amounts of serotonin, dopamine and norepinephrine, as well as the ratio of 5-HIAA/5-HT, were reduced from 20% to 50%. KXS2012 at all doses restored the levels of serotonin, dopamine and norepinephrine, as well as the ratio of 5-HIAA/5-HT in the striatum (Fig. 2C). Imipramine (20 mg/kg/day) was used as a positive control, and non-stressed rats were set as another control.
Figure 2. KXS2012 restores the levels of neurotransmitters in CMS-induced depressive rat brain.
(A) The CMS-induced depressive rats were randomly divided into six groups as the protocol in Fig. 1. After drug treatment, the hippocampus was collected. The amounts of norepinephrine (NE), serotonin (5-HT) and ratio of 5-HIAA/5-HT in the extracts of hippocampus were calculated, as described in method session. Similar procedures were applied onto cerebral cortex (B) and striatum (C) of CMS-induced depressive rats. Additionally, the level of dopamine in striatum (C) was determined (bottom right). Dopamine level was too low to be measured in hippocampus and cortex. Values are expressed in μg/g or the number of ratio, Mean ± SEM, n = 8, *p < 0.05, **p < 0.01 and ***p < 0.001 compared to the corresponding control.
KXS2012 up-regulates neurotrophic factor expressions in CMS-induced depressive rat brain
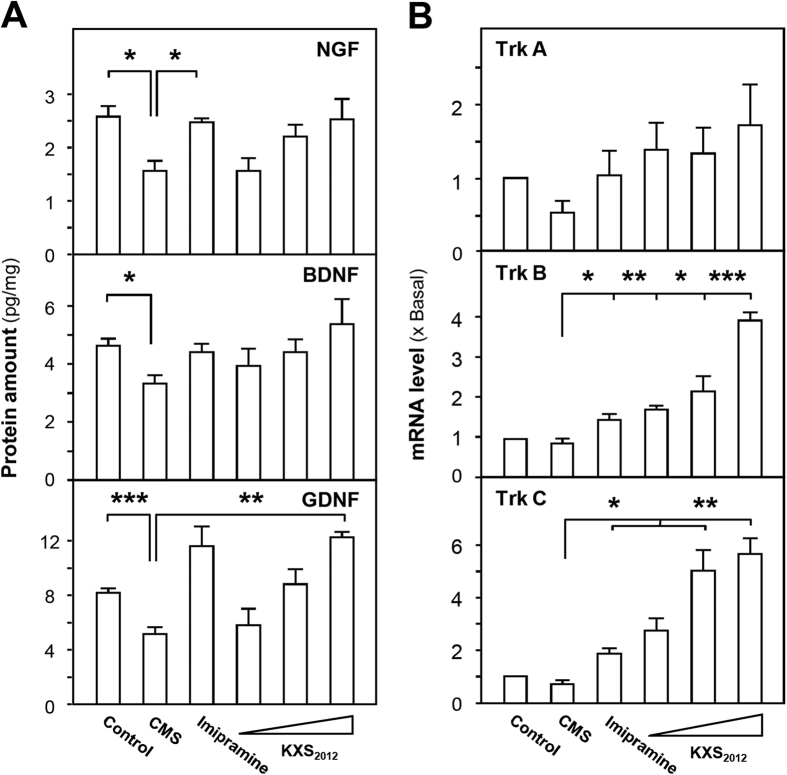
The deficiency of neurotrophic factors is one of the indicative pathogens during depression. Here, the protein levels of nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and glia cell-derived neurotrophic factor (GDNF) in depressive rat cerebral cortex were detected by ELISA. The standard curves for calibration were: Y = 0.002X + 0.026 (NGF), R2 = 0.998; Y = 0.001X−0.044 (BDNF), R2 = 0.990; Y = 0.000516X + 0.00648 (GDNF), R2 = 1.000 (Fig. S3). The protein levels of NGF, BDNF and GDNF in cortex were markedly decreased in CMS-induced depressive rat cortex; however, this reduction was alleviated after KXS2012 treatment (Fig. 3A). In the same rat samples, the CMS-reduced mRNA expressions of neurotrophic factor receptors, including tropomyosin receptor kinase (Trk) A, Trk B and Trk C, were restored by the treatment of KXS2012 (Fig. 3B). In particularly, KXS2012 at medium and high doses largely increased the mRNA expression levels of Trk B and Trk C, indicating that KXS2012 might exert anti-depression through up-regulating neurotrophic factor signaling pathway (Fig. 3B). The induction of Trk receptors by KXS2012 was robust from 2 to 6 folds as compared to the control, which was much better than the positive control imipramine at 20 mg/kg/day.
Figure 3. KXS2012 restores the levels of neurotrophic factors and their receptors in CMS-induced depressive rat brain.
(A) The CMS-induced depressive rats were randomly divided into six groups as the protocol as in Fig. 1. After drug treatment, the protein levels of neurotrophic factors (NGF, BDNF and GDNF) in depressive rat cerebral cortex were analyzed by ELISA. The calibration curves of protein amounts were from Fig. S3. (B) The mRNA expression levels of neurotrophic factor receptors (Trk A, Trk B and Trk C) in depressive rat cerebral cortex were analyzed by real-time quantitative PCR. Values are expressed in pg/mg of protein or the number of fold to control (x Basal), Mean ± SEM, n = 4, *p < 0.05, **p < 0.01 and ***p < 0.001 compared to the corresponding control.
KXS2012 promotes neurogenesis in cultured neurons
The impairment of the brain’s ability to promote neurogenesis underlies depression. As expected, the cellular study of KXS2012 on primary cultured rat cortical neurons and hippocampal neurons were employed to investigate the function of KXS2012 on neurogenesis. Cultured cortical neurons could undergo the progress of differentiation from day in vitro (DIV) 1 to DIV 30. Thus, DIV 5 and DIV 15 were chosen as early and middle stage of neuron differentiation. Synapse formation involves pairing of the pre- and post-synaptic partners at a specific neuro-spatial coordinate21,22,23. The optimized dose of KXS2012 in cultured cortical neurons was determined by MTT assays, i.e. the dose did not affect the cell number, as shown in Fig. S4A. In DIV 5 of cortical neurons, KXS2012 increased the expression of synaptic vesicle protein, synaptotagmin: the maximal induction could reach over 4 folds (Fig. 4A). Synaptotagmin is a Ca2+ sensor regulating neurotransmitter release and hormone secretion. The expression of PSD95, a post-synaptic marker, however was not altered. In DIV 15 of hippocampal neurons, KXS2012 increased the dendritic spine density (number of spine per 10 μm segment) at post-synaptic sites by ~4 folds, which was higher than the positive control BDNF application (Fig. 4B,C). The KXS2012-induced dendritic spine density could serve as an indicator of increased possible contacts between neurons. In addition, a remarkable increase of synaptotagmin expression was revealed indicating the potential function of KXS2012 in synaptogenesis. Thus, the function of KXS2012 against depression might be mediated by promoting neurogenesis in the brain.
Figure 4. KXS2012 promotes neurogenesis in cultured neurons.
