Abstract
Patients undergoing total knee arthroplasty (TKA) report low satisfaction with postoperative pain control. The purpose of this study is to examine if there is a difference in post-operative pain for TKA patients without femoral nerve block receiving an intra-operative pericapsular injection of bupivacaine liposome suspension (EXPAREL; Pacira Pharmaceuticals, Inc., San Diego, California) versus a concentrated multi drug cocktail. Seventy TKA patients were randomly assigned to either the bupivacaine liposome or the multi-drug cocktail. Post-operative pain scores, morphine sulfate equivalence consumption values, adverse events, and overall pain control satisfaction scores were collected. Patients reported significantly higher pain level for the cocktail group on post-op day 1 (p < .05) and post-op day 2 (p < .01) versus the bupivacaine liposome group. This same trend was found for morphine sulfate equivalence consumption in the PACU (p < .01) and post-op day 2 (p < .01). Higher satisfaction in pain control (p < .001) and overall experience (p < .01) was also found in the bupivacaine liposome group. Finally, significantly more adverse events were found in the multi-drug group versus the bupivacaine liposome group (p < .05). The study findings demonstrated a non-inferior difference, albeit not a clinically significant difference, in patient-perceived pain scores, morphine sulfate equivalence consumption, adverse events, and overall satisfaction.
Keywords: Total knee arthroplasty, Pain, Pericapsular injection
Introduction
Postoperative pain can have a major effect on patient recovery through decreased patient satisfaction and prolonged rehabilitation. Although pain is a predictable part of the postoperative experience, inadequate management of pain is common and can have profound implications. Despite guidelines and recommendations from pain management societies, postoperative pain continues to be viewed as a major health care concern [1].
Total knee arthroplasty (TKA) is a common surgical procedure which can cause a considerable amount of pain. Multimodal pain management reduces postoperative pain in the majority of TKA patients 2, 3, 4, 5, 6, 7, 8, 9, 10 yet too many patients are still dissatisfied with overall pain control [8] and adverse drug reactions to neuroleptic and opioid medications prescribed. Additionally, the common use of femoral nerve blocks as a pain control measure has demonstrated to have several concerning issues such as hindering early physical therapy, contributing to increased fall rates [11], and new neurological symptoms associated with a block [12].
Pain management therapy, which includes surgical site infiltration of an anesthetic, is one of the latest methods being used to decrease postoperative pain [8]. Several agents have been used for this method; however a new method using liposomal bupivacaine has recently been introduced. Liposomal bupivacaine suspension is a non-opioid medication using Depofoam technology which allows for extended release of pericapsular injected bupivacaine for up to 72 hours postoperatively 13, 14. There is one recent randomized control trial in TKA patients, as well as a pooled analysis of five different surgical procedures, that favorably compared pericapsular injection with bupivacaine liposome suspension versus bupivacaine hydrochloride (HCL) 15, 16. The liposome suspension provided a considerable longer amount of pain relief when compared to the hydrochloride 15, 17. To date, the clinical use and published evidence supports bupivacaine liposome suspension in patients undergoing bunionectomy or hemorrhoidectomy [16], however with a paucity of available data, future research is desperately needed.
We suspect hospitalized TKA patients without a pre-operative femoral nerve block will experience a non-inferior postoperative pain control and MSO4 (morphine sulfate) equivalence consumption when receiving an injection of bupivacaine liposome suspension (EXPAREL; Pacira Pharmaceuticals, Inc., San Diego, California) versus a concentrated multi drug cocktail.
