Key Points
Merestinib blocks Mnk kinase activity in acute myeloid leukemia cells.
Merestinib suppresses human leukemic progenitors and exhibits potent antileukemic effects in a xenograft mouse model.
Abstract
Mitogen-activated protein kinase interacting protein kinases (Mnks) play important roles in the development and progression of acute myeloid leukemia (AML) by regulating eukaryotic translation initiation factor 4E (eIF4E) activation. Inhibiting Mnk1/2-induced phosphorylation of eIF4E may represent a unique approach for the treatment of AML. We provide evidence for antileukemic effects of merestinib, an orally bioavailable multikinase inhibitor with suppressive effects on Mnk activity. Our studies show that merestinib effectively blocks eIF4E phosphorylation in AML cells and suppresses primitive leukemic progenitors from AML patients in vitro and in an AML xenograft model in vivo. Our findings provide evidence for potent preclinical antileukemic properties of merestinib and support its clinical development for the treatment of patients with AML.
Introduction
Aberrant activation of multiple signaling pathways has been implicated in the pathogenesis of acute myeloid leukemia (AML).1,2 The selective targeting of these pathways could improve the outcome of the currently available, generally unsatisfactory, treatments for patients with AML.3-5 The mitogen-activated protein kinase (MAPK) pathways regulate multiple cellular processes including leukemic cell proliferation, differentiation, and apoptosis.1,6 Two key effectors of MAPK pathways are the MAPK interacting protein kinases 1 and 2 (Mnk1/2), which are activated downstream of MAP kinases and regulate the activation of eukaryotic translation initiation factor 4E (eIF4E). eIF4E is a key component of the cap-binding complex required for mRNA translation of mitogenic proteins, including cyclins, c-Myc, and Bcl-xl, and its activity has been linked to leukemogenesis and malignant cell proliferation.7-9 The phosphorylation and activation of eIF4E by Mnk1/2 on serine 209 (Ser209) is critical for its oncogenic activity.10,11 As Mnk1/2 double knockout mice have a normal phenotype,12 Mnk1/2 are attractive targets for cancer therapy as their inhibition could conceivably target selectively malignant cells.
Merestinib, an orally bioavailable small-molecule multikinase inhibitor, suppresses Mnk1/2 activity13 and inhibits tumor growth and metastasis in models of non–small lung cancer.14,15 In this study, we investigated whether merestinib has antileukemic properties. For this purpose, we used in vitro and in vivo models of AML.
Study design
The MV4-11 human leukemia cell line was obtained from ATCC. MM6 cells were purchased from DSMZ. Peripheral blood or bone marrow from patients with AML were collected after obtaining written informed consent as approved by the institutional review board of Northwestern University. Merestinib (LY2801653) was from Eli Lilly and Company (Indianapolis, IN). All animal studies were approved by the Northwestern University Institutional Animal Care and Use Committee. Details about experimental procedures can be found in supplemental Materials and methods, available on the Blood Web site.
Results and Discussion
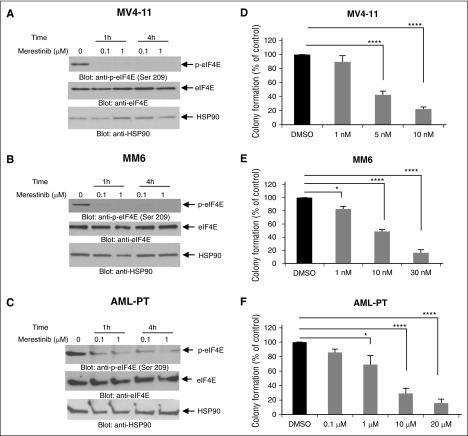
In initial studies, we examined the effects of merestinib on eIF4E phosphorylation in AML cells. Treatment of MV4-11 (Figure 1A) or MM6 (Figure 1B) cells with merestinib blocked phosphorylation of eIF4E on Ser209. Similarly, merestinib treatment decreased eIF4E phosphorylation on Ser209 in a dose- and time-dependent manner in patient-derived primary AML cells (Figure 1C). Next, to assess whether inhibition of eIF4E phosphorylation results in inhibitory effects on cap-dependent mRNA translation, polysomal fractionation analysis was carried out. Treatment with merestinib resulted in suppression of polysomal peaks (supplemental Figure 1A, left). In addition, merestinib significantly inhibited the polysomal mRNA expression of MCL-1, BCL-2, MYC,and cyclin B1 (CCNB1) in MV4-11 cells (supplemental Figure 1A, right). Consistent with these findings, MCL-1 protein levels were found to be reduced after 4-hour treatment with merestinib (supplemental Figure 1B).