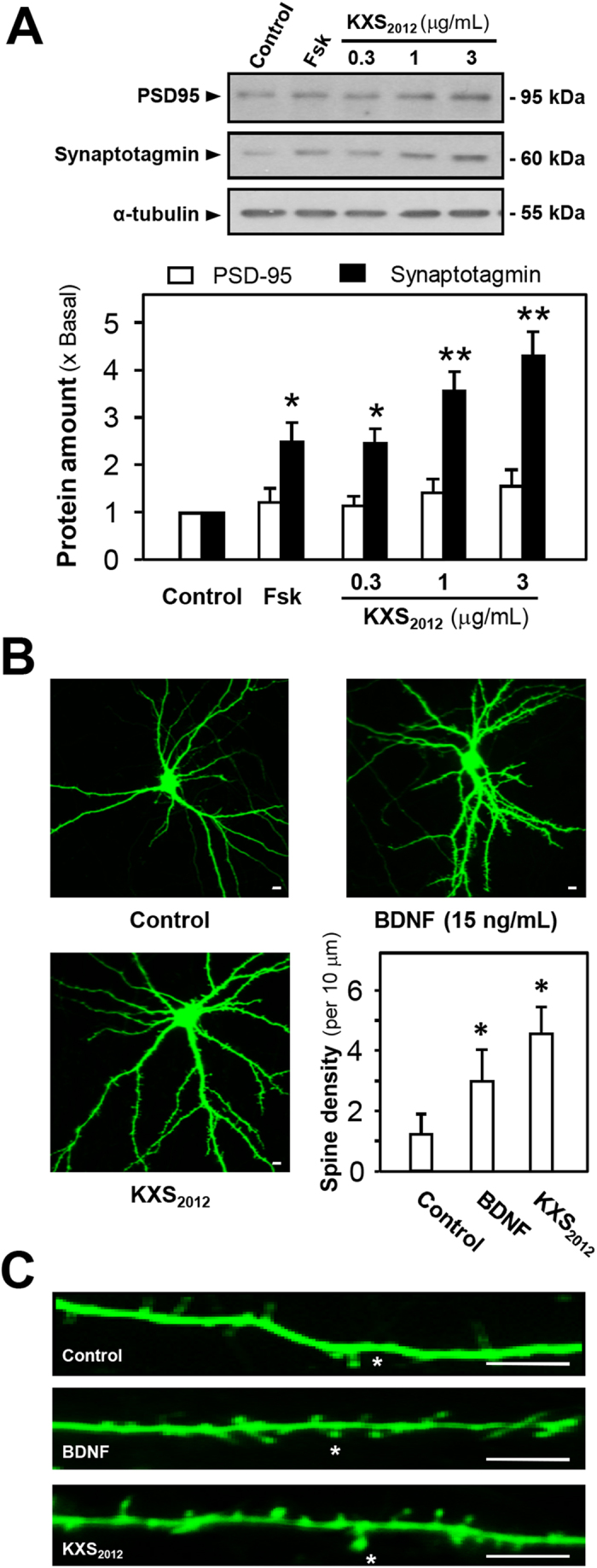
(A) KXS2012 (0.3 to 3 μg/mL) was applied onto cultured rat cortical neurons (DIV 5) for 96 h. The protein levels of PSD-95 (~95 kDa) and synaptotagmin (~60 kDa) were measured by Western blot assay (upper panel). α-Tubulin (~55 kDa) served as a loading control. Forskolin (Fsk, 50 nM) was used as a positive control. The amount of protein level was quantified (lower panel). (B) KXS2012 (3 μg/mL) and BDNF (15 ng/mL) were applied onto cultured hippocampal neurons (DIV 15) for 96 h before pEGFP-N1 transfection. The treatment significantly increased the spine density of control neurons (9–16 dendrites from 6–10 neurons per 10 μm were measured for each condition). The quantification plot was shown in lower right. (C) Representative images show that KXS2012 (3 μg/mL) and BDNF (15 ng/mL) increased the spine density compared to control. The counted spine density was indicated by asterisk. Scale bars = 10 μm. Values are expressed the fold of change as compared to control (x Basal) or number, Mean ± SEM, n = 4–10. *p < 0.05 and **p < 0.01 compared to the control.
KXS2012 up-regulates the expressions of neurotrophic factors in normal and H2O2-stressed astrocytes
Neurotrophic factors secreted from astrocytes play key roles in neurogenesis, and which are down-regulated in brain during depression. The optimized dose of KXS2012 in cultured astrocytes was determined by MTT assays, i.e. the dose did not affect the cell number, as shown in Fig. S4B. In cultured astrocytes, application of KXS2012 greatly increased the mRNA and protein levels of NGF, BDNF and GDNF: the maximal induction was at ~2 folds (Fig. 5A). For the underlying mechanism, the expressions of neurotrophic factors are mediated by cAMP-protein kinase A (PKA) and mitogen-activated protein kinases (MAPK) signaling pathways in astrocytes24,25,26. The phosphorylation levels of cAMP response element-binding protein (CREB) and extracellular regulated protein kinases (Erk) 1/2 were determined. In cultured astrocytes, KXS2012 increased the phosphorylation of Erk1/2 (~1.5 folds) and CREB (~2 folds) in a time-dependent manner (Fig. 5B,C). Then, H89 and U0126, the inhibitors of PKA and MEK1/2, were selected for further study. In all scenarios, application of both inhibitors in cultured astrocytes blocked the KXS2012-induced mRNA levels of NGF, BDNF and GDNF (Fig. 5D). The KXS2012-mediated phosphorylation of CREB was fully blocked by H89, and reduced by ~60% with U0126. On the other hand, U0126 fully blocked KXS2012-mediated phosphorylation of Erk1/2, while H89 did not show any effect (Fig. S5). These results of inhibitors therefore suggested the activation of KXS2012 should be firstly at PKA, then to Erk1/2. Erk1/2, which was reported to be located downstream of PKA, participated in the PKA-mediated CREB phosphorylation27. In addition, the condition medium derived from KXS2012-treated astrocytes did not significantly increase the expressions of synaptotagmin and PSD-95 in cultured cortical neurons (Fig. S6). These results suggested that the potential functional relationship of KXS2012 between neurons and astrocytes might be complicated and need further study.
Figure 5. KXS2012 up-regulates neurotrophic factor expressions in cultured astrocytes.