Material and methods
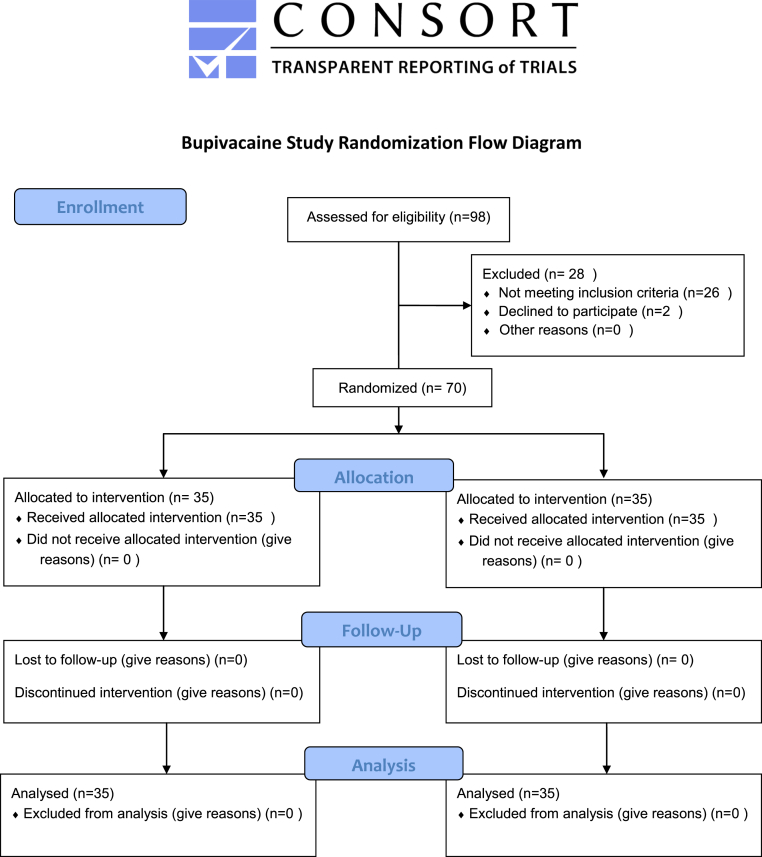
This prospective randomized, double blind controlled trial was conducted at a large community hospital with approval from the internal Institutional Review Board. Subjects were at least 18 years of age and received a TKA for degenerative joint disease, due to failing all other non-operative treatments to control knee pain. Subjects were excluded from the study if they were found to have a history of orthopedic and medical co-morbidities that would prevent postoperative pain control (i.e. extra-articular pathology with referred pain to the knee associated with spinal stenosis, neuropathy, and ipsilateral hip disease), severe knee deformity, post-traumatic, and inflammatory arthritis, BMI >40, were unable to receive multimodal pain remitting agents, had active knee sepsis, had remote sites of active infection, had diabetes with A1C > 7, American Society of Anesthesiologists class (ASA) > III, cardiac disease failing medical clearance, severe liver disease, peripheral artery disease (PAD) with ankle arm index (AAI) < .75, seizure disorder, allergic to any pain remitting agent, alcohol abuse, or smoking abuse (Fig. 1).
Figure 1.
CONSORT 2010 Bupivacaine Study Randomization Flow Diagram. This diagram displays the progress of all subjects throughout the study.
This study was registered with ClinicalTrials.gov, record 13055-13-034. The protocol and all other study related documents are retained at the research facility responsible for conducting the study.
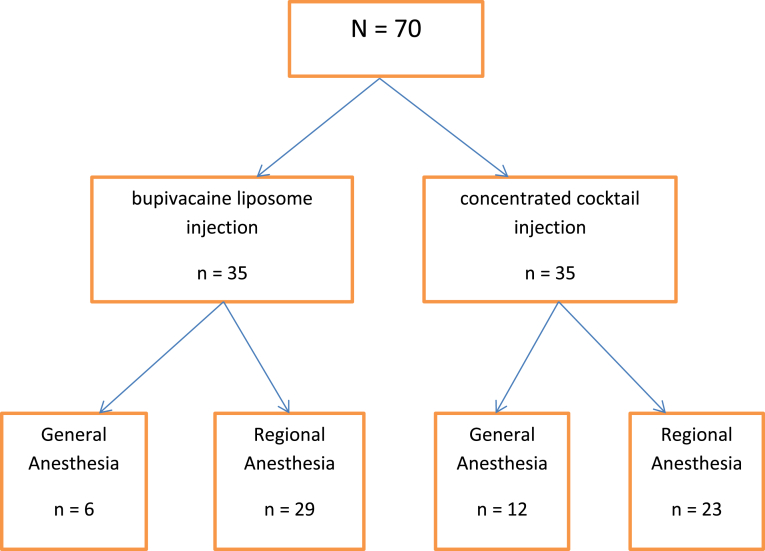
Once subjects were able to understand the study purpose and the potential risks and benefits of each medication potentially being administered, written informed consent was obtained per the research nurse. After enrollment, subjects were randomized at a 1:1 ratio per permuted block randomization table to either the bupivacaine liposomal suspension injection or the concentrated cocktail mixture injection by a pharmacist (Fig. 2). Neither the patient nor the surgeon were aware of to which group each subject had been randomized.