Figure 1.
Merestinib blocks phosphorylation of eIF4E and suppresses growth of primary leukemic progenitors from AML patients. (A) MV4-11 cells, (B) MM6 cells, and (C) AML patient-derived cells (AML-PT) were incubated with merestinib (LY2801653) at final concentrations of either 0.1 or 1 µM for 1 and 4 hours. Cell lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with an antibody against the phosphorylated form of eIF4E on serine 209. The same blots were stripped and reprobed with an antibody against eIF4E. The immunoblots were also probed for HSP90 as a loading control. (D) MV4-11 and (E) MM6 cells were plated in clonogenic assays in methylcellulose with increasing concentrations of merestinib (LY2801653), as indicated. Data are expressed as percentage of colony formation of control vehicle-treated cells, and bar graphs represent means ± standard error (SE) of 5 independent experiments. (F) Dose-dependent suppression of primary leukemic precursors from AML patients by merestinib in clonogenic assays in methylcellulose. Data are expressed as percentage of colony formation of control vehicle-treated cells. Bar graphs represent means ± SE from 5 independent experiments, using cells from 5 different patients with AML. One-way ANOVA analysis followed by Tukey’s test was used to evaluate statistically significant differences: *P < .05, ****P < .0001.
In subsequent studies, merestinib treatment resulted in dose-dependent suppression of cell viability of MV4-11 and MM6 cells in water-soluble tetrazolium salt-1 assays (supplemental Figure 2), suggesting potent antileukemic properties. This prompted further studies, aimed to determine the effects of merestinib on primitive leukemic precursors. Merestinib-treatment resulted in potent inhibition of MV4-11 or MM6-derived leukemic progenitor colony formation (Figure 1D-E). It also resulted in inhibitory effects on primary leukemic progenitors from different patients with AML (Figure 1F). There were also suppressive effects on normal CD34+-derived colony-forming unit–granulocyte/macrophage, but these were only statistically significant at higher concentrations (supplemental Figure 3).
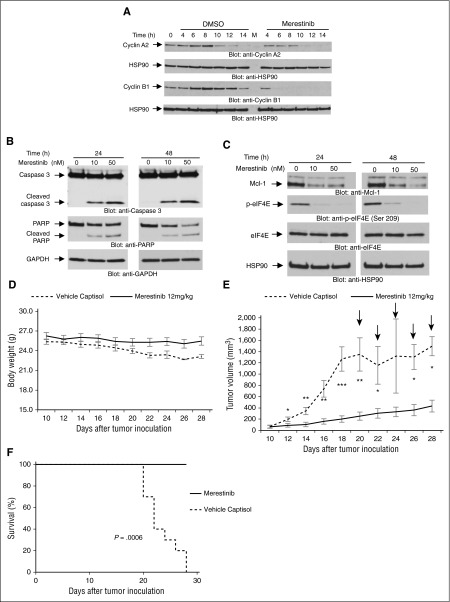
To understand the mechanisms by which this agent exhibits antileukemic properties, its effects on cell cycle progression were assessed. Short-term exposure to merestinib blocked cell cycle progression into the G2/M phase (supplemental Figure 4) and inhibited cyclin A2 and cyclin B1 protein expression in AML cells (Figure 2A), consistent with cell cycle arrest. This arrest was followed by leukemic cell apoptosis after long-term merestinib treatment and was associated with continuous suppression of eIF4E phosphorylation (Figure 2B-C; supplemental Figure 5).
Figure 2.