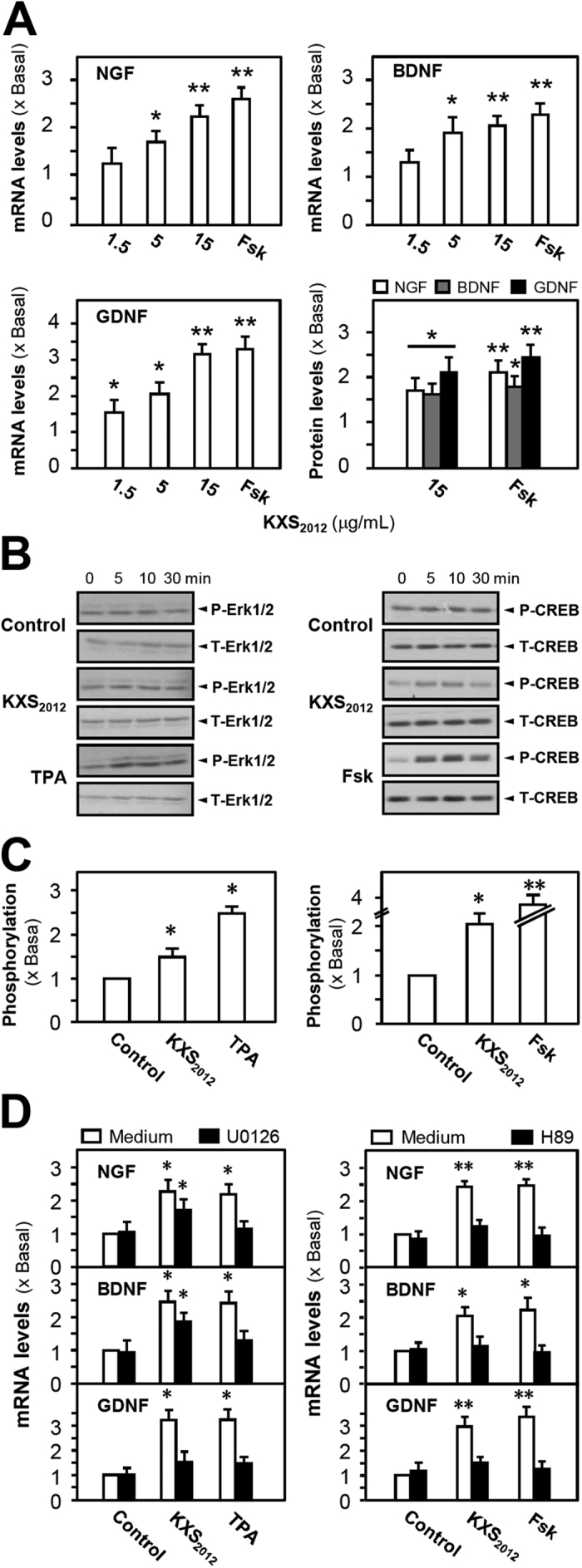
(A) The mRNA and protein levels of neurotrophic factors in cultured astrocytes were analyzed by real-time quantitative PCR and ELISA kits (calibration curves of protein amounts were in Fig. S3). The cultured astrocytes were treated with KXS2012 (1.5 to15 μg/mL) for 48 h. Forskolin (Fsk, 50 nM) was used as a positive control. (B) Cultured astrocytes were serum starved for 5 h. Then KXS2012 (15 μg/mL) was applied onto the cultures. Fsk (50 nM) or TPA (50 nM) served as a positive control, respectively. Total Erk1/2, phosphorylated Erk1/2 (both at ~42/44 kDa), total CREB and phosphorylated CREB (both at ~40 kDa) were revealed by using specific antibodies at different time. (C) Quantification plot of the phosphorylation level at 10 min was shown. (D) U0126 (20 μM) and H89 (5 μM) were applied onto astrocytes 3 h before the drug treatment. Quantification plot of mRNA and protein levels of neurotrophic factors was shown. Fsk (50 nM) or TPA (50 nM) served as a positive control, respectively. Values are expressed as the fold of change as compared to control (x Basal), where control value is set as 1, Mean ± SEM, n = 4, *p < 0.05 and **p < 0.01 compared to the control.
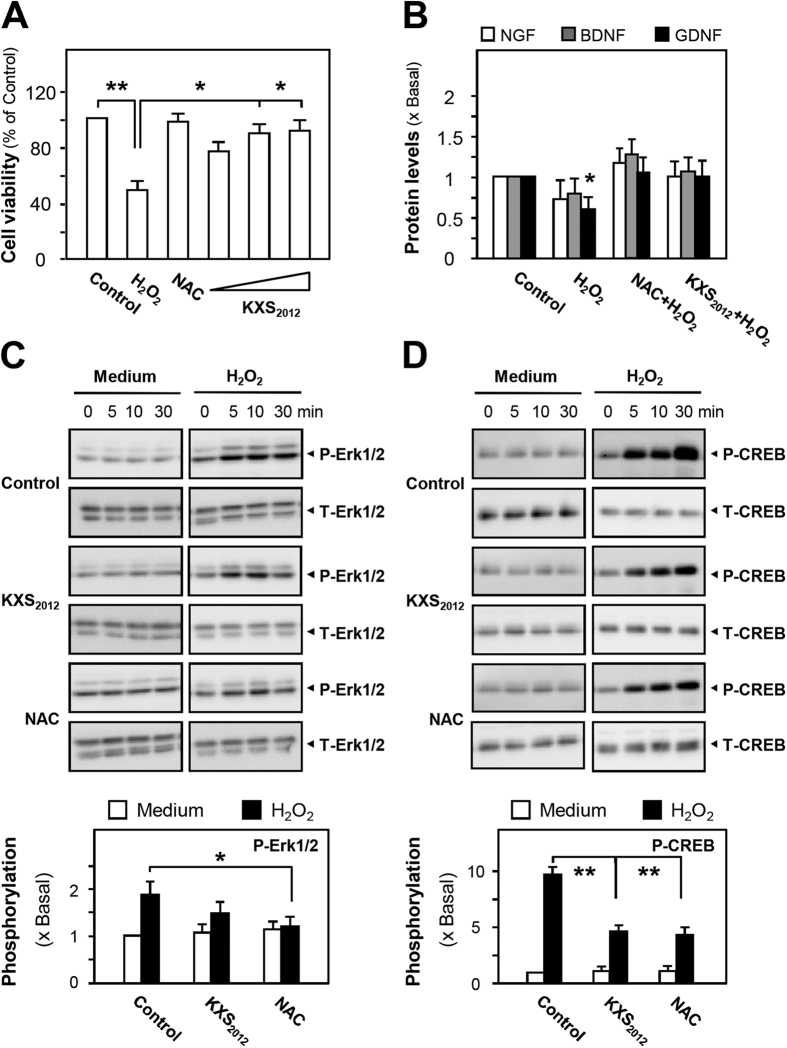
Oxidative stress is considered as a common mechanism associated with most neurodegenerative diseases, e.g. depression. Thus, the protective role of KXS2012 against oxidative stress was further demonstrated in cultured astrocytes. In H2O2-stressed astrocytes, the H2O2-induced toxicity was alleviated by the pre-treatment with KXS2012 (Fig. 6A). The H2O2-induced loss of NGF, BDNF and GDNF proteins were reversed in the presence of KXS2012 (Fig. 6B). N-acetyl-L-cysteine (NAC) served as a positive control here. Moreover, the application of KXS2012 reduced H2O2-induced phosphorylations of CREB and Erk1/2 in cultured astrocytes (Fig. 6C,D), which might be associated with the function of neuronal adaptation based on changes in gene expression; these changes of gene expression were shown to be linked to stress28,29. The differential gene expression related to stress, e.g. induction of apoptotic protein, subsequently down-regulates the expressions of neurotrophic factors. These findings demonstrated that in stressed astrocytes, KXS2012 could effectively prevent the loss of neurotrophic factors so as to support neurogenesis, which showed similarity to that of KXS2012 in depressive rats.
Figure 6. KXS2012 up-regulates neurotrophic factor expressions in H2O2-stressed astrocytes.
(A) Cultured astrocytes were pre-treated with KXS2012 (1.5–15 μg/mL) for 24 h before the addition of H2O2 (400 μM) for 24 h. The protective effect of herbal extracts by cell viability assay was shown. N-acetyl-L-cysteine (NAC, 1 mM) served as a positive control. The viability of astrocytes is in percentage to normal control. (B) Cultured astrocytes were pre-treated with KXS2012 (15 μg/mL) for 24 h before the addition of H2O2 (400 μM) for 24 h. The protein levels of NGF, BDNF and GDNF were determined using ELISA kits (calibration curves of protein amounts were in Fig. S3). NAC (1 mM) served as a positive control. (C) Cultured astrocytes were pre-treated with KXS2012 (15 μg/mL) for 24 h before the addition of H2O2 (400 μM). NAC (1 mM) served as a positive control. Total Erk1/2, phosphorylated Erk1/2 (both at ~42/44 kDa) were revealed by using specific antibodies at different time. Quantification plot of the phosphorylation level at 10 min was shown in lower panel. (D) Similar procedures were carried out, as described in (C). Total CREB and phosphorylated CREB (both at ~40 kDa) were revealed by using specific antibodies at different time. Quantification plot of the phosphorylation level at 30 min was shown in lower panel. Values are expressed as the fold of change as compared to control (x Basal), where control value is set as 1, Mean ± SEM, n = 4, *p < 0.05 and **p < 0.01 compared to the control.