Figure 2.
Subject Randomization Flowchart. This flowchart depicts how subjects were randomized at a 1:1 ratio to either the bupivacaine liposomal suspension injection or the concentrated cocktail mixture injection. Further detail shows the type of anesthesia each group received.
On the day of surgery, the randomized drug was prepared in pharmacy and brought to the operating room. Due to the dissimilar color of the medications used, the drug was transferred to a sterile syringe covered in opaque coban to blind the surgeon from subjects' randomization group. Subjects were anesthetized by either regional spinal anesthesia consisting of .75% ropivicaine or general anesthesia consisting of a 1% propofol (10 mg/ml) continuous drip (Table 1). The randomized pericapsular injection was delivered during the surgery. Thirty percent of the medication was slowly injected into the post capsular region of the knee using an 18 gauge needle prior to the cementation of the implants. The remaining 70% of the medication was then equally infiltrated into the surrounding soft tissue using an 18 gauge needle and a precise delivery technique to evenly deposit the drug. Once surgery was complete, the patient was transferred to PACU where postoperative pain assessments began using a numerical rating scale (NRS) ranging from 0 (No pain) to 10 (Worst possible pain) [18]. Both verbal pain scores and total opioid (morphine equivalence) consumption were collected for each day. Additionally, postoperative overall pain control satisfaction scores were collected using a 5 point Likert-type scale ranging from 1 (Not at all satisfied) to 5 (Extremely satisfied) at the 10 day follow-up visit with the surgeon. Adverse events were also collected and documented for each subject.
Table 1.
Periarticular injection.
| Bupivacaine liposome injection | Concentrated multi drug injection |
|---|---|
|
|
Statistical analysis was performed using IBM SPSS Statistics for Windows version 21 (Armonk, NY: IBM Corp.). Univariate chi-square analysis was incorporated for all categorical variables and for expected cell counts <5, a Fisher's exact test was employed. For continuous variables, independent-samples t-test was performed and for non-normal continuous variables, a Mann–Whitney U test was used for mean comparison.
There was no external source of funding for this study. All medications administered for study purposes were provided by the research institution's hospital pharmacy.
Results
A total of 126 patients were screened against inclusion/exclusion criteria for participation in the study, but a total of 70 were included in the analysis (Fig. 1). This was enough to justify using an independent-sample t-test given this tests ability to handle smaller sample sizes in it's a priori assumptions with a reasonable expected difference between groups. In addition, the non-parametric Mann–Whitney U has even less assumptions, so this was the default test for non-normal distributions. Majority of the subjects were white (98.1%) male (52.8%) with a mean age of 66.4 years (9.1 years) and a mean BMI of 30.9 (4.5). About 55% of patients had an ASA score of II and 74.3% of patients received spinal anesthesia. With regards to comorbidities, 8.57% of patients had coronary artery disease, 20% were diabetic, 57.14% were hypertensive, and just over 1.43% suffered from atrial fibrillation. A statistical significance difference of p < .05 was considered significant. There were no statistically significant differences reported in the above demographics, anesthesia scores, anesthesia type, and comorbidities, thus indicating homogenous groups. The only adverse event reported was nausea, which 40% of subjects reported. Of this 40%, 67.9% were in the concentrated multi-drug cocktail group versus only 32.1% in the bupivacaine liposomal suspension group. This difference was statistically significant (p < .05). These and other descriptive statistics can be found in Table 2.
Table 2.