Antileukemic properties of merestinib in vitro and in vivo. (A) Expression of cell cycle markers in merestinib-treated MV4-11 cells. Cells were treated with or without merestinib (10 nM) for the indicated times. Whole cell lysates were evaluated by western blot analysis with the indicated antibodies. (B-C) MV4-11 cells were incubated for 24 and 48 hours in the presence or absence of merestinib (LY2801653) at the indicated doses. Whole cell lysates were analyzed by western blot with the indicated antibodies. (D-F) MM6 cells were injected subcutaneously into the left flank of nu/nu mice (n = 10). Once tumors reached a measurable size, mice were divided into control (vehicle-Captisol) and merestinib (LY2801653) (12 mg/kg)-treated groups. (D) Mice body weight was recorded throughout the study. (E) Average of tumor volumes treated with vehicle or merestinib. Data are means ± SE of tumor volumes. Mann-Whitney test was used to assess statistically significant differences between the 2 treatment groups (*P < .05, **P < .01, ***P < .001). The arrow symbols indicate that mice were killed when significant morbidity was observed as described in the Study design. (E) Kaplan-Meier survival analysis of control and merestinib-treated mice, P = .0006, using a log-rank (Mantel-Cox) test.
To determine whether merestinib exhibits antileukemic properties in vivo, its effects on an AML xenograft model were determined. No statistically significant differences were noted in body weight between vehicle and merestinib-treated mice (Figure 2D). On the other hand, merestinib-treatment potently suppressed AML tumor growth compared with the control group (Figure 2E). Additionally, daily merestinib treatment prolonged survival compared with the vehicle-treated control group (Figure 2F), establishing that this agent exhibits potent antileukemic properties in vivo.
Mnks are important components of MAPK pathways, which are constitutively activated in hematologic malignancies and play key roles in malignant hematopoietic cell survival.16,17 Mnk-dependent eIF4E phosphorylation has been associated with cancer initiation and metastasis in in vitro and in vivo tumorigenesis models,10,11 making eIF4E an attractive therapeutic target for the treatment of malignancies.18-20 We previously reported that cercosporamide, a natural antifungal agent with potent Mnk1/2 inhibitory effects, suppresses Mnk-induced phosphorylation of eIF4E in human AML cell lines and that this correlates with decreased cell proliferation/viability in vitro and in vivo.21 Others have shown that ribavirin, an antiviral guanosine analog, reduces eIF4E activity and induces responses in some patients with M4/M5 refractory AML in a clinical trial.19,22 Together, these studies support that specific targeting of Mnk-dependent eIF4E phosphorylation and/or eIF4E are potentially highly promising approaches for the treatment of AML.
In the present study, we sought to evaluate the antileukemic properties of merestinib, an orally bioavailable agent.13 This multikinase inhibitor blocks cell proliferation and tumor growth in multiple in vitro and in vivo cancer models.13-15 We report that merestinib rapidly and effectively inhibits eIF4E phosphorylation in AML cell lines and in patient-derived cells, leading to suppression of cellular proliferation and cell viability in vitro. Notably, our study demonstrates that merestinib results in negative regulatory effects on mRNA translation of genes encoding mitogenic proteins. We also demonstrate that merestinib blocks cell cycle progression, possibly by impairing the protein expression of key cell cycle regulators in G2 and M phase, such as cyclin B1.23 Moreover, we provide evidence that inhibition of eIF4E phosphorylation by merestinib is associated with induction of apoptosis and we establish that merestinib exhibits anti-leukemic properties in a human AML xenograft model. It is important to note that merestinib inhibits several other kinases, including FLT3 (FMS-like tyrosine kinase receptor-3) and the MET receptor kinase.13 FLT3 activating mutations are expressed in some AML cells,24 including MV4-11 cells that express FLT3-internal tandem duplication (ITD).25 Of the FLT3 mutations, the ITD in AML patients is associated with poor prognosis compared with patients with FLT3 wild-type gene.25-27 The function of eIF4E can be controlled by 2 major pathways that play a critical role in leukemogeniesis, the MAPK and mammalian target of rapamycin pathways,28 which can be activated downstream of FLT3 receptor.29,30 Our work demonstrates that inhibition of eIF4E results in antileukemic responses in both FLT3-mutated cell lines and FLT3 WT AML patient-derived cells. In addition, a recent study has implicated activation of MET receptor kinase as a target for the treatment of a subset(s) of AML patients.31 It remains to be seen whether this mechanism also contributes to merestinib regulatory effects, possibly by inactivation of MET-dependent engagement of the Mnk/eIF4E pathway. Independently of the precise mechanism, the current report establishes merestinib as a potent Mnk-eIF4E inhibitor with important antileukemic effects in AML progenitors and provides a rationale for clinical studies to assess the effects of this inhibitor in patients with refractory AML.