Discussion
Depression is a serious mental illness with high incidence in clinics. It causes mood disorder, cognitive and behavioral disturbances, and even increases suicidal tendency1. Current treatment of depression is hindered due to the lack of effective agents. Chinese medicine has been widely used in cases of shock, low mood, forbid forgetfulness, anhedonia and dizziness that share similar syndromes to depression. In TCM, depression is manifested as mental disease caused by insufficiency of “xin-qi” and exuberance of “dampness”. KXS is one of the common Chinese herbal formulae for the treatment of depression. In KXS, the two paired-herbs (GR-PR and ATR-PO) with the function of enhancing “xin-qi” and eliminating “dampness” could promise superior efficacy against depression. However, the make-up of these two paired-herbs has been commonly revised according to syndrome differentiation and treatment variation. In addition, traditional herbal formulae do not have intellectual property that hinders the possible development of herbal extract in developing as a treatment for anti-depression. Thus, KXS2012 was developed here by optimizing the pairing of GR-PR and ATR-PO on cell study14, and we investigated the functions of KXS2012 against depression in vivo and in vitro.
The CMS rat model is probably the most valid animal model of depression, which aims to model a chronic depression-like state that develops gradually over time in response to stress with more natural induction15,16. Based on our findings, KXS2012 could significantly alleviate depression-like symptoms in CMS-induced depressive rat model. Depression is closely related to decreased levels of neurotransmitters in the brain30,31. The treatment of KXS2012 could restore the low levels of neurotransmitters, i.e. norepinephrine, serotonin, dopamine and 5-HIAA/5-HT, in cerebral cortex, hippocampus and striatum of CMS-induced depressive rats. Abundant evidence indicates that neurotrophic factors play important roles against depression32,33. According to the results, the most striking feature was that the expressions of neurotrophic factors as well as their receptors could be up-regulated in KXS2012-adminstrated group, as compared to CMS depressive group. Our findings implied that KXS2012 attenuated depression through up-regulating the levels of neurotransmitters and neurotrophic factors.
The neurogenesis hypothesis suggests that depression is largely caused by an impairment of nerve growth in the brain, which also helps to explain that anti-depressants may take several weeks for restoring neurogenesis to exert an effect17,18. Here, the potential drug targets and global cellular mechanism on neurogenesis could be addressed through cell study on primary cultured neurons and astrocytes. Our results indicated that KXS2012 increased the expression of synaptic vesicle protein, synaptotagmin, at pre-synaptic neurons, and induced the dendritic spine density at post-synaptic neurons. In this regard, KXS2012 may serve to promote the possible synapse development between neurons. The development of synapse is also dependent on neurotrophic factors, e.g. BDNF34,35. In line to this notion, KXS2012 increased the expressions of neurotrophic factors in astrocytes through up-regulating cAMP-PKA signaling pathway. Erk1/2, a downstream effector of PKA, was also shown to be participated in the KXS2012-induced gene expressions. In addition, the condition medium derived from KXS2012-treated astrocytes did not significantly increase the expressions of synaptotagmin and PSD-95 in cultured neurons, suggesting that the potential functional relationship of KXS2012 between neurons and astrocytes might be complicated and need further study.
Oxidative stress is considered as a common mechanism associated with most neurodegenerative diseases, e.g. depression. H2O2 is a potent reactive oxygen species28. In H2O2-stressed astrocyte model, application of KXS2012 relieved the H2O2-induced neurotrophic factor loss and restored the stress-caused phosphorylations of CREB and Erk1/2, suggesting that KXS2012 might restore the levels of neurotrophic factors in stressed astrocytes, as to support neurophysiological functions, e.g. neurogenesis. These protective effects were illustrated both in vitro and in vivo here. Interestingly, Erk1/2 and CREB can be activated not only in response to the pro-growth and pro-survival stimuli, but in response to oxidative stress in neurons. The sustained phosphorylations of Erk1/2 and CREB correlate well with cell death and apoptotic protein expression, which subsequently down-regulate the expressions of neurotrophic factors. Our results implied that KXS2012 could down-regulate the phosphorylations of Erk1/2 and CREB in stressed astrocytes, as to improve neuron adaptation29.
Considering the major ingredients in KXS2012 herbal extract should be crucial for anti-depression, different chemicals within the herbs are known for specific functions. Ginsenosides derived from GR were the main chemicals with strong neurotrophic and neuroprotective effects in vitro and vivo36,37,38,39. 3,6′-Disinapoyl sucrose derived from PR was reported to show obvious anti-depressive effect on depressive animal models, and the efficiency was mediated by increasing BDNF levels and CREB phosphorylation via the CaMKII and ERK1/2 pathway40,41,42. In parallel, ATR-derived α- and β-asarones were shown to reverse learning and memory abilities in dementia mice43. Pachymic acid of PO regulated the expression of 5-HT3A receptors in Xenopus oocytes44. Among all the active ingredients, asarones were considered as toxic. β-Asarone shows genotoxic, hepatocarcinogens and carcinogenicity in rodents. In our previous study, the making up of KXS2012 should decrease the toxicity by conversion of β-asarone to a less toxic form α-asarone, as to enhance therapeutic efficacy14. Meanwhile, due to compatibility of paired-herbs in TCM that the combination of PO and PR with ATR might help reduce side or adverse effects. Therefore, it is reasonable that KXS2012 could be used as a new regimen for anti-depression for the new make-up could exert robust effect against depression through multiple targets, whereas the combination could eliminate toxicity and side/adverse effects.
Materials and Methods
Preparation of herbal extracts
The plant materials from Qinping Market in Guangzhou China were morphologically authenticated by one of the authors, Dr. Tina T. X. Dong. The corresponding voucher specimens were deposited in Center for Chinese Medicine of The Hong Kong University of Science and Technology. The herbs were tested to be qualified according to the requirements of Chinese Pharmacopeia (2015 Edition) and Hong Kong Materia Medica Standards. GR-PR was boiled together in a weight ratio of 1:1, and ATR-PO was in 1:2, under moderate heating in 8 volumes of water. In preparing the herbal extracts, 20 g of herb mixture was boiled in 160 mL of water for 2 h and extracted twice. The extracts were filtered and combined, dried under vacuum and stored at −80 °C. KXS2012 was obtained by mixing the water extracts of GR-PR and ATR-PO together in 1:5 weight ratio.