Patient demographics.
| Total sample n (%) N = 70 |
Concentrated multi-drug cocktail n (%) N = 35 |
Bupivacaine liposomal suspension n (%) N = 35 |
p-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (yrs), mean (SD)d | 66.54 (9.14) | 65.57 (7.89) | 67.31 (10.27) | .429 |
| BMI, mean (SD)d | 30.98 (4.45) | 31.29 (4.70) | 30.68 (4.23) | .575 |
| Genderc | ||||
| Male | 37 (52.9) | 15 (42.9) | 22 (62.9) | .094 |
| Female | 33 (47.1) | 20 (57.1) | 13 (37.1) | .094 |
| Raceb | ||||
| Caucasian | 69 (98.60) | 34 (97.10) | 35 (100.00) | 1.000 |
| African American | 1 (1.40) | 1 (2.90) | 0 (.00) | 1.000 |
| Anesthesia | ||||
| ASA scorec | .969 | |||
| I | 4 (5.70) | 2 (5.71) | 2 (5.71) | |
| II | 39 (55.7) | 20 (57.14) | 19 (54.29) | |
| III | 27 (38.6) | 13 (37.1) | 14 (40.00) | |
| Anesthesia typec | .101 | |||
| General | 18 (25.71) | 12 (34.29) | 6 (17.14) | |
| Spinal | 52 (74.29) | 23 (65.71) | 29 (82.86) | |
| Comorbidities | ||||
| CADb | 6 (8.57) | 2 (5.71) | 4 (11.43) | .673 |
| Diabetesb | 7 (20.00) | 4 (11.43) | 3 (8.57) | .690 |
| Hypertensionb | 40 (57.14) | 18 (51.43) | 22 (62.86) | .334 |
| A-Fibb | 1 (1.43) | 0 (.00) | 1 (2.86) | 1.000 |
| Adverse event | ||||
| Nauseac | 28 (40.00) | 19 (54.29) | 9 (25.71) | .011a |
CA = Coronary artery disease, A-fib = Atrial fibrillation.
Statistically significant at the p < .05 level.
Fisher's exact Test.
Univariate chi-square analysis.
Independent-samples t-test.
Patient-reported NRS pain scores were collected immediately post op in the PACU and throughout the patient's hospitalization. In the PACU patients reported a significantly lower score in the bupivacaine liposomal suspension M = 2.11 (2.68) versus the concentrated multi-drug cocktail group M = 3.49 (2.87) (p < .05).
Postoperatively, patients also reported lower scores in the bupivacaine liposomal suspension group versus the concentrated multi-drug cocktail group on Postoperative day (POD)#0, POD#1, and POD#2, with significant differences on POD#1 and POD#2, p < 05, p < .01, respectively. Statistical tests could not be performed for POD#3, due to lack of statistical testing assumptions being made, specifically with regards to homogeneity of variance. No patients were in the hospital 4 days postoperatively, so no data were reported. More objective measurements were also used in addition to patient reported pain scores, specifically using the morphine sulfate equivalence consumption tables for all patients. Patients in the bupivacaine liposomal suspension group consumed less narcotics in the PACU, as well as everyday postoperatively (POD#0-POD#2) when compared to patients in the concentrated multi-drug cocktail group with significant differences reported in the PACU (p < .01) and on POD#2 (p < .01). Statistical testing was also not performed for MS04 equivalency scores on POD#3 or POD#4 for reasons stated above. Patient reported NRS pain scores and morphine sulfate equivalency comparisons can be found in Table 4.
Table 4.
Correlations between patient reported NRS scores and MSO4 equivalency scores.
| MS04 equivalency scores |
||||
|---|---|---|---|---|
| MS04 total score PACU | MS04 total score POD#0 | MS04 total score POD#1 | MS04 total score#2 | |
| NRS pain scores | ||||
| Post-op pain score PACUa | r = .785 (.0001) | |||
| POD#0 Pain scorea | r = .511 (.0001) | |||
| POD#1 Pain scorea | r = .687 (.0001) | |||
| POD#2 Pain scorea | r = .577 (.0001) | |||
Statistically significant at the p < .0001 level.
Patient satisfaction with in-hospital pain control, as well as 10 days postoperative pain control was assessed using a 5 point Likert-type scale ranging from 1 (not at all satisfied) to 5 (extremely satisfied). Patients assigned to the bupivacaine liposomal suspension group reported both higher satisfaction with pain control overall M = 4.57 (SD = .70) as well as satisfaction with pain control while they were in the hospital M = 4.91 (SD = .37) with significant values of (p < .01) and (p < .0001), respectively (see Table 3).