Acknowledgments
This work was supported by National Institutes of Health, National Cancer Institute grants CA155566, CA77816, and CA121192 and grant I01CX000916 from the Department of Veterans Affairs. D.S. was also supported by National Institutes of Health, National Cancer Institute training grant T32 CA080621. The Northwestern University Flow Cytometry Facility is supported by Cancer Center Support grant CA060553.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.M.K., D.S., F.E., and L.C.P. designed research; E.M.K., D.S., B.K, E.M.B., F.E., G.T.B., S.M.A., and J.K.A. performed research; E.M.K., D.S., B.K., E.M.B., F.E., F.J.G., and L.C.P. analyzed data/interpreted experimental results; and E.M.K., D.S., F.E., F.J.G., and L.C.P. wrote/edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Leonidas C. Platanias, Robert H. Lurie Comprehensive Cancer Center, 303 East Superior St, Lurie 3-125, Chicago, IL 60611; e-mail: l-platanias@northwestern.edu.
References
- 1.Gilliland DG, Tallman MS. Focus on acute leukemias. Cancer Cell. 2002;1(5):417–420. doi: 10.1016/s1535-6108(02)00081-8. [DOI] [PubMed] [Google Scholar]
- 2.Dash A, Gilliland DG. Molecular genetics of acute myeloid leukaemia. Best Pract Res Clin Haematol. 2001;14(1):49–64. doi: 10.1053/beha.2000.0115. [DOI] [PubMed] [Google Scholar]
- 3.Carneiro BA, Kaplan JB, Altman JK, Giles FJ, Platanias LC. Targeting mTOR signaling pathways and related negative feedback loops for the treatment of acute myeloid leukemia. Cancer Biol Ther. 2015;16(5):648–656. doi: 10.1080/15384047.2015.1026510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saleiro D, Platanias LC. Intersection of mTOR and STAT signaling in immunity. Trends Immunol. 2015;36(1):21–29. doi: 10.1016/j.it.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beauchamp EM, Platanias LC. The evolution of the TOR pathway and its role in cancer. Oncogene. 2013;32(34):3923–3932. doi: 10.1038/onc.2012.567. [DOI] [PubMed] [Google Scholar]
- 6.Sakamoto KM, Grant S, Saleiro D, et al. Targeting novel signaling pathways for resistant acute myeloid leukemia. Mol Genet Metab. 2015;114(3):397–402. doi: 10.1016/j.ymgme.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonenberg N, Morgan MA, Merrick WC, Shatkin AJ. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5′-terminal cap in mRNA. Proc Natl Acad Sci USA. 1978;75(10):4843–4847. doi: 10.1073/pnas.75.10.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diab S, Kumarasiri M, Yu M, et al. MAP kinase-interacting kinases—emerging targets against cancer. Chem Biol. 2014;21(4):441–452. doi: 10.1016/j.chembiol.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Joshi S, Platanias LC. Mnk kinases in cytokine signaling and regulation of cytokine responses. Biomol Concepts. 2015;6(1):85. doi: 10.1515/bmc-2011-2000. [DOI] [PubMed] [Google Scholar]
- 10.Wendel HG, Silva RL, Malina A, et al. Dissecting eIF4E action in tumorigenesis. Genes Dev. 2007;21(24):3232–3237. doi: 10.1101/gad.1604407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furic L, Rong L, Larsson O, et al. eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression. Proc Natl Acad Sci USA. 2010;107(32):14134–14139. doi: 10.1073/pnas.1005320107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S, Fukunaga R. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol Cell Biol. 2004;24(15):6539–6549. doi: 10.1128/MCB.24.15.6539-6549.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan SB, Peek VL, Ajamie R, et al. LY2801653 is an orally bioavailable multi-kinase inhibitor with potent activity against MET, MST1R, and other oncoproteins, and displays anti-tumor activities in mouse xenograft models. Invest New Drugs. 2013;31(4):833–844. doi: 10.1007/s10637-012-9912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawada I, Hasina R, Arif Q, et al. Dramatic antitumor effects of the dual MET/RON small-molecule inhibitor LY2801653 in non-small cell lung cancer. Cancer Res. 2014;74(3):884–895. doi: 10.1158/0008-5472.CAN-12-3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu W, Bi C, Credille KM, et al. Inhibition of tumor growth and metastasis in non-small cell lung cancer by LY2801653, an inhibitor of several oncokinases, including MET. Clin Cancer Res. 2013;19(20):5699–5710. doi: 10.1158/1078-0432.CCR-13-1758. [DOI] [PubMed] [Google Scholar]