The quality control of herbal extracts was described in previous study7. An Agilent HPLC coupled with a diode array detector (DAD) system was performed for quality control of the herbal extracts. The samples were analyzed on an Agilent DAD detector G1315C with wavelength of 258 nm and 330 nm. An Agilent zorbax XDB C18 column (4.6 mm × 50 mm, 1.8 μm) maintained at ambient room temperature was selected. The mobile phase condition was recorded as follows: 0.1% formic acid in acetonitrile (A) and 0.1% formic acid in water (B). The flow rate was 0.4 mL/min with injection volume of 5 μL. 0–14 min, linear gradient 20.0–42.0% (A); 14–17 min, linear gradient 42.0–75.0% (A); 17–18 min, isocratic gradient 75.0% (A); 18–25 min, linear gradient 75.0–85.0% (A). A pre-equilibration period of 5 min was used between each sample. 3,6′-disinapoyl sucrose, α-asarone and β-asarone were identified by comparison of their retention time (Rt) with those of known standards from the chromatograms. The effluent was further analyzed by an Agilent QQQ-MS/MS system (Agilent QQQ-MS/MS 6410A with an ESI ion source in negative mode). The drying gas temperature was 325 °C; drying gas flow: 10 L/min; nebulizer pressure: 35 psig; capillary voltage: 4000 V; delta electro multiplier voltage: 400 V. The determinations of ginsenoside Rb1, Rd, Re, Rg1 and pachymic acid were conducted, where astragaloside IV was taken as an internal control. The mass spectra properties of the chemicals were listed in Table S2. Agilent Mass Hunter workstation software version B.01.00 was used for data acquisition and processing. All values are in Mean ±SD, n = 3. The established chemical parameters served as the control for repeatability of biochemical analyses.
Animal experiments
Male Sprague-Dawley (SD) rats (150–180 g, 7-week-old) were obtained from Shanghai SipprBK Laboratory Animals Ltd (Shanghai, China). Animals were hosted on a 12 h light/dark cycle (lights on at 6:00 a.m. and off at 6:00 p.m.) under controlled temperature (22 ± 2 °C) and humidity (50 ± 10%), with standard diet and water ad libitum. They were feed for 7 days before use to adapt to the new environment. The experimental procedures had been approved by The Animal Experimentation Ethics Committee of China Pharmaceutical University (No. 2015–0254 in 01/03/2015 for Animal Ethics Approval) and under the guidelines of “Principles of Laboratory Animal Care” (NIH publication No. 80–23, revised 1996). All the efforts were to reduce the suffering of animals. The behavioral tests were conducted in quiet room with soft light and all references stayed the same location.
The procedures of CMS were conducted with some adjustments11,45. Briefly, a series of stressors were applied onto the animals: (1) water deprivation for 24 h, (2) stroboscopic illumination for 10 h, (3) cage tilt (45°) for 7 h, (4) noise for 10 h, (5) soiled cage (200 mL water in 100 g sawdust bedding) for 12 h, (6) exposure to an empty bottle for 1 h, (7) forced swimming at 8 °C for 6 min, (8) tail-clipping restraint for 6 min and (9) food deprivation for 21 h. These stressors were randomly arranged in one-week and repeated for 6 weeks. The rats in control group were left undisturbed in their home cages except for house maintaining such as cage cleaning. At the end of CMS procedures, sucrose preference test was determined to evaluate the CMS model.
The SD rats were randomly divided into six groups (n = 12). The control animals and CMS model were given with saline. For the other four groups, KXS2012 at low dose (60.9 mg/kg/day), medium dose (182.7 mg/kg/day), high dose (548.1 mg/kg/day) and imipramine (20 mg/kg/day) were intra-gastrically given 30 min before stress exposure for 6 weeks. Sucrose preference test was carried out at the end of CMS procedures11. In Brief, rats in each group were learned to adapt to 2 bottles of 1% sucrose solution (w/v) 72 h before the test, and 24 h later, one bottle of 1% sucrose solution (w/v) was replaced with tap water for 24 h. Then, rats were deprived of water and food for 24 h. Sucrose preference test was conducted at 17:00 p.m., where rats were kept in individual cages with two bottles, one with 100 mL of 1% sucrose solution (w/v) and the other with 100 mL of water. After 3 h, the volumes of consumed sucrose solution and water were recorded and the sucrose preference was calculated by the following formula: sucrose preference = sucrose consumption/(water consumption + sucrose consumption)×100%45.
Open field test was carried out at the end of herbal treatment. Briefly, the open field apparatus consisted of a square arena (100 cm × 100 cm × 50 cm). The floor was divided equally into 25 squares. In the test, a single rat was placed in the center of the arena and allowed to explore freely. The time that rats spent into the central area was recorded during a test period of 5 min. This apparatus was cleaned with a detergent and dried after occupancy by each rat. The open field test was started at 9: 00 a.m. in a quiet room45.
Forced swimming test was carried out at the end of herbal treatment. Rats in each group were placed in large glass cylinders (50 cm height and 20 cm diameter) with 30 cm height water at 22 ± 2 °C, so that rats were not able to support themselves by hind limbs. The test consisted of two parts: the first 15 min was for pre-swimming and then 24 h later, the swimming behavior was observed in 5 min, and the latency to float was measured and analyzed46.
Measurement of neurotransmitters
The brain tissues were dissected from the manipulated rats. In brief, the rats were sacrificed by decapitation, and the cortex, hippocampus and striatum were separated, respectively, rapidly frozen in liquid nitrogen and kept in −80 °C for storage. The procedure was described: the tissue samples (1 g in 5 mL) were treated by tissue lysate (0.6 mol/L perchloric acid, 0.5 mM Na2EDTA and 0.1 g/L L-cysteine), and centrifuged (14,000 g, 15 min, 4 °C) twice. The supernatant was collected and treated with perchloric acid precipitation agent (0.6 mol/L perchloric acid, 1.2 M K2HPO4 and 2 mM Na2EDTA). After centrifugation (14,000 g, 15 min, 4 °C) and filtration, the samples were challenged by HPLC-fluorescence detector (HPLC-FLD, Shimadzu, Japan)47.
The chromatographic separation for monoamine neurotransmitters was performed on a Shimadzu HPLC-RF series which was equipped with a degasser, a binary pump, an auto-sampler and a thermo-stated column compartment. The tissue samples were separated on an Agilent zorbax SB-C18 (150 mm × 4.6 mm, 5 μm) column. The mobile phase composed of citric acid-sodium acetate buffer (50 mM citric acid, 50 mM sodium acetate, 0.5 mM 1-heptane sulfonate, 5 mM triethylamine, 0.5 mM Na2EDTA) (A)-methanol (B) (95: 5, v/v) (pH 3.8); flow rate of 1.0 mL/min; injection volume of 5 μL; emission wavelength of 330 nm, excitation wavelength of 280 nm19,20,48.
Measurement of neurotrophic factors
The levels of neurotrophic factors were determined by commercial ELISA kits (Abfrontier, Korea) according to the manufacturer’s instructions. The samples were added onto a 96-well plate with coating of anti-rat NGF/BDNF/GDNF antibody and incubated at 37 °C for 90 min. Then, the samples were replaced by biotinylated anti-rat NGF/BDNF/GDNF antibody and incubated at 37 °C for 60 min. After washing four times with PBS, avidin-biotin-peroxidase complex solution was added and incubated at 37 °C for 90 min, which was replaced by tetra-methylbenzidine solution with another incubation of 30 min at 37 °C. At last, 1 M sulfuric acid was added to stop the reaction and absorbance of 450 nm was measured immediately. The absorbance of non-specific binding was taken consideration for sample analysis and each sample in duplicate was employed to minimize inter-assay variation49.