Table 3.
Reported pain scores and morphine sulfate equivalency between groups.
| Total sample n (%) N = 70 |
Concentrated multi-drug cocktail n (%) N = 35 |
Bupivacaine liposomal suspension n (%) N = 35 |
p-value | |
|---|---|---|---|---|
| NRS scores | ||||
| PACU, Mean (SD)d | 2.80 (2.84) | 3.49 (2.87) | 2.11 (2.68) | .033a |
| POD#0d | 3.24 (1.81) | 3.60 (1.77) | 2.89 (1.81) | .114 |
| POD#1d | 2.94 (1.45) | 3.31 (1.55) | 2.57 (1.27) | .023a |
| POD#2d | 2.96 (1.60) | 3.51 (1.54) | 2.40 (1.48) | .002 |
| POD#3 | 4.00 (1.31) | 4.00 (1.83) | 4.00 (.82) | – |
| MS04 equivalency scores (mg) | ||||
| PACUd | 4.92 (6.51) | 6.85 (6.57) | 2.99 (5.93) | .002a |
| POD#0d | 7.81 (7.04) | 8.73 (7.48) | 6.89 (6.56) | .267 |
| POD#1d | 13.24 (9.65) | 15.57 (10.91) | 10.91 (7.67) | .079 |
| POD#2d | 10.00 (10.66) | 13.11 (13.30) | 6.89 (5.79) | .005b |
| POD#3 | 13.75 (10.94) | 16.25 (16.01) | 11.25 (2.50) | – |
| Post op 10 day Likert score | ||||
| Hospital Pain control Satisfactiond | 4.51 (.78) | 4.11 (.87) | 4.91 (.37) | .0001c |
| Overall Pain control Satisfactiond | 4.27 (.83) | 3.97 (.86) | 4.57 (.70) | .001b |
POD = Postoperative day.
Statistically significant at the p < .05 level.
Statistically significant at the p < .01 level.
Statistically significant at the p < .001 level.
Mann–Whitney U Test.
Correlational analysis was performed for patient reported NRS pain scores, as well as morphine sulfate equivalency scores in the PACU, POD#0, POD#1, and POD#2. All correlations were significant at the p < .0001 level, thus demonstrating a strong relationship between patient reported pain and the pain narcotics administered to the patient (see Table 4).
Discussion
We suspected that hospitalized TKA patients without a pre-operative femoral nerve block will experience a non-inferior postoperative pain control and MSO4 (morphine sulfate) equivalence consumption when receiving an injection of bupivacaine liposome. Bupivacaine liposome pericapsular injection, as part of a pain management approach, was shown to be non-inferior.
This study demonstrated a difference in patient-reported pain scores, morphine sulfate equivalency consumption, adverse events, and overall satisfaction for patients receiving a pericapsular injection of bupivacaine liposome versus a multi-drug cocktail. Individual differences between study groups were accounted for by showing no statistically significant differences, thus making our findings valid.
Nausea and vomiting were significantly less prominent in the bupivacaine liposome group versus the concentrated multi-drug cocktail group, which has the potential to help improve overall patient satisfaction with the operative and recovery experience. Pain control has a direct bearing on patient satisfaction by limiting opioid consumption which can reduce the potential for adverse drug reactions and a prolonged recovery [19]. In addition; less reliance on nerve blocks could reduce the amount of postoperative falls and allow for accelerated ambulation [20]. Early mobilization and positive patient outlook are arguably some of the more influential factors contributing to good postoperative TKA rehabilitation. In preparation for shorter hospital stays for total joint arthroplasty programs, prevention and effective relief of postoperative pain is needed and could subsequently contribute to advanced healing, rapid mobilization, and reduced health care costs [21].