- 16.Towatari M, Iida H, Tanimoto M, Iwata H, Hamaguchi M, Saito H. Constitutive activation of mitogen-activated protein kinase pathway in acute leukemia cells. Leukemia. 1997;11(4):479–484. doi: 10.1038/sj.leu.2400617. [DOI] [PubMed] [Google Scholar]
- 17.Landon AL, Muniandy PA, Shetty AC, et al. MNKs act as a regulatory switch for eIF4E1 and eIF4E3 driven mRNA translation in DLBCL. Nat Commun. 2014;5:5413. doi: 10.1038/ncomms6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topisirovic I, Guzman ML, McConnell MJ, et al. Aberrant eukaryotic translation initiation factor 4E-dependent mRNA transport impedes hematopoietic differentiation and contributes to leukemogenesis. Mol Cell Biol. 2003;23(24):8992–9002. doi: 10.1128/MCB.23.24.8992-9002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Assouline S, Culjkovic B, Cocolakis E, et al. Molecular targeting of the oncogene eIF4E in acute myeloid leukemia (AML): a proof-of-principle clinical trial with ribavirin. Blood. 2009;114(2):257–260. doi: 10.1182/blood-2009-02-205153. [DOI] [PubMed] [Google Scholar]
- 20.Kentsis A, Topisirovic I, Culjkovic B, Shao L, Borden KL. Ribavirin suppresses eIF4E-mediated oncogenic transformation by physical mimicry of the 7-methyl guanosine mRNA cap. Proc Natl Acad Sci USA. 2004;101(52):18105–18110. doi: 10.1073/pnas.0406927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altman JK, Szilard A, Konicek BW, et al. Inhibition of Mnk kinase activity by cercosporamide and suppressive effects on acute myeloid leukemia precursors. Blood. 2013;121(18):3675–3681. doi: 10.1182/blood-2013-01-477216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borden KL. Targeting the oncogene eIF4E in cancer: From the bench to clinical trials. Clin Invest Med. 2011;34(6):E315. doi: 10.25011/cim.v34i6.15889. [DOI] [PubMed] [Google Scholar]
- 23.Rhind N, Russell P. Signaling pathways that regulate cell division. Cold Spring Harb Perspect Biol. 2012;4(10):a005942. doi: 10.1101/cshperspect.a005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17(9):1738–1752. doi: 10.1038/sj.leu.2403099. [DOI] [PubMed] [Google Scholar]
- 25.Quentmeier H, Reinhardt J, Zaborski M, Drexler HG. FLT3 mutations in acute myeloid leukemia cell lines. Leukemia. 2003;17(1):120–124. doi: 10.1038/sj.leu.2402740. [DOI] [PubMed] [Google Scholar]
- 26.Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005;353(2):172–187. doi: 10.1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]
- 27.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99(12):4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 28.Kosciuczuk EM, Saleiro D, Platanias LC. Dual targeting of eIF4E by blocking MNK and mTOR pathways in leukemia [published online ahead of print April 16, 2016]. Cytokine. doi:10.1016/j.cyto.2016.01.024. [DOI] [PMC free article] [PubMed]
- 29.Hayakawa F, Towatari M, Kiyoi H, et al. Tandem-duplicated Flt3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene. 2000;19(5):624–631. doi: 10.1038/sj.onc.1203354. [DOI] [PubMed] [Google Scholar]
- 30.Chen W, Drakos E, Grammatikakis I, et al. mTOR signaling is activated by FLT3 kinase and promotes survival of FLT3-mutated acute myeloid leukemia cells. Mol Cancer. 2010;9:292. doi: 10.1186/1476-4598-9-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGee SF, Kornblau SM, Qiu Y, et al. Biological properties of ligand-dependent activation of the MET receptor kinase in acute myeloid leukemia. Leukemia. 2015;29(5):1218–1221. doi: 10.1038/leu.2014.348. [DOI] [PMC free article] [PubMed] [Google Scholar]