Real-time quantitative PCR
Total RNA was isolated from cell cultures by RNAzol RT reagent (Molecular Research Center, Cincinnati, OH) according to the manufacture’s instruction12,14. The amounts of RNAs were detected by UV absorbance at 260 nm. The total RNA was used to perform the reverse transcription with Moloney Murine Leukemia Virus (MMLV) reverse transcriptase (Life Technologies, Grand Island, NY), according to the protocol provided by the manufacturer. Real-time PCR was performed using FastStart SYBR Green Master (Roche, Indianapolis, IN). The SYBR green signal was detected by Mx3000PTM muitiplex quantitative PCR machine (BD Biosciences Clontech, San Jose, CA). Primers used were: NGF-S: CAC TCT GAG GTG CAT AGC GTA ATG TC; NGF-AS: CTG TGA GTC CTG TTG AAG GAG ATT GTA C; BDNF-S: GAG CTG AGC GTG TGT GAC AGT ATT AG; BDNF-AS: ATT GGG TAG TTC GGC ATT GCG AGT TC; GDNF-S: GCG CTG ACC AGT GAC TCC AAT ATG; GDNF-AS: CGC TTC ACA GGA ACC GCT ACA ATA TC; TrkA-S: ACC TCA ACC GTT TCC TCC GGT C; TrkA-AS: CTC GAT CGC CTC AGT GTT GGA GA; TrkB-S: CGG GAG CAT CTC TCG GTC TAT G; TrkB-AS: CAA ATG TGT CCG GCT TGA GCT GG; TrkC-S: CAC TGT CTA CTA CCC TCC ACG TG; TrkC-AS: CTC TCT GGA AAG GGC TCC TTA AGG; Synaptotagmin-S: GCT GGG TGA CAT CTG TAC CTC C; Synaptotagmin-AS: CAC CTG GAC TTT CTG GAT CTG CTC; PSD-95-S: TGG TGA CGA AGA GTG GTG GCA AG; PSD-95-AS: CAA AGT GGT AAT CCC GGC CGT C; 18S-S: TGT GAT GCC CTT AGA TGT CC; 18S-AS: GAT AGT CAA GTT CGA CCG TC.
Cell culture
Cortical and hippocampal neurons, from SD rat embryos at day 18, were isolated and cultured as described previously50 with modifications. Briefly, the whole brain was dissected in Hank’s Balanced Salt Solution (Sigma, St. Louis, MO) supplemented with 1 mM sodium pyruvate (Life Technologies) and 10 mM HEPES (Sigma, pH 7.4) without Ca2+ and Mg2+ to obtain cortex. The tissues were treated with trypsin (Life Technologies, 2.5%) for 20 min, followed by centrifugation at 1,500x g for 4 min or washing with plating medium (DMEM with 10% HS, supplemented with 0.5 mM GlutaMAX, 25 μM monosodium glutamic acid, 100 U/mL penicillin and 100 μg/mL streptomycin) to remove trypsin. Then, the tissues were triturated for several times to get single cells. The number of cells were counted using trypan blue (Life Technologies), and the cells were seeded on a poly-L-lysine (Sigma, 100 μg/mL) coated 60-mm culture plates (106 cells/plate) and incubated at 37 °C in 5% CO2. Plating medium was changed to culturing medium (Neurobasal supplemented with 1 × B27, Life Technologies) in 24 h after plating. Cytosine arabinoside (Ara-C, Sigma, 2.5 μM), a mitotic inhibitor was applied onto the cultures on the third day in vitro (DIV) to eliminate glial cells. One third of the medium was replaced by fresh culture medium every 5 day afterwards. At DIV 5 or 15, the medium was changed by neurobasal supplemented with 0.1× B27 for 3 h before herbal treatment. The time of herbal treatment was 96 h. Forskolin (Sigma, 50 nM) and BDNF (Alomone lab, Israel, 15 ng/mL) were used as positive controls, respectively51.
Astrocytes, from postnatal SD rat at day 1, were isolated and cultured as described previously52. The cortex was dissected in Hank’s Balanced Salt Solution without Ca2+ and Mg2+. After being trypsinized for 20 min, the cortex was washed with culture medium and triturated several times. The culture medium was DMEM supplemented with 10% FBS, 100 units/mL penicillin and 100 μg/mL streptomycin. The cells were centrifuged at 1,000× g for 4 min. The cell pellet was re-suspended in the culture medium. The cells were seeded on plastic culture plates with the density of 2 × 104 cell/cm2 and incubated at 37 °C in 5% CO2 for 48 h before medium change. The culture medium was changed twice a week. Every time, the astrocytes were pipetted up and down several times to get rid of any loosely adherent oligodendrocytes, microglia and neurons53. In herbal treatment, drugs were diluted by DMEM with 0.5% FBS and 100 units/mL penicillin and 100 μg/mL streptomycin, and the medium was changed by fresh DMEM with 0.5% FBS and 100 units/mL penicillin and 100 μg/mL streptomycin 3 h before herbal treatment. The time of herbal treatment was 48 h. H89 (Sigma, 5 μM) and U0126 (Sigma, 20 μM) were applied onto cultures 3 h before herbal treatment, respectively. Forskolin (Sigma, 50 nM) and tetra-decanoylphorbol acetate (TPA, Sigma, 50 nM) were used as positive controls, respectively. In H2O2-stressed astrocytes, the cells were pre-treated with herbal extracts for 24 h before exposure to H2O2 (400 μM) for another 24 h. N-acetyl-L-cysteine (NAC, Sigma, 1 mM) was used as a positive control.
Cell viability assay
Cell viability was assessed by MTT [3-(4,5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2 H-tetrazolium bromide] assay. Cells were plated in 96-well plate for 24 h and treated with drugs for 48 h before adding MTT. In H2O2-stressed astrocytes, cells were pre-treated with herbal extracts for 24 h before exposure to H2O2 (400 μM) for another 24 h. NAC (Sigma, 1 mM) were used as a positive control. Then the cells were incubated with MTT for another 3 h at 37 °C. After that, absorbance of 570 nm was measured in a microplate reader (Thermo Scientific, Fremont, CA).
Protein phosphorylation
Primary cultures of astrocytes were seeded onto 12-well plate to reach the confluence of above 90%, the culture medium was changed to DMEM without serum for 5 h. The inhibitors were added 3 h before herbal treatment. The cells were treated with herbal extracts at different time points (0 min, 5 min, 10 min and 30 min). In stressed astrocyte culture, herbal extracts were applied onto the cells for 24 h, followed by H2O2 (400 μM) at different time points (0 min, 5 min, 10 min and 30 min). Then, the medium was expelled, and the cells were digested with 2x direct lysis buffer. The degrees of phosphorylation were determined by their specific anti-phospho-kinase and total kinase antibodies. Results are presented as intensities of phospho-bands relative to total bands and expressed as x Basal54.
SDS-PAGE and immunoblotting
The cell lysate was collected, and protein content was determined by Bradford method. Proteins (~20 μg) were separated on 8% SDS-polyacrylamide gels and transferred to a nitrocellulose. Transfer and equal loading of the samples was confirmed by staining the ponceau-S. The nitrocellulose was blocked with 5% fat-free milk in Tris-buffer saline/0.1% Tween 20 (TBS-T), and then incubated in the primary antibodies diluted in 2.5% fat-free milk in TBS-T over night at 4 °C. The primary antibodies were: anti-synaptotagmin (Gift from Prof. H. B. Peng, HKUST), anti-PSD-95 antibody (BD Biosciences), anti-phospho-Erk1/2 (Cell Signaling, Banvers, MA), anti-total Erk1/2 (Cell Signaling), anti-phospho-CREB (Cell Signaling) and anti-total CREB (Cell Signaling). After that, the nitrocellulose was rinsed with TBS-T and incubated for 2 h at room temperature in peroxidase (HRP)-conjugated anti-mouse secondary antibody, or peroxidase (HRP)-conjugated anti-rabbit secondary antibody (Life Technologies), diluted in 2.5% fat-free milk in TBS-T. After intensive washing with TBS-T, the immune complexes were visualized using the enhanced chemiluminescence (ECL) method (GE Healthcare, Piscataway, NJ). The intensities of bands in control and samples, run on the same gel and under strictly standardized ECL conditions, were compared on an image analyzer, using a calibration plot constructed from a parallel gel with serial dilutions of one of the sample49,54.