Limitations are recognized within this study. The results can only be applied to the study population using the methods and procedures described for this study. Postoperative pain control cannot be generalized to every type of surgical procedure; though possibly all extremity surgery patients might benefit. Other methods must be in place in order to help with those patients whose pain might not be controlled with the pericapsular injection alone, such as the option to use rescue opioid pain medications. Also, different anesthesia techniques are used on patients based on past medical history and anatomy. Some patients receive neuraxial anesthesia while others undergo general anesthesia, thus missing the pre-emptive pain remitting benefit of neuraxial anesthesia. In these patients, bridging soft tissue Marcaine HCL injections might be beneficial.
This study demonstrated a novel approach for examining the differences between bupivacaine liposomal injection versus a multi-drug cocktail suspension standard of care approach on patient perceived pain narcotic consumption and overall patient satisfaction around a TKA. Due to the study limitations and minimal clinical significance, more research is needed to clarify the differences in pain scores and patient satisfaction with overall pain control in various combinations of anesthesia and pericapsular injections. Also, bupivacaine liposome injection techniques must be scrutinized to determine if injection methods vary and if variances could adversely affect pericapsular injection pain control. This study was one of the first of its kind to examine the Bupivacaine liposomal suspension for patients receiving a TKA without a femoral nerve block. It is the first known study that used a level 1 double-blinded randomized control research method to investigate, in total knee patients, and to prove the benefit of bupivacaine liposome over a commonly utilized multi-drug injection method.
Conclusions
In light of patient dissatisfaction with postoperative pain management and the enlarging national narcotic abuse crisis, it is arguably the primary burden for innovative surgeons to further enhance multimodal pain management strategies. Pre-emption of surgical pain, non-narcotic analgesic and neuroleptic medications, accelerated physical therapy, optimized patient expectations, and avoidance of excessive pain and almost all complications will remain the targets for evidence-based research discoveries and translations into clinical practice. Surgeons' sole reliance on narcotic control of postoperative pain was never the best way to ensure patient satisfaction and safety, particularly since narcotics are among the medications most likely to contribute to short- and long-term adverse events.
Pericapsular injections with prolonged acting agents, coupled with tourniquet-less and tissue-sparing surgical techniques might well emerge as some of the brightest stars on our joint reconstructive horizon. We must lead with evidence-based research designs and publications so that broader adoption can benefit most joint arthroplasty patients. Value-based health care improvements will always benefit from safer and more effective postoperative pain control strategies that avoid prolonged patient recoveries and narcotic addictions.
Footnotes
One or more of the authors of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field which may be perceived to have potential conflict of interest with this work. For full disclosure statements refer to http://dx.doi.org/10.1016/j.artd.2015.05.005.
Appendix. Supplementary data
References
- 1.Ranawat A.S., Ranawat C.S. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22(7):12. doi: 10.1016/j.arth.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 2.Duncan C.M., Long K.H., Warner D.O., Pagnano J.R. The economic implications of a multimodal analgesic regimen for patient's major orthopedic surgery: a comparative study of direct costs. Reg Anesth Pain Med. 2009;34(4):301. doi: 10.1097/AAP.0b013e3181ac7f86. [DOI] [PubMed] [Google Scholar]
- 3.Lavernia C.J., Alcerro J.C., Rossi M.D. Fear in arthroplasty surgery: the role of race. Clin Orthop Relat Res. 2010;486:547. doi: 10.1007/s11999-009-1101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riddle D.L., Wade J.B., Jiranek W.A., Xiangrong K. Preoperative pain catastrophizing predicts pain outcome after knee arthroplasty. Clin Orthop Relat Res. 2010;468:798. doi: 10.1007/s11999-009-0963-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parvizi J., Miller A., Gandhi K. Multimodal pain management after total joint arthroplasty. J Bone Joint Surg. 2011;93(11):1075. doi: 10.2106/JBJS.J.01095. [DOI] [PubMed] [Google Scholar]
- 6.Franklin P.D., Karbosi J.A., Li W., Yang W., Ayers P.C. Reduction in narcotic use after primary total knee arthroplasty and association with patient pain relief and satisfaction. J Arthroplasty. 2010;25(6 Suppl.):12. doi: 10.1016/j.arth.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Meunier A., Lisander B., Good L. Effects of celecoxib on blood loss, pain, and recovery of function after total knee replacement; a randomized placebo-controlled trial. Acta Orthop. 2007;78(5):661. doi: 10.1080/17453670710014365. [DOI] [PubMed] [Google Scholar]
- 8.Busch C.A., Shore B.J., Bhandari R. Efficacy of a multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg Am. 2006;88:959. doi: 10.2106/JBJS.E.00344. [DOI] [PubMed] [Google Scholar]
- 9.Schroeer W.C., Diesfeld P.J., LeMarr A.R., Reedy M.E. Feb 19, 2011. Six-week Postoperative COX-2 Inhibitor Use Improves TKA Recovery: a Double-blind, Placebo-controlled Study. Symposium Lll: TKA Perioperative Management. The Knee Society 2011 Annual Meeting at AAOS. San Diego, California. [Google Scholar]
- 10.Tiippana E.M., Hamunen K., Kontinen V.K., Kalso E. Do surgical patients benefit from perioperative gabapentin/pregabalin? A systematic review of efficacy and safety. Anesth Analg. 2007;104:1545. doi: 10.1213/01.ane.0000261517.27532.80. [DOI] [PubMed] [Google Scholar]
- 11.Fredrickson M.J., Kilfoyle D.H. Neurological complication analysis of 1000 ultrasound guided peripheral nerve blocks for elective orthopaedic surgery: a prospective study. Anesthesia. 2009;64(8):836. doi: 10.1111/j.1365-2044.2009.05938.x. [DOI] [PubMed] [Google Scholar]
- 12.Chaumeron A., Audy D., Drolet P., Lavigne M., Vendittoli P.A. Injection in knee arthroplasty improves quadriceps function. Clin Orthop. 2013;471:2284. doi: 10.1007/s11999-013-2928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergese S.D., Onel E., Portillo J. Evaluation of DepoFoam bupivacaine for the treatment of postsurgical pain. Pain Manag. 2011;1(6):539. doi: 10.2217/pmt.11.62. [DOI] [PubMed] [Google Scholar]
- 14.Baxter R., Bramlett K., Onel E., Daniels S. Impact of local administration of liposome bupivacaine for postsurgical analgesia on wound healing: a review of data from ten prospective controlled clinical trials. Clin Ther. 2013;35(3):312. doi: 10.1016/j.clinthera.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Dasta J., Ramamoorthy S., Patou G., Sinatra R. Bupivacaine liposome injectable suspension compared with bupivacaine HCL for the reduction of opioid burden in the postsurgical setting. Curr Med Res Opin. 2012;28(10):1609. doi: 10.1185/03007995.2012.721760. [DOI] [PubMed] [Google Scholar]
- 16.Golf M., Daniels S.E., Onel E. A phase 3, randomized, placebo-controlled trial of DepoFoam bupivacaine (extended-release bupivacaine local analgesic) in bunionectomy. Adv Ther. 2011;28(9):776. doi: 10.1007/s12325-011-0052-y. [DOI] [PubMed] [Google Scholar]
- 17.Bramlett K., Onel E., Viscusi E.R., Jones K. A randomized, double-blind, dose–ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCL for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530. doi: 10.1016/j.knee.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Salaffi F., Stancati A., Silvestri C., Ciapetti A., Grassi W. Minimally clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004;8(4):283. doi: 10.1016/j.ejpain.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Kessler E.R., Shah M., Gruschkus S.K., Raju A. Cost and quality implications of opioid –based postsurgical pain control using administrative claims data from a large health system: opioid related adverse events and their impact on clinical and economic outcomes. Pharmacoptherapy. 2013;33(4):383. doi: 10.1002/phar.1223. [DOI] [PubMed] [Google Scholar]
- 20.Mohanasundaram K., Kinninmonth A., Sarungi M., Baines J. Femoral nerve block for total knee replacement – a word of caution. Knee. 2009;16(2):98. doi: 10.1016/j.knee.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Peters C.L., Shirley B., Erickson J. The effect of a new multimodal perioperative anesthetic regimen on postoperative pain, side effects, rehabilitation, and length of hospital stay after total joint arthroplasty. J Arthroplasty. 2006;21(6 Suppl. 2):132. doi: 10.1016/j.arth.2006.04.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.