Spine density analysis
Rat hippocampal neurons were seeded on 18-mm coverslips (1.5 × 105 cells per coverslip) coated with poly-D-lysine (1 mg/mL). At DIV 19, hippocampal neurons were changed by neurobasal medium supplemented with 1× B27 1 h before transfecting with pEGFP-N1 (Clontech, Mountain View, CA) using calcium phosphate precipitation, as previously described55. For imaging of dendritic spines in EGFP-transfected hippocampal neurons, the images were acquired by Zeiss LSM710 confocal microscope with a 40X oil-immersion objective using z serial scanning mode, and image analyses were performed with Imaris software. For quantification, two to three dendrite segments from each neuron were analyzed. The basal threshold values for the background of all images in each experiment were measured. The average of these threshold values was then applied to all images in the same experiment56.
Statistical analysis
All data were analyzed using one-way ANOVA followed by the Students t-test. Statistically significance were classed as * where P < 0.05; ** where P < 0.01; *** where P < 0.001.
Additional Information
How to cite this article: Yan, L. et al. A Chinese herbal decoction, reformulated from Kai-Xin-San, relieves the depression-like symptoms in stressed rats and induces neurogenesis in cultured neurons. Sci. Rep. 6, 30014; doi: 10.1038/srep30014 (2016).
Supplementary Material
Acknowledgments
This research was supported by Hong Kong Research Grants Council Theme-based Research Scheme (T13-607/12 R), GRF (6662911, 660411, 663012, 662713, M-HKUST604/13), TUYF12SC03, TUYF15SC01, The Hong Kong Jockey Club Charities Trust (HKJCCT12SC01) and Foundation of The Awareness of Nature (TAON12SC01) to Karl Tsim, National Natural Science Foundation of China (81403087) to Wenchuan Bi and National Natural Science Foundation of China (81303190) to Yue Zhu.
Footnotes
Author Contributions L.Y. and K.W.K.T. conceived and designed the experiments, L.Y. and Q.H. performed the experiments, L.Y., Q.H., M.S.H.M., S.L.X. and J.L. analyzed the data, H.W., Y.Z., C.W.C.B. and T.T.X.D. contributed reagents, L.Y. and K.W.K.T. contributed to the writing of the manuscript.
References
- Barlow D. H. & Durand V. M. Abnormal psychology: An integrated approach 248–249 (Wadsworth Publishing, 2011). [Google Scholar]
- Krishnan V. & Nestler E. J. The molecular neurobiology of depression. Nature 455, 894–902 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald S. D., George R. H. & Eric J. N. A molecular and cellular theory of depression. Arch Gen Psychiatry 54, 597–606 (1997). [DOI] [PubMed] [Google Scholar]
- Lee A. L., Ogle W. O. & Sapolsky R. M. Stress and depression: Possible links to neuron death in the hippocampus. Bipolar Disord 4, 117–128 (2002). [DOI] [PubMed] [Google Scholar]
- Rosenzweig-Lipson S. et al. Differentiating antidepressants of the future: Efficacy and safety. Pharmacol Therapeut 113, 134–153 (2007). [DOI] [PubMed] [Google Scholar]
- Sun S. M. Beiji Qianjin Yaofang 147 (People’s Medical Publishing House, 1997). [Google Scholar]
- Zhu K. Y. et al. Quality assessment of a formulated Chinese herbal decoction, Kaixinsan, by using rapid resolution liquid chromatography coupled with mass spectrometry: A chemical evaluation of different historical formulae. J Sep Sci 33, 3666–3674 (2010). [DOI] [PubMed] [Google Scholar]
- Liu P. et al. Research on anti-depression and compatibility of Kai-Xin-San. Chin J Tradit Chin Med Pharm 20, 279–282(2005). [PubMed] [Google Scholar]
- Wang J. L. Research on anti-depressive material basis and mechanism of Kai-Xin-San., (Date of access: http://202.119.248.240/KNS50/detail.aspx?QueryID=0&CurRec=1) (2005).
- Liu M. Y. Research on the anti-depression and neuroprotection of Kai-Xin-San and its main active ingredient: 3,6'-disinapoyl sucrose on CREB-BDNF signaling pathway. (Date of access: http://cdmd.cnki.com.cn/Article/ CDMD-10092-1014142821.htm) (2013).
- Zhu K. Y. et al. A standardized Chinese herbal decoction, kai-xin-san, restores decreased levels of neurotransmitters and neurotrophic factors in the brain of chronic stress-induced depressive rats. Evid Based Complement Alternat Med 149256 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K. Y. et al. Kai-xin-san, a Chinese herbal decoction containing Ginseng Radix et Rhizoma, Polygalae Radix, Acori Tatarinowii Rhizoma, and Poria, stimulates the expression and secretion of neurotrophic factors in cultured astrocytes. Evid Based Complement Alternat Med 731385 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y. et al. Antidepressant and neuroprotective effect of the Chinese herb kaixinsan against lentiviralsh RNA knockdown brain-derived neurotrophic factor-induced injury in vitro and in vivo. Neuropsychobiology 69, 129–139 (2014). [DOI] [PubMed] [Google Scholar]
- Yan L. et al. Optimizing the compatibility of paired-herbs in an ancient Chinese herbal decoction Kai-Xin-San in activating neurofilament expression in cultured PC12 cells. J Ethnopharmacol 162, 155–162 (2015). [DOI] [PubMed] [Google Scholar]
- Krishnan V. & Nestler E. J. Animal models of depression: Molecular perspectives. Curr Top Behav Neurosci 7, 121–147 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp M. Current Protocols in Pharmacology Unit.5.9, 1–11 (John Wiley & Sons, 2012). [Google Scholar]
- Sahay A. & Hen R. Adult hippocampal neurogenesis in depression. Nat Rev Neurosci 10, 1110–1115 (2007). [DOI] [PubMed] [Google Scholar]
- Eisch A. J. & Petrik D. Depression and hippocampal neurogenesis: A road to remission? Science 338, 72–75 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Lu Y. J., Deng T. L. & Zheng X. X. Measurement of monoamine transmitters in the brains of ovariectomized mice using high performance liquid chromatography with fluorescent detection and gradient flow rate elution. Chinese Journal of Chromatography 23, 76–78 (2005). [PubMed] [Google Scholar]
- Zhao Y. Y. et al. Simultaneous detection of 8 monoamine neurotransmitters in the different sections of mouse brains by high performance liquid chromatography with fluorescent detection. Chinese Journal of Chromatography 29, 146–151 (2011). [DOI] [PubMed] [Google Scholar]
- Chao D. L., Ma L. & Shen K. Transient cell-cell interactions in neural circuit formation. Nat Rev Neurosci 10, 262–271 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colón-Ramos D. A. Synapse formation in developing neural circuits. Curr Top Dev Biol 87, 53–79 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K. & Cowan C. Guidance molecules in synapse formation and plasticity. CSH Perspect Biol 2, a001842 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condorelli D. F., Dell’ Albani P., Mudo G., Timmusk T. & Belluardo N. Expression of neurotrophins and their receptors in primary astroglial cultures: Induction by cyclic AMP-elevating agents. J Neurochem 63, 509–516 (1994). [DOI] [PubMed] [Google Scholar]
- Hisaoka K. et al. Antidepressant drug treatments induce glial cell line-derived neurotrophic factor (GDNF) synthesis and release in rat C6 glioblastoma cells. J Neurochem 79, 25–34 (2001). [DOI] [PubMed] [Google Scholar]
- Takano K., Yamasaki H., Kawabe K., Moriyama M. & Nakamura Y. Imipramine induced brain-derived neurotrophic factor mRNA expression in cultured astrocytes. J Pharmacol Sci 120, 176–186 (2012). [DOI] [PubMed] [Google Scholar]
- Boer U. et al. CRE/CREB-driven up-regulation of gene expression by chronic social stress in CRE-luciferase transgenic mice: Reversal by antidepressant treatment. PLoS ONE 2, e431. doi: 10.1371/journal.pone.0000431 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossthwaite A. J., Hasan S. & Williams R. J. Hydrogen peroxide-mediated phosphorylation of ERK1/2, Akt/PKB and JNK in cortical neurones: Dependence on Ca(2+) and PI3-kinase. J Neurochem 80, 24–35 (2002). [DOI] [PubMed] [Google Scholar]
- Lonze B. E. & Ginty D. D. Function and regulation review of CREB family transcription factors in the nervous system. Neuron 35, 605–623 (2002). [DOI] [PubMed] [Google Scholar]
- Manji H. K., Drevets W. C. & Charney D. S. The cellular neurobiology of depression. Nat Med 7, 541–547 (2001). [DOI] [PubMed] [Google Scholar]
- Nestler E. J. et al. Neurobiology of depression. Neuron 34, 13–25 (2002). [DOI] [PubMed] [Google Scholar]
- Manji H. K., Moore G. J. & Chen G. Clinical and preclinical evidence for the neurotrophic effects of mood stabilizers: Implications for the pathophysiology and treatment of manic-depressive illness. Biol Psychiat 48, 740–754 (2000). [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Shimizu E. & Iyo M. Critical role of brain-derived neurotrophic factor in mood disorders. Brain Res Rev 45, 104–114 (2004). [DOI] [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem 10, 86–98 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. & Poo M. M. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14, 7–23 (2013). [DOI] [PubMed] [Google Scholar]
- Chen X. C. et al. Ginsenoside Rg1 attenuates dopamine-induced apoptosis in PC12 cells by suppressing oxidative stress. Eur J Pharmacol 473, 1–7 (2003). [DOI] [PubMed] [Google Scholar]
- Ji Z. N. et al. Ginsenoside Re attenuate beta-amyloid and serum-free induced neurotoxicity in PC12 cells. J Ethnopharmacol 107, 48–52 (2006). [DOI] [PubMed] [Google Scholar]
- Xu L., Chen W. F. & Wong M. S. Ginsenoside Rg1 protects dopaminergic neurons in a rat model of Parkinson’s disease through the IGF-I receptor signaling pathway. Brit J Pharmacol 158, 138–148 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J. M. et al. Ginsenosides attenuate methylglyoxal-induced impairment of insulin signaling and subsequent apoptosis in primary astrocytes. Neuropharmacology 85, 215–223 (2014). [DOI] [PubMed] [Google Scholar]
- Hu Y. et al. Antidepressant-like effects of 3,6′-disinapoyl sucrose on hippocampal neuronal plasticity and neurotrophic signal pathway in chronically mild stressed rats. Neurochem Int 56, 461–465 (2010). [DOI] [PubMed] [Google Scholar]
- Hu Y. et al. Possible mechanism of the antidepressant effect of 3,6′-disinapoyl sucrose from Polygala tenuifolia Willd. J Pharm Pharmacol 63, 869–874 (2011). [DOI] [PubMed] [Google Scholar]
- Hu Y., Liu M. Y., Liu P., Dong X. & Boran A. D. Neuroprotective effects of 3,6′-disinapoyl sucrose through increased BDNF levels and CREB phosphorylation via the CaMKII and ERK1/2 pathway. J Mol Neurosci 53, 600–607 (2014). [DOI] [PubMed] [Google Scholar]
- Guo J. H. et al. Effects of active components of Rhizoma Acori Tatarinowii and their compatibility at different ratios on learning and memory abilities in dementia mice. Traditional Chinese Drug Research and Clinical Pharmacology 23, 144–147 (2012). [Google Scholar]
- Lee J. H. et al. Effects of triterpenoids from Poria cocos Wolf on the serotonin type 3A receptor-mediated ion current in Xenopusoocytes. Eur J Pharmacol 615, 27–32 (2009). [DOI] [PubMed] [Google Scholar]
- Mao Q. Q. et al. Effects of SYJN, a Chinese herbal formula, on chronic unpredictable stress-induced changes in behavior and brain BDNF in rats. J Ethnopharmacol 128, 336–341 (2010). [DOI] [PubMed] [Google Scholar]
- Ma Z. Q. et al. Metabonomic study on the antidepressant-like effects of Banxia Houpu decoction and its action mechanism. Evid Based Complement Alternat Med 213739 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X., Cheng G., Pan Y., Xia Z. H. & Kong L. D. Behavioral, neurochemical and neuroendocrine effects of the ethanolic extract from Curcuma longa L. in the mouse forced swimming test. J Ethnopharmacol 110, 356–363 (2007). [DOI] [PubMed] [Google Scholar]
- Xu Q. et al. Antidepressant-like effects of the mixture of honokiol and magnolol from the barks of Magnolia officinalis in stressed rodents. Prog Neuro-psychoph 32, 715–725 (2008). [DOI] [PubMed] [Google Scholar]
- Xu S. L. et al. Flavonoids induce the synthesis and secretion of neurotrophic factors in cultured rat astrocytes: A signaling response mediated by estrogen receptor. Evid Based Complement Alternat Med 127075 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer G. J. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J Neurosci Res 42, 674–683 (1995). [DOI] [PubMed] [Google Scholar]
- Meberg P. J. & Miller M. W. Culturing hippocampal and cortical neurons. Methods Cell Biol 71, 111–127 (2003). [DOI] [PubMed] [Google Scholar]
- Pekny M. & Nilsson M. Astrocyte activation and reactive gliosis. Glia 50, 427–434 (2005). [DOI] [PubMed] [Google Scholar]
- Lepore A. C. et al. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci 11, 1294–1301 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S. L. et al. Flavonoids induce the expression of synaptic proteins, synaptotagmin, and postsynaptic density protein-95 in cultured rat cortical neuron. Planta Med 79, 1710–1714 (2013). [DOI] [PubMed] [Google Scholar]
- Fu W. Y. et al. Cdk5 regulates EphA4-mediated dendritic spine retraction through an ephexin1-dependent mechanism. Nat Neurosci 10, 67–76 (2007). [DOI] [PubMed] [Google Scholar]
- Lai K. O. et al. Trk B phosphorylation by Cdk5 is required for activity dependent structural plasticity and spatial memory. Nat Neurosci 15, 1506–1515 